Abstract
Pancreatic cancer is an aggressive, drug-resistant disease; its first-line chemotherapeutic, gemcitabine, is only marginally effective. Intracellular depletion of glutathione, a major free-radical scavenger, has been associated with growth arrest and reduced drug resistance (chemosensitization) of cancer cells. In search of a new therapeutic approach for pancreatic cancer, we sought to determine whether specific inhibition of the plasma membrane xc− cystine transporter could lead to reduced uptake of cysteine, a key precursor of glutathione, and subsequent glutathione depletion. Sulfasalazine (approximately 0.2 mmol/L), an anti-inflammatory drug with potent xc−-inhibitory properties, markedly reduced l-[14C]-cystine uptake, glutathione levels, and growth and viability of human MIA PaCa-2 and PANC-1 pancreatic cancer cells in vitro. These effects were shown to result primarily from inhibition of cystine uptake mediated by the xc− cystine transporter and not from inhibition of nuclear factor κB activation, another property of sulfasalazine. The efficacy of gemcitabine could be markedly enhanced by combination therapy with sulfasalazine both in vitro and in immunodeficient mice carrying xenografts of the same cell lines. No major side effects were observed in vivo.
The results of the present study suggest that the xc− transporter plays a major role in pancreatic cancer by sustaining or enhancing glutathione biosynthesis, and as such, represents a potential therapeutic target. Sulfasalazine, a relatively nontoxic drug approved by the U.S. Food and Drug Administration, may, in combination with gemcitabine, lead to more effective therapy of refractory pancreatic cancer.
Keywords: Pancreatic cancer, xc−cystine transporter, sulfasalazine, cystine, cysteine, glutathione, gemcitabine resistance, nfκb
1. INTRODUCTION
Pancreatic cancer is one of the most aggressive and drug-resistant cancers and the fourth-leading cause of cancer-related deaths in North America 1,2. It is characterized by a lack of detectable symptoms in early stages of the disease, leading to late diagnosis and rapid development into metastatic, drug-resistant cancer 2. Although gemcitabine (gem) is the first-line chemotherapeutic for pancreatic cancer, it increases survival only marginally; most cases, if not all, are palliative 3. It is therefore crucial to identify new therapeutic targets and approaches for treatment of this deadly disease.
Glutathione is a major antioxidant considered essential for protection of cells from oxidative stress. It also plays an important role as a detoxifier and is known to underlie drug resistance 4,5. Oxidative stress is particularly generated in cancer cells because of their relatively high metabolism, and glutathione depletion has been suggested as a therapeutic approach for a variety of cancers, in particular to reduce resistance to conventional anticancer agents 5–7.
Intracellularly, glutathione is present mainly in a reduced form—that is, as a linear tripeptide thiol [consisting of glutamate, cysteine, and glycine (gsh)] with a short half-life. Cysteine is a rate-limiting precursor of gsh, and sustenance of adequate intracellular cysteine levels is critical for maintaining adequate gsh levels, cell growth, and viability 4. For some cancers, this sustenance depends on uptake of cysteine or cystine (the oxidized form of the amino acid) from the microenvironment, as indicated by the cystine/cysteine growth requirements of cell lines derived from lymphomas, gliomas, and pancreatic cancers, among others 8–12.
Evidence is increasing to suggest that the xc− plasma membrane cystine/glutamate antiporter plays an important role in the maintenance of intracellular cysteine/cystine levels by mediating direct uptake of cystine and in vivo cysteine supply by stromal cells (for example, activated macrophages, fibroblasts). Such somatic cells can use the xc− transporter to pick up cystine (the predominant extracellular form), reduce the cystine intracellularly to cysteine, and secrete surplus cysteine (which neighbouring cancer cells can readily take up using the ubiquitous ASC transport system) 13. This particular growth-promoting function of stromal cells has been well established using co-cultures of fibroblasts and lymphoma cells that do not express a cystine transporter: Acting as feeder layers, the fibroblasts supply cysteine essential for growth of the lymphoma cells 14,15. In view of these mechanisms, inhibition of the xc− transporter, leading to cystine/cysteine starvation and subsequent glutathione depletion, has been proposed as a potential therapeutic approach in a variety of cancers 7–13.
We recently observed that, in pancreatic ductal adenocarcinoma tissue from patients, the xc− transporter was overexpressed relative to normal pancreatic tissue from the same patients. We also obtained evidence that human pancreatic cancer cell lines, such as MIA PaCa-2 and PANC-1, critically depend on cystine uptake via the xc− transporter for growth and viability and that gem resistance in PANC-1 cells is associated with elevated xc− expression 11. Together, these findings suggest that specific inhibition of the xc− transporter, aimed at glutathione depletion (with subsequent growth arrest and reduced drug resistance of target cells), could provide a new approach for targeted therapy of pancreatic cancer.
In the present study, we targeted the xc− cystine transporter to examine the effect on growth and gem resistance in MIA PaCa-2 and PANC-1 cells in vitro and as xenografts in immunodeficient mice. As an xc− inhibitor, we used sulfasalazine, a well-established anti-inflammatory drug with potent xc− inhibitory properties, which is also relatively non-toxic 9,16.
2. MATERIALS AND METHODS
2.1. Materials, Animals, Cultures and Cell Viability and Proliferation Assay
Chemicals, dyes, solvents, and solutions were obtained from Sigma–Aldrich Canada (Oakville, ON) unless otherwise indicated. The BC Cancer Research Centre Animal Resource Centre, BC Cancer Agency, Vancouver, bred the 8- to 10-week-old male Rag-2M mice used in the study. Animal care and experiments were carried out in accordance with the guidelines of the Canadian Council on Animal Care. The human pancreatic cancer cell lines MIA PaCa-2 and PANC-1 were originally obtained from the American Type Culture Collection (Manassas, VA, U.S.A.) and were maintained as monolayer cultures in minimum essential medium containing 0.1 mmol/L cystine (StemCell Technologies, Vancouver, BC), supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, U.S.A.) and 3.6 g/L glucose, as previously described 11.
Cell proliferation and viability were determined by neutral red uptake assays using 96-well plates initially containing about 1000 cells per well. After a 4-hour incubation of treated cells with 100 μL 0.0025% neutral red dye in culture medium, intracellular neutral red dye was measured by absorbance at 550 nm using a VersaMax microplate reader (Molecular Devices, Sunnyvale, CA, U.S.A.), as previously described 11.
2.2. Drug Preparations, xc− Function, and Glutathione Assays
Gemcitabine (Eli Lilly and Company, Indianapolis, IN, U.S.A.) was dissolved in 0.9% NaCl (33 mmol/L) for in vitro studies and in phosphate-buffered saline [pbs (12 mg/mL)] for in vivo studies. Sulfasalazine solutions were freshly prepared and used as previously described 9. Cystine uptake activity of the Na+-independent xc− transporter in cultures was measured using a buffer solution free of Na+ ions (to exclude the contribution of Na+-dependent transporters), supplemented with 112 nmol/L l-[14C]-cystine [300 mCi/mmol (Perkin Elmer and Analytical Sciences, Waltham, MA, U.S.A.)] in the presence or absence of 1 μmol/L non-labelled amino-acid competitors (l-glutamate, l-cystine), a non-competitor (l-leucine), sulfasalazine (0.2 mmol/L) or 2-mercaptoethanol [2-ME (66 μmol/L)] for 20 minutes at 37°C, as previously described 11. Total glutathione (gsh+gssg) levels were measured using the ApoGSH GSH Colorimetric Detection Kit (BioVision, Mountain View, CA, U.S.A.) following the manufacturer’s recommendations.
2.3. Reporter Assay for Nuclear Factor κB
To determine nuclear factor κB (nfκb) activity, MIA PaCa-2 or PANC-1 cells were transfected with nfκb luciferase reporter constructs. In 24-well plates, cells were plated at 4000 cells per well and incubated overnight. They were then co-transfected overnight with 300 ng/well pGL3-empty luciferase reporter vector (control) or pGL3-nfκb promoter luciferase reporter (Promega, Madison, WI, U.S.A.) and 7.5 ng/well pRL-cytomegalovirus [internal control vector (Promega)] using 3 μL/well of ExGen 500 in vitro transfection reagent (Fermentas, Burlington, ON), according to the manufacturer’s recommendations.
One day post transfection, the medium was removed, and the cells were incubated for 3 hours with 10 ng/mL tumour necrosis factor α (tnfα—to induce nfκb activity) or with culture medium (control). The medium was then removed, and the cells were further incubated for 24 hours with various concentrations of sulfasalazine or msg, with or without 2-ME (66 μmol/L), after which the cells were washed, and nfκb luciferase activity was determined using the Dual-Luciferase Reporter Assay System (Promega), following the manufacturer’s recommendations.
2.4. In Vivo Drug Testing
PANC-1 or MIA PaCa-2 cells (3x106 cells/100 μL) were injected subcutaneously into both dorsal flanks of Rag-2M mice. The developing xenografts were allowed to grow for about 3 weeks, until the tumours reached an average volume of approximately 50 mm3. The mice were randomized into control and treatment groups (6 mice per group). For 14 days (a period considered long enough to develop significant differences in tumour volume between treated and untreated animals), the mice were treated with pbs (control), sulfasalazine (250 mg/kg intraperitoneally twice daily), gem (120 mg/kg intraperitoneally once weekly), or a combination of sulfasalazine and gem. The gem dosage was obtained from other studies 17; the sulfasalazine dosages used were previously found to be effective for rats 9. Calliper measurements were used to assess tumour volume pre- and post-treatment, using the formula
The mice were provided with food and water ad libitum, and their health was monitored daily for signs of stress, including weight loss and abnormal behaviour.
2.5. Statistical Analysis
The Student t-test was used to determine statistical significance. Results at p ≤ 0.05 were considered significant.
3. RESULTS
3.1. Role of the xc− Transporter in Cystine Uptake: Effect of Sulfasalazine
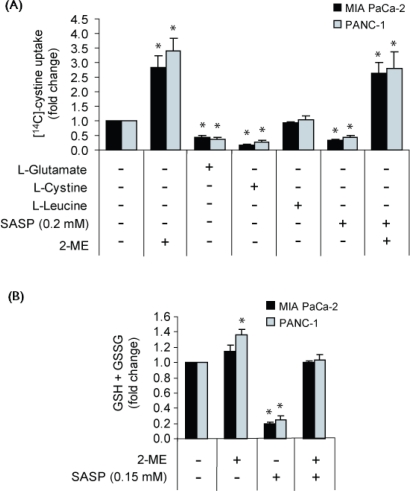
As shown in Figure 1(A), uptake of l-[14C]-cystine by MIA PaCa-2 and PANC-1 cells was markedly inhibited—to about the same extent (approximately 60%–80%)—by l-glutamate and l-cystine, basic substrates of the xc− cystine/glutamate antiporter featuring similar affinities for the transporter 18. In contrast, l-leucine, a non-substrate, had no effect on l-[14C]-cystine uptake [Figure 1(A)]. The data show that, under the Na+-free conditions used, uptake of cystine by both cell lines was primarily mediated by the xc− transporter.
Figure 1.
Effect of sulfasalazine (sasp) on xc− function and glutathione levels of MIA PaCa-2 and PANC-1 cells. (a) l-[14C]-cystine 112 nmol/L uptake by cells incubated for 24 hours under Na+-free conditions with sasp 0.2 mmol/L in the absence or presence of 2-mercaptoethanol (2-ME) 66 μmol/L, competitive substrate (1 μmol/L l-glutamate or l-cystine), or non-substrate (1 μmol/L l-leucine); (B) Intracellular total glutathione (gsh+gssg) in cells incubated for 24 hours in maintenance medium (containing cystine approximately 0.1 mmol/L) with or without sasp 0.15 mmol/L and 2-ME 66 μmol/L in the combinations indicated. Data represent the mean ± standard error from three independent experiments. mM = mol/L. * p ≤ 0.05.
Sulfasalazine, used under the same conditions, also markedly decreased uptake of l-[14C]-cystine by the cells (approximately 60%–70%), similar to glutamate and cystine, indicating that its reduction of l-[14C]-cystine uptake reflected an ability to markedly inhibit the function of the xc− transporter, as previously established in other systems 9. Furthermore, sulfasalazine did not affect messenger rna expression of the xCT and 4F2hc subunits of the xc− transporter in the two cell lines (data not shown)—supporting existing evidence that the drug inhibits the function of the transporter rather than its expression.
Figure 1(A) also shows that, in cultures containing 2-ME 66 μmol/L (that is, control + 2-ME and sulfasalazine + 2-ME), l-[14C]-cystine uptake was much higher than it was in counterpart cultures containing no 2-ME. These results were expected on the basis of the well-established cystine uptake–enhancing ability of 2-ME which, at 50–100 μmol/L, allows cellular uptake of cystine via a transport system not normally used for cystine uptake. Thus, interaction of 2-ME and cystine has been reported to produce a mixed disulfide of 2-ME and cysteine that cells can take up using the L transport system; the disulfide is then split intracellularly into cysteine and 2-ME. The cysteine accumulates in the cells, but the 2-ME escapes rapidly back into the medium to again react with cystine for a continued supply of cysteine to the cells 19.
3.2. Effect of Sulfasalazine on Glutathione Levels
MIA PaCa-2 and PANC-1 cells were incubated in maintenance medium for 24 hours with 0.15 mmol/L sulfasalazine in the absence and presence of 2-ME 66 μmol/L. As shown in Figure 1(B), sulfasalazine led to a marked reduction of the glutathione levels (70%–80%), which could be completely prevented by inclusion in the medium of cystine uptake-enhancing 2-ME 66 μmol/L. At this concentration, 2-ME does not affect the chemical structure of sulfasalazine, as indicated by high-performance liquid chromatography of sulfasalazine samples treated with the thiol for extended periods (Buckley AR and colleagues. University of Cincinnati Academic Health Center. Personal communication) 12. Taken together, the results in Figure 1 indicate that sulfasalazine-induced inhibition of xc−-mediated cystine uptake can, within 24 hours, lead to a major reduction of the glutathione levels in the two cell lines.
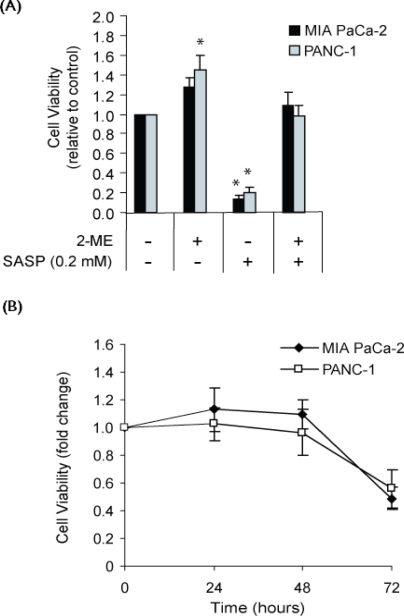
3.3. Growth-Inhibitory Effect of Sulfasalazine
Sulfasalazine inhibited in vitro growth of MIA PaCa-2 and PANC-1 cell lines (measured using neutral red uptake assay) at half maximal inhibitory concentrations of 0.01 mmol/L and 0.05 mmol/L respectively (data not shown), indicating that the PANC-1 cells were less sensitive to the drug. Sulfasalazine at 0.2 mmol/L, a patient-tolerated plasma concentration 20, markedly decreased cell viability of MIA PaCa-2 and PANC-1 cell lines—that is, by 87% and 80% respectively [Figure 2(A)]. This growth inhibition could be largely prevented by including 2-ME 66 μmol/L in the culture medium [Figure 2(A)]. The sulfasalazine-induced growth arrest therefore appears to be primarily attributable to inhibition of cystine uptake. As shown in Figure 2(B), sulfasalazine-induced loss of cell viability manifested itself after about 48 hours. It may be noted that the concentration of cystine used in the culture medium (about 0.1 mmol/L) approximates that observed in human blood plasma 21.
Figure 2.
Effect of sulfasalazine (sasp) on growth and cell viability of MIA PaCa-2 and PANC-1 cell cultures in maintenance medium (containing cystine approximately 0.1 mmol/L) as measured by neutral red uptake assay. (A) Viability of cells incubated for 72 hours with sasp 0.2 mmol/L and 2-mercaptoethanol (2-ME) 66 μmol/L in the combinations indicated. (B) Proliferation of cells incubated with sasp 0.2 mmol/L for 24, 48, and 72 hours. Data represent the mean ± standard error from three independent experiments. mM = mol/L. * p ≤ 0.05.
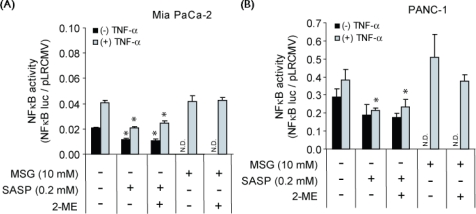
3.4. Effect of Sulfasalazine on NFκB Activation: Relationship to xc−-Inhibitory and Growth-Inhibitory Activity
Sulfasalazine is also known to inhibit activation of the nfκb transcription factor, and its growth-inhibitory activity in certain experimental systems has been related to nfκb inhibition 22 as distinct from cystine starvation 9,10. We therefore sought to determine whether growth arrest of the two cell lines by sulfasalazine [Figure 2(A)] also involved inhibition of nfκb activation.
As shown in Figure 3(A), a 24-hour incubation of MIA PaCa-2 cells (transfected with nfκb luciferase constructs) with sulfasalazine 0.2 mmol/L reduced nfκb activity by about 40%–50% for both tnfα-treated and -untreated cells. Inclusion of 2-ME 66 μmol/L in the culture medium did not prevent the sulfasalazine-induced decline in nfκb activity. Similar results were obtained with PANC-1 cells [Figure 3(B)]. These data contrast with those showing sulfasalazine-induced inhibition of cystine uptake [about 60%–70%, Figure 1(A)], reduction of glutathione levels [70%–80% after 24 hours, Figure 1(B)], and population growth [Figure 2(A)], all of which could be prevented by 2-ME 66 μmol/L because of enhancement of cystine uptake [Figure 1(A)].
Figure 3.
Activity of nuclear factor κb (nfκb) in MIA PaCa-2 and PANC-1 cells as affected by sulfasalazine (sasp) and monosodium glutamate (msg). Activity of nfκb was measured by nfκb-promoter luciferase assay in (A) MIA PaCa-2 cells and (B) PANC-1 cells, untreated or pretreated with tumour necrosis factor α (tnfα) for 3 hours to induce nfκb activation, followed by a 24-hour incubation with sasp 0.2 mmol/L or msg 10 mmol/L in maintenance medium in the absence or presence of 2-mercaptoethanol 66 μmol/L. Data represent the mean ± standard error from three independent experiments. N.D. = not determined; mM = mol/L. * p < 0.05 with respect to control.
Taken together, the results (Figures 1–3) show that cultures containing both sulfasalazine 0.2 mmol/L and 2-ME 66 μmol/L exhibited normal glutathione levels and growth despite markedly reduced nfκb activity. This finding strongly suggests that the growth inhibition of the two cell lines by sulfasalazine 0.2 mmol/L [Figure 2(A)] was not based on inhibition of nfκb activation. Furthermore, as shown in Figure 3, nfκb activation in the two cell lines was not inhibited by msg 10 mmol/L, which is a highly specific and rapid inhibitor of xc−-mediated cystine uptake 8,9; and, as previously found, msg at 4 mmol/L fully arrested growth of MIA-PaCa-2 and PANC-1 cell cultures 11. The lack of effect of msg on nfκb activation indicates that the reduction in nfκb activity by sulfasalazine 0.2 mmol/L was not related to its interference with cystine uptake—that is, the function of the xc− transporter.
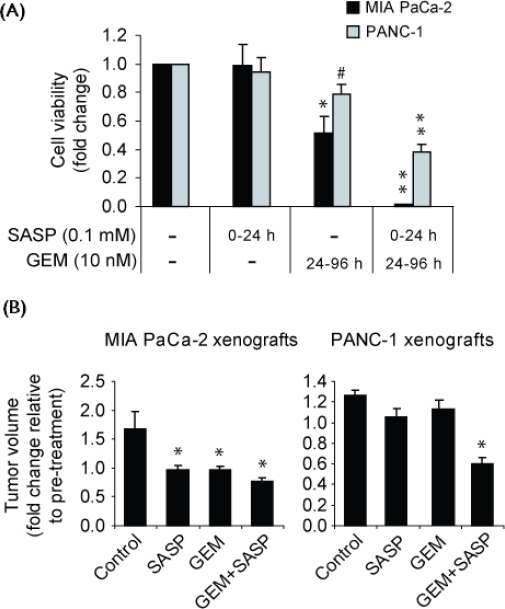
3.5. Effect of Sulfasalazine on GEM Sensitivity of Cells In Vitro and In Vivo
As shown in Figure 4(A), incubation with sulfasalazine 0.1 mmol/L alone for just 24 hours (0–24 h) did not affect survival of the cell lines as measured 72 hours later by neutral red uptake assay (at hour 96); in contrast, treatment with gem 10 nmol/L alone (24–96 h) caused significant cell death in the MIA PaCa-2 cell line, but much less in the PANC-1 cell line, previously shown to be highly resistant to gem 11 [Figure 4(A)]. The lack of effect of 24-hour treatment with sulfasalazine alone (ended by removal of the drug-containing medium) shows that the cells, which were still viable at hour 24 [Figure 2(B)], can recover following removal of the drug. Importantly, greatly reduced cell survival was evident in cultures of both cell lines that had been subjected to a 24-hour treatment with sulfasalazine 0.1 mmol/L (0–24 h), followed by a 72-hour treatment with gem 10 nmol/L (24–96 h). The growth of the MIA PaCa-2 cell line was essentially abrogated and the relatively gem-resistant PANC-1 line was greatly affected by the combined sequential use of the two drugs [Figure 4(A)]. The results indicate that treatment with sulfasalazine can increase pancreatic cancer cell sensitivity to gem in vitro.
Figure 4.
Sulfasalazine (sasp)–induced sensitization of MIA PaCa-2 and PANC-1 cells to gemcitabine (gem) in vitro and in vivo. (A) Cell viability, as measured by neutral red uptake assay, of cells incubated in vitro for 96 hours in maintenance medium while subjected to treatment with no drugs (control); sasp 0.1 mmol/L (0–24 h); gem 10 nmol/L (24–96 h); and sasp 0.1 mmol/L (0–24 h) followed by gem 10 nmol/L (24–96 h). Data represent the mean ± standard error from three independent experiments. mM = mol/L. # p ≤ 0.05, * p ≤ 0.01, and ** p < 0.001. (B) Tumour volumes of subcutaneous xenografts in four groups of Rag-2M mice (each n = 6) after 2-weeks of treatment with intraperitoneal injections of phosphate-buffered saline (control, twice daily), sasp 250 mg/kg (twice daily), gem 120 mg/kg (once weekly), or gem+sasp. * p < 0.05.
We next determined whether sulfasalazine could enhance pancreatic cancer cell sensitivity to gem in vivo. To that end, mice bearing actively growing MIA PaCa-2 and PANC-1 subcutaneous xenografts were treated for 2 weeks with pbs (control), sulfasalazine alone, gem alone, or sulfasalazine and gem in combination. All treatments were administered by intraperitoneal injection, particularly aiming to avoid intra-intestinal cleavage of sulfasalazine to sulfapyridine and 5-amino-salicylic acid 16, both of which lack xc−-inhibitory activity 9. Xenografts of MIA PaCa-2 treated with either sulfasalazine alone (twice daily) or gem alone (once weekly) showed marked inhibition of tumour growth. Significantly greater inhibition was found when the two drugs were used in combination [Figure 4(B)]. Xenografts of the gem-resistant PANC-1 cells, receiving the same protocol, were relatively resistant to treatment with gem alone and also to sulfasalazine alone; in contrast, therapy with combined sulfasalazine and gem resulted in much enhanced growth inhibition [Figure 4(B)]. These treatments in the mice did not lead to major side effects. The data indicate that sulfasalazine can act as an anticancer drug and as a chemosensitizing agent in vivo.
4. DISCUSSION
Pancreatic cancer is characterized by aggressive metastatic behaviour and resistance to conventional chemotherapy 1,2. Development of novel, more effective approaches—particularly those aimed at overcoming drug resistance—is therefore of critical importance. In a previous study, we obtained evidence suggesting that pancreatic cancer growth and viability could critically depend on uptake of cystine/cysteine from the micro-environment and that the xc− cystine/glutamate antiporter played a major role in uptake of the amino acid 11. In the present study, the function of the xc− transporter in pancreatic cancer cells was further examined, particularly in relation to sulfasalazine, which, as previously observed with lymphoma cells 9, acted as an xc− inhibitor in both MIA PaCa-2 and PANC-1 cell cultures. The high sulfasalazine-induced inhibition of l-[14C]-cystine uptake by cells in Na+-free medium was similar to that found for cystine and glutamate, two highly specific xc− inhibitors [Figure 1(A)]. The substantial sulfasalazine-induced decreases in glutathione levels and in growth and viability, which were specifically based on cystine uptake inhibition [because they could be prevented by 2-ME–enhanced cystine uptake, Figures 1(B) and 2(A)], indicate that the xc− transporter is indeed an important mediator of cystine uptake essential for the growth and viability of both studied pancreatic cancer cell lines.
Growth inhibition resulting from glutathione depletion has been observed in a variety of experimental systems, including those involving pancreatic cancer 23 and glioma 10 cells, and can be overcome by inclusion in the culture medium of glutathione ethyl ester, a membrane-permeable form of glutathione 10. The sulfasalazine-induced growth inhibition of MIA PaCa-2 and PANC-1 cell cultures [Figure 2(A)] was likely also attributable to marked reductions in the levels of glutathione, as reported for sulfasalazine-treated glioma cells 10. Thus sulfasalazine-induced loss of cell viability started after about 48 hours of incubation [Figure 2(B)], at a time when glutathione levels had been severely reduced for at least 24 hours [Figure 1(B)]. Moreover, enhancement of cellular cystine uptake by 2-ME 66 μmol/L prevented reductions both in cell viability [Figure 2(A)] and in glutathione level [Figure 1(B)]. In vivo, sulfasalazine on its own also inhibited growth of pancreatic cancer cell xenografts, in particular xenografts of MIA PaCa-2 cells [Figure 4(A)], consistent with the greater sensitivity of those cells to sulfasalazine in vitro. It may be noted that sulfasalazine treatment of xenografts of human glioma and lung cancer cells has been reported to lead to a reduction both of tumour growth and of glutathione content 10,12.
Growth arrest of cancer cells by sulfasalazine has also been reported to stem from inhibition of nfκb, a transcription factor considered to be a major protector against apoptosis 24. Sulfasalazine 0.2 mmol/L indeed reduced nfκb activity in the two studied cell lines by 40%–50% (Figure 3), but the reduced nfκb activity could not account for the sulfasalazine-induced growth arrest. Although inclusion of 2-ME 66 μmol/L in the culture medium did not prevent reduction of nfκb activity (Figure 3), it did allow for normal growth in the presence of the drug [Figure 2(A)]. That finding, and the additional finding that msg, a specific xc− inhibitor, did not affect nfκb activity (Figure 3), suggests that the action of sulfasalazine on nfκb activity was not directly related to interference with xc− transporter function. Inhibition of xc− function and inhibition of nfκb activation appear to represent two distinct mechanisms of action of sulfasalazine. Taken together, the results show that growth arrest of pancreatic cancer cells by sulfasalazine used at relatively low, patient-tolerated concentrations (for example, 0.2 mmol/L), is primarily attributable to inhibition of xc− transporter function rather than to inhibition of nfκb activation. Support for this conclusion has come from a recent publication reporting that sulfasalazine inhibits the growth of primary brain tumours independent of nfκb 25.
Glutathione depletion has also been linked to chemosensitization of various cell types 4,5,7,26, which, as indicated by the present study, may also include pancreatic cancer cells. Thus MIA PaCa-2 and gem-resistant PANC-1 cells, both showing glutathione depletion after a 24-hour treatment with sulfasalazine [Figure 1(B)], were more sensitive in vitro to gem when pretreated with sulfasalazine than when no pretreatment occurred [the MIA PaCa-2 cells in particular, Figure 4(A)]. Sulfasalazine-induced sensitization was also apparent in vivo and markedly reduced the gem resistance of the PANC-1 cell xenografts [Figure 4(B)]. It is not clear why the MIA PaCa-2 cells in vivo were not as responsive to sulfasalazine-induced sensitization as they were in vitro (Figure 4).
The basis for the sulfasalazine-induced sensitization of pancreatic cancer cells to gem is not clearly understood. Apparently, gem neither forms conjugates with glutathione, nor is it detoxified by this tripeptide thiol. However, there is evidence that gem can cause reactive oxygen intermediates (rois) in pancreatic cancer cells 27. Elimination of such rois by glutathione could contribute to the drug resistance of the cells. In such a case, sulfasalazine-induced reduction of glutathione levels could lead to reduced drug resistance.
Sensitization of pancreatic cancer cells by sulfasalazine has also been reported by other researchers. Enhancement of etoposide sensitivity, for example, has been investigated in relation to sulfasalazine as an inhibitor of nfκb activity 28. Drug resistance has been linked to elevated glutathione levels, but it has also been associated with constitutive nfκb activity 24, and chemosensitization by sulfasalazine could involve not only glutathione depletion but also nfκb inhibition.
5. CONCLUSIONS
The results of the present study suggest that the xc− transporter plays a major role in pancreatic cancer as a mediator of cystine uptake for the biosynthesis of glutathione important for cell growth, viability, and drug resistance. As such, the xc− transporter represents a potential therapeutic target in this disease. Sulfasalazine, a relatively nontoxic drug approved by the U.S. Food and Drug Administration, may, in combination with gem, lead to more effective therapy of refractory pancreatic cancer.
6. CONFLICT OF INTEREST DISCLOSURES
This study was supported by grants from the National Cancer Institute of Canada–Canadian Cancer Society (VL), the Canadian Institutes of Health Research (PWG/YZ) and the British Columbia Cancer Foundation (PWG). M. Lo held a Doctoral Research Award from the Canadian Institutes of Health Research.
7. ACKNOWLEDGMENTS
The authors thank Rebecca Wu for excellent technical assistance and Yuwei Wang, Hui Xue, and Jun Guan—all at the BC Cancer Research Centre—for helpful suggestions regarding animal work.
8. REFERENCES
- 1.Zalatnai A, Molnár J. Review. Molecular background of chemoresistance in pancreatic cancer. In Vivo. 2007;21:339–47. [PubMed] [Google Scholar]
- 2.Freelove R, Walling AD. Pancreatic cancer: diagnosis and management. Am Fam Physician. 2006;73:485–92. [PubMed] [Google Scholar]
- 3.Wolff RA. Chemotherapy for pancreatic cancer: from metastatic disease to adjuvant therapy. Cancer J. 2007;13:175–84. doi: 10.1097/PPO.0b013e318074e6c3. [DOI] [PubMed] [Google Scholar]
- 4.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27:922–35. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 5.Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43:143–81. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 6.Vanhoefer U, Cao S, Minderman H, et al. d,l-Buthionine-(S,R)-sulfoximine potentiates in vivo the therapeutic efficacy of doxorubicin against multidrug resistance protein–expressing tumors. Clin Cancer Res. 1996;2:1961–8. [PubMed] [Google Scholar]
- 7.Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Sulfasalazine-induced reduction of glutathione levels in breast cancer cells: enhancement of growth-inhibitory activity of doxorubicin. Chemotherapy. 2007;53:210–17. doi: 10.1159/000100812. [DOI] [PubMed] [Google Scholar]
- 8.Gout PW, Kang YJ, Buckley DJ, Bruchovsky N, Buckley AR. Increased cystine uptake capability associated with malignant progression of Nb2 lymphoma cells. Leukemia. 1997;11:1329–37. doi: 10.1038/sj.leu.2400739. [DOI] [PubMed] [Google Scholar]
- 9.Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the xc− cystine transporter: a new action for an old drug. Leukemia. 2001;15:1633–40. doi: 10.1038/sj.leu.2402238. [DOI] [PubMed] [Google Scholar]
- 10.Chung WJ, Lyons SA, Nelson GM, et al. Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci. 2005;25:7101–10. doi: 10.1523/JNEUROSCI.5258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo M, Ling V, Wang YZ, Gout PW. The xc− cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. Br J Cancer. 2008;99:464–72. doi: 10.1038/sj.bjc.6604485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan J, Lo M, Dockery P, et al. The xc− cystine/glutamate antiporter as a potential therapeutic target for small-cell lung cancer: use of sulfasalazine. Cancer Chemother Pharmacol. 2009;64:463–72. doi: 10.1007/s00280-008-0894-4. [DOI] [PubMed] [Google Scholar]
- 13.Lo M, Wang YZ, Gout PW. The xc− cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol. 2008;215:593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- 14.Ishii T, Hishinuma I, Bannai S, Sugita Y. Mechanism of growth promotion of mouse lymphoma L1210 cells in vitro by feeder layer or 2-mercaptoethanol. J Cell Physiol. 1981;107:283–93. doi: 10.1002/jcp.1041070215. [DOI] [PubMed] [Google Scholar]
- 15.Gout PW, Simms CR, Robertson MC. In vitro studies on the lymphoma growth-inhibitory activity of sulfasalazine. Anticancer Drugs. 2003;14:21–9. doi: 10.1097/00001813-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Klotz U. Clinical pharmacokinetics of sulphasalazine, its metabolites and other prodrugs of 5-aminosalicylic acid. Clin Pharmacokinet. 1985;10:285–302. doi: 10.2165/00003088-198510040-00001. [DOI] [PubMed] [Google Scholar]
- 17.Solorzano CC, Hwang R, Baker CH, et al. Administration of optimal biological dose and schedule of interferon alpha combined with gemcitabine induces apoptosis in tumor-associated endothelial cells and reduces growth of human pancreatic carcinoma implanted orthotopically in nude mice. Clin Cancer Res. 2003;9:1858–67. [PubMed] [Google Scholar]
- 18.Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem. 1986;261:2256–63. [PubMed] [Google Scholar]
- 19.Ishii T, Bannai S, Sugita Y. Mechanism of growth stimulation of L1210 cells by 2-mercaptoethanol in vitro. Role of the mixed disulfide of 2-mercaptoethanol and cysteine. J Biol Chem. 1981;256:12387–92. [PubMed] [Google Scholar]
- 20.Guastavino E, Litwin NH, Heffes Nahmod L, Licastro R. Ulcerative colitis in children. Levels of salicylazosulfapyridine and sulfapyridine during treatment [Spanish] Acta Gastroenterol Latinoam. 1988;18:107–13. [PubMed] [Google Scholar]
- 21.Chawla RK, Lewis FW, Kutner MH, Bate DM, Roy RG, Rudman D. Plasma cysteine, cystine, and glutathione in cirrhosis. Gastroenterology. 1984;87:770–6. [PubMed] [Google Scholar]
- 22.Robe PA, Bentires–Alj M, Bonif M, et al. In vitro and in vivo activity of the nuclear factor-κb inhibitor sulfasalazine in human glioblastomas. Clin Cancer Res. 2004;10:5595–603. doi: 10.1158/1078-0432.CCR-03-0392. [DOI] [PubMed] [Google Scholar]
- 23.Schnelldorfer T, Gansauge S, Gansauge F, Schlosser S, Beger HG, Nussler AK. Glutathione depletion causes cell growth inhibition and enhanced apoptosis in pancreatic cancer cells. Cancer. 2000;89:1440–7. [PubMed] [Google Scholar]
- 24.Olivier S, Robe P, Bours V. Can nf-κb be a target for novel and efficient anti-cancer agents? Biochem Pharmacol. 2006;72:1054–68. doi: 10.1016/j.bcp.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 25.Chung WJ, Sontheimer H. Sulfasalazine inhibits the growth of primary brain tumors independent of nuclear factor-κb. J Neurochem. 2009;110:182–93. doi: 10.1111/j.1471-4159.2009.06129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudin CM, Yang Z, Schumaker LM, et al. Inhibition of glutathione synthesis reverses Bcl-2–mediated cisplatin resistance. Cancer Res. 2003;63:312–18. [PubMed] [Google Scholar]
- 27.Ng SSW, Tsao MS, Chow S, Hedley DW. Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells. Cancer Res. 2000;60:5451–5. [PubMed] [Google Scholar]
- 28.Muerkoster S, Arlt A, Witt M, et al. Usage of the nf-κb inhibitor sulfasalazine as sensitizing agent in combined chemotherapy of pancreatic cancer. Int J Cancer. 2003;104:469–76. doi: 10.1002/ijc.10963. [DOI] [PubMed] [Google Scholar]