Abstract
In the first trimester the extravillous cytotrophoblast cells occlude the uterine spiral arterioles creating a low oxygen environment early in pregnancy, which is essential for pregnancy success. Paradoxically, shallow trophoblast invasion and defective vascular remodelling of the uterine spiral arteries in the first trimester may result in impaired placental perfusion and chronic placental ischemia and hypoxia later in gestation leading to adverse pregnancy outcomes. The hypoxia inducible factors (HIFs) are key mediators of the response to low oxygen. We aimed to elucidate mechanisms of regulation of HIFs and the role these may play in the control of placental differentiation, growth and function in both normal and pathological pregnancies. The Pubmed database was consulted for identification of the most relevant published articles. Search terms used were oxygen, placenta, trophoblast, pregnancy, HIF and hypoxia. The HIFs are able to function throughout all aspects of normal and abnormal placental differentiation, growth and function; during the first trimester (physiologically low oxygen), during mid-late gestation (where there is adequate supply of blood and oxygen to the placenta) and in pathological pregnancies complicated by placental hypoxia/ischemia. During normal pregnancy HIFs may respond to complex alterations in oxygen, hormones, cytokines and growth factors to regulate placental invasion, differentiation, transport and vascularization. In the ever-changing environment created during pregnancy, the HIFs appear to act as key mediators of placental development and function and thereby are likely to be important contributors to both normal and adverse pregnancy outcomes.
Keywords: oxygen, hypoxia, placenta, hypoxia inducible factor, trophoblast
Introduction
Placentation requires the complex regulation of trophoblast proliferation, differentiation and invasion. This enables the trophoblasts to breach the uterine luminal epithelium and colonize the endometrium and its vasculature in order to sequester a blood supply for the developing placenta. Impaired placental invasion, development and function have been implicated in several complications of pregnancy, such as unexplained miscarriage (Khong et al., 1987), pre-eclampsia, intrauterine growth restriction (IUGR) (Khong et al., 1986), placental abruption (Dommisse and Tiltman, 1992), pre-term labour with intact membranes (Kim et al., 2003), premature rupture of the membranes (Kim et al., 2002) and stillbirth (Smith et al., 2004).
In humans, endovascular cytotrophoblasts (CTBs) invade and initially plug the uterine spiral arterioles, so there is little to no maternal blood flow to the intervillous space (IVS). Paradoxically, despite oxygen being essential for late gestation placental function and fetal growth, the early placenta and embryo differentiate in a relatively low oxygen environment. However, at about 11 weeks of gestation the arteriole plugs are displaced and maternal blood flow commences (Burton et al., 1999). Extravillous CTBs (EVTs) replace the maternal endothelium and the spiral arterioles are remodelled, dramatically increasing in diameter and compliance and are no longer responsive to vasoactive molecules in the maternal circulation. This and expansion of the uterine vascular tree, permit the 12-fold increase in uterine blood flow that occurs from the non-pregnant state to provide for the demands of the growing fetus later in gestation (Martin, 1965). However, if trophoblast differentiation, invasion and/or vascular remodelling are impaired, pregnancy complications can occur. In pre-eclampsia and some miscarriages, for example, insufficient trophoblast invasion and vascular remodelling in the first trimester paradoxically promote premature blood flow into the IVS leading to reduced maternal blood flow to the placenta later in pregnancy (Khong et al., 1987; Rinkenberger et al., 1997; Zhou et al., 1998; Jauniaux et al., 2003a, b). Hypoxia is therefore implicated as a key regulator of placental morphogenesis and function. In normal pregnancy, a low oxygen environment in the placenta is physiological and necessary in the first trimester but when it occurs later in gestation it is pathological and associated with common complications of pregnancy.
Hypoxia inducible factors (HIFs) are key regulators of placental vascularization and invasion as well as trophoblast differentiation and thus are essential to placental development. It is well known that HIFs mediate the response to hypoxia, however, it is increasingly apparent that this regulation is highly complex and, furthermore, that these transcription factors can be regulated by non-hypoxic stimuli. Therefore, in addition to being potential mediators of the effects of variation in oxygen supply on placentation, HIFs may also be involved in the regulation of placental development through their interactions with hormones, growth factors and cytokines. This review will examine what is known about the regulation of placental development and function by oxygen in both normal and abnormal pregnancies. Furthermore, it will elucidate mechanisms of acute and prolonged hypoxic regulation, as well as non-hypoxic regulation, of HIFs and what role these may play in the control of placental differentiation, growth and function in both normal and pathological pregnancies. In addition, we will identify novel interactions which may regulate placental development and suggest new areas for research.
Methods
Hypoxic regulation of HIF activity in other systems has been extensively reviewed over many years. Therefore, we provide a brief overview of this area. An extensive online search of published articles on HIFs and oxygen in the placenta was undertaken. The Pubmed database was consulted using combinations of the following search terms: placenta, trophoblast, pregnancy, HIF, oxygen, hypoxia, non-hypoxic regulation, angiogenesis, invasion and transport. Where possible we have limited publications to those involving human placentation. The articles were then selected based on relevance and quality after discussion between the authors. References of selected articles were also hand searched for additional relevant citations. HIF is thought to be regulated at the post-translational level and thus articles reporting HIF nuclear localization or DNA binding were given greater weighting. However, as reported in this review, emerging evidence suggests that HIF may also be regulated at the transcriptional level and we felt that these reports, although scarce, were highly novel and worth inclusion, despite the lack of data on HIF nuclear localization or DNA binding.
Hypoxia inducible factors
HIFs are a family of basic Helix–Loop–Helix transcription factors that mediate the response to changes in cellular oxygen. HIF-1 is a heterodimer consisting of a HIF-1α subunit bound to the aryl hydrocarbon nuclear translocator (ARNT), which is also known as HIF-1β (Wang and Semenza, 1995; Safran and Kaelin, 2003). Following hypoxia-induced nuclear translocation and dimerization, HIF is able to bind to the hypoxia response element (HRE) of target genes, which has the core consensus sequence 5′-CGTG-3′, in responsive genes (Rajakumar and Conrad, 2000). HIFs mediate the expression of a wide variety of genes that play critical roles in erythropoiesis, angiogenesis, glucose transport and glycolysis (Wenger and Gassmann, 1999; Safran and Kaelin, 2003; Rocha, 2007). In mammals, three genes have been shown to encode HIF-α subunits. The expression of HIF-1α is ubiquitous whereas the expression of its paralogs, HIF-2α (Ema et al., 1997; Flamme et al., 1997; Tian et al., 1997) and HIF-3α (Gu et al., 1998) are more restricted (Wenger and Gassmann, 1999; Semenza, 2000; Daikoku et al., 2003). These data indicate that HIF-1 plays a general role in homeostasis whereas HIF-2 and HIF-3 may play more specialized roles (Wenger and Gassmann, 1999; Semenza, 2000).
Oxygen-regulated HIF stabilization
The process of HIF-1α activation has been studied extensively over the past few years and includes both enhanced protein stability and transcriptional activity. In well-oxygenated cells, HIF-1α has a half-life of <5 min (Wang et al., 1995). Under normoxic conditions the α subunit is hydroxylated at conserved proline residues (HIF-1α Pro564 and Pro402) (Ivan et al., 2001; Jaakkola et al., 2001; Masson et al., 2001) contained within a unique oxygen-dependent degradation domain that critically controls protein stability (Huang et al., 1998; Srinivas et al., 1999; Huang and Bunn, 2003; Chan et al., 2005). The enzymes promoting the hydroxylation of these residues are prolyl-4-hydroxylases (PHDs) of which there are three isoforms, PHD1, PHD2 and PHD3 (Bruick and McKnight, 2001; Epstein et al., 2001; Jaakkola et al., 2001). These enzymes have an absolute requirement for Fe(II) as a cofactor, and molecular oxygen as a co-substrate, but do not require ATP or NAD (P) (Jaakkola et al., 2001). The requirement of the PHD for molecular oxygen means that this reaction can act as an ‘oxygen sensor’ (Mole et al., 2002).
Once the proline residues of HIF-1α are hydroxylated, the von Hippel–Lindau (VHL) tumour suppressor gene product (pVHL) is able to bind (Ivan et al., 2001; Jaakkola et al., 2001; Mole et al., 2002). pVHL is then able to regulate HIF-1α proteolysis (Maxwell et al., 1999) by acting as the recognition component of an E3 ubiquitin protein ligase complex (Lisztwan et al., 1999; Cockman et al., 2000; Ohh et al., 2000) to mediate polyubiquitination and proteasomal degradation of HIF-1α under normoxic conditions (Salceda and Caro, 1997; Huang et al., 1998; Kallio et al., 1999; Tanimoto et al., 2000).
Regulation of HIF activity
The second means of HIF inhibition during normoxia involves the hydroxylation of a conserved asparagine residue (Lando et al., 2002a, b) by factor inhibiting HIF-1 (FIH-1), an asparaginyl hydroxylase (Mahon et al., 2001; Hewitson et al., 2002; Lando et al., 2002a, b). Therefore, the hypoxic regulation of HIF requires firstly the stabilization of the HIF-1α subunit and secondly its activation (Minet et al., 2000). Like other known hydroxylase enzymes, FIH-1 is an Fe(II)-dependent enzyme that uses molecular oxygen to modify its substrate (Lando et al., 2002a, b). When oxygen is available, FIH-1 hydroxylates the α subunit, inhibiting the interaction of HIF-1α and HIF-2α with the transcriptional co-activators p300/CBP whereas during hypoxia the non-hydroxylated HIF is free to bind to p300/CBP (Arany et al., 1996; Dames et al., 2002; Freedman et al., 2002; Lando et al., 2002a, b).
In addition to FIH-1, other co-factors can impact on HIF function. Specifically, p300/CBP interacting transactivator with ED-rich tail 2 (CITED2) is a negative regulator of HIF function. CITED2 actively competes with HIF-1 for p300/CBP binding (Freedman et al., 2003). Furthermore, Cited2 expression is activated through a HRE in its promoter under hypoxic conditions (Bhattacharya et al., 1999) which suggests a possible negative feedback to HIF transcriptional activity. CITED2 may play an important role in regulating HIF function during placentation as CITED2 null mice have impaired trophoblast differentiation and fetal vascularization of the placenta (Withington et al., 2006).
Placental oxygenation
There has been much debate in recent years as to whether the term hypoxia should be used when discussing the oxygen tension in the placenta. Therefore, it is important to outline how we perceive hypoxia and normoxia during placental development. For the purposes of this review we have used the term hypoxia only when the oxygen concentration is lower than that to which the placenta is normally exposed. For example, as will be discussed, the first trimester placenta normally develops in a low oxygen environment and so in this case the oxygen tension is physiologically normoxic, even though it is lower than that experienced later in gestation. This is in contrast to, for example, a term placenta that is experiencing abnormally low oxygen levels and is therefore classified as hypoxic or ischemic.
Physiologically low oxygen in early placental development
In 1987, Hustin and Schaaps reported that maternal blood flow to the IVS was not fully established until ∼ 11–12 weeks of gestation (Hustin and Schaaps, 1987). The use of Doppler ultrasound during pregnancy confirmed that there is very little blood flow within the IVS until 11–12 weeks of gestation (Foidart et al., 1992; Coppens et al., 1996) due to CTBs initially plugging the maternal spiral arterioles. This is consistent with acid-base and respiratory gas values in the fetal circulation and placental tissue during the first trimester (Jauniaux et al., 2001). At about 11 weeks of gestation the arteriole plugs are displaced and blood flow commences (Hustin and Schaaps, 1987; Burton et al., 1999). The onset of maternal blood flow is progressive, it starts at the periphery of the placenta and gradually extends towards the centre (Jauniaux et al., 2003a, b). As a result, the oxygen tension within the IVS rises from 18 mmHg (2.5%) at 8 weeks to ∼60 mmHg (8.5%) at 12 weeks (Rodesch et al., 1992; Jauniaux et al., 2000; Burton and Caniggia, 2001), resulting in a burst of oxidative stress in the placenta, particularly in the syncytiotrophoblast (STB) (Jauniaux et al., 2000). This switch to a well-oxygenated environment serves a physiological role in stimulating trophoblast differentiation. However, early onset of maternal blood flow into the IVS results in a premature increase in placental oxidative stress (Jauniaux et al., 2003a, b) and is associated with early pregnancy loss (Hustin et al., 1990; Jauniaux et al., 1994; Jauniaux et al., 2003a, b). This premature onset of blood flow occurs prior to the establishment of robust mechanisms to ameliorate oxidative stress since the placenta only contains low levels of antioxidant enzymes at 8–10 weeks of gestation (Watson et al., 1997; Watson et al., 1998). Therefore, adequate trophoblast invasion and ‘plugging’ of the uterine arterioles resulting in a low oxygen environment in early pregnancy are essential for normal embryonic and placental development.
The above data suggest that trophoblast invasion early in pregnancy determines placental oxygenation. The question then is what causes inadequate trophoblast invasion in pathological pregnancies? Indeed there are many potential regulators of trophoblast invasion including trophoblast genotype, various growth factors and cytokines and interactions with maternal immune cells. Perhaps to some extent it is also true that the oxygen environment determines optimal trophoblast invasion. Oxygen concentration is a major influence on the balance between CTB proliferation and invasion in cultured explants of early gestation human placentas.
Culturing 5–8-week-old placental villous explants in a low oxygen environment (2 or 3% O2) stimulates EVT outgrowth associated with increased trophoblast proliferation compared with explants in a well-oxygenated environment (20% O2) (Genbacev et al., 1997; Caniggia et al., 2000a, b). It has therefore been proposed that early in the first trimester (prior to 8 weeks) a low oxygen environment causes CTBs to proliferate but prevents their differentiation along the invasive pathway (Genbacev et al., 1997; Caniggia et al., 2000a, b; Caniggia and Winter, 2002). Similar conclusions were drawn from using villous explants from 8 to 14 weeks gestation cultured at 2–3, 8 or 20% oxygen (Genbacev et al., 1996; Seeho et al., 2008). In contrast, culture of placental samples from 8–10, 12–14 to 16–18 weeks gestation in 3% O2 inhibited EVT invasion, with no change in cell proliferation, and increased apoptosis at 3 and 8% oxygen compared with 20% O2 (Lash et al., 2006). Similarly, culture of 8–12 week villous explants in 1.5% oxygen resulted in fewer explants producing EVT outgrowths than those in 8% O2 and a decrease in the area of outgrowth and number of cells that these explants produced (James et al., 2006).
Utilizing a different in vitro technique involving culture of myometrial spiral arteries with fluorescently labelled EVTs, culture in 3% oxygen suppressed EVT invasion regardless of the route (interstitial or endovascular) compared with those cultured in 17% oxygen (Crocker et al., 2005). However, there was more endovascular EVT invasion than interstitial invasion in 17% oxygen. These studies demonstrate conflicting data on the role of oxygen in the regulation of trophoblast function particularly regarding trophoblast proliferation, apoptosis and invasion. This may result from differences in media composition, the substrate the samples are grown on, oxygen levels used in the experiment or the gestational age of the trophoblast. In the future we would recommend that at a minimum, researchers include a group exposed to 5–10% oxygen in all studies. This is physiological for the placenta from 10 to 12 weeks onwards and will make results from different studies easier to interpret. Having only a control group exposed to 20% oxygen, which although a standard culture condition, is actually hyperoxic, and comparing this to a low oxygen tension, such as 2%, is only relevant when comparing results with the literature and makes data much more difficult to interpret and relate to the in vivo situation.
Furthermore, some studies suggest that culture of HTR-8/SVneo cells (a human first trimester cytotrophoblast cell line) in low (1%) oxygen conditions promotes invasion of these cells when compared with culture under standard conditions (20% oxygen) (Graham et al., 1998). In contrast, there has also been a report that culture of HTR-8/SVneo cells in 2% oxygen decreases trophoblast invasion and increases cell proliferation compared with those cultured in 20% oxygen (Kilburn et al., 2000). In a similar study, the invasiveness of HTR-8/SVneo cells was increased after 24 h but inhibited by 72 h in culture (Lash et al., 2007). The presence of serum, the addition of plasminogen or other factors, the reduced oxygen levels, or the use of a different invasion assay in the Graham et al. (1998) study may account for the different results observed. Microarray and real-time RT–PCR analyses of HTR-8/SVneo cells exposed to either 20 or 1% oxygen have revealed several genes that encode proteins involved in cell migration, angiogenesis and apoptosis, as well as anti-invasive factors which are up-regulated in low oxygen (Koklanaris et al., 2006), suggesting that a low oxygen environment may ‘fine-tune’ trophoblast differentiation.
Importantly, it is difficult to relate these in vitro observations to the situation that occurs in vivo, especially when there is such an array of conflicting evidence in the literature. It is clear that in vivo, invading trophoblast cells are exposed to an oxygen gradient as they migrate from the trophoblast cell column into the maternal decidua. Invading CTBs respond to this increased oxygen availability by altering the expression of various cell adhesion molecules and proteases, promoting further migration. We suggest that future research efforts are directed toward assessing whether these current in vitro models truly reflect the environment in vivo, by matching gestational age and oxygen concentration as much as possible. Additionally, a much more detailed characterization of placental development as it occurs in vivo is required (including trophoblast differentiation, invasion, proliferation and vasculogenesis) as well as of the molecules involved in the regulation of these events and their interaction (e.g. growth factors, cytokines, oxygen levels). Of course this has proven difficult, since there is a lack of early gestation samples for which the pregnancy outcome is known.
Placental hypoxia late in pregnancy
Insufficient trophoblast invasion and defective vascular remodelling of the uterine spiral arteries in the first trimester can lead to increased rate of blood flow, which damages the villous architecture, and an increased risk of spontaneous vasoconstriction and ischemia-reperfusion injury resulting in oxidative stress (Burton et al., 2009). This causes impaired placental perfusion and hence has been assumed to result in chronic placental ischemia and hypoxia later in gestation, which is detrimental to pregnancy success and may lead to pre-eclampsia and/or IUGR, and in some cases will result in preterm delivery (Chaddha et al., 2004; Viero et al., 2004). Although the preeclamptic and IUGR placentas are thought to be hypoxic, to date there have been no direct measurements to support this assumption. Interestingly, in pregnancies at high altitude, where placental hypoxia has been considered to be exacerbated, infant birthweight is decreased, primarily due to a slowing of fetal growth in the third trimester (reviewed in Zamudio, 2003; Moore et al., 2004), and these pregnancies have an increased incidence of pre-eclampsia. Subsequently, there is also an increased incidence of perinatal and infant mortality and morbidity in pregnancies at high altitude. Importantly, placentas from uncomplicated pregnancies at high altitude show no indication of significant hypoxic stress at term compared with placentas from pregnancies at low altitude and appear to have successfully adapted to the lower oxygen atmosphere (Tissot van Patot et al., 2004; Zamudio et al., 2007a, b). It is unclear then whether these placentas can actually be considered hypoxic and it will be important in future to determine why some placentas apparently adapt to the hypoxic environment and others do not.
Thus, oxygen appears to play a critical role in the development of pregnancy complications and is a key mediator of placental development and function. During pregnancy a low oxygen environment can result in different outcomes depending on the timing of exposure in pregnancy, the severity of the hypoxic exposure and the duration. The exact mechanism by which trophoblasts sense oxygen tension is currently unclear. However, several potential pathways have been identified: these pathways often utilize redox-sensitive transcription factors, of which the HIF family is the best characterized in trophoblasts.
Expression and function of HIFs in the placenta
Both HIF-1α and HIF-2α proteins are constitutively expressed in the human placenta. In the first trimester, HIF-1α and -2α have been localized in the STB, villous CTB and fetoplacental vascular endothelium (Rajakumar and Conrad, 2000; Genbacev et al., 2001), consistent with the low oxygen environment of the placenta during the first trimester of pregnancy, but their abundance decreases significantly with gestational age (Caniggia et al., 2000a, b; Rajakumar and Conrad, 2000; Caniggia and Winter, 2002). More specifically Ietta et al. (2006) have described in detail the dynamic expression of HIF and HIF regulators during placental development. HIF-1α and VHL mRNA and protein are present throughout gestation, with levels peaking at 7–10 weeks of gestation, when the oxygen tension is low, and declining thereafter (Ietta et al., 2006).
During early pregnancy, HIF-1α can be immunolocalized to CTBs and EVTs while VHL is present in STB. By 10–12 weeks, HIF-1α disappears, coinciding with an increase in expression of VHL in the CTB and an increase in VHL:HIF-1α binding throughout the placenta (Ietta et al., 2006). The expression of ARNT and HIF-2α mRNA and protein are present and stable throughout gestation (Ietta et al., 2006). The PHD enzymes (PHD1, 2, 3) are also dynamically expressed in an oxygen dependent fashion, peaking at 10–12 weeks gestation (Ietta et al., 2006). Previous reports by others have found slightly different results. In 2000, Rajakumar et al. reported that the levels of Hif-1α mRNA in the placenta do not change across gestation, while Hif-2α mRNA abundance increases as gestation proceeds (Rajakumar and Conrad, 2000). However, it would be most pertinent to quantify HIF-1α and HIF-2α proteins and determine their cytoplasmic versus nuclear localization and DNA binding in the placenta across gestation in order to understand HIF-1 activity.
In a preliminary report examining pregnancies at high altitude, HIF-1 DNA binding (measured by enzyme-linked immunosorbent assay) and HIF-associated proteins were unchanged compared with placentae from low altitude pregnancies (Tissot van Patot et al., 2004), leading to the conclusion that there was placental adaption to the hypoxic environment. However, in a more recent and comprehensive study, HIF-1α and VHL mRNA and protein levels were significantly increased in placentae from high altitude pregnancies, whereas Hif-2α and Arnt levels are unchanged (Zamudio et al., 2007a, b). Similarly, HIF-1α and HIF-2α are over-expressed in the villous placenta of women with pre-eclampsia compared with normal term pregnancies (Rajakumar et al., 2001; Rajakumar et al., 2004), with the expression of HIF-1α in villous and extravillous trophoblast cells being similar to the expression in trophoblasts prior to exposure to increased oxygen in first trimester (Caniggia and Winter, 2002). Oxygen-dependent down-regulation of HIF-1α and -2α protein is impaired in pre-eclampsia (Rajakumar et al., 2003), likely a result of both increased formation and decreased degradation owing to proteosome dysfunction (Rajakumar et al., 2008). Consistent with this, others have reported enhanced placental expression of HIF target genes, including Vegf, in pregnancies complicated by pre-eclampsia and IUGR (Helske et al., 2001), which maybe suggestive of a vasculo-adaptative response to chronic hypoxia at the fetoplacental–maternal interface. Both HIF-1α and HIF-2α are therefore in an ideal position to regulate placental development throughout gestation in both normal and pathological pregnancies.
Differential regulation of HIF-1α and HIF-2α in acute versus prolonged hypoxia
The degradation of the HIF-α protein and its stabilization under hypoxia has been the focus of much research in the last 10 years. In particular, it is apparent that HIF-1α and HIF-2α may respond differently depending on the length and severity of hypoxic exposure. HIF-2α is expressed to a higher degree than HIF-1α in a number of endothelial, fibroblast-like and epithelial cell lines in normoxic conditions (Wiesener et al., 1998). In addition, HIF-2α is increased to a greater extent than HIF-1α following exposure to mild hypoxia (5% O2) (Wiesener et al., 1998; Holmquist-Mengelbier et al., 2006). It has now been proposed that HIF-1α stabilization in hypoxia (1% O2) is an acute response, which is diminished under prolonged periods of low oxygen exposure (reviewed in Lofstedt et al., 2007). Conversely, HIF-2α protein levels are increased under mild hypoxia (5% O2) and continue to increase with time, thereby playing an important role in mediating effects on gene expression under conditions of prolonged exposure to low oxygen. In addition, it is likely that a different set of HIF targets are up-regulated in response to acute versus prolonged hypoxia but this phenomenon is yet to be examined in the placenta.
Differential regulation of the mRNA for the α subunits has been reported in response to acute and chronic hypoxia. For instance, in rats, Hif-3α, but not Hif-1α or Hif-2α, mRNA levels increase in the cerebral cortex, hippocampus, lung, heart, liver and kidney after 2 h of in vivo hypoxic exposure but no change was seen within 30 min (Heidbreder et al., 2003). In contrast, a previous study had shown that Hif-1α mRNA in rat and mouse brain, kidney and lung was up-regulated within 30 min of hypoxia treatment in vivo, but returned to baseline after 4 h (Wiener et al., 1996). Similarly, Wang et al. showed that hypoxia induces the expression of Hif-1 mRNA in vitro but this is diminished by longer periods of hypoxia (Wang et al., 1995; Uchida et al., 2004). These studies suggest that Hif-1α mRNA is transiently increased in response to hypoxic stimuli. Consistent with this, others have reported unaltered Hif-1α mRNA levels in mice in response to 4 h of hypoxia in vivo (Wenger et al., 1996).
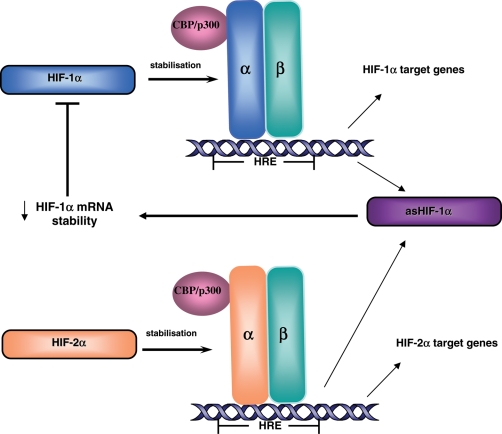
In 1999, a naturally occurring antisense transcript of HIF-1α (asHif-1α) was discovered in human renal cancer (Thrash-Bingham and Tartof, 1999). More recently, asHif-1α was identified in the kidney, liver, testis, brain, lung, spleen, heart and skeletal muscle of rodents (Rossignol et al., 2004) and specifically in the mouse decidua and trophoblasts early in gestation (Pringle et al., 2007). It has been suggested that asHIF-1α exposes AU rich elements in the 3′untranslated region of Hif-1α mRNA which decreases the stability of Hif-1α mRNA (Fig. 1), impairing HIF-1α protein translation and expression (Rossignol et al., 2002). Conversely, Hif-2α mRNA is not destabilized by asHIF-1α owing to the lack of AU rich elements (Uchida et al., 2004). In fact, Hif-2α transcription can increase in hypoxia when it increases its own promoter activity (Sato et al., 2002). Since asHif-1α contains a putative HRE it is assumed that both HIF-1 and HIF-2 are involved in the regulation of asHif-1α expression thus creating a negative feedback loop of HIF regulation under prolonged hypoxia (Fig. 1) (Rossignol et al., 2002; Uchida et al., 2004).
Figure 1.
Mechanism of HIF regulation under acute and prolonged hypoxia. Under acute hypoxia, HIF-1α and HIF-2α are stabilized and can induce the transcription of their target genes. Following exposure to prolonged hypoxia, HIF-1α and HIF-2α can up-regulate the expression of the antisense transcript of HIF-1α (asHIF-1α) which, in turn, destabilizes HIF-1α mRNA thereby reducing HIF-1α protein levels and HIF-1α mediated gene transcription. Therefore under prolonged hypoxia HIF-2α mediated transcription is more abundant. HRE: hypoxia response element.
In the placenta, some evidence of differing responses to acute versus prolonged hypoxic exposure can be found but to date no studies have examined this directly. Human placental villous explants from 5 to 8 weeks gestation, cultured at 3% oxygen demonstrated significantly greater mRNA expression of Hif-1α when compared with explants cultured at 20% oxygen, after 24 h of culture (Caniggia et al., 2000a, b). First trimester or term villous explants exposed to 2% oxygen for 3–24 h showed increases in HIF-1α and HIF-2α protein expression, with no change in their mRNA levels, and increased HIF-1 DNA binding activity (Rajakumar and Conrad, 2000). However, in cultured 6–8 week placenta it was HIF-2α protein that dramatically increased following 48 h exposure to 2% oxygen, whereas HIF-1α levels were the same in those explants cultured under hypoxic and normoxic conditions (Genbacev et al., 2001). These in vitro studies have yielded inconclusive results, which may result from the use of different oxygen tensions, culture conditions or duration of exposure.
In the future it will be important to investigate the effect of different oxygen concentrations and prolonged versus acute hypoxic exposure on HIF mRNA and protein and their targets throughout placental development in more detail. In addition, asHIF-1α expression and localization in the human placenta has yet to be elucidated. In the mouse, we have found that culture of early trophoblasts in 1% oxygen for 3 days significantly decreases Hif-2αmRNA abundance whereas Hif-1αmRNA levels remain unchanged (Pringle et al., 2007). Furthermore, our data suggest that the differential effects on Hif-1 and 2α mRNA levels may be mediated by an increase in asHif-1α mRNA in trophoblasts cultured in 1% oxygen. Research in this area may be of critical importance when considering the role of HIFs in prolonged hypoxic exposure of the placenta, such as occurs in pre-eclampsia and pregnancy at high altitude.
Non-hypoxic induction of HIF-1α
HIF-1α can also be induced by factors other than hypoxia, including hormones, cytokines and growth factors (Table I), many of which are expressed in the placenta or are increased in the maternal circulation during pregnancy. Indeed, HIF-1α protein is up-regulated in a well-oxygenated environment (∼20% oxygen) during Matrigel induced endovascular differentiation in a human EVT cell line (Fukushima et al., 2008). The mechanisms, however, are unknown.
Table I.
Non-hypoxic regulators of HIFs.
| Non-hypoxic stimuli | Action | Proposed mechanism | Found in placenta (Yes/No) | HIF target gene (Yes/No) | References |
|---|---|---|---|---|---|
| Progesterone | Increased Hif-1α mRNA, or HIF-1α and HIF-2α protein | ? | Yes | No | Daikoku et al. (2003), Song et al. (2008) |
| Estrogen | Increased Hif-2α mRNA | ? | Yes | No | Daikoku et al. (2003) |
| Prostaglandin E2 | Increased HIF-1α protein | ERK, AKT, C-SRC tyrosine kinase activity | Yes | No | Fukuda et al. (2003) |
| Angiotensin II | Increased HIF-1α mRNA and protein | AT1R, PKC, ROS activation | Yes | No | Richard et al. (2000), Page et al. (2002), Araki-Taguchi et al. (2008) |
| Thrombin | Increased HIF-1α mRNA, protein and activity | AT1R, PKC, ROS activation | Yes | No | Richard et al. (2000), Gorlach et al. (2001), Page et al. (2002) |
| Platelet derived growth factor | Increased HIF-1α protein and activity | Via ROS activation | Yes | No | Richard et al. (2000), Gorlach et al. (2001) |
| Transforming growth factor-β1 | Increased HIF-1α protein via inhibiting HIF-1α degradation | Decreased VHL mRNA and protein via Smad pathway | Yes | Yes | Gorlach et al. (2001), McMahon et al. (2006) |
| Endothelin-1 | Increased HIF-1α protein | ? | Yes | Yes | Spinella et al. (2002) |
| Epidermal Growth Factor | Increased HIF-1α protein | Via PI3K | Yes | No | Jiang et al. (2001) |
| RhoA | Increased HIF-1α protein | ? | Yes | No | Hayashi et al. (2005) |
| Mechanical stretch | Increased HIF-1α mRNA and protein | Via p42/p44 MAPK pathway | Chang et al. (2003) | ||
| Interleukin-1β | Increased HIF-1 protein | ERK 1/2 activation | Yes | Yes | Hellwig-Burgel et al. (1999), Karmakar and Das (2002), Qian et al. (2004) |
| Tumor Necrosis Factor-α | Increased HIF-1 DNA binding | Activation of HIF co-activators, NFκB pathway | Yes | No | Zhou and Bondy (1992), Hellwig-Burgel et al. (1999), Huber et al. (2006) |
| Insulin | Increased Hif-3α mRNA, HIF-1α and HIF-3α protein | Via PI3 and MAPK pathways | Yes | No | Zelzer et al. (1998), Feldser et al. (1999), Jiang et al. (2001), Heidbreder et al. (2003), Treins et al. (2002) |
| Insulin-like Growth Factor-I | Increased HIF-1α and HIF-2α protein | Via PI3 and MAPK pathways or through PHD2 | Yes | No | Zelzer et al. (1998), Feldser et al. (1999), Chavez and LaManna (2002), Richard et al. (2000) |
| Insulin-like Growth Factor-II | Increased HIF-1α protein, decreased Hif-2α mRNA | Via Mdm2 and decreased p53 | Yes | Yes | Feldser et al. (1999), Kwon et al. (2004), Pringle et al. (2007) |
Hif-1α mRNA can be regulated by progesterone in the mouse uterus whereas estrogen has the ability to regulate Hif-2α mRNA (Daikoku et al., 2003). In sheep, progesterone treatment increases HIF-1α and HIF-2α protein but not mRNA in endometrial luminal and superficial glandular epithelium (Song et al., 2008). Similarly, exposure of colon cancer cells to prostaglandin E2 (PGE2) induces the expression of vascular endothelial growth factor (VEGF) via increased HIF-1α (Fukuda et al., 2003). HIF-1α protein levels in vascular smooth muscle cells are strongly increased in normal oxygen conditions when cells are stimulated with angiotensin II (Ang II), thrombin, platelet derived growth factor (PDGF) or Transforming Growth Factor-β1 (TGF-β1) (Richard et al., 2000; Gorlach et al., 2001; Page et al., 2002). More recently, it was found that TGF-β1 decreases mRNA and protein levels of PHD2, through the Smad signalling pathway, thereby inhibiting the degradation of HIF-1α (McMahon et al., 2006). Other non-hypoxic inducers of HIF-1α include Endothelin 1 (Spinella et al., 2002), epidermal growth factor (Jiang et al., 2001), Rho A which regulates actin stress fibres (Hayashi et al., 2005) and even cyclical mechanical stretch (Chang et al., 2003).
Of particular interest is a recent study demonstrating that in first trimester placental villous explants Ang II mimics the effects of hypoxia by increasing HIF-1α mRNA and protein (Araki-Taguchi et al., 2008). In addition, Ang II increased EVT outgrowth and the number of cells in CTB cell columns and increased Ki67 suggesting an increase in proliferation (Araki-Taguchi et al., 2008). Ang II has also been shown to increase plasminogen activator inhibitor (PAI)-1 synthesis and secretion in human trophoblasts through the type 1 angiotensin receptor (AT1R) and is associated with decreased trophoblast invasion (Xia et al., 2002; Araki-Taguchi et al., 2008). Interestingly, it has been proposed that Ang II and the renin angiotensin system may play a role in the development of pre-eclampsia. Ang II levels in the placental bed are significantly elevated in women with pre-eclampsia (Anton et al., 2009), and may act on the placental villi, which display an up-regulation of AT1Rs (Anton et al., 2008),
Cytokines also regulate HIF in normoxia. For example, interleukin (IL)-1β and tumour necrosis factor (TNF)-α increased HIF-1 DNA binding in human hepatoma cells (Hellwig-Burgel et al., 1999). IL-1β increased HIF-1α nuclear protein, whereas the positive effect of TNF-α on HIF-1 DNA binding was suggested to result from activation of HIF co-activator proteins (Hellwig-Burgel et al., 1999). Similarly, a more recent study found that IL-1β induces HIF-1α-mediated VEGF secretion in normal human trophoblast cells and this may be a result of ERK 1/2 activation (Qian et al., 2004). Interestingly, matrix metalloproteases (MMP-2, MMP-9) and urokinase plasminogen activator (uPA), which are abundantly expressed by CTBs and degrade the extracellular matrix (ECM), were also up-regulated by IL-1β in human trophoblasts (Karmakar and Das, 2002). In contrast, HIF-1α protein accumulation induced by TNFα requires the NFκB pathway (Zhou et al., 2003). In addition, TNFα inhibits trophoblast migration through elevation of PAI-1 in first trimester villous explant cultures (Bauer et al., 2004; Huber et al., 2006) likely by activation of NFκB (Huber et al., 2006). Indeed a recent review by Redman and Sargent (2009) emphasizes the role of inflammatory stimuli in HIF accumulation. This may be important in both normal pregnancies, where a systemic inflammatory response is evident, and in pathological pregnancies, such as pre-eclampsia, where inflammation is exaggerated. To date there have been few studies on the role of HIF in the placental inflammatory response but research in this area may provide novel insights in the future.
Although insulin-like growth factor (IGF)-II is known to be a target gene of HIF-1 (Feldser et al., 1999), insulin, IGF-I and IGF-II are all able to induce HIF-1α protein expression in various cell lines (Zelzer et al., 1998; Feldser et al., 1999; Jiang et al., 2001; Chavez and LaManna, 2002; Treins et al., 2002; Thomas and Kim, 2008; Alam et al., 2009). Similarly, both insulin and 2-deoxy-d-glucose treatment resulted in a widespread increase in HIF-3α mRNA and protein (Heidbreder et al., 2007). IGF-I can also induce VEGF expression in human osteoblast-like cells through transcriptional activation involving the HIF-2α/ARNT complex (Akeno et al., 2002). However, the magnitude of the increase in HIF protein induced by insulin or IGFs in vitro is far less than that caused by hypoxia. In contrast, IGF-II treatment of mouse trophoblasts in vitro has been shown to decrease Hif-2α, asHif-1α, Glut-1 and Vegf mRNA levels following culture in prolonged hypoxia, but not in well oxygenated cells (Pringle et al., 2007). This suggests that the response to growth factors varies depending on the oxygen availability.
Many, if not all, of the non-hypoxic regulators of HIF protein abundance reported to date are present and active in the placenta (Table I). To date, the role of HIF in mediating their effects in the placenta has been little studied. As an initial step, it will be important in the future to identify which of these non-hypoxic regulators of HIF (outlined in Table I) are playing a similar role in the placenta. From there we can start to examine the role of non-hypoxic HIF regulation in placental development and when this may occur. This would be a novel approach to research on placental HIF and will provide exciting insights into the regulation of placental development. In addition, it is important to consider possible interactions between hypoxic and non-hypoxic HIF regulators and the effects these may have. Importantly, it will be essential to also examine the role of both hypoxic and non-hypoxic HIF regulation in pathological pregnancies, such as pre-eclampsia, and whether these interact.
Regulation of placental development and function by HIF target genes
HIF target genes may therefore be regulated by either hypoxic or non-hypoxic stimuli and many of these genes have critical roles in placental development and function. Interestingly, some HIF target genes, such as IGF-II and TGF-β, are also non-hypoxic regulators of HIF (Table I) whereas others can feed back to regulate HIF in prolonged hypoxia. Therefore, these molecules may provide novel co-regulatory responses or feedback pathways in the regulation of placental development. Many other HIF target genes have not yet been investigated in this way but further research may provide interesting insights into the complexity of interrelationships between HIF and its target genes. Here, we outline just some of the numerous roles that HIF-regulated genes have in placental development and how they could interact to regulate its differentiation, growth and function through HIF dependent or independent mechanisms.
Trophoblast invasion and migration
Hif1α −/− Hif2α −/− mice display a 17% reduction in trophoblast invasion compared with wild type placentae (Cowden Dahl et al., 2005). Several pro- and anti-invasive factors that are expressed by the trophoblast cells themselves or by the maternal decidua are HIF target genes. Furthermore, these factors seem to interact in such a way that they are able to tightly coordinate the extent and timing of trophoblast invasion and migration.
uPA system
uPA, the uPA receptor (uPAR) and uPA inhibitors play a central role in trophoblast migration and invasion. EVTs have been shown to exhibit a highly polarised distribution of uPAR-bound uPA at the invasive front (Multhaupt et al., 1994). uPA is secreted as an inactive proenzyme (pro-uPA) which, upon binding to its specific uPAR on the cell surface, is cleaved to its active form. Following activation, cell membrane bound uPA can catalyse the conversion of the inactive zymogen plasminogen, to the active proteinase plasmin (Kjoller et al., 1997) which can then go on to activate MMPs and directly degrade certain ECM components required for invasion and remodelling of the maternal decidua. This process is modulated by tissue inhibitors of matrix metalloproteinases (TIMPs) and PAI-1 which are predominantly synthesized by the decidua but also by the invading trophoblasts (Yebra et al., 1996; Floridon et al., 2000). Interestingly, the uPA:uPAR interaction can stimulate EVT migration independently of uPA catalytic action, via the mitogen activated protein kinase (MAPK) pathway and calcium signalling events that require phosphatidylinositol-3 kinase and phospholipase C (Liu et al., 2003).
Culture of HTR-8/SVneo trophoblasts less than 1% oxygen results in elevated expression of uPAR at the mRNA and cell surface bound protein levels and promotes trophoblast invasion (Graham et al., 1998). However, a more recent study has shown that culture in 3% oxygen inhibits early gestation EVT invasion, increases uPAR and PAI-2 expression and decreases uPAR activity (Lash et al., 2006). Although direct experimental evidence for HIF regulation of uPA in placenta is currently lacking, PAI-1 mRNA and protein levels have been shown to increase in HTR-8/SVneo cells in response to hypoxia via HIF-1α or HIF-2α [determined by small interfering RNA (siRNA) against HIF-1α and -2α] (Fitzpatrick and Graham, 1998; Meade et al., 2007) and may provide a mechanism for the control of trophoblast invasion.
Insulin-like growth factor-II
IGF-II is regulated by HIF (Feldser et al., 1999) among other factors and is thought to play an important role in placental development. Maternal circulating concentrations of IGF-II increase during early pregnancy in humans (Wilson et al., 1982; Gargosky et al., 1990). In addition, presumed in vivo hypoxia at term has been associated with increased levels of placental Igf2 mRNA (Trollmann et al., 2007) whereas placental IGF-II protein is elevated in preeclamptic placenta (Gratton et al., 2002). Direct evidence for HIF regulation of IGF-II in the placenta has not yet been reported but warrants further investigation. Indeed, IGF-II has been shown to promote migration of human EVT cells (Irving and Lala, 1995; Hamilton et al., 1998; McKinnon et al., 2001), increase proliferation in first trimester villous CTBs in vitro (Hills et al., 2004; Forbes et al., 2008) and decrease CTB apoptosis (Forbes et al., 2008). IGF-II induced migration of EVT cells in vitro is thought to be mediated by the IGF2R using a Gi-coupled receptor and (MAPK) intracellular signalling pathway (McKinnon et al., 2001).
The bioavailability and biological actions of the IGFs are regulated by a family of 6 IGF binding proteins (IGFBPs 1-6). Interestingly, IGFBP-1, -2 and -3 are also HIF regulated genes (Tazuke et al., 1998; Feldser et al., 1999). In blood, ∼75% of IGFs circulate as a complex with IGFBP-3 or IGFBP-5 and the acid-labile subunit, which acts as a reservoir (Mohan and Baylink, 2002). The remaining IGFs in the circulation bind to one of the remaining IGFBPs enabling them to cross the endothelium, increasing their availability to local tissues. Binding of IGFs to IGFBPs can result in either inhibition or enhancement of IGF actions. The affinity of IGFBPs for IGFs is higher than that of the receptors for IGFs, therefore binding of IGFBPs to IGFs prevents interactions with the IGF receptors (Clemmons, 1998; Baxter, 2000; Mohan and Baylink, 2002; Rosenzweig, 2004; Duan and Xu, 2005). Conversely, several studies have also shown that a number of IGFBPs are able to stimulate IGF actions in a variety of cell types. Indeed, the same IGFBP can act to enhance or inhibit IGF actions depending on the cell type, abundance of IGFBP and post-translational modifications such as glycosylation or phosphorylation, which result in altered affinity for IGFs (Baxter, 2000; Mohan and Baylink, 2002; Duan and Xu, 2005). Furthermore, IGF-II can alter the phosphorylation state of IGFBP-1 at the feto-maternal interface promoting its own actions (Westwood et al., 1997). Several IGFBPs are also associated with the cell surface or ECM, reducing their affinity for the IGFs, and hence can also modulate IGF actions (Clemmons, 1998; Baxter, 2000; Mohan and Baylink, 2002).
Transforming growth factor-β
The TGF-β isoforms are believed to be major factors regulating CTB behaviour and are commonly thought to inhibit cell proliferation and invasion (Bischof, 2001). TGFβ3 expression is induced by hypoxia both in vivo and in vitro and is directly regulated by HIF-1 in trophoblast cells (determined by siRNA knockdown of HIF-1α) (Caniggia et al., 2000a, b; Schaffer et al., 2003; Nishi et al., 2004). Interestingly, both HIF-1α and TGF-β3 are overexpressed in preeclamptic placentae (Nishi et al., 2004). However, during normal pregnancy, the decidua is the major source of TGF-β. TGF-β1 inhibits EVT invasion via the RhoA/Rho-associated kinase (ROCK) pathway (Fafet et al., 2008) by up-regulating integrin expression (Irving and Lala, 1995) and reducing uPA activity in vitro via decreasing the secretion of uPA and increasing the production and secretion of PAI-1 (Graham, 1997; Fitzpatrick and Graham, 1998). Similarly, TGF-β1 can up-regulate TIMP-1 and -2, PAI-1 and -2 (Karmakar and Das, 2002) and deposition of ECM components (Feinberg et al., 1994) and adhesive cell surface molecules (Meisser et al., 1999; Karmakar and Das, 2004) in human trophoblasts in vitro. TGF-β1 is also known to inhibit steroidogenesis in human trophoblasts (Luo et al., 2002) and promotes syncytium formation in isolated first trimester trophoblast primary cultures by increasing surface expression of cadherin-11 (Getsios et al., 1998). In contrast to this, first trimester villous explants, exposed to either antisense TGF-β3 oligonucleotide or TGF-β3 antibody (but not those for TGF-β1 or TGF-β2), displayed prominent EVT outgrowth from the distal end of the villous tip and an increased number of cells migrating into the surrounding matrix (Caniggia et al., 1999).
TGF-β is secreted in a latent pro-form in complex with a latency associated peptide (LAP) and has the ability to bind the IGF2 receptor (IGF2R) (via M6P residues in the LAP) which interacts with the uPAR on the cell surface (Godar et al., 1999). uPA binding to uPAR allows uPA to cleave plasminogen into plasmin which can then go on to cleave the LAP, and active TGF-β1 is released. This provides a novel interaction between the uPA, IGF-II, TGF-β and HIF systems whereby, depending on the relative abundance of IGF-II and/or TGF-β, the uPAR/IGF2R interaction can be utilized to either promote or inhibit trophoblast migration and invasion. Importantly, hypoxia-induced HIF regulation of IGF-II, TGF-β, PAI-1, uPAR and uPA will also impact on this system and ultimately, trophoblast behaviour. In addition, non-hypoxic regulation of HIFs by IGF-II and TGF-β may provide additional feedback pathways.
Placental vascularization
It is thought that the low oxygen environment in the placenta controls CTB vascular remodelling and acts as a major regulator of placental angiogenesis. Indeed, Hif1α −/− Hif2α −/− mice have impaired placental vascularization (Cowden Dahl et al., 2005). The VEGFs are key molecules involved in angiogenesis. The VEGF family consists of VEGF (VEGF-A), VEGF-B, VEGF-C, VEGF-D, VEGF-E and placental growth factor (PlGF), the latter of which is closely related to VEGF-A. There are three VEGF family receptors, VEGFR-1 (Flt-1), VEGFR-2 (KDR, called Flk-1 in mice) and VEGFR-3, as well as a soluble form of Flt-1 (sFlt-1) that is generated by alternative splicing and lacks transmembrane and cytoplasmic domains (Kendall and Thomas, 1993). VEGF is thought to act locally via its receptors, VEGFR-1 and VEGFR-2, to establish the fetoplacental circulation. VEGF is hypoxia inducible and is a well-known HIF target gene (Wang and Semenza, 1995). Circulating sFlt-1 binds VEGF and PlGF with high affinity thereby decreasing their availability for the transmembrane receptors VEGFR-1 and VEGFR-2. Although not members of the VEGF family, the angiopoietins are also involved in placental vasculogenesis through their interaction with tyrosine kinase receptor (Tie-2) and are influenced by oxygen availability (Zhang et al., 2001). It appears that rather than solely their concentration at the feto-maternal interface, the ratio and interaction of VEGF family members, angiopoietins and growth factors orchestrate the development of the placental vasculature and all in all, this is influenced by oxygen (Charnock-Jones and Burton, 2000).
Placental villous and extravillous trophoblasts secrete sFlt-1 during pregnancy (Clark et al., 1998) and sFlt-1 levels in the maternal circulation increase with increasing gestational age (Koga et al., 2003). Interestingly, sFlt-1 mRNA levels and sFlt-1 secretion into culture media are up-regulated following exposure to a low oxygen environment in villous explants from first trimester and uncomplicated and preeclamptic pregnancies at term (Rajakumar et al., 2009). HIF-1α knockdown using antisense oligonucleotides has demonstrated that this up-regulation is mediated by HIF-1α in first trimester villous explants (Nevo et al., 2006). In addition, placental sFlt-1 is up-regulated in pre-eclampsia, leading to increased maternal circulating concentrations of sFlt-1 that precede symptoms and fall after delivery (Koga et al., 2003; Maynard et al., 2003; Rajakumar et al., 2005; Rajakumar et al., 2007; Rajakumar et al., 2009).
Recently, multiple isoforms of sFlt-1 have been identified in the circulation of women with pre-eclampsia as well as in uncomplicated pregnancies (Rajakumar et al., 2009). Vegf and its receptor Flt-1 mRNA levels are also increased in preeclamptic placenta compared with controls (Tsatsaris et al., 2003; Rajakumar et al., 2004; Rajakumar et al., 2005; Rajakumar et al., 2007). Conversely, membrane bound Flt-1 is decreased in the placental bed of patients with pre-eclampsia (Tsatsaris et al., 2003). Pre-eclampsia is associated with an increase in total circulating VEGF, but decreased free VEGF and PlGF a result of increased sFlt-1 (Maynard et al., 2003; Tsatsaris et al., 2003) and this is thought to contribute to endothelial dysfunction seen in pre-eclampsia. Indeed, administration of sFlt-1 to pregnant rats induces hypertension, proteinuria and glomerular endotheliosis (Maynard et al., 2003). Of considerable interest is the observation that experimental induction of utero-placental insufficiency (ischaemia with restricted transport function) by uterine artery ligation in baboons resulted in a pre-eclampsia-like syndrome with hypertension and proteinuria and, notably, elevated placental and maternal circulating monocyteFlt-1 mRNA and increased sFlt-1 protein in plasma (Makris et al., 2007).
Recently, sFlt-1 was shown to be up-regulated in pregnant mice lacking catechol-O-methyltransferase (Comt −/− mice) (Kanasaki et al., 2008). COMT generates 2-methoxyoestradiol (2-ME) from hydroxyoestradiol, a potent inhibitor of angiogenesis (Fotsis et al., 1994; Mabjeesh et al., 2003). These Comt −/− pregnant mice display a pre-eclampsia-like phenotype, including endothelial damage in the decidual arteries and renal glomeruli, gestational hypertension and proteinuria (Kanasaki et al., 2008). Importantly, this pre-eclampsia-like phenotype is restored by the administration of 2-ME. This is in accordance with human preeclamptic pregnancies which display decreased levels of 2-ME and COMT (Kanasaki et al., 2008). Additionally, Comt −/− mice display signs of hypoxia (as determined by hypoxyprobe localization), elevated nuclear HIF-1α in the placenta and increased circulating sFlt-1, which was prevented by 2-ME administration. 2-ME is a negative regulator of HIF-1α (Mabjeesh et al., 2003) and was further shown to suppress hypoxia-induced HIF-1α and sFlt-1 in HTR-8 trophoblast cells (Kanasaki et al., 2008). This very elegant study by Kanasaki et al. provides very strong evidence that disruption of COMT/2-ME resulting in elevated HIF-1α and subsequent sFlt-1 leads to angiogenic dysfunction, placental insufficiency and ultimately, the preeclamptic phenotype in mice.
Placental transport
Glucose transport is up-regulated by hypoxia in a number of cell types and is crucial for fetoplacental growth. In term placental villous explants, in vitro hypoxic exposure increases Glut1 and Glut3 mRNA levels and significantly increases glucose consumption (Esterman et al., 1997). It was subsequently shown that hypoxic induction of Glut1 mRNA and protein in human BeWo and rat Rcho-1 trophoblast cell lines was a result of HIF-1α interacting with a consensus HRE site in the Glut-1 promoter (Hayashi et al., 2004). Furthermore, in human BeWo cells and first trimester explants, hypoxic induction of GLUT1 and GLUT3 was inhibited by asHIF-1α, and culture in 3% oxygen was shown to increase transepithelial glucose transport (Baumann et al., 2007). Paradoxically, expression of placental GLUT1 is decreased in pregnancies at high altitude (Zamudio et al., 2006).
The placental barrier is also a site of iron transport and recently hypoxia and HIF have been implicated in this process. Iron in serum is mainly transported by transferrin (Dunn et al., 2007) which interacts with the transferrin receptor (TfR) and this complex is internalized by receptor-mediated endocytosis, enabling iron to enter the cell. It has been previously shown that transferrin is up-regulated in response to hypoxia and this is mediated by HIF in hepatoma cells (Rolfs et al., 1997). Similarly, culture of human Hep3B hepatoma cells increases TfR mRNA expression via HIF-1 dependent mechanisms (Tacchini et al., 1999). Surprisingly, expression of TfR is decreased in the placentae of pregnancies at high altitude (Zamudio et al., 2006) and in pregnancies complicated by pre-eclampsia (Khatun et al., 2003). However, there is currently no direct evidence for HIF regulation of tranferrin or TfR in the placenta. There are very conflicting results between in vitro and in vivo studies regarding the effect of hypoxia on GLUTs and TfR in the placenta, perhaps, in part, because of the chronic exposure to hypoxia in pregnancies at high altitude compared with the short-term in vitro experiments. Additionally, other factors involved in the regulation of these transporters in vivo may be missing from the in vitro system, which may warrant further investigation.
The future of placental HIF research
While oxygen regulation of placental development and function and the role of HIFs in these processes is an area of keen interest, the currently available data are highly confusing. In vitro studies examining the effects of oxygen are all diverse in methodology in terms of media composition, cell line versus primary trophoblasts, first trimester versus term placental samples, presence or absence of ECM substrate and timing of exposure. Most studies use 20% oxygen, which is hyperoxic, as a control or comparison group and is irrelevant when considering the physiology of the placenta and all but a few tissues for that matter: it is only useful in attempts to compare it with previous observations in the literature. How then can these data be truly interpreted and related to the in vivo situation? It is clear that what is needed is a systematic study looking at the effects of low oxygen (1–3%) compared with physiological control (5–10%) using placental villi from different gestational ages. In addition, given that the effects of prolonged versus acute hypoxia in the placenta are yet to be explored, it will be of great interest to examine this in the future.
Secondly, publication of articles that only report HIF mRNA or protein levels will no longer be sufficient. Ideally, studies examining the regulation of HIF should, where possible, include data on mRNA, protein and DNA binding for both HIF-1 and HIF-2. It is only when more data, such as these for placenta, are available that we will be able to draw accurate conclusions about the role of HIFs in placental biology. In addition, the regulation of HIFs is complex, and involves factors influencing stability, degradation and transcriptional activity of the HIFs. Investigation of other components of the HIF regulatory network, such as asHIF-1α, may offer valuable insights into placental HIF regulation in both normal and pathological pregnancies. Finally, HIF can no longer be considered simply as a transcription factor which is regulated by hypoxia. Future studies examining the regulation of HIF by oxygen and other factors are essential if we are to move forward in this field.
Conclusion
The HIFs are able to function throughout all aspects of normal and abnormal placental differentiation, growth and function; during the first trimester (physiologically low oxygen), during mid-late gestation (where there is adequate maternal blood supply and placental oxygenation) and in pathological pregnancies complicated by placental hypoxia/ischemia. The identity of HIFs as simply hypoxia-responsive transcription factors is an oversimplification and very misleading, as they do much more. During normal pregnancy HIFs respond to complex alterations in oxygen, hormones, cytokines and growth factors to precisely regulate placental invasion, differentiation, transport and vascularization. In the ever-changing environment created during pregnancy, the HIFs appear to act as key mediators of placental development and function and thereby are likely to be important contributors to both normal and adverse pregnancy outcomes.
Authors' Role
K.G.P., K.L.K., A.N.S.-P., J.G.T. and C.T.R. were all involved in reviewing the literature and the preparation of the manuscript.
Funding
We acknowledge funding from the National Health and Medical Research Council of Australia. Funding to pay the Open Access publication charges for this article was provided by the National Health and Medical Research Council, Australia.
References
- Akeno N, Robins J, Zhang M, Czyzyk-Krzeska MF, Clemens TL. Induction of vascular endothelial growth factor by IGF-I in osteoblast-like cells is mediated by the PI3K signaling pathway through the hypoxia-inducible factor-2alpha. Endocrinology. 2002;143:420–425. doi: 10.1210/endo.143.2.8639. [DOI] [PubMed] [Google Scholar]
- Alam H, Weck J, Maizels E, Park Y, Lee EJ, Ashcroft M, Hunzicker-Dunn M. Role of the PI3-kinase and ERK pathways in the induction of HIF-1 activity and the HIF-1 target VEGF in ovarian granulosa cells in response to follicle stimulating hormone. Endocrinology. 2009;150:915–928. doi: 10.1210/en.2008-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton L, Merrill DC, Neves LA, Stovall K, Gallagher PE, Diz DI, Moorefield C, Gruver C, Ferrario CM, Brosnihan KB. Activation of local chorionic villi angiotensin II levels but not angiotensin (1–7) in preeclampsia. Hypertension. 2008;51:1066–1072. doi: 10.1161/HYPERTENSIONAHA.107.103861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton L, Merrill DC, Neves LA, Diz DI, Corthran J, Valdes G, Stovall K, Gallagher PE, Moorefield C, Gruver C, et al. The uterine placental bed renin-angiotensin system in normal and preeclamptic pregnancy. Endocrinology. 2009;150:4316–4325. doi: 10.1210/en.2009-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki-Taguchi M, Nomura S, Ino K, Sumigama S, Yamamoto E, Kotani-Ito T, Hayakawa H, Kajiyama H, Shibata K, Itakura A, et al. Angiotensin II mimics the hypoxic effect on regulating trophoblast proliferation and differentiation in human placental explant cultures. Life Sci. 2008;82:59–67. doi: 10.1016/j.lfs.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, Livingston DM. An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci USA. 1996;93:12969–12973. doi: 10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knofler M. Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab. 2004;89:812–822. doi: 10.1210/jc.2003-031351. [DOI] [PubMed] [Google Scholar]
- Baumann MU, Zamudio S, Illsley NP. Hypoxic upregulation of glucose transporters in BeWo choriocarcinoma cells is mediated by hypoxia-inducible factor-1. Am J Physiol Cell Physiol. 2007;293:C477–C485. doi: 10.1152/ajpcell.00075.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278:E967–E976. doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Michels CL, Leung MK, Arany ZP, Kung AL, Livingston DM. Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev. 1999;13:64–75. doi: 10.1101/gad.13.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof P. Endocrine, paracrine and autocrine regulation of trophoblastic metalloproteinases. Early Pregnancy. 2001;5:30–31. [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Caniggia I. Hypoxia: implications for implantation to delivery-a workshop report. Placenta. 2001;22:S63–S65. doi: 10.1053/plac.2001.0642. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol. 1999;181:718–724. doi: 10.1016/s0002-9378(99)70518-1. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PG, Novak JF, Yanosick TB, McMaster JH. Involvement of the plasmin system in dissociation of the insulin-like growth factor-binding protein complex. Endocrinology. 1992;130:1401–1412. doi: 10.1210/endo.130.3.1371448. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Winter JL. Adriana and Luisa Castellucci Award lecture 2001. Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies—a review. Placenta. 2002;23:S47–S57. doi: 10.1053/plac.2002.0815. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Grisaru-Gravnosky S, Kuliszewsky M, Post M, Lye SJ. Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J Clin Invest. 1999;103:1641–1650. doi: 10.1172/JCI6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J Clin Invest. 2000a;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000b;21:S25–S30. doi: 10.1053/plac.1999.0522. [DOI] [PubMed] [Google Scholar]
- Chaddha V, Viero S, Huppertz B, Kingdom J. Developmental biology of the placenta and the origins of placental insufficiency. Semin Fetal Neonatal Med. 2004;9:357–369. doi: 10.1016/j.siny.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Chan DA, Sutphin PD, Yen SE, Giaccia AJ. Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha. Mol Cell Biol. 2005;25:6415–6426. doi: 10.1128/MCB.25.15.6415-6426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Shyu KG, Wang BW, Kuan P. Regulation of hypoxia-inducible factor-1 alpha by cyclical mechanical stretch in rat vascular smooth muscle cells. Clin Sci (Lond) 2003;105:447–456. doi: 10.1042/CS20030088. [DOI] [PubMed] [Google Scholar]
- Charnock-Jones DS, Burton GJ. Placental vascular morphogenesis. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:953–968. doi: 10.1053/beog.2000.0137. [DOI] [PubMed] [Google Scholar]
- Chavez JC, LaManna JC. Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: potential role of insulin-like growth factor-1. J Neurosci. 2002;22:8922–8931. doi: 10.1523/JNEUROSCI.22-20-08922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R, Charnock-Jones DS. A Vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod. 1998;59:1540–1548. doi: 10.1095/biolreprod59.6.1540. [DOI] [PubMed] [Google Scholar]
- Clemmons DR. Role of insulin-like growth factor binding proteins in controlling IGF actions. Mol Cell Endocrinol. 1998;140:19–24. doi: 10.1016/s0303-7207(98)00024-0. [DOI] [PubMed] [Google Scholar]
- Cockman ME, Masson N, Mole DR, Jaakkola P, Chang GW, Clifford SC, Maher ER, Pugh CW, Ratcliffe PJ, Maxwell PH. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel–Lindau tumor suppressor protein. J Biol Chem. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- Coppens M, Loquet P, Kollen M, De Neubourg F, Buytaert P. Longitudinal evaluation of uteroplacental and umbilical blood flow changes in normal early pregnancy. Ultrasound Obstet Gynecol. 1996;7:114–121. doi: 10.1046/j.1469-0705.1996.07020114.x. [DOI] [PubMed] [Google Scholar]
- Cowden Dahl KD, Fryer BH, Mack FA, Compernolle V, Maltepe E, Adelman DM, Carmeliet P, Simon MC. Hypoxia-Inducible Factors 1 alpha and 2 alpha Regulate Trophoblast Differentiation. Mol Cell Biol. 2005;25:10479–10491. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker IP, Wareing M, Ferris GR, Jones CJ, Cartwright JE, Baker PN, Aplin JD. The effect of vascular origin, oxygen, and tumour necrosis factor alpha on trophoblast invasion of maternal arteries in vitro. J Pathol. 2005;206:476–485. doi: 10.1002/path.1801. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Matsumoto H, Gupta RA, Das SK, Gassmann M, DuBois RN, Dey SK. Expression of hypoxia-inducible factors in the peri-implantation mouse uterus is regulated in a cell-specific and ovarian steroid hormone-dependent manner. Evidence for differential function of HIFs during early pregnancy. J Biol Chem. 2003;278:7683–7691. doi: 10.1074/jbc.M211390200. [DOI] [PubMed] [Google Scholar]
- Dames SA, Martinez-Yamout M, De Guzman RN, Dyson HJ, Wright PE. Structural basis for Hif-1 alpha/CBP recognition in the cellular hypoxic response. Proc Natl Acad Sci USA. 2002;99:5271–5276. doi: 10.1073/pnas.082121399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommisse J, Tiltman AJ. Placental bed biopsies in placental abruption. Br J Obstet Gynaecol. 1992;99:651–654. doi: 10.1111/j.1471-0528.1992.tb13848.x. [DOI] [PubMed] [Google Scholar]
- Duan C, Xu Q. Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. Gen Comp Endocrinol. 2005;142:44–52. doi: 10.1016/j.ygcen.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Dunn LL, Rahmanto YS, Richardson DR. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2007;17:93–100. doi: 10.1016/j.tcb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein ACR, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Esterman A, Greco MA, Mitani Y, Finlay TH, Ismail-Beigi F, Dancis J. The effect of hypoxia on human trophoblast in culture: morphology, glucose transport and metabolism. Placenta. 1997;18:129–136. doi: 10.1016/s0143-4004(97)90084-9. [DOI] [PubMed] [Google Scholar]
- Fafet P, Rebouissou C, Maudelonde T, Vignais ML. Opposite Effects of Transforming Growth Factor-{beta} Activation and Rho-Associated Kinase Inhibition on Human Trophoblast Migration in a Reconstituted Placental-Endometrial Coculture System. Endocrinology. 2008;149:4475–4485. doi: 10.1210/en.2008-0253. [DOI] [PubMed] [Google Scholar]
- Feinberg RF, Kliman HJ, Wang CL. Transforming growth factor-beta stimulates trophoblast oncofetal fibronectin synthesis in vitro: implications for trophoblast implantation in vivo. J Clin Endocrinol Metab. 1994;78:1241–1248. doi: 10.1210/jcem.78.5.8175984. [DOI] [PubMed] [Google Scholar]
- Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59:3915–3918. [PubMed] [Google Scholar]
- Fitzpatrick TE, Graham CH. Stimulation of plasminogen activator inhibitor-1 expression in immortalized human trophoblast cells cultured under low levels of oxygen. Exp Cell Res. 1998;245:155–162. doi: 10.1006/excr.1998.4240. [DOI] [PubMed] [Google Scholar]
- Flamme I, Frohlich T, von Reutern M, Kappel A, Damert A, Risau W. HRF, a putative basic helix–loop–helix-PAS-domain transcription factor is closely related to hypoxia-inducible factor-1[alpha] and developmentally expressed in blood vessels. Mech Dev. 1997;63:51–60. doi: 10.1016/s0925-4773(97)00674-6. [DOI] [PubMed] [Google Scholar]
- Floridon C, Nielsen O, Holund B, Sweep F, Sunde L, Thomsen SG, Teisner B. Does plasminogen activator inhibitor-1 (PAI-1) control trophoblast invasion? A study of fetal and maternal tissue in intrauterine, tubal and molar pregnancies. Placenta. 2000;21:754–762. doi: 10.1053/plac.2000.0573. [DOI] [PubMed] [Google Scholar]
- Foidart JM, Hustin J, Dubois M, Schaaps JP. The human placenta becomes haemochorial at the 13th week of pregnancy. Int J Dev Biol. 1992;36:451–453. [PubMed] [Google Scholar]
- Forbes K, Westwood M, Baker PN, Aplin JD. Insulin-like growth factor I and II regulate the life cycle of trophoblast in the developing human placenta. Am J Physiol Cell Physiol. 2008;294:C1313–C1322. doi: 10.1152/ajpcell.00035.2008. [DOI] [PubMed] [Google Scholar]
- Fotsis T, Zhang Y, Pepper MS, Adlercreutz H, Montesano R, Nawroth PP, Schweigerer L. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature. 1994;368:237–239. doi: 10.1038/368237a0. [DOI] [PubMed] [Google Scholar]
- Freedman SJ, Sun ZY, Poy F, Kung AL, Livingston DM, Wagner G, Eck MJ. Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proc Natl Acad Sci USA. 2002;99:5367–5372. doi: 10.1073/pnas.082117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman SJ, Sun ZY, Kung AL, France DS, Wagner G, Eck MJ. Structural basis for negative regulation of hypoxia-inducible factor-1alpha by CITED2. Nat Struct Biol. 2003;10:504–512. doi: 10.1038/nsb936. [DOI] [PubMed] [Google Scholar]
- Fukuda R, Kelly B, Semenza GL. Vascular endothelial growth factor gene expression in colon cancer cells exposed to prostaglandin E2 is mediated by hypoxia-inducible factor 1. Cancer Res. 2003;63:2330–2334. [PubMed] [Google Scholar]
- Fukushima K, Murata M, Hachisuga M, Tsukimori K, Seki H, Takeda S, Asanoma K, Wake N. Hypoxia inducible factor 1 alpha regulates matrigel-induced endovascular differentiation under normoxia in a human extravillous trophoblast cell line. Placenta. 2008;29:324–331. doi: 10.1016/j.placenta.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Gallicchio MA, Kaun C, Wojta J, Binder B, Bach LA. Urokinase type plasminogen activator receptor is involved in insulin-like growth factor-induced migration of rhabdomyosarcoma cells in vitro. J Cell Physiol. 2003;197:131–138. doi: 10.1002/jcp.10352. [DOI] [PubMed] [Google Scholar]
- Gargosky SE, Moyse KJ, Walton PE, Owens JA, Wallace JC, Robinson JS, Owens PC. Circulating levels of insulin-like growth factors increase and molecular forms of their serum binding proteins change with human pregnancy. Biochem Biophys Res Commun. 1990;170:1157–1163. doi: 10.1016/0006-291x(90)90514-n. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97:540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Krtolica A, Kaelin W, Fisher SJ. Human cytotrophoblast expression of the von Hippel–Lindau protein is downregulated during uterine invasion in situ and upregulated by hypoxia in vitro. Dev Biol. 2001;233:526–536. doi: 10.1006/dbio.2001.0231. [DOI] [PubMed] [Google Scholar]
- Getsios S, Chen GT, Huang DT, MacCalman CD. Regulated expression of cadherin-11 in human extravillous cytotrophoblasts undergoing aggregation and fusion in response to transforming growth factor beta 1. J Reprod Fertil. 1998;114:357–363. doi: 10.1530/jrf.0.1140357. [DOI] [PubMed] [Google Scholar]
- Godar S, Horejsi V, Weidle UH, Binder BR, Hansmann C, Stockinger H. M6P/IGFII-receptor complexes urokinase receptor and plasminogen for activation of transforming growth factor-beta1. Eur J Immunol. 1999;29:1004–1013. doi: 10.1002/(SICI)1521-4141(199903)29:03<1004::AID-IMMU1004>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Gorlach A, Diebold I, Schini-Kerth VB, Berchner-Pfannschmidt U, Roth U, Brandes RP, Kietzmann T, Busse R. Thrombin activates the hypoxia-inducible factor-1 signaling pathway in vascular smooth muscle cells: Role of the p22(phox)-containing NADPH oxidase. Circ Res. 2001;89:47–54. doi: 10.1161/hh1301.092678. [DOI] [PubMed] [Google Scholar]
- Graham CH. Effect of transforming growth factor-beta on the plasminogen activator system in cultured first trimester human cytotrophoblasts. Placenta. 1997;18:137–143. doi: 10.1016/s0143-4004(97)90085-0. [DOI] [PubMed] [Google Scholar]
- Graham CH, Fitzpatrick TE, McCrae KR. Hypoxia stimulates urokinase receptor expression through a heme protein-dependent pathway. Blood. 1998;91:3300–3307. [PubMed] [Google Scholar]
- Gratton RJ, Asano H, Han VK. The regional expression of insulin-like growth factor II (IGF-II) and insulin-like growth factor binding protein-1 (IGFBP-1) in the placentae of women with pre-eclampsia. Placenta. 2002;23:303–310. doi: 10.1053/plac.2001.0780. [DOI] [PubMed] [Google Scholar]
- Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr. 1998;7:205–213. [PMC free article] [PubMed] [Google Scholar]
- Hamilton GS, Lysiak JJ, Han VK, Lala PK. Autocrine-paracrine regulation of human trophoblast invasiveness by insulin-like growth factor (IGF)-II and IGF-binding protein (IGFBP)-1. Exp Cell Res. 1998;244:147–156. doi: 10.1006/excr.1998.4195. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Sakata M, Takeda T, Yamamoto T, Okamoto Y, Sawada K, Kimura A, Minekawa R, Tahara M, Tasaka K, et al. Induction of glucose transporter 1 expression through hypoxia-inducible factor 1alpha under hypoxic conditions in trophoblast-derived cells. J Endocrinol. 2004;183:145–154. doi: 10.1677/joe.1.05599. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Sakata M, Takeda T, Tahara M, Yamamoto T, Minekawa R, Isobe A, Tasaka K, Murata Y. Hypoxia up-regulates hypoxia-inducible factor-1{alpha} expression through RhoA activation in trophoblast cells. J Clin Endocrinol Metab. 2005;90:1712–1719. doi: 10.1210/jc.2004-1547. [DOI] [PubMed] [Google Scholar]
- Heidbreder M, Frohlich F, Johren O, Dendorfer A, Qadri F, Dominiak P. Hypoxia rapidly activates HIF-3α mRNA expression. FASEB J. 2003;17:1541–1543. doi: 10.1096/fj.02-0963fje. [DOI] [PubMed] [Google Scholar]
- Heidbreder M, Qadri F, et al. Non-hypoxic induction of HIF-3[alpha] by 2-deoxy-d-glucose and insulin. Biochem Biophy Res Commun. 2007;352:437–443. doi: 10.1016/j.bbrc.2006.11.027. [DOI] [PubMed] [Google Scholar]
- Hellwig-Burgel T, Rutkowski K, Metzen E, Fandrey J, Jelkmann W. Interleukin-1beta and tumor necrosis factor-alpha stimulate DNA binding of hypoxia-inducible factor-1. Blood. 1999;94:1561–1567. [PubMed] [Google Scholar]
- Helske S, Vuorela P, Carpen O, Hornig C, Weich H, Halmesmaki E. Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol Hum Reprod. 2001;7:205–210. doi: 10.1093/molehr/7.2.205. [DOI] [PubMed] [Google Scholar]
- Hewitson KS, McNeill LA, Riordan MV, Tian YM, Bullock AN, Welford RW, Elkins JM, Oldham NJ, Bhattacharya S, Gleadle JM, et al. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J Biol Chem. 2002;277:26351–26355. doi: 10.1074/jbc.C200273200. [DOI] [PubMed] [Google Scholar]
- Hills FA, Elder MG, Chard T, Sullivan MH. Regulation of human villous trophoblast by insulin-like growth factors and insulin-like growth factor-binding protein-1. J Endocrinol. 2004;183:487–496. doi: 10.1677/joe.1.05867. [DOI] [PubMed] [Google Scholar]
- Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H, Pietras A, Vallon-Christersson J, Borg A, Gradin K, et al. Recruitment of HIF-1 alpha and HIF-2 alpha to common target genes is differentially regulated in neuroblastoma: HIF-2 alpha promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Huang LE, Bunn HF. Hypoxia-inducible factor and its biomedical relevance. J Biol Chem. 2003;14:14. doi: 10.1074/jbc.R200030200. [DOI] [PubMed] [Google Scholar]
- Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. PNAS. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AV, Saleh L, Bauer S, Husslein P, Knofler M. TNF alpha-mediated induction of PAI-1 restricts invasion of HTR-8/SVneo trophoblast cells. Placenta. 2006;27:127–136. doi: 10.1016/j.placenta.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Hustin J, Schaaps JP. Echographic and anatomic studies of the maternotrophoblastic border during the first trimester of pregnancy. Am J Obstet Gynecol. 1987;157:162–168. doi: 10.1016/s0002-9378(87)80371-x. [DOI] [PubMed] [Google Scholar]
- Hustin J, Jauniaux E, Schaaps JP. Histological study of the materno-embryonic interface in spontaneous abortion. Placenta. 1990;11:477–486. doi: 10.1016/s0143-4004(05)80193-6. [DOI] [PubMed] [Google Scholar]
- Ietta F, Wu Y, Winter J, Xu J, Wang J, Post M, Caniggia I. Dynamic HIF-1alpha regulation during human placental development. Biol Reprod. 2006;75:112–121. doi: 10.1095/biolreprod.106.051557. [DOI] [PubMed] [Google Scholar]
- Irving JA, Lala PK. Functional role of cell surface integrins on human trophoblast cell migration: regulation by TGF-beta, IGF-II, and IGFBP-1. Exp Cell Res. 1995;217:419–427. doi: 10.1006/excr.1995.1105. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al. Targeting of HIF-alpha to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- James JL, Stone PR, Chamley LW. The effects of oxygen concentration and gestational age on extravillous trophoblast outgrowth in a human first trimester villous explant model. Hum Reprod. 2006;21:2699–2705. doi: 10.1093/humrep/del212. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Zaidi J, Jurkovic D, Campbell S, Hustin J. Pregnancy: Comparison of colour Doppler features and pathological findings in complicated early pregnancy. Hum Reprod. 1994;9:2432–2437. doi: 10.1093/oxfordjournals.humrep.a138465. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E, Watson A, Burton G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks' gestation. Am J Obstet Gynecol. 2001;184:998–1003. doi: 10.1067/mob.2001.111935. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Greenwold N, Hempstock J, Burton GJ. Comparison of ultrasonographic and Doppler mapping of the intervillous circulation in normal and abnormal early pregnancies. Fertil Steril. 2003a;79:100–106. doi: 10.1016/s0015-0282(02)04568-5. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Hempstock J, Greenwold N, Burton GJ. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am J Pathol. 2003b;162:115–125. doi: 10.1016/S0002-9440(10)63803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 2001;12:363–369. [PubMed] [Google Scholar]
- Kallio PJ, Wilson WJ, O'Brien S, Makino Y, Poellinger L. Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. J Biol Chem. 1999;274:6519–6525. doi: 10.1074/jbc.274.10.6519. [DOI] [PubMed] [Google Scholar]
- Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–1121. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- Karmakar S, Das C. Regulation of trophoblast invasion by IL-1beta and TGF-beta1. Am J Reprod Immunol. 2002;48:210–219. doi: 10.1034/j.1600-0897.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- Karmakar S, Das C. Modulation of ezrin and E-cadherin expression by IL-1[beta] and TGF-[beta]1 in human trophoblasts. J Reprod Immunol. 2004;64:9–29. doi: 10.1016/j.jri.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatun R, Wu Y, Kanenishi K, Ueno M, Tanaka S, Hata T, Sakamoto H. Immunohistochemical Study of Transferrin Receptor Expression in the Placenta of Pre-eclamptic Pregnancy. Placenta. 2003;24:870–876. doi: 10.1016/s0143-4004(03)00138-3. [DOI] [PubMed] [Google Scholar]
- Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- Khong TY, Liddell HS, Robertson WB. Defective haemochorial placentation as a cause of miscarriage: a preliminary study. Br J Obstet Gynaecol. 1987;94:649–655. doi: 10.1111/j.1471-0528.1987.tb03169.x. [DOI] [PubMed] [Google Scholar]
- Kilburn BA, Wang J, Duniec-Dmuchowski ZM, Leach RE, Romero R, Armant DR. Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biol Reprod. 2000;62:739–747. doi: 10.1095/biolreprod62.3.739. [DOI] [PubMed] [Google Scholar]
- Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, Thaler HT, Romero R. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187:1137–1142. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]
- Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–1069. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- Kjoller L, Kanse SM, Kirkegaard T, Rodenburg KW, Ronne E, Goodman SL, Preissner KT, Ossowski L, Andreasen PA. Plasminogen activator inhibitor-1 represses integrin- and vitronectin-mediated cell migration independently of its function as an inhibitor of plasminogen activation. Exp Cell Res. 1997;232:420–429. doi: 10.1006/excr.1997.3540. [DOI] [PubMed] [Google Scholar]
- Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348–2351. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- Koklanaris N, Nwachukwu JC, Huang SJ, Guller S, Karpisheva K, Garabedian M, Lee MJ. First-trimester trophoblast cell model gene response to hypoxia. Am J Obstet Gynecol. 2006;194:687–693. doi: 10.1016/j.ajog.2006.01.067. [DOI] [PubMed] [Google Scholar]
- Kreiling JL, Byrd JC, Deisz RJ, Mizukami IF, Todd RF, 3rd, MacDonald RG. Binding of urokinase-type plasminogen activator receptor (uPAR) to the mannose 6-phosphate/insulin-like growth factor II receptor: contrasting interactions of full-length and soluble forms of Q6 uPAR. J Biol Chem. 2003;278:20628–20637. doi: 10.1074/jbc.M302249200. [DOI] [PubMed] [Google Scholar]
- Kwon YW, Kwon KS, Moon HE, Park JA, Choi KS, Kim YS, Jang HS, Oh CK, Lee YM, Kwon YG, et al. Insulin-like growth factor-II regulates the expression of vascular endothelial growth factor by the human keratinocyte cell line HaCaT. J Invest Dermatol. 2004;123:152–8. doi: 10.1111/j.0022-202X.2004.22735.x. [DOI] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick R. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002a;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002b;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- Lash GE, Otun HA, Innes BA, Bulmer JN, Searle RF, Robson SC. Low oxygen concentrations inhibit trophoblast cell invasion from early gestation placental explants via alterations in levels of the urokinase plasminogen activator system. Biol Reprod. 2006;74:403–409. doi: 10.1095/biolreprod.105.047332. [DOI] [PubMed] [Google Scholar]
- Lash GE, Hornbuckle J, Brunt A, Kirkley M, Searle RF, Robson SC, Bulmer JN. Effect of low oxygen concentrations on trophoblast-like cell line invasion. Placenta. 2007;28:390–398. doi: 10.1016/j.placenta.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lisztwan J, Imbert G, Wirbelauer C, Gstaiger M, Krek W. The von Hippel–Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev. 1999;13:1822–1833. doi: 10.1101/gad.13.14.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chakraborty C, Graham CH, Barbin YP, Dixon SJ, Lala PK. Noncatalytic domain of uPA stimulates human extravillous trophoblast migration by using phospholipase C, phosphatidylinositol 3-kinase and mitogen-activated protein kinase. Exp Cell Res. 2003;286:138–151. doi: 10.1016/s0014-4827(03)00089-2. [DOI] [PubMed] [Google Scholar]
- Lofstedt T, Fredlund E, Holmquist-Mengelbier L, Pietras A, Ovenberger M, Poellinger L, Pahlman S. Hypoxia inducible factor-2alpha in cancer. Cell Cycle. 2007;6:919–926. doi: 10.4161/cc.6.8.4133. [DOI] [PubMed] [Google Scholar]
- Luo S, Yu H, Wu D, Peng C. Transforming growth factor-beta 1 inhibits steroidogenesis in human trophoblast cells. Mol Hum Reprod. 2002;8:318–325. doi: 10.1093/molehr/8.4.318. [DOI] [PubMed] [Google Scholar]
- Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71:977–984. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- Martin CB., Jr Uterine blood flow and placental circulation. Anesthesiology. 1965;26:447–459. doi: 10.1097/00000542-196507000-00009. [DOI] [PubMed] [Google Scholar]
- Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. Embo J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon T, Chakraborty C, Gleeson LM, Chidiac P, Lala PK. Stimulation of human extravillous trophoblast migration by IGF-II is mediated by IGF type 2 receptor involving inhibitory G protein(s) and phosphorylation of MAPK. J Clin Endocrinol Metab. 2001;86:3665–3674. doi: 10.1210/jcem.86.8.7711. [DOI] [PubMed] [Google Scholar]
- McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming growth factor beta 1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem. 2006;281:24171–24181. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- Meade ES, Ma YY, Guller S. Role of hypoxia-inducible transcription factors 1alpha and 2alpha in the regulation of plasminogen activator inhibitor-1 expression in a human trophoblast cell line. Placenta. 2007;28:1012–1019. doi: 10.1016/j.placenta.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisser A, Chardonnens D, Campana A, Bischof P. Effects of tumour necrosis factor-alpha, interleukin-1 alpha, macrophage colony stimulating factor and transforming growth factor ß on trophoblastic matrix metalloproteinases. Mol Hum Reprod. 1999;5:252–260. doi: 10.1093/molehr/5.3.252. [DOI] [PubMed] [Google Scholar]
- Minet E, Michel G, Remacle J, Michiels C. Role of HIF-1 as a transcription factor involved in embryonic development, cancer progression and apoptosis. Int J Mol Med. 2000;5:253–259. doi: 10.3892/ijmm.5.3.253. [DOI] [PubMed] [Google Scholar]
- Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- Mole DR, Pugh CW, Ratcliffe PJ, Maxwell PH. Regulation of the HIF pathway: enzymatic hydroxylation of a conserved prolyl residue in hypoxia-inducible factor alpha subunits governs capture by the pVHL E3 ubiquitin ligase complex. Adv Enzyme Regul. 2002;42:333–347. doi: 10.1016/s0065-2571(01)00037-1. [DOI] [PubMed] [Google Scholar]
- Moore LG, Shriver M, Bemis L, Hickler B, Wilson M, Brutsaert T, Parra E, Vargas E. Maternal adaptation to high-altitude pregnancy: an experiment of nature—a review. Placenta. 2004;25:S60–S71. doi: 10.1016/j.placenta.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Multhaupt HA, Mazar A, Cines DB, Warhol MJ, McCrae KR. Expression of urokinase receptors by human trophoblast. A histochemical and ultrastructural analysis. Lab Invest. 1994;71:392–400. [PubMed] [Google Scholar]
- Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, Zamudio S, Caniggia I. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1085–1093. doi: 10.1152/ajpregu.00794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi H, Nakada T, Hokamura M, Osakabe Y, Itokazu O, Huang LE, Isaka K. Hypoxia-inducible factor-1 transactivates transforming growth factor-beta 3 in trophoblast. Endocrinology. 2004;145:4113–4118. doi: 10.1210/en.2003-1639. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Christensen EI, Vorum H, Hager H, Petersen CM, Røigaard H, Min HY, Vilhardt F, Møller LB, Kornfeld S. Mannose 6-phosphate/insulin-like growth factor-II receptor targets the urokinase receptor to lysosomes via a novel binding interaction. J Cell Biol. 1998;141:815–828. doi: 10.1083/jcb.141.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel–Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- Olson LJ, Yammani RD, Dahms NM, Kim JJ. Structure of uPAR, plasminogen, and sugar-binding sites of the 300 kDa mannose 6-phosphate receptor. Embo J. 2004;23:2019–2028. doi: 10.1038/sj.emboj.7600215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page EL, Robitaille GA, Pouyssegur J, Richard DE. Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. J Biol Chem. 2002;277:48403–48409. doi: 10.1074/jbc.M209114200. [DOI] [PubMed] [Google Scholar]
- Pringle KG, Kind KL, Thompson JG, Roberts CT. Complex interactions between hypoxia inducible factors, insulin-like growth factor-II and oxygen in early murine trophoblasts. Placenta. 2007;28:1147–1157. doi: 10.1016/j.placenta.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Qian D, Lin H-Y, Wang H-M, Zhang X, Liu D-L, Li Q-L, Zhu C. Normoxic Induction of the Hypoxic-inducible factor-1 alpha by interleukin-1 beta involves the extracellular signal-regulated kinase 1/2 pathway in normal human cytotrophoblast cells. Biol Reprod. 2004;70:1822–1827. doi: 10.1095/biolreprod.103.025031. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, Conrad KP. Expression, ontogeny, and regulation of hypoxia-inducible transcription factors in the human placenta. Biol Reprod. 2000;63:559–569. doi: 10.1095/biolreprod63.2.559. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, Whitelock KA, Weissfeld LA, Daftary AR, Markovic N, Conrad KP. Selective overexpression of the hypoxia-inducible transcription factor, HIF-2alpha, in placentas from women with preeclampsia. Biol Reprod. 2001;64:499–506. doi: 10.1093/biolreprod/64.2.499. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, Doty K, Daftary A, Harger G, Conrad KP. Impaired oxygen-dependent reduction of HIF-1alpha and -2alpha proteins in pre-eclamptic placentae. Placenta. 2003;24:199–208. doi: 10.1053/plac.2002.0893. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, Brandon HM, Daftary A, Ness R, Conrad KP. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta. 2004;25:763–769. doi: 10.1016/j.placenta.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26:563–573. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, Jeyabalan A, Markovic N, Ness R, Gilmour C, Conrad KP. Placental HIF-1 alpha, HIF-2 alpha, membrane and soluble VEGF receptor-1 proteins are not increased in normotensive pregnancies complicated by late-onset intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2007;293:R766–R774. doi: 10.1152/ajpregu.00097.2007. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, Michael HM, Daftary A, Jeyabalan A, Gilmour C, Conrad KP, et al. Proteasomal activity in placentas from women with preeclampsia and intrauterine growth restriction: implications for expression of HIF-alpha proteins. Placenta. 2008;29:290–299. doi: 10.1016/j.placenta.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, Powers RW, Hubel CA, Shibata E, von Versen-Höynck F, Plymire D, Jeyabalan A. Novel soluble Flt-1 isoforms in plasma and cultured placental explants from normotensive pregnant and preeclamptic women. Placenta. 2009;30:25–34. doi: 10.1016/j.placenta.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30:S38–S42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J Biol Chem. 2000;275:26765–26771. doi: 10.1074/jbc.M003325200. [DOI] [PubMed] [Google Scholar]
- Rinkenberger JL, Cross JC, Werb Z. Molecular genetics of implantation in the mouse. Dev Genet. 1997;21:6–20. doi: 10.1002/(SICI)1520-6408(1997)21:1<6::AID-DVG2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Roberts CT, Sferruzzi-Perri AN, Owens JA, Ng P. Distinct effects of insulin-like growth factors (IGF) on placental invasion and function. Placenta. 2007;28:A5. P13. [Google Scholar]
- Rocha S. Gene regulation under low oxygen: holding your breath for transcription. Trends Biochem Sci. 2007;32:389–397. doi: 10.1016/j.tibs.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- Rolfs A, Kvietikova I, Gassmann M, Wenger RH. Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J Biol Chem. 1997;272:20055–20062. doi: 10.1074/jbc.272.32.20055. [DOI] [PubMed] [Google Scholar]
- Rosenzweig SA. What's new in the IGF-binding proteins? Growth Horm IGF Res. 2004;14:329–336. doi: 10.1016/j.ghir.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol F, Vache C, Clottes E. Natural antisense transcripts of hypoxia-inducible factor 1alpha are detected in different normal and tumour human tissues. Gene. 2002;299:135–140. doi: 10.1016/s0378-1119(02)01049-1. [DOI] [PubMed] [Google Scholar]
- Rossignol F, de Laplanche E, Mounier R, Bonnefont J, Cayre A, Godinot C, Simonnet H, Clottes E. Natural antisense transcripts of HIF-1alpha are conserved in rodents. Gene. 2004;339:121–130. doi: 10.1016/j.gene.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Safran M, Kaelin WG., Jr HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111:779–783. doi: 10.1172/JCI18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1 alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- Sato M, Tanaka T, Maeno T, Sando Y, Suga T, Maeno Y, Sato H, Nagai R, Kurabayashi M. Inducible expression of endothelial PAS domain protein-1 by hypoxia in human lung adenocarcinoma A549 Cells. Role of Src family kinases-dependent pathway. Am J Respir Cell Mol Biol. 2002;26:127–134. doi: 10.1165/ajrcmb.26.1.4319. [DOI] [PubMed] [Google Scholar]
- Schaffer L, Scheid A, Spielmann P, Breymann C, Zimmermann R, Meuli M, Gassmann M, Marti HH, Wenger RH. Oxygen-regulated expression of TGF-beta 3, a growth factor involved in trophoblast differentiation. Placenta. 2003;24:941–950. doi: 10.1016/s0143-4004(03)00166-8. [DOI] [PubMed] [Google Scholar]
- Seeho SKM, Park JH, Rowe J, Morris JM, Gallery EDM. Villous explant culture using early gestation tissue from ongoing pregnancies with known normal outcomes: the effect of oxygen on trophoblast outgrowth and migration. Hum Reprod. 2008;23:1170–1179. doi: 10.1093/humrep/den066. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- Smith GC, Crossley JA, Aitken DA, Pell JP, Cameron AD, Connor JM, Dobbie R. First-trimester placentation and the risk of antepartum stillbirth. J Am Med Assoc. 2004;292:2249–2254. doi: 10.1001/jama.292.18.2249. [DOI] [PubMed] [Google Scholar]
- Song G, Kim J, Bazer FW, Spencer TE. Progesterone and interferon tau regulate hypoxia-inducible factors in the endometrium of the ovine uterus. Endocrinology. 2008;149:1926–1934. doi: 10.1210/en.2007-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinella F, Rosano L, Di Castro V, Natali PG, Bagnato A. Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1alpha in ovarian carcinoma cells. J Biol Chem. 2002;277:27850–27855. doi: 10.1074/jbc.M202421200. [DOI] [PubMed] [Google Scholar]
- Srinivas V, Zhang LP, Zhu XH, Caro J. Characterization of an oxygen/redox-dependent degradation domain of hypoxia-inducible factor alpha (HIF-alpha) proteins. Biochem Biophys Res Commun. 1999;260:557–561. doi: 10.1006/bbrc.1999.0878. [DOI] [PubMed] [Google Scholar]
- Tacchini L, Bianchi L, Bernelli-Zazzera A, Cairo G. Transferrin receptor induction by hypoxia. HIF-1 mediated transcriptional activation and cell-specific post-transcriptional regulation. J Biol Chem. 1999;274:24142–24146. doi: 10.1074/jbc.274.34.24142. [DOI] [PubMed] [Google Scholar]
- Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel–Lindau tumor suppressor protein. Embo J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazuke SI, Mazure NM, Sugawara J, Carland G, Faessen GH, Suen LF, Irwin JC, Powell DR, Giaccia AJ, Giudice LC. Hypoxia stimulates insulin-like growth factor binding protein 1 (IGFBP-1) gene expression in HepG2 cells: a possible model for IGFBP-1 expression in fetal hypoxia. Proc Natl Acad Sci USA. 1998;95:10188–10193. doi: 10.1073/pnas.95.17.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Kim MH. A HIF-1alpha-dependent autocrine feedback loop promotes survival of serum-deprived prostate cancer cells. The Prostate. 2009;69:263–275. doi: 10.1002/pros.20885. [DOI] [PubMed] [Google Scholar]
- Thrash-Bingham CA, Tartof KD. aHIF: a natural antisense transcript overexpressed in human renal cancer and during hypoxia. J Natl Cancer Inst. 1999;91:143–151. doi: 10.1093/jnci/91.2.143. [DOI] [PubMed] [Google Scholar]
- Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- Tissot van Patot MC, Bendrick-Peart J, Beckey VE, Serkova N, Zwerdlinger L. Greater vascularity, lowered HIF-1/DNA binding, and elevated GSH as markers of adaptation to in vivo chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2004;287:L525–L532. doi: 10.1152/ajplung.00203.2003. [DOI] [PubMed] [Google Scholar]
- Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem. 2002;277:27975–27981. doi: 10.1074/jbc.M204152200. [DOI] [PubMed] [Google Scholar]
- Trollmann R, Klingmuller K, Schild RL, Rascher W, Dotsch J. Differential gene expression of somatotrophic and growth factors in response to in vivo hypoxia in human placenta. Am J Obstet Gynecol. 2007;197:601.e1–601.e6. doi: 10.1016/j.ajog.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Tsatsaris V, Goffin F, Munaut C, Brichant J-F, Pignon M-R, Noel A, Schaaps J-P, Cabrol D, Frankenne F, Foidart J-M. Overexpression of the Soluble Vascular Endothelial Growth Factor Receptor in Preeclamptic Patients: Pathophysiological Consequences. J Clin Endocrinol Metab. 2003;88:5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- Uchida T, Rossignol F, Matthay MA, Mounier R, Couette S, Clottes E, Clerici C. Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression in lung epithelial cells: implication of natural antisense HIF-1alpha. J Biol Chem. 2004;279:14871–14878. doi: 10.1074/jbc.M400461200. [DOI] [PubMed] [Google Scholar]
- Viero S, Chaddha V, Alkazaleh F, Simchen MJ, Malik A, Kelly E, Windrim R, Kingdom JCP. Prognostic value of placental ultrasound in pregnancies complicated by absent end-diastolic flow velocity in the umbilical arteries. Placenta. 2004;25:735–741. doi: 10.1016/j.placenta.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang B, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-Helix–Loop–Helix-PAS heterodimer regulated by cellular O2 tension. PNAS. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AL, Palmer ME, Jauniaux E, Burton GJ. Variations in expression of copper/zinc superoxide dismutase in villous trophoblast of the human placenta with gestational age. Placenta. 1997;18:295–299. doi: 10.1016/s0143-4004(97)80064-1. [DOI] [PubMed] [Google Scholar]
- Watson AL, Skepper JN, Jauniaux E, Burton GJ. Changes in concentration, localization and activity of catalase within the human placenta during early gestation. Placenta. 1998;19:27–34. doi: 10.1016/s0143-4004(98)90095-9. [DOI] [PubMed] [Google Scholar]
- Wenger R, Gassmann M. HIF-1 and the molecular response to hypoxia in mammals. In: Storey K, editor. Environmental Stress and Gene Regulation. Washington, DC: BIOS scientific publishers; 1999. pp. 25–40. [Google Scholar]
- Wenger RH, Rolfs A, Marti HH, Guenet J-L. Nucleotide sequence, chromosomal assignment and mRNA expression of mouse hypoxia-inducible factor-1[alpha] Biochem Biophys Res Commun. 1996;223:54–59. doi: 10.1006/bbrc.1996.0845. [DOI] [PubMed] [Google Scholar]
- Westwood M, Gibson JM, White A. Purification and characterization of the insulin-like growth factor-binding protein-1 phosphoform found in normal plasma. Endocrinology. 1997;138:1130–1136. doi: 10.1210/endo.138.3.5020. [DOI] [PubMed] [Google Scholar]
- Wiener CM, Booth G, Semenza GL. In vivo expression of mrnas encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun. 1996;225:485–488. doi: 10.1006/bbrc.1996.1199. [DOI] [PubMed] [Google Scholar]
- Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL, Wood SM, Gatter KC, Harris AL, Pugh CW, et al. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood. 1998;92:2260–2268. [PubMed] [Google Scholar]
- Wilson DM, Bennett A, Adamson GD, Nagashima RJ, Liu F, DeNatale ML, Hintz RL, Rosenfeld RG. Somatomedins in pregnancy: a cross-sectional study of insulin-like growth factors I and II and somatomedin peptide content in normal human pregnancies. J Clin Endocrinol Metab. 1982;55:858–861. doi: 10.1210/jcem-55-5-858. [DOI] [PubMed] [Google Scholar]
- Withington SL, Scott AN, Saunders DN, Lopes Floro K, Preis JI, Michalicek J, Maclean K, Sparrow DB, Barbera JPM, Dunwoodie SL. Loss of Cited2 affects trophoblast formation and vascularization of the mouse placenta. Dev Biol. 2006;294:67–82. doi: 10.1016/j.ydbio.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Xia Y, Wen HY, Kellems RE. Angiotensin II inhibits human trophoblast invasion through AT1 receptor activation. J Biol Chem. 2002;277:24601–24608. doi: 10.1074/jbc.M201369200. [DOI] [PubMed] [Google Scholar]
- Yebra M, Parry GC, Stromblad S, Mackman N, Rosenberg S, Mueller BM, Cheresh DA. Requirement of receptor-bound urokinase-type plasminogen activator for integrin alphavbeta5-directed cell migration. J Biol Chem. 1996;271:29393–29399. doi: 10.1074/jbc.271.46.29393. [DOI] [PubMed] [Google Scholar]
- Zamudio S. The placenta at high altitude. High Alt Med Biol. 2003;4:171–191. doi: 10.1089/152702903322022785. [DOI] [PubMed] [Google Scholar]
- Zamudio S, Baumann MU, Illsley NP. Effects of chronic hypoxia in vivo on the expression of human placental glucose transporters. Placenta. 2006;27:49–55. doi: 10.1016/j.placenta.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio S, Kovalenko O, Vanderlelie J, Illsley NP, Heller D, Belliappa S, Perkins AV. Chronic hypoxia in vivo reduces placental oxidative stress. Placenta. 2007a;28:846–853. doi: 10.1016/j.placenta.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio S, Wu Y, Ietta F, Rolfo A, Cross A, Wheeler T, Post M, Illsley NP, Caniggia I. Human placental hypoxia-inducible factor-1alpha expression correlates with clinical outcomes in chronic hypoxia in vivo. Am J Pathol. 2007b;170:2171–2179. doi: 10.2353/ajpath.2007.061185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. Embo J. 1998;17:5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang EG, Smith SK, Baker PN, Charnock-Jones DS. The regulation and localization of angiopoietin-1, -2, and their receptor Tie2 in normal and pathologic human placentae. Mol Med. 2001;7:624–635. [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Bondy C. Insulin-Like Growth Factor-II and Its Binding Proteins in Placental Development. Endocrinol. 1992;131:1230–11240. doi: 10.1210/endo.131.3.1380437. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Genbacev O, Damsky CH, Fisher SJ. Oxygen regulates human cytotrophoblast differentiation and invasion: implications for endovascular invasion in normal pregnancy and in pre-eclampsia. J Reprod Immunol. 1998;39:197–213. doi: 10.1016/s0165-0378(98)00022-9. [DOI] [PubMed] [Google Scholar]
- Zhou J, Schmid T, Brune B. Tumor necrosis factor-alpha causes accumulation of a ubiquitinated form of hypoxia inducible factor-1alpha through a nuclear factor-kappaB-dependent pathway. Mol Biol Cell. 2003;14:2216–2225. doi: 10.1091/mbc.E02-09-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]