Abstract
BACKGROUND
Female cancer patients are offered ‘banking’ of gametes before starting fertility-threatening cancer therapy. Transplants of fresh and frozen ovarian tissue between healthy fertile and infertile women have demonstrated the utility of the tissue banked for restoration of endocrine and fertility function. Additional methods, like follicle culture and isolated follicle transplantation, are in development.
METHODS
Specialist reproductive medicine scientists and clinicians with complementary expertise in ovarian tissue culture and transplantation presented relevant published literature in their field of expertise and also unpublished promising data for discussion. As the major aims were to identify the current gaps prohibiting advancement, to share technical experience and to orient new research, contributors were allowed to provide their opinioned expert views on future research.
RESULTS
Normal healthy children have been born in cancer survivors after orthotopic transplantation of their cryopreserved ovarian tissue. Longevity of the graft might be optimized by using new vitrification techniques and by promoting rapid revascularization of the graft. For the in vitro culture of follicles, a successive battery of culture methods including the use of defined media, growth factors and three-dimensional extracellular matrix support might overcome growth arrest of the follicles. Molecular methods and immunoassay can evaluate stage of maturation and guide adequate differentiation. Large animals, including non-human primates, are essential working models.
CONCLUSIONS
Experiments on ovarian tissue from non-human primate models and from consenting fertile and infertile patients benefit from a multidisciplinary approach. The new discipline of oncofertility requires professionalization, multidisciplinarity and mobilization of funding for basic and translational research.
Keywords: fertility preservation, tissue culture, transplantation, oocyte, follicle
Introduction
The banking of ovarian tissue has become widespread in countries that have already well-established fertility centres (Jeruss and Woodruff, 2009). Some research programmes are exploring small adaptations of conventional cryopreservation methods applied specifically to ovarian tissue. Despite the fact that there has been a growing interest in banking ovarian tissue, with samples collected from several hundred patients, there are very few research initiatives on how to ensure the function of the thawed tissue once patients return with fertility treatment requests. Major advances in this technology have occurred in the past 6 months and a number of transplants in cohorts of infertility patients provide a rationale for banking cancer tissue and transplants (Silber et al., 2005, 2008b; Silber and Gosden, 2007). Thus far, approximately 30 autotransplantations have led to the birth of eight healthy children, which is a very encouraging evolution of the technique (Donnez et al., 2004; Meirow et al., 2005; Demeestere et al., 2007; Andersen et al., 2008; Silber et al., 2008b). The advantage of this technology is that the patient will have restoration of both endocrine and fertility function. The disadvantage is that some cancers have an elevated risk of metastatic spread to the ovary which may re-introduce aggressive cancer cells to the recent survivor of their disease. Thus, additional methods for use of the stored ovarian tissue are needed to minimize risk of cancer potentiation, whereas maximizing the likelihood of a take home baby.
In contrast, research efforts to establish efficient in vitro culture methods for growing follicles at various stages have lagged, primarily due to the inability to access primate (human and non-human) tissues. There were very few original research articles published in the last 2–3 years on this topic, which suggests that barriers to tissue acquisition, low levels of research funding and gaps in our knowledge about early follicle growth contribute to this phenomenon. Indeed, the number of institutes worldwide focusing on the culture of human tissue can be counted on one hand and addressing the barriers will significantly increase the pace and quality of research that is urgently needed to improve the future fertility of cancer patients (Jeruss and Woodruff, 2009). The most important need is for robust in vitro follicle culture methods as an alternative to homologous transplantation, as the risk for re-introducing cancer cells via the transplant can pose significant and undue risk to the patient. The growth of follicles in vitro, starting from a primordial follicle and ending in the production of an ovulatory follicle with a developmentally competent oocyte, has only been achieved in mice thus far. The mouse model demonstrates that long-term culture in vitro is compatible with healthy offspring (O'Brien et al., 2003). Proactive experiments in mice using different in vitro culture methodology have shown that long-term culture of follicles does not harm the primary imprinting process (Anckaert et al., 2009a). The mouse model has also been very valuable for understanding some basic principles of follicle assembly, initiation of follicle growth and the role of specific growth and differentiation factors (for reviews see Van den Hurk and Zhao, 2005; Picton et al., 2008).
However, the physical and anatomical differences in the ovaries of humans, cows, sheep, pigs and mice make it very difficult to apply the successful culture method used for growing mouse primordial (Eppig and O'Brien, 1996) or primary follicles (Lenie et al., 2004) to larger species (Smitz and Cortvrindt, 2002). Overcoming the growth arrest and follicle death observed in current in vitro culture systems requires a better comprehension of the physiology of follicle growth initiation, the interplay of important growth factors, the basic metabolic needs of early growing follicles and the physical environment of the follicle.
Due to the limited availability of human and primate ovarian tissue for research, follicle culture technology has progressed more quickly in some domestic animals, such as the cow, sheep, goat and pig, than in primates and humans. The data generated from these animal models are valuable for translational and clinical research, as human tissue is less accessible. Research in non-human primate models is also scarce, although it will be necessary in order to identify the optimal culture protocol for human follicles. Similarities in the physiology of folliculogenesis and in the aetiology of common gynaecological diseases in primates (D'Hooghe et al., 2009) could justify their preferential use as a model to more rapidly advance the work done with human tissues.
Outside of their natural environment, follicles must cope with physical restrictions such as oxygen tension, temperature and uptake of nutrients. Such complex problems have pushed ovarian follicle culturists to seek help from tissue engineers. Furthermore expertise in basic physiology and biochemistry is of great value when designing and implementing artificial matrices and monitoring basic metabolite consumption from conditioned media. Some very promising initial data on the use of new matrices in human and non-human primate follicle culture are presented in this synopsis (Xu et al., 2009a, b). Observations from the most recent experiments using this matrix have introduced new concepts in follicle biology; in particular, these studies have challenged the premise that hormones are the only factors that control follicle development; rather, that the three-dimensional (3D) architecture of the oocyte and surrounding somatic cells are critical to the appropriate development of the oocyte. Further, this work shows that the physical environment of the ovary is a regulating force and this biomechanical signal can be pheno-copied by an appropriate support matrix (West-Farrell et al., 2009).
This manuscript brings together data and opinions from directors of well-established American and European research laboratories who are recognized leaders in their field. It gives a factual overview of where female fertility preservation and oncofertility research stands today and identifies challenges and new directions for the field in the near future.
Methods
Contributors to this article agreed to focus on one particular area of their expertise within the larger topics of either follicle culture/oocyte maturation or ovarian tissue transplantation. The main goal of this article was not to extensively review all existing literature on each topic, but rather to provide a concise overview of where the field stands today and the current opinion of experts in the field. Contributors were asked to pinpoint some relevant research areas for the near future and were encouraged to discuss any new unpublished data from their own research. The article aims to provide ESHRE and its readership with an unbiased view of where the field stands today and to indicate new directions that might advance the field.
All contributors have been performing research in the area of ovarian function and assisted reproductive technologies and have published extensively on their research. The panel members were representatives of European and American Academic Reproductive Research Centres and their work reflects current practise in their home countries. It was decided that the article would include rich and diverse expertise from scientists working with large mammalian models (cow, sheep, primate, cat) as the basis for developing translational and clinical methodologies, and in order to discuss the potential use of current technologies for the preservation of endangered animal species. All members of the panel had been invited by ESHRE's Task Force on Basic Reproductive Science.
Although it is recognized that the work on rodent species has, and is, playing a tremendously important role for our understanding of reproductive physiology, the organizers chose not to include experts working with this model as it was a pre-set goal of the panel to focus on particular problems with culture and transplantation of human ovarian tissue. Nevertheless, proper referencing of work performed with the mouse model and with certain knockout models was included in this article. Although the technologies described in this article are intended to be used on tissues and cells that will be cryopreserved, the panel preferred not to expand on cryopreservation or vitrification technologies, as most members of the panel are self-described users of the technology rather than experts in this research area. Where applicable, appropriate referencing of cryopreservation research was used.
Results
Ovarian tissue culture techniques
Models for ovarian cortical tissue culture: from mouse to large mammalian species
The production of one live mouse in 1996 from an oocyte grown entirely in vitro showed that oocyte development from the primordial follicle stage to the stage of developmental competence is possible in in vitro culture (Eppig and O'Brien, 1996). This inspired the development of methods for culturing primordial follicles from cattle and baboons (Wandji et al., 1996a, b; Braw-Tal and Yossefi, 1997; Wandji et al., 1997). Eppig and O'Brien used a two-step culture system, comprised of an 8-day culture of whole newborn mouse ovaries, with primordial follicles just beginning to form, followed by enzymatic dissociation of the ovary, and isolation and further culture of oocyte–granulosa cell complexes from the secondary follicles that had developed during the 8-day organ culture. Subsequent efforts improved the culture system and produced multiple, normal offspring (O'Brien et al., 2003). These techniques work well for mice because their follicles form during the first few days after birth, providing an ovary with a fairly uniform population of primordial follicles. In addition, the ovaries are small enough to be cultured intact, and soft enough to be dissociated enzymatically.
In contrast, in primates and most domestic species, follicles form over a number of weeks during fetal life, the ovaries are too large to be organ-cultured, and the stromal tissue is tough, making enzymatic dissociation without damage to oocytes difficult (Wandji et al., 1996a). To avoid these problems, an in vitro system was developed for culturing small pieces of ovarian cortex obtained from fetal bovine and baboon ovaries during the last third of gestation. Fetal ovarian cortex is rich in primordial follicles, since massive follicular attrition has yet to occur, and the tissue is much softer than adult ovarian tissue, facilitating dissection of the cortex from the inner medulla. Cortical pieces were cultured in Waymouth's MB 752/1 supplemented with antibiotics, pyruvic acid (25 mg/l) and ITS+ [6.25 µg insulin, 6.25 µg transferrin and 6.25 ng selenium plus 1.25 mg bovine serum albumin (BSA) and 5.35 µg linoleic acid/ml]. In this medium, primordial follicles of fetal baboons and fetal or adult cattle activate to become primary follicles within the first 2 days of culture and follicular and oocyte growth ensues (Wandji et al., 1996b, 1997; Braw-Tal and Yossefi, 1997). Initially this activation was considered to be ‘spontaneous’ because the same medium and culture conditions promoted an apparently normal percentage of follicles to be activated in the study by Eppig and O'Brien (1996). Because almost all the bovine and baboon follicles activated in medium with ITS+, this system is not appropriate for studying factors that might promote activation in vivo, but it was used to show that anti-Mullerian hormone (AMH) can inhibit activation and growth of primary follicles in bovine cortical pieces (Cushman and Fortune, 2003 and unpublished results). In more recent studies, culturing bovine cortical pieces in TS+ (i.e. ITS without insulin) maintained follicles at the primordial stage for at least 10 days, implicating insulin as an activator of primordial follicles in cattle. Culturing cortical pieces in TS+ has provided a new culture system that allows the testing of other putative follicle activators and use of this system has thus far suggested a role for kit ligand in follicle activation in cattle (Muruvi and Fortune, 2009).
Although bovine and baboon follicles activate readily in the cortical culture systems detailed above, very few primary follicles progress to the secondary follicle stage (i.e. follicles with a theca layer surrounding two or more layers of granulosa cells with no antral cavity). Attempts to stimulate the primary to secondary follicle transition by adding fetal bovine serum (FBS), follicle-stimulating hormone (FSH) or activin to the culture medium or by lowering the oxygen tension in the system detailed above were found not to be effective (Fortune et al., 1998, 1999, 2000; Gigli et al., 2006). These negative results led to the development of an ‘in ovo’ culture system, in which cortical pieces from fetal cattle or baboons were grafted beneath the chorioallantoic membrane (CAM) of 6-day-old chick embryos, to test the hypothesis that they would become vascularized and that this would allow not only activation, but also development to the secondary stage. Although the grafts vascularized rapidly and remained healthy until their removal 10 days later, follicle activation did not occur (Cushman et al., 2002). Further transplantation studies with whole mouse ovaries provided evidence that the AMH secreted by the gonads of male and female chick embryos (Hutson et al., 1981; Teng, 1987; di Clemente et al., 1992) inhibits activation of mouse primordial follicles in ovo, since follicles in mouse ovaries lacking the AMH type II receptor activated in ovo, whereas follicles in wild type ovaries did not (Gigli et al., 2005). CAM-grafting of bovine cortical pieces after gonadectomy of the host chick embryo also allowed activation, providing indirect evidence that AMH is responsible for the lack of activation in bovine and baboon cortical pieces in ovo (Gigli et al., 2005). In the future, the in ovo model could be used to test putative stimulators of activation to determine which can overcome the inhibitory effect of AMH when applied to a CAM graft of ovarian tissue, but such experiments are technically difficult.
In summary, a new in vitro culture system for bovine ovarian cortical pieces has been developed that can maintain follicles in the primordial stage. The culture system can be used to identify follicle activators, and insulin and kit ligand have thus far been identified. In vivo activation is probably regulated by a balance between stimulatory and inhibitory factors impinging on an individual follicle and the in vitro culture system can also be used to test the effects of putative inhibitors, such as AMH, on the action of various stimulators. The in vitro system is not very effective at promoting development to the secondary follicle stage; however, an important goal is to determine how that might be achieved. Follicles in whole newborn mouse ovaries quickly reach the secondary stage under similar culture conditions, so it is important to study species that are good models for human follicular development, such as cattle and non-human primates. In the in ovo culture system, follicles are held at the primordial stage by circulating AMH and this system could be used to test various factors for their ability to overcome the inhibition by AMH and to stimulate the primary to secondary transition. The drawbacks of this culture system are that it is only practical to leave the grafts in place for about 10 days because of developmental changes to the CAM, and the techniques are difficult to master.
Growing human oocytes from primordial follicles
The development of a culture system for human tissue has always been perceived as being problematic because of the prolonged period of follicle development required. Recent work from Telfer's lab (Telfer et al., 2008) has shown that human primordial follicles grow well within mechanically loosened cortical pieces, which contain growing follicles with the underlying stroma removed, and can develop to multilaminar pre-antral (secondary) stages within 6 days. The culture conditions in this new system differ from those described in previous studies (Hovatta et al., 1997, 1999) as no serum is present and no supporting matrix is used. Most of the underlying stromal tissue is removed so that the cultured pieces consist of predominantly ovarian cortex containing primordial and primary follicles. Multilaminar (pre-antral) follicles grown within human ovarian cortical strips can be isolated and have the potential to grow to the antral stage of development if cultured individually within a total culture period of 10 days (Telfer et al., 2008). This time scale makes the complete in vitro development of oocytes from human tissue a practical and viable prospect. However, whether this altered growth rate affects subsequent oocyte development is a question that needs to be addressed.
It is assumed that complete follicle development from primordial to the pre-ovulatory stage in human takes up to 8 months and Gougeon (1986) calculated the time needed for a follicle to grow from the primary to the pre-ovulatory stage to be 84 days. However, there is no good evidence to show that this is a continuous period of growth; indeed, it is likely that follicles grow in vivo in a ‘start-stop’ manner in response to local influences. This hypothesis is supported by studies showing that advanced-stage follicles can arrest the growth of follicles at the pre-antral stage without compromising their subsequent ability to proceed further (Mizunuma et al., 1999). Studies on the underlying molecular mechanisms regulating the initiation of follicle development (Foxo3 and Pten knockout studies) have shown that despite global activation of follicles, the rate at which follicles develop differs (i.e. some initiated follicles take days to reach antral stages although others take weeks or even months; Castrillon et al., 2003; Reddy et al., 2008). Studies in which the rate of granulosa cell division has been altered in vivo (e.g. by deletion of Pten in murine granulosa cells), resulted in rapid follicular growth but had no effect on fertility (Fan et al., 2008). Therefore, it appears that oocyte development can be supported within a rapidly developing follicle. Indeed, this ‘accelerated’ growth has been demonstrated in other (non-human) culture systems and fully grown oocytes have been obtained (porcine: Wu et al., 2001; bovine: Telfer et al., 2000; Thomas et al., 2003a for review). The challenge now is to define the in vitro conditions that facilitate a rate of growth that supports normal oocyte development, and current work in Telfer's lab is focusing on this (Johnson et al., 2009; Telfer et al., 2009).
Cortical strip culture removes follicles from the in vivo endocrine and paracrine processes regulating growth rate; however, follicles will still be subject to the effect of follicle interactions and the influence of stromal cell factors. It is clear that tissue shape and stromal density are important factors that regulate follicle growth initiation in vitro, as solid cubes of cortical tissue show a lower rate of growth initiation (Hovatta et al., 1997). In contrast, when stromal cells are removed and the tissue is cultured as flattened ‘sheets’, the initiation rate is greater and follicles grow faster (Telfer et al., 2008). The physical environment of the follicles within the cortical tissue affects their response to stimulatory and inhibitory factors and therefore influences their ability to grow (McLaughlin and McIver, 2009).
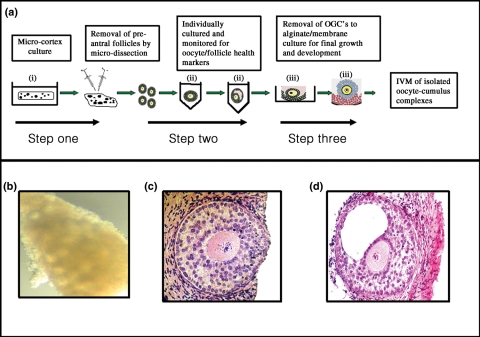
Once follicle growth is initiated within the strip, the follicles can develop to multilaminar stages; at this point, the cortical strip environment becomes inhibitory to further growth. Therefore, strip culture cannot support optimal development of all stages, and a multi-step culture system is required to support complete development (Fig. 1). Pre-antral follicles can be mechanically isolated from the cortex culture system after 6 days and placed within an individual culture system for further development to antral stages (Telfer et al., 2008). Conditions to support development of isolated follicles have been defined in bovine systems (McCaffery et al., 2000; Thomas et al., 2001, 2003a, b; Walters et al., 2006; Thomas et al., 2007) and this work formed the basis for optimising culture conditions for isolated human follicles (Telfer et al., 2008). Key factors at this stage are activin A and FSH (Telfer et al., 2008) and it will be important to determine the optimal timing and concentrations of their combined delivery during the culture period.
Figure 1.
Multi-step culture system to support human follicle development in vitro. (a) Diagram showing steps involved in the culture of human primordial follicles to antral stages (i–ii) and removal of oocyte granulosa cell complexes (OGCs) for placement in alginate bead/membrane (iii) for further growth and development and subsequent IVM [as proposed by Telfer's lab (Telfer and McLaughlin, 2007)]. Human follicles can be developed from primordial (b) to pre-antral (c) and antral stages (d) in serum free medium (Telfer et al., 2008).
Further study is required to evaluate whether manipulating key signalling pathways such as PTEN and mTOR might be an original way to maximize in vitro yields of follicles from an ovarian tissue piece.
Essential hormones and growth factors for growing human follicles in vitro
Human ovarian follicle culture techniques have been developed since the early 1990s, with the ultimate goal of achieving mature oocytes from thawed tissue collected and frozen before initiation of fertility-threatening therapies, such as chemotherapy or radiation therapy. Research on the in vitro growth of follicles and maturation of oocytes has offered the additional opportunity to study the factors controlling human follicular development (for review, see Van Den Hurk and Zhao, 2005; Sadeu et al., 2008). Effects of hormones and growth factors tested in in vitro follicle culture are shown in Table I.
Table I.
Hormones and growth factors used in the culture of follicles from fresh and frozen-thawed human ovarian tissue.
| Article | Hormone/growth factor | Fresh versus frozen | Stage in culture | Culture duration | Observed outcomes |
|---|---|---|---|---|---|
| Roy and Treacy (1993) | FSH, FCS | Fresh | Pre-antral to early antral | 4 days | Increased growth, steroid production, antrum formation |
| Hovatta et al. (1997) | FSH, LH, insulin, human serum | Fresh and frozen | Primordial to early antral and secondary | 15–21 days | Increased survival and growth |
| Abir et al. (1997) | FSH, LH, human serum | Fresh | Pre-antral to early antral | 14 days | Improved survival |
| Roy and Kole (1998) | FSH, EGF, TGFβ, FCS | Fresh | Pre-antral | 2 days | Growth increase by FSH and EGF |
| Wright et al. (1999) | FSH, ITS, HSA | Fresh | Primordial to pre-antral in cortex | 10 days | Growth increase |
| Louhio et al. (2000) | Insulin, IGF-I, IGF-II, HSA | Fresh | Primordial to early pre-antral in cortex | 21 days | Increased survival and growth |
| Hreinsson et al. (2002) | Rat recombinant GDF9, ITS, HSA | Fresh | Primordial to pre-antral in cortex | 14 weeks | Increased development of secondary follicles and survival |
| Schmidt et al. (2005) | rhAMH, FSH, LH, testosterone, ITS, HSA | Frozen | Primordial to pre-antral within cortex | 28 days | Increased initiation of growth and survival of the follicles |
| Carlsson et al. (2006a) | Kit ligand, FSH, ITS, anti c-kit antibody, HSA | Fresh | Primordial to early antral | 28 days | Oocyte death after blocking Kit receptor |
| Carlsson et al. (2006b) | rhAMH, ITS, HSA | Fresh | Primordial in cortex | 7 days | Inhibition of initiation of growth |
| Sadeu and Smitz (2008) | HSA | Frozen | Primordial to early antral in cortex | 28 days | Expression of GDF9 and AMH |
| Telfer et al. (2008) | Activin A, ascorbic acid, HSA | Vitrified | Primordial in cortex, then isolated | Two-step culture: first 6 days in cortex, then 4 days as isolated follicles with activin A | Significant growth and maturation from primordial to antral stage |
| Aghajanova et al. (2009) | rhAMH, blocking antibody and receptor blocker, ITS, HSA | Fresh | Primordial to secondary | 14 days | Increased development and survival with GDF9, blocking proved the role of endogenous GDF9 |
| Garor and Abir (2009) | FGF2, FCS or HSA, FSH | Frozen | Primordial to early antral | 28 days | Increased E2 production, no effect on follicle growth |
| Aghajanova et al. (2009) | HSA, ITS, TSH or T4 | Fresh | Primordial and primary | 3 days | No effect on follicles, cAMP or ERK phosphorylation |
Culture conditions
In the earliest studies, FBS or human serum-containing medium was used, but for safety reasons it was soon replaced by defined media substituted with human serum albumin (HSA) and a combination of insulin, selenium and transferrin (ITS). Many basal culture media were compared in the early studies, and since then, alpha minimal essential medium (MEM) has been used predominantly. For clinical maturation cultures, a good manufacturing practices (GMP) grade culture medium is needed. A clinical grade keratinocyte medium was used by Sadeu and Smitz (2008). The human recombinant forms of hormones and growth factors should be used when available: a central repository of hormones and grow factors would facilitate the comparability of results from different laboratories. Factors that prevent apoptosis have also been added, such as ascorbic acid, cyclic GMP and cyclic AMP (Scott et al., 2004a).
As the vast majority of oocytes exist within primordial follicles in ovarian cortical tissue, the culture setup should be designed to support growth initiation from this earliest follicle stage. As in animal experiments, the initiation of growth of follicles in cortical tissue is generally successful (Hovatta et al., 1997). Isolation of the follicles after growth initiation is a possible strategy (Telfer et al., 2008). Isolated follicle culture has been performed in small inserts with an extracellular cellular matrix (ECM) coating (human placental Matrigel) or without matrix, or isolated follicles may also be cultured on plastic in 24-well plates. For clinical purposes, clinical grade xeno-free, serum-free medium has to be used and follicle growth in this medium has been shown to be feasible (Scott et al., 2004b).
Animal models, particularly the sheep and bovine models, have provided a basis for human follicle culture studies (Newton et al., 1999; Gutierrez et al., 2000). For setting up human ovarian tissue culture models, researchers have utilized various sources of tissue as listed in Table II. No difference in culture results has been observed between fresh or frozen-thawed tissue. Culture of ovarian tissue is a suitable way to evaluate the quality of the freezing and thawing procedures. It remains unclear whether it is worthwhile to use ovarian tissue from women over 38 years of age and the same question remains as to the utility of ovarian tissue that had already been treated for 1–2 weeks by chemotherapy.
Table II.
Sources of human ovarian tissue for evaluation of growth factors.
| Source | Fresh/frozen | Advantages | Disadvantages |
|---|---|---|---|
| Oophorectomy specimens, Abir et al. (1997) | Fresh and frozen | Large samples may be obtained | Advanced age of the donor, often few follicles |
| Part of the patient's frozen tissue, Xu et al. (2009c) | Fresh or frozen | Often good numbers of follicles obtained | Reduces the tissue left for clinical use |
| Transsexuals' tissue, Van den Broecke et al. (2001) | Fresh or frozen | Many follicles obtained | Androgen-treated |
| Donated biopsy specimens in tubal ligation or other laparoscopies, Scott et al. (2004a, b) | Fresh or frozen | Relatively easy to obtain | Varying numbers of follicles, infertile women, no ideal patient group |
| Tissue from donors who have died, Schmidt et al. (2005) | Frozen | Often good numbers of follicles obtained | Rare samples |
| Donated biopsy specimens from Caesarean sections, Carlsson et al. (2006a, b; 2009) | Fresh or frozen | Frequently performed operation, fertile women, good numbers of follicles obtained | Careful bloodless collection procedure required, small pieces |
Evaluation of cultured follicle outcomes
Histological evaluation has always been the primary approach to assessing cultured follicles. Optimal fixation of the ovarian tissue can be achieved with Bouin's fixative, which fixes the oocytes without causing shrinkage and enables evaluation of the tissue and oocyte general morphology. Parameters of histological evaluation include: assessing follicular density per square mm of tissue; measuring the diameter of the oocyte and follicle and the thickness of the zona pellucida; counting the layers of granulosa cells; and noting the presence of theca cells. If immunohistochemistry (IHC) is intended, a portion of the tissue can be fixed in paraformaldehyde. Electron microscopy gives a more accurate evaluation of the tissue after freezing and culture, but is extremely laborious. Functionality can be tested using cell proliferation stains by IHC. Hormone production, particularly estradiol (E2), can be measured in the culture medium. Follicle development can now be evaluated at the molecular level with gene expression analyses, quantitative real-time polymerase chain reaction (PCR) and microarrays.
Conclusions: growth factors and hormones for follicle culture
FSH, insulin, activin A and growth and differentiation factor 9 (GDF9) promote follicular development and survival. AMH inhibits the initiation of growth of primordial follicles. Multi-step culture followed by in vitro maturation (IVM) of the cumulus-enclosed oocyte will be needed to obtain fertilizable oocytes (Fig. 1).
The importance of extracellular matrix in in vitro follicle culture
Introduction to ECM composition and its regulation
In discussions of factors that drive ovarian follicle development, hormones and growth factors acting by endocrine, paracrine and autocrine mechanisms are typically the first to be mentioned; however, the ECM within and around the follicle regulate numerous cellular processes associated with follicle development (Irving-Rodgers and Rodgers, 2006; Irving-Rodgers et al., 2009). The ECM is composed of a variety of molecules, which can include collagens, laminin, fibronectin, proteoglycans and polysaccharides, though the characterization of the ECM within the ovary is incomplete (for review Berkholtz et al., 2006a). In the mouse, collagen I is present throughout the ovary, with higher concentrations in the ovarian surface epithelium (OSE) and follicular compartments (for review Berkholtz et al., 2006b). Collagen IV is abundant in the theca cell compartment with low-level expression in the stroma and granulosa cells. Fibronectin is present in the stroma and theca cell compartment and increased throughout follicle development, although its presence in the granulosa cell compartment is decreased. Laminin is localized primarily to the theca cell compartment, with a defined ring at the exterior of the follicular granulosa cells marking the basement membrane. Low levels of laminin are also apparent in the stroma and granulosa cell compartment. The ECM influences a variety of cellular processes, such as cell morphology, aggregation and communication, proliferation, survival and steroidogenesis. Additionally, the turnover and remodelling of the ECM during folliculogenesis suggests that autocrine and paracrine signalling by secreted ECM molecules can regulate the transitions from one stage of development to another and ultimately the follicle fate (Kreeger et al., 2003, 2006).
Principles for choosing an ECM
The complete recapitulation of follicle growth and oocyte maturation in vitro is a goal that has profound implications both for discovery-based reproductive science and for patient health. The process of follicle development is remarkably similar between small and large animals having short and long reproductive cycles resulting in one or more mature oocytes. Follicles isolated from mouse, rat, sheep, cow, pig, non-human primate and human ovaries begin at a modest size, at roughly 35 µm in diameter for primordial follicles and 120 µm in diameter for two-layer secondary structures (for review: Hsueh et al., 2000; McGee and Hsueh, 2000). The final follicle diameter around ovulation is 700 µm in the mouse and around 15–22 mm in the human. The mature oocyte from these mammalian species is also of similar size ranging from 82 to 110 µm. FSH-dependent follicle maturation from the secondary stage to a mature follicle takes 72 h in rodents (estrus cycle) and 10–12 days from the small antral stage to the pre-ovulatory stage in the human (follicular phase of the menstrual cycle) with remarkably similar patterns of steroid and peptide hormone regulation. The question is: what are the in vitro ‘design principles’ that are most important to the development of in vitro follicle maturation systems and how these principles might be altered depending on the species from which the immature follicle is isolated?
A major goal of in vitro follicle culture in follice culture systems centred around the use of ECM is to maintain the connections between the oocyte and the somatic cells. We know that the oocyte depends on the surrounding granulosa cells for metabolic regulation, pH balance and other small molecules. Thus, the first design principle for this type of approach is the use of a follicle culture matrix that provides sufficient support to ensure that the somatic cells do not ‘abandon’ the oocyte. A second design principle is that the material supporting the follicle should be permeable to the media, permitting hormones to access the follicle structure and factors secreted by the follicle to be released. A final design principle is that the surrounding material should be amenable to modification, including its rigidity and ECM characteristics. The material we elected to work with is alginate, a product of seaweed, which has each of these characteristics and has been used successfully to produce live, healthy offspring in mice born from in vitro matured follicles and to support growth of non-human primate and human follicles with the coordinate maturation of the oocyte (Xu et al., 2009a, b).
Alginate, a translational culture system
The development of culture systems for follicle development should account for the functions of the ECM, which are categorized as structural and biochemical (i.e. signalling). The ECM provides mechanical support to the follicle, which is essential for maintaining cell–cell contacts and paracrine signalling between the cellular compartments. Natural (e.g. collagen) and synthetic (e.g. alginate) hydrogels have been employed to maintain the 3D architecture, and have been referred to as 3D culture systems to differentiate them from traditional 2D culture on flat tissue culture surface. Three-dimensional culture systems may have an advantage in overcoming the difficulty of maintaining follicular structure in early-stage follicles or in follicles from large species. In the case of human follicles, culture in a 3D collagen gel promoted follicle growth, whereas follicles on a 2D collagen coated surface maintained their original size (Abir et al., 2001). In the alginate system, however, secondary mouse, monkey and human follicles have been able to grow (Pangas et al., 2003), produce fluid-filled antral cavities (Xu et al., 2006a) and produce meiotically competent oocytes (Kreeger et al., 2005, 2006; Xu et al., 2009a, b), which were successfully fertilized and implanted to yield multiple live births of healthy mouse pups (Xu et al., 2006b). The mechanical properties of the 3D matrix have a significant role in supporting follicle development (Xu et al., 2006b). The hydrogel must have sufficient rigidity to maintain the 3D structure of the follicle, yet must also allow for expansion due to oocyte growth, granulosa cell proliferation and antrum formation (Xu et al., 2006a, b; West et al., 2007; West-Farrell et al., 2009). Forces are generated by follicles in 3D matrices because the growing follicle exerts an outward force on its surrounding matrix. The matrix also exerts a force on the follicle, which may affect actin organization, likely activating one or more of these mechanoresponsive pathways. In a 3D system, cell–cell and cell–matrix interactions are maintained; therefore, a mechanical force applied to the cells at the exterior of the follicle will be transmitted to all cells within the follicle to influence development and maturation.
The signals necessary for follicle development must be presented within the context of the biomaterial. Diffusible signals (i.e. hormones, growth factors) can be added to the culture media, and are generally able to transport through the hydrogel. The ECM regulates cellular function and differentiation within the follicle in vivo, processes which are disrupted during follicle isolation. Providing a particular ECM in an in vitro system allows for an analytical approach to cell and tissue culture and could therefore lead to a better understanding of factors determining the quality of the follicle in culture (Kong et al., 2003; West et al., 2007). Natural materials, such as collagen, are themselves composed of ECM proteins and provide innate interactions with encapsulated cells. Other matrices, such as alginate, do not possess these innate interactions and must be modified to better mimic the ECM in vivo. In the case of follicle development, the incorporation of ECM proteins (collagen I and IV, fibronectin and laminin) or the arginine–glycine–aspartic acid (RGD) peptide into an alginate matrix affected follicle growth and differentiation, as well as oocyte quality (Kreeger et al., 2006). The ECM in the 3D matrix may be useful in maintaining a gradient of paracrine growth factors essential to polarized growth. Note that the adhesivity of the hydrogel must be balanced to limit cell migration from the follicle and into the hydrogel, which can disrupt follicle structure and its growth potential. Taken together, determining the structural and biochemical design of the ECM for each stage of follicle environment will facilitate the growth of follicles during 3D culture.
Experience with alginate in mouse and human follicle culture
Secondary follicles have been isolated from mouse, rat (Heise et al., 2005), non-human primate (Xu et al., 2009a) and human (Amorim et al., 2009) ovaries and grown in alginate beads. The hypothesis was that the microvilli and transzonal projections between the oocyte and somatic cells would be maintained if follicles were cultured in alginate (Albertini et al., 2001). Indeed, it has been shown that these membrane extensions are maintained in 3D alginate cultures (Pangas et al., 2003). The reason why high quality cultured oocytes from the mouse and healthy germinal vesicle-containing oocytes from the human are obtained is due to the maintenance of these vital corridors of communication between the two cellular compartments in the alginate matrix. Heise et al. (2005) demonstrated the presence of connexions between granulosa cells and oocytes in isolated pre-antral rat follicles cultured in alginate.
New horizons offered by 3D follicle culture systems: Since mouse follicles can be routinely cultured in this 3D in vitro system, it becomes possible to address some basic questions about follicle differentiation in relation to oocyte quality. First, what are the genes that control follicle selection and activation? What are the signalling pathways that exist between the oocyte and somatic cells during terminal follicle development? What signals antrum formation? What is the earliest follicle stage at which oocytes attain nuclear and cytoplasmic maturation? What is the role of the somatic cell in follicle development? What controls hormone secretion and what controls the rate of hormone production? What factors are essential for oocyte development? Very few tissues are able to mature independent of a vascular supply; indeed, the follicle is the only known example of autonomous tissue function. Many new and important discoveries in follicle biology are made possible by a follicle culture system that faithfully recapitulates the main feature of the ovary that contributes to follicle growth, its actual structure as an organ. Simultaneously, the alginate system is being applied for human follicle culture (Xu et al., 2009c).
Until recently the work has focused on the secondary follicles stage, which is in less abundance in adult ovaries but in sufficient prevalence to be useful to the cancer patient (Xu et al., 2009c). On the basis of these studies we now know that follicles can be grown autonomous of the surrounding stroma if sufficient physical support is provided to them. Whether primordial or primary follicles are independent of their cellular environment is a question that is currently being addressed. It is known that the oocyte and somatic cells need constant communication in order for the oocyte to mature (Albertini et al., 2001). Finally, these studies show that a rationally designed tissue engineered approach to follicle maturation is providing new insights into the physiology of the follicle which can be applied to the cancer patient setting. Current challenges are to understand better the role of the gonadotrophins, steroid and peptide hormones in follicle growth and the integration of hormones with the physical environment. These fundamental studies will be of great impact for the development of a robust system for human follicle development and oocyte maturation.
Experience with alginate in rhesus monkey follicle culture
Deficits in our understanding of the processes and factors controlling the onset, progression and maturation of the human follicle limit our ability to provide ovary-based options for preserving fertility, including for women experiencing cancer therapies. Various culture systems mentioned earlier in this review and, notably, recent advances that encapsulate individual follicles within a permeable matrix (Xu et al., 2006a, b), have been shown to support mouse follicle development in vitro and achieve oocyte maturation required for fertilization and production of live offspring. Whether such culture systems will support similar development of primate follicles has received little attention. Pre-antral follicles from the marmoset, a New World monkey, developed to antral follicles and yielded mature oocytes during two dimensional culture (Nayudu et al., 2003). Old World macaques, such as the rhesus monkey (Macaca mulatta), are a valuable model for studying the primate ovary, as many of the characteristics and regulation of cyclic ovarian function are comparable to those in women. For example, rhesus macaques are monovular and display menstrual cycles of a month duration, with a 2-week follicular phase. Rhesus monkeys provide an opportunity to address issues specific to primate follicle development relative to rodent follicles, e.g. large differences in the duration of follicle growth to obtain mature ovulatory follicles and possible differences in hormonal and metabolic requirements. For obvious practical and ethical reasons, it is possible to address issues in macaques that can only be indirectly studied with human tissue or in an associative manner.
Therefore, studies were initiated (Zelinski et al., 2008; Xu et al., 2009a, b) to determine if secondary follicles isolated from adult female rhesus monkeys, encapsulated in alginate hydrogels and cultured individually in αMEM media supplemented with BSA, bovine fetuin, insulin, transferrin and selenium, would survive and grow in vitro. Initial studies addressed three questions: (i) does the stage of the ovarian cycle at follicle isolation impact follicle development in vitro? (ii) does the rigidity of the alginate hydrogel influence follicle survival and growth? and (iii) do follicles require in vitro exposure to pituitary gonadotropic hormones?
Our results indicate that secondary follicles isolated from prepubertal monkeys gave a better survival than from adult monkeys. Surprisingly the follicles from the early follicular phase of the menstrual cycle have a higher survival rate than those collected during the luteal phase. This difference correlated positively, and may be related to, the larger mean diameter of secondary follicles in the early follicular versus luteal phase (270 versus 191 µm). Follicles survived and grew in two hydrogel conditions (0.5 and 0.25% alginate). The alginate scaffold concentration affected follicle survival rates and the proliferation and differentiation of theca and granulosa cells. The higher tested alginate concentrations such as 1.5 and 1.0% hindered follicle development (Xu et al., 2006a, b). Follicles did not survive in culture in the absence of FSH (recombinant human FSH; Organon). However, follicle survival up to 30 days in vitro did not differ between follicles exposed to FSH alone or FSH plus recombinant human luteinizing hormone (LH; Merck Serono). Follicles exposed to either FSH alone or FSH plus LH demonstrated continuous growth (increased follicular diameter), and approached 1 mm diameter with an extracellular compartment reminiscent of the antrum within 30 days of culture (Fig. 2). Unexpectedly, follicles cultured with FSH alone had a greater mean diameter than those cultured with FSH and LH (750 versus 550 µm; P < 0.05). To consider the steroidogenic function of these encapsulated follicles during growth in vitro, media samples were assayed for androstenedione (A), E2 and progesterone (P) content. Steroid levels were low during the first week of culture, but increased 5–10-fold (P < 0.05) by 30 days of culture in the presence of FSH alone or FSH plus LH. Addition of LH tended to increase E and A levels at Day 30 (P = 0.07), and decrease P levels (P < 0.05). Thus, an alginate hydrogel matrix supports the 3D structure of individual secondary follicles from macaques, and permits follicle growth to the small antral stage within 30 days of culture. FSH, but not LH, is required for follicle survival and growth, and increased steroidogenesis is associated with antral development between Days 20 and 30 of culture. LH increased E and A and reduced P production in the follicle. The effects of LH activity observed were compatible with data addressing the needs for LH activity in human in vitro fertilization (IVF) showing a similar effect upon A/E balance and reduced pre-ovulatory progesterone production (Smitz et al., 2007).
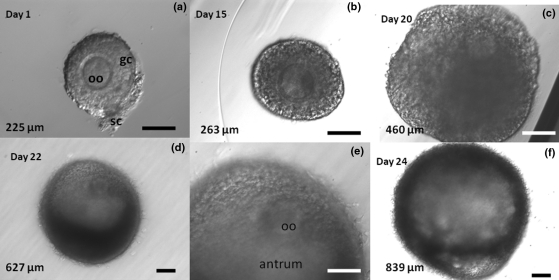
Figure 2.
Rhesus monkey secondary follicles grown in alginate hydrogel over 3 weeks. Photomicrographs illustrate the morphological and size changes in follicles during the 24-day culture period. (a) Upon initial isolation and encapsulation in alginate, the oocyte (oo) is centrally located within a single layer of granulosa cells (gc). A few stromal cells (sc) remain with the follicle. (b) After 15 days in culture, the follicle has increased almost 20% in diameter. (c) By Day 20, the follicle has more than doubled its original size and a developing antrum can be observed. The oocyte is no longer centrally located within the follicle. (d, e) By Day 22, the follicle has further increased in diameter and a well-defined antrum can be seen. (f) On Day 24, the follicle has increased in diameter almost 4-fold, yet retains its spherical morphology and antral cavity, both of which are supported by the alginate hydrogel. Some follicles (≈30–50% of those surviving depending upon the culture conditions) achieve diameters typical of a small antral follicle in the macaque by the fourth week in vitro. Upper left = day of culture; lower left = follicle diameter; lower right bars = 100 µm. Images on Days 1, 15, and 20 (top row) and of the antrum and oocyte on Day 22 (middle, bottom row) were taken at 20×. Images on Days 22 and 24 were taken at 10×. Images from M. Zelinski.
Ongoing studies are addressing additional questions: (i) will this 3D follicle culture system support the growth of follicles from young (prepubertal, ≈4–5 years of age), and older (≥12 years of age) adult macaques; (ii) does sequential exposure to FSH, followed by FSH plus LH, promote further follicular growth; (iii) are paracrine factors, such as AMH, produced by macaque follicles during 3D culture; (iv) what is the effect of the FSH/LH balance upon oocyte developmental capacity?; (v) what is the role of theca during in vitro follicle growth on antrum formation and on steroid production; (vi) what oxygen tension should be applied taking into account the fast effects of oxidative stress and (vii) how could one evaluate oocyte maturity in a non-invasive manner?
Initial evidence suggests that a greater percentage of secondary follicles from prepubertal animals survive for 30 days in vitro compared with those from adult monkeys (77 versus 59%; P < 0.05). At collection, follicle diameters did not differ between prepubertal and adult animals, but by Day 30, the diameters of growing follicles were larger if obtained from adults compared with prepubertal animals. However, an antrum developed by Day 25 regardless of age. Notably, compared with FSH alone, addition of LH after Day 30 culture increased the diameter of follicles from prepubertal animals (P < 0.05), but did not affect those from adults (Zelinski et al., 2008). Further analyses indicated that surviving follicles could be assigned to three categories according to diameters achieved by Day 40: no growth (<300 µm); slow growth (301–600 µm) and fast growth (>600 µm). Although follicle diameters in these groups did not differ during early culture (Days 1–15), AMH levels in the media after 1 week were higher for fast growth follicles compared with no growth follicles (P < 0.05), and after 2 weeks were higher in fast growth versus slow growth follicles (P < 0.05; Bernuci et al., 2008). AMH levels peaked at 2 weeks in the presence of FSH and declined to low levels by 4 weeks of culture (P < 0.05). Thus, AMH is produced by macaque pre-antral follicles and is associated with more rapid follicle growth in vitro.
This model could become valuable for understanding the role of gonadotrophins, steroids and peptide hormones in follicle development and its link to oocyte developmental capacity. Once achieved, this knowledge might be of benefit to human follicle culture and could be combined with other assisted reproductive technologies to restore fertility to women, including cancer survivors.
Transplantation of ovarian tissue
Follicle isolation and selection
For patients at risk of ovarian metastatic involvement (e.g. leukemia, neuroblastoma, breast cancer), alternatives to ovarian tissue fragment reimplantation need to be considered. However, ovarian tissue fragments can be reimplanted when the risk of cancerous involvement of the ovary is absent or minimal, and when autografting would thus present little or no danger. Indeed, studies evaluating the safety of cryopreserved human ovarian tissue reimplantation have suggested that ovarian tissue grafting in Hodgkin's disease may be considered safe (Meirow et al., 1998; Kim et al., 2001; Seshadri et al., 2006). However, in leukemia, malignant cells are present in the bloodstream and are at risk of being transferred along with the cryopreserved tissue (Jahnukainen et al., 2001). For this reason, some alternatives have been under development. Ovarian tissue could be in vitro cultured for subsequent in vitro follicle culture or used for isolation of pre-antral follicles from the surrounding stroma that may potentially contain cancerous cells. These healthy follicles could then be safely retransplanted to patients (Dolmans et al., 2007). Obtaining individual isolated follicles has been studied in mice (Gosden, 1990; Eppig, 1994), sheep (Amorim et al., 2006), goats (Lucci et al., 1999), cows (Figueiredo et al., 1993), pigs (Greenwald and Moor, 1989) and humans (Oktay et al., 1997; Abir et al., 1999; Dolmans et al., 2006), but only one study, in mice, demonstrated fertility restoration after transplantation of isolated follicles (Carroll and Gosden, 1993).
Since human ovarian cortex is extremely tough to handle, this rules out mechanical isolation to separate intact primordial follicles. Enzymatic tissue digestion using collagenase is currently used for human ovarian follicle isolation (Oktay et al., 1997; Abir et al., 1999). However, since collagenase may contain high levels of endotoxins, which could severely impair culture and grafting outcomes, a protocol to digest human ovarian cortex using a purified endotoxin-poor enzyme (Liberase) has been described to isolate primordial and primary follicles from ovarian cortical tissue (Dolmans et al., 2006). Recovery of the isolated follicles is then facilitated by passing the suspension through a Ficoll gradient prior to microscopic examination (Martinez-Madrid et al., 2004). This technique allows isolation of large numbers of primordial follicles, with high viability, unaltered morphology and a well preserved ultrastructure (Dolmans et al., 2006). The viability of these isolated human follicles has been tested by vital fluorescent dyes (live/dead assays) and in vivo xenografting in a fibrin clot to the nude mouse ovarian bursa. Their survival and growth after transplantation was evidenced by their morphologically normal structure, their progression to more advanced follicular stages, and their positive staining for human Ki-67 1 week post-transplantation (Dolmans et al., 2007). Furthermore, recent data indicate that human isolated follicles grafted for 6 months can reach antral stages, demonstrating the capacity of oocytes to survive and grow further (Dolmans et al., 2008). Isolation of human follicles could be done directly after tissue harvesting on fresh ovarian tissue, as it has been done in experimental studies so far. These isolated follicles could then be cryopreserved and banked until use, as described previously with sheep follicles (Amorim et al., 2007). Although the easiest and most logical way to select is probably to cryopreserve the ovarian biopsy, in strips or fragments, and thaw them the day of isolation and grafting, it needs to be investigated whether there could be an advantage in cryopreserving follicles over tissue. The cryoprotectant permeation might be more efficacious through a cell suspension than through tissue blocks.
As this approach has been shown to successfully restore fertility in mice (Carroll and Gosden, 1993), optimization of follicle isolation and recovery protocols will allow us to consider its development for humans, in cases where the risk of reintroducing malignant cells into cured cancer patients by ovarian tissue autografting cannot be excluded. Further focus should be given to the development of sensitive and rapid detection methods for cancer detection in ovarian stroma. Reseeding purified follicle fractions, free of cancer cells, requires new surgical approaches to increase efficiency of graft recovery. New artificial matrices might play a role in this.
Transplantation of ovarian tissue to orthotopic sites: case studies
Cryopreservation of ovarian tissue has proved to be a valuable option for fertility preservation in young patients at risk of premature ovarian failure. It is the only option available for prepubertal girls, as well as women who cannot delay the start of chemotherapy and/or undergo ovarian stimulation with embryo cryopreservation (Gosden et al., 1994a; Donnez and Bassil, 1998; Dolmans et al., 2005; Donnez et al., 2006a).
Approximately 30 cases of autotransplantation of cryopreserved ovarian tissue to orthotopic sites have been reported worldwide and eight live births have been published to date (Donnez et al., 2004; Meirow et al., 2005; Demeestere et al., 2007; Andersen et al., 2008; Silber et al., 2008b; Piver et al., 2009). Donnez et al. reported the first successful transplantation of frozen-thawed ovarian tissue resulting in a pregnancy and live birth in 2004. From his series of seven published autotransplantations of cryopreserved ovarian tissue, all patients recovered ovarian function within a period of 4–5 months post-grafting (Donnez et al., 2008). Two techniques were successfully used to reimplant frozen-thawed ovarian tissue in an orthotopic site: either in a specially created window on the peritoneum (two steps technique, Donnez et al., 2004) or on the remaining ovary (Donnez et al., 2006a). When using large tissue strips (10 × 4 mm), the ovarian fragments can be sutured onto the remaining ovary after removal of the native cortex. In case of small cubes (2 × 2 mm), they can be placed on the decorticated medulla and held in place with an absorbable adhesion barrier, itself sutured to the remaining cortex of the native ovary (Donnez et al., 2008).
Although several live births have been reported after cryopreserved ovarian tissue transplantation, some important questions remain. Experimental studies show a considerable decrease in the number of primordial follicles in grafted tissue. This may be due to hypoxia and the delay that occurs before reimplanted cortical tissue becomes revascularized. The loss of primordial follicles in frozen-thawed ovarian tissue after transplantation is estimated to be 50–65% in some studies (Baird et al., 1999; Nisolle et al., 2000) and >90% in one study (Aubard, 1999; Nottola et al., 2008). On the other hand, the primordial follicle pool also appears to be depleted by follicular activation after grafting. Experimental studies demonstrated a significant increase in the proportion of growing follicles, from <20% in ovaries before grafting to >70% after grafting in sheep (Baird et al., 2004), monkeys (Gougeon and Busso, 2000) and humans (Dolmans et al., 2007). According to Baird et al. (2004), this massive recruitment of primordial follicles, which also occurs in cultured fragments, suggests the removal of some inhibitory mechanisms regulating FSH. Following autotransplantation, the number of antral follicles and inhibin A secretion are reduced, resulting in raised basal levels of FSH that may account for the massive recruitment, although the early stage of folliculogenesis can occur in the absence of FSH and LH. Other factors such as significantly reduced circulating AMH levels, might contribute to the underlying rapid depletion similar to that described in the AMH knock-out model (Lie Fong et al., 2008, 2009).
A crucial question is how to prevent this premature activation of the transplanted follicular pool and whether active angiogenesis can be induced to accelerate the process of neovascularization in grafted tissue. Research is actually conducted to evaluate, by oximetry and electro-paramagnetic resonance, the reoxygenation and revascularization of human ovarian xenografts in order to have objective parameters to monitor the revascularization process (Van Eyck et al., 2009a, b). Indeed, these studies have shown that revascularization of avascular grafts are only complete on Day 10 post-grafting, and that the follicle pool remains anoxic for ∼5 days.
Another important question is how to improve oocyte quality in frozen-thawed transplanted tissue. A study recently presented by Dolmans et al. (2009) reported a higher risk of empty follicles, abnormal or immature oocytes and low embryo transfer rates in patients with cryopreserved autotransplanted ovarian tissue. In this IVF series, 21 oocyte retrievals yielded 16 oocytes, 10 of which were in metaphase II (MII). Five MII oocytes successfully fertilized with normal subsequent embryo development, but no pregnancy occurred. Women who have undergone grafting of frozen-thawed ovarian tissue have reduced ovarian reserves and elevated FSH concentrations, and there is frequently a failure to recover oocytes in the aspirates from follicles from these patients. This might arise from the inappropriate activation of granulosa cells and oocytes by increased FSH concentrations, which could provoke an asynchronous maturation. Another hypothesis to explain the prevalence of empty follicles is that the oocytes have been damaged by the cryopreservation, thawing and transplantation procedures.
Finally, the standard method for human ovarian tissue cryopreservation is slow programmed freezing, using HSA-containing medium with either propanediol, dimethylsulphoxide or ethylene glycol as a cryoprotectant, combined or not with sucrose. Vitrification is still at the experimental stage for human tissue, but appears to yield promising results (Kagawa et al., 2009; Keros et al., 2009).
In conclusion, live births obtained after transplantation of frozen-thawed ovarian tissue in humans give hope to young cancer patients, but the techniques can be improved at several levels. Research programmes need to determine the ideal fragment size for cryopreservation and transplantation, whether active angiogenesis can be induced to accelerate the process of neovascularization in grafted tissue, and whether oocyte quality is affected by the freezing and grafting procedures applied.
Techniques for safeguarding fertility in rare animal species
All felid species in the wild are near extinction
Aside from their application to fertility preservation in humans, the studies described above raise the hope that advances in culture and cryopreservation of ovarian tissue may provide a valuable tool for assisted reproduction in rare and endangered species.
Of the 37 felid species, all but the domestic cat are threatened worldwide and face extinction in all or part of their native habitats (CITES-appendices, www.cites.org). In some species, a greater number of individuals are present in zoo facilities than in their natural habitats. Advanced reproductive techniques (e.g. IVF and embryo transfer) are undoubtedly powerful tools for safeguarding rare and endangered felid species (Wildt et al., 1992; Jewgenow et al., 1997; Pope et al., 2006), but the most critically limiting factor to their application is the lack of mature and fertilizable female germ cells (Jewgenow and Paris, 2006). This problem could be addressed by accessing the large source of oocytes available within the pre-antral and primordial follicles in the ovarian cortex and culturing them to maturity in vitro. In this respect, ovarian tissue from wild cats that must be spayed or that either die suddenly or be euthanized for medical reasons, may serve as an oocyte pool for rescuing the valuable genetic potential of females and subsequently aid in the propagation of these species.
The domestic cat as a model for in vitro culture and transplantation
The full-term culture of primordial follicles to a mature stage and the birth of viable offspring after IVF and embryo transfer have not yet been achieved in a felid species (Jewgenow and Pitra, 1993; Jewgenow, 1996, 1998; Jewgenow and Paris, 2006). Therefore, transplantation of ovarian tissue in a xenogeneic host is discussed as an long-term alternative method for follicle growth and development (Jewgenow and Paris, 2006). Gosden et al. (1994b) first reported the xenotransplantation of fresh ovarian tissue of domestic cat in SCID mice. Transplant survival as well as the development of antral follicles was documented up to 9 months after grafting at autopsy. More recently, Bosch et al. (2004) showed antral follicle stages following xenografting of cortex fragments from frozen/thawed domestic cat ovaries in SCID mice by post-mortem histology 2 months after grafting. Fassbender et al. (2007) demonstrated that fresh ovarian cortex from domestic cats survived xenotransplantation in athymic nude rats and that high resolution ultrasonography provided a reliable method to follow xenograft survival and follicular development within the grafts. It turned out that this technique is suitable to assess the efficiency of hormonal treatments and to narrow the optimal time frame for oocyte retrieval in living recipients. The success of all ovarian tissue transplantation, independent of the tissue status (fresh or frozen) is highly dependent on the time point of oocyte retrieval to ensure maturation and subsequent IVF trails, particularly if the donor ovary originated from a rare and valuable animal. Unfortunately, the existing protocols for ovarian cryopreservation and grafting are species-specific and most protocols only apply to just a few species (humans and laboratory animals). A network of research in a broad diversity of species is required to develop and apply cryobanking technology to felid conservation. Development of cryopreservation protocols specifically for felids will impact a number of other threatened cat species worldwide, and a concerted, integrated effort to conserve critically endangered European carnivores is urgently needed.
Discussion
Surveys of cancer survivors have identified an increased risk of emotional distress in those who become infertile because of their treatment (Berkowitz K., 2003; Partridge et al., 2004; Carter et al., 2005). Loss of fertility potential is something difficult to understand for young children, but is potentially traumatic to them as adults. It is considered good clinical practise that all cancer patients of reproductive age be informed about the possibility of treatment-related infertility. Professional societies recommend that oocyte and ovarian tissue cryopreservation be performed only in centres with demonstrated expertise using only IRB-approved protocols [British Fertility Society (BFS): http://www.britishfertilitysociety.org.uk; European Society of Human Reproduction and Embryology (ESHRE): http://www.eshre.com; American Society for Reproductive Medicine (ASRM): http://www.asrm.org; American Society for Clinical Oncologists (ASCO): http://www.asco.org). Guidance documents stress that this field of medicine is still considered to be experimental. Although the practice of oocyte and ovarian tissue cryopreservation has been spreading rapidly, there has been almost no research effort directed towards future use of this tissue.
In view of the rapid introduction of fertility preservation in clinical practice, organization of the field in a more professional way has become a high priority.
As cancer treatments evolve rapidly, so should current understanding on their impact on female fertility, so that clinicians can provide their patients with the most accurate information about their risk of infertility related to cancer treatment (Jeruss and Woodruff, 2009). Most of the available literature documenting cancer treatment-related infertility risks reports rates of azoospermia and amenorrhea, which are surrogate markers of limited practical value. There are better biochemical markers of fertility, such as AMH and non-invasive imaging techniques (ultrasound, Doppler, magnetic resonance) that allow a more precise evaluation of the damage to the reproductive organs (ovary, testicle, uterus; van Beek et al., 2007). A follow-up of all cancer patients that receive potentially gonadotoxic drugs with these investigative tools must be recommended in order to gather precise information on the following questions: what cancers directly affect fertility? Which cancer drugs should be avoided in girls and premenopausal women because of their gonadal toxicity? Which combination of drugs should be banned for negative effects on the ovary and uterus?
In most countries, patients are followed up by oncology but receive no expert advice from a fertility specialist as part of the patient management protocol. Newer techniques, such as AMH measurements, would allow a better appreciation of post-treatment fertility potential (Lie Fong et al., 2008, 2009). Providing adequate support and funding for regional or national ‘fertility preservation banks’ would allow better organization and coordination of patient follow-up.
Restoration of fertility after cancer by transplantation techniques
It was estimated that ∼20% of grafted ovarian tissue cases led to childbirth in the first reported series worldwide, which is an encouraging figure. However, there is still a lot to do towards improving the techniques for grafting of ovarian tissue or isolated primordial follicles. More specifically, new approaches to the revascularization of the grafted tissue are needed, with the goal of reducing the number of follicles that die within the first hours after transplantation by ischemia. As the clinical benefit of transplanted ovarian cortical pieces is directly proportional to their longevity, useful information can be gained from recent work by Silber and Gosden (2007) and by Silber et al. (2008a, b). Ovarian cortex transplantation between monozygotic twin sisters, where ovarian tissue transplants had not been frozen before grafting of the thinned cortex using a microsurgical technique provide evidence that it is now technically possible to restore ovarian function within 4 months post-surgery, to obtain pregnancies within a median period of 15 months and for healthy babies to be born. Two to four years post-transplantation of on average 30% of cortical tissue from the donated ovary, the acceptors were still menstruating and some patients had even had a second child from the graft.
Pioneering work on transplantation of previously cryopreserved human ovarian tissue in a heterotopic site such as a subcutaneous location (Oktay et al., 2001; Callejo et al., 2001; Oktay et al., 2003) or in the space between the rectus sheath and rectus muscle (Kim et al., 2009) has proven that follicular function can be recovered and this research has led to the development of several technical improvements. However, until now only a few intact oocytes could be recovered over several ‘cycles’ from the developed follicles in the transplant (Oktay et al., 2004; Kim et al., 2009). These oocytes fertilized normally and a few embryos developed in vitro. Of the few patients that had consented to undergo heterotopic grafting, none have become pregnant.
Many other challenges are remaining in the field of transplantation, to determine the optimal site for grafting ovarian tissue or its isolated follicles (Donnez et al., 2006b; Demeestere et al., 2009), and to restore ovarian function after transplantation of frozen-thawed whole ovary (Jeremias et al., 2002; Salle et al., 2002; Arav et al., 2005; Courbiere et al., 2005; Imhof et al., 2006; Martinez-Madrid et al., 2007).
Until today, only a few patients (±1%) who have been declared cancer-free have come back with a request for reimplantation of ovarian tissue, so our experience is still very limited. The amount of data on the quality of oocytes after the combined procedures of cryopreservation and transplantation is lacking. Basic knowledge regarding neovascularization, reinnervation and paracrine and autocrine regulation is still very scarce, and basic research efforts in these areas requires support in order to overcome some of the challenges that limit the success of the transplantation.
Advances and challenges with tissue and follicle culture techniques
Significant progress has been made in improving the methods for in vitro growth and maturation of ovarian follicles from a range of species including rodents, ruminants, primates and humans (Xu et al., 2006b; Nogueira et al., 2008; Picton et al., 2008; Sadeu et al., 2008; Xu et al., 2009a, b). A strategy to maximize the reproductive potential of fresh and cryopreserved human ovarian tissue used in follicle culture strategies must be orientated around the activation and sustained in vitro growth of primordial, primary or secondary follicles, as these follicles are all in the human ovarian cortex. In this context, although long-term ruminant and primate follicle culture systems are a better model for human follicle culture than shorter term rodent follicle culture systems, ultimately the best model for the therapeutic derivation of fertile human oocytes is a human follicle culture system. Human follicle culture systems must work equally effectively in both fresh and cryopreserved ovarian tissues (Newton et al., 1999; Muruvi et al., 2005, 2009; Xu et al., 2009b).
Progress in the development of systems for the derivation of mature human oocytes from immature follicles in vitro is hampered by the very limited availability of accurate biological information on the genes and growth factors that initiatal follicle growth and support follicle and oocyte development to maturity in vivo in humans (Picton, 2001; Xu et al., 2006a; West et al., 2007). A non-limitative list of current knowledge gaps on basic biological mechanisms related to in vitro oocyte growth is shown in Table III. Making significant progress in understanding which factors regulate the early stages of follicle development requires the availability of costly high-tech infrastructure, including laser capture microdissection, quantitative PCR, confocal microscopy and DNA microarrays. It is important that the consequences of extended follicle culture and the use of assisted reproductive technologies on key programming events during oogenesis and embryogenesis can be apprehended (Pesty et al., 2007; Huntriss and Picton, 2008; Anckaert et al., 2009a, b). Research also needs to evaluate the metabolic requirements that support acceptable rates of somatic and oocyte growth in vitro and how these demands change as oocyte and follicle development progress (Harris et al., 2007, 2009; Harris and Picton, 2007). Ultimately, the information gained from this fundamental research must be applied to the biological and practical problems associated with human follicle culture.
Table III.
Current gaps and opportunities in follicle and oocyte in vitro culture.
| Component | Controversy |
|---|---|
| Follicle harvest strategy | Isolated follicle culture versus in-situ or in ovo culture systems |
| Follicle attachment system | Use of adherent versus non-adherent culture systems |
| Follicle growth strategy | Use of linear (2D) versus spherical (3D) culture systems |
| Culture matrix | Inclusion versus exclusion of matrix and/or scaffolds to support 3D culture and antrum formation |
| Culture system continuity | Use of consistent system for all stages of follicle growth development versus sequential, follicle phase-dependent systems |
| Base media | Optimization of base media and buffering systems to suit species and culture strategy |
| Oxygen tension | Use of high (20%) versus low (5–6%) oxygen tensions |
| Media supplementation | Use of serum-based versus defined serum-free media |
| Developmental timeframes | Use of accelerated versus protracted growth systems |
| Growth additives | Supplementation of media with pharmacological versus physiological doses of growth additives |
| Hormonal additives | Supplementation of growth media with FSH and/or LH and estrogen and/or androgens |
| Metabolic requirements | Use of continuous versus sequential systems that modify the culture environment to meet the changing metabolic requirements of follicles and oocytes during in vitro growth and maturation |
| Culture starting and end points | Optimal initial and terminal size for oocytes and follicles at the start and end of follicle culture, species dependent |
| End-point measures | Agreement on best end-point measures, including morphology, viability assays, follicle and oocyte dimensions, growth rates, hormone secretion, cell signalling, molecular markers, IVM, fertilization, embryo production and live births |
| Normality testing | Agreement on how and what markers to measure to definitively test the normality, methylation and epigenetic programming, fertility and developmental competence of in vitro cultured oocytes and the embryos they produce |
Typical biopsies obtained for research are often from women over 35 years with gynecological pathologies and these tissues are inhomogeneous due to age-related follicle depletion. It is difficult to obtain cortical ovarian tissue from young patients for experimentation. Active recruitment of young and fertile tissue donors (e.g. at the time of Caesarean section) should be encouraged and the spare tissues and cells from IVF laboratories should be collected and properly stored for use in research (Schubert et al., 2008). Other possible sources of research material for culture are human fetal ovarian tissue (therapeutic abortion material obtained from prenatal diagnostic units) and ovarian tissue from consenting cancer patients (Sadeu et al., 2006). This material can be obtained via collaboration with a prenatal diagnosis unit and through operative laparoscopists.
Today, a number of different culture systems have been developed that have been partially or fully optimized for a range of different animal models (Picton et al., 2008). Although some systems are more advanced than others, no one system is fully optimized for the complete in vitro growth and maturation of human follicles and oocytes. The variety in system development is vital at this stage in the evolution of human follicle culture as each of the different published systems have strengths and weaknesses that may be usefully exploited to meet the challenges of human follicle culture. A good example of this is the recent development of the alginate systems detailed in this review that have been shown to be an effective vehicle to support the three dimensional growth of pre-antral to antral staged murine follicles with the production of live offspring (Xu et al., 2006b; West et al., 2007). In contrast, a physiological, serum-free culture system has also been shown to be highly effective in supporting antral cavity formation and the production of mature, fertile ovine follicles and oocytes in the absence of any ECM support (Newton et al., 1999). It is likely that the great majority of the advances made in follicle culture with small animals will not prove to be robust enough to be scaled-up to meet the challenges of extended human follicle growth in vitro. At the time of writing, many fundamental biological approaches (Table III) are being scrutinized. For example, there is no clear agreement between follicle culture practitioners within or across species on the efficacy and safety of serum-based culture systems supplemented with pharmacological levels of growth additives (Picton et al., 2008) or of the more challenging, but consistent, serum-free culture systems supplemented with low doses of additives (Newton et al., 1999), based on the physiological requirements to support somatic cell interactions and differentiation (Campbell et al., 1996; Picton et al., 1999a, b) and gamete maturation in vitro (Wynn et al., 1998; Nogueira et al., 2003, 2006; Sutton et al., 2003; Gilchrist and Thompson, 2007; Romero and Smitz, 2008, 2009) in a number of species.
Challenges to fertility preservation research funding in Europe
Studies on early embryogenesis in humans should be allowed as this is the only way to reliably evaluate current fertility preservation techniques. Spare oocytes from human IVF/ICSI practises should be canalized to a dedicated research laboratory to make optimal use of this rare material. In light of the few human oocytes available and the associated ethical considerations, difficult molecular techniques may be used to evaluate genetic and epigenetic damage on well-validated animal models, including mice and larger animals such as the cow, sheep, goat or pig.
A major hurdle which remains to be tackled in the context of human follicle culture is the need for comprehensive testing of human oocytes derived by in vitro growth and maturation. The molecular and epigenetic programming and chromosomal health of in vitro derived human MII oocytes, as well as the fertility of these gametes and evaluation of all aspects of the health, molecular and genetic normality and developmental competence of the embryos they produce, must be tested to quantify the risks of long-term culture. The testing of the health of human oocytes and the embryos they produce is fraught with technical and ethical challenges that must be overcome before in vitro derived human gametes can be used safely to treat patients. Until further extensive research has been conducted, the potential of in vitro oocyte growth and maturation technologies remains to be realized.
A template for the European program is the U.S.-based Oncofertility Consortium, which includes experts from Europe on its advisory board and is involved in all aspects of fertility preservation research from the fundamentals of follicle maturation to the ethics, legal and economic complexities facing the patients and providers. The development of a sister-EU based Oncofertility Consortium will provide worldwide strength by bringing experts together to solve common problems and reduce the time to development of strong technologies. Indeed, the first step in this process has occurred with the ESHRE and ASRM liaison board agreeing to support the first ever meeting on the topic of Oncofertility at the World Health Organization (WHO) to be held September 2010 at Northwestern University in Chicago. This unification of purpose will advance the pace and quality of research in a significant manner and ensure that the patients who are storing their tissues today will have an opportunity to use them in the future.
Development of a central European database on ‘assisted reproduction after cancer’ and a Web site with the addresses of the Centres with expertise in the field should be accessible to all professional bodies and patients from the European Community. A European equivalent to the US Web site ‘www.myoncofertility.com’, which is sponsored by the NIH, should be organized on a multinational level. ESHRE, as the largest European Society fostering advances in the field assisted reproduction, will need to take the initiative in driving a multidisciplinary multinational collaboration via its Task Forces in ‘Basic Science in Reproduction’ and ‘Fertility Preservation’ and involve most of its Special Interest Groups (www.eshre.com).
Conclusion
In short, we have convened to discuss a matter of urgency for young women facing a life-preserving but fertility-threatening cancer treatment. We have identified the opportunities and the gaps in our field both at the basic and translational levels. We have clear ways to tackle the problems and take advantage of the opportunities. Central to this program is the strong collaboration of a global team of investigators and clinicians working on the maturation of the ovarian follicle. This unprecedented team has convened at an unprecedented time—more and more young cancer patients are surviving their disease. Our work will ensure that not only do we learn more about follicle development, but we provide appropriate options to these people who are banking their ovaries on the notion that we will succeed in our work.
Funding
The research performed above was sponsored by the National Institutes of Health, Award Numbers: UL1DE019587, RL1HD058293, RL1HD058294, RL1HD058295, PL1EB008542 (the Oncofertility Consortium) NIH Award Number P51RR00163, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) through cooperative agreement as part of the Specialized Cooperative Center Program in Reproduction and Infertility Research (Grant Numbers U54HD41857 and U54HD18185). The content of this research is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NICHD.
Acknowledgements
All authors greatly acknowledge ESHRE for organising this brainstorming meeting in Brussels (Belgium), in January 2009, on the topics ovarian cortex transplantation and in vitro follicle culture, attended by a multidisciplinary group of European and North American Scientists and Clinicians.
References
- Abir R, Franks S, Mobberly M, Moore P, Margara R, Winston RML. Mechanical isolation and in-vitro growth of preantral and small antral human follicles. Fertil Steril. 1997;68:682–688. doi: 10.1016/s0015-0282(97)00264-1. [DOI] [PubMed] [Google Scholar]
- Abir R, Roizman P, Fisch B, Nitke S, Okon E, Orvieto R, Ben Rafael Z. Pilot study of isolated early human follicles cultured in collagen gels for 24 hours. Hum Reprod. 1999;14:1299–1301. doi: 10.1093/humrep/14.5.1299. [DOI] [PubMed] [Google Scholar]
- Abir R, Fisch B, Nitke S, Okon E, Raz A, Ben Rafael Z. Morphological study of fully and partially isolated early human follicles. Fertil Steril. 2001;75:141–146. doi: 10.1016/s0015-0282(00)01668-x. [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Lindeberg M, Carlsson IB, Stavreus-Evers A, Zhang P, Scott JE, Hovatta O, Skjöldebrand-Sparre L. Receptors for thyroid-stimulating hormone and thyroid hormones in human ovarian tissue. Reprod Biomed Online. 2009;18:337–347. doi: 10.1016/s1472-6483(10)60091-0. [DOI] [PubMed] [Google Scholar]
- Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121:647–653. doi: 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- Amorim CA, Rondina D, Lucci CM, Gonçalves PB, Figueiredo JR, Giorgetti A. Permeability of ovine primordial follicles to different cryoprotectants. Fertil Steril. 2006;85:1077–1081. doi: 10.1016/j.fertnstert.2005.09.041. [DOI] [PubMed] [Google Scholar]
- Amorim CA, Rondina D, Lucci CM, Giorgetti A, de Figueiredo JR, Gonçalves PB. Cryopreservation of sheep primordial follicles. Reprod Domest Anim. 2007;42:53–57. doi: 10.1111/j.1439-0531.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- Amorim CA, Van Langendonckt A, David A, Dolmans MM, Donnez J. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in-vitro culture in a calcium alginate matrix. Hum Reprod. 2009;24:92–99. doi: 10.1093/humrep/den343. [DOI] [PubMed] [Google Scholar]
- Anckaert E, Adriaenssens T, Romero S, Dremier S, Smitz J. Unaltered imprinting establishment of key imprinted genes in mouse oocytes after in-vitro follicle culture under variable follicle-stimulating hormone exposure. Int J Dev Biol. 2009a;53:541–548. doi: 10.1387/ijdb.082619ea. [DOI] [PubMed] [Google Scholar]
- Anckaert E, Adriaenssens T, Romero S, Smitz J. Ammonium accumulation and use of mineral oil overlay do not alter imprinting establishment at three key imprinted genes in mouse oocytes grown and matured in a long-term follicle culture. Biol Reprod. 2009b;81:666–673. doi: 10.1095/biolreprod.109.076810. [DOI] [PubMed] [Google Scholar]
- Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, Schmidt KL, Andersen AN, Ernst E. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–2272. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- Arav A, Revel A, Nathan Y, Bor A, Gacitua H, Yavin S, Gavish Z, Uri M, Elami A. Oocyte recovery, embryo development and ovarian function after cryopreservation and transplantation of whole sheep ovary. Hum Reprod. 2005;20:3554–3559. doi: 10.1093/humrep/dei278. [DOI] [PubMed] [Google Scholar]
- Aubard Y. Ovarian tissue graft: from animal experiment to practice in human. Eur J Obstet Gynecol Reprod Biol. 1999;86:1–3. doi: 10.1016/s0301-2115(99)00027-5. [DOI] [PubMed] [Google Scholar]
- Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196°C. Endocrinol. 1999;140:462–471. doi: 10.1210/endo.140.1.6453. [DOI] [PubMed] [Google Scholar]
- Baird DT, Campbell B, de Souza C, Telfer E. Long-term ovarian function in sheep after ovariectomy and autotransplantation of cryopreserved cortical strips. Eur J Obstet Gynecol Reprod Biol. 2004;113:S55–S59. doi: 10.1016/j.ejogrb.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Berkholtz CB, Shea LD, Woodruff TK. Extracellular matrix functions in follicle maturation. Semin Reprod Med. 2006a;24:262–269. doi: 10.1055/s-2006-948575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkholtz CB, Lai BE, Woodruff TK, Shea LD. Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem Cell Biol. 2006b;126:583–592. doi: 10.1007/s00418-006-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz KR. Subfecundity and anxiety in a nationally representative sample. Soc Sci Med. 2003;56:739–751. doi: 10.1016/s0277-9536(02)00069-2. [DOI] [PubMed] [Google Scholar]
- Bernuci MP, Stouffer RL, Lawson M, Yeoman RR, Zelinski MB. Production of anti-Müllerian hormone (AMH) as a function of rhesus monkey follicle fate and growth rate during three-dimensional culture. Fertil Steril. 2008;90:S274. (P-496) [Google Scholar]
- Bosch P, Hernandez-Fonseca HJ, Miller DM, Wininger JD, Massey JB, Lamb SV, Brackett BG. Development of antral follicles in cryopreserved cat ovarian tissue transplanted to immunodeficient mice. Theriogenology. 2004;61:581–594. doi: 10.1016/s0093-691x(03)00244-9. [DOI] [PubMed] [Google Scholar]
- Braw-Tal R, Yossefi S. Studies in vivo and in vitro on the initiation of follicle growth in the bovine ovary. J Reprod Fertil. 1997;109:165–171. doi: 10.1530/jrf.0.1090165. [DOI] [PubMed] [Google Scholar]
- Callejo J, Salvador C, Miralles A, Vilaseca S, Lailla JM, Balasch J. Long-term ovarian function evaluation after autografting by implantation with fresh and frozen-thawed human ovarian tissue. J Clin Endocrinol Metab. 2001;86:4489–4494. doi: 10.1210/jcem.86.9.7871. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Scaramuzzi RJ, Webb R. Induction and maintenance of oestradiol and immunoreactive inhibin production with FSH by ovine granulosa cells in serum-free media. J Reprod Fertil. 1996;106:7–16. doi: 10.1530/jrf.0.1060007. [DOI] [PubMed] [Google Scholar]
- Carlsson IB, Laitinen MP, Louhio H, Velentzis L, Tuuri T, Aaltonen J, Ritvos O, Winston RML, Hovatta O. Kit ligand and c-kit are expressed during early human ovarian folliculogenesis and their interaction is required for the survival of follicles in long-term culture. Reproduction. 2006a;131:641–649. doi: 10.1530/rep.1.00868. [DOI] [PubMed] [Google Scholar]
- Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen APN, Hovatta O. Anti-Müllerian hormone inhibits initiation of growth of human primordial ovarian follicles in-vitro. Hum Reprod. 2006b;21:2223–2227. doi: 10.1093/humrep/del165. [DOI] [PubMed] [Google Scholar]
- Carroll J, Gosden RG. Transplantation of frozen-thawed mouse primordial follicles. Hum Reprod. 1993;8:1163–1167. doi: 10.1093/oxfordjournals.humrep.a138221. [DOI] [PubMed] [Google Scholar]
- Carter J, Rowland K, Chi D, Brown C, Abu-Rustum N, Castiel M, Barakat R. Gynecologic cancer treatment and the impact of cancer-related infertility. Gynecol Oncol. 2005;97:90–95. doi: 10.1016/j.ygyno.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, et al. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Courbiere B, Massardier J, Salle B, Mazoyer C, Guerin JF, Lornage J. Follicular viability and histological assessment after cryopreservation of whole sheep ovaries with vascular pedicle by vitrification. Fertil Steril. 2005;84:1065–1071. doi: 10.1016/j.fertnstert.2005.03.079. [DOI] [PubMed] [Google Scholar]
- Cushman RA, Fortune JE. Anti-Mullerian hormone inhibits the growth of bovine primary follicles in-vitro. Biol Reprod. 2003;68:322. doi: 10.1093/molehr/gax010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman RA, Wahl CM, Fortune JE. Bovine ovarian cortical pieces grafted to chick embryonic membranes: a model for studies on the activation of primordial follicles. Human Reprod. 2002;17:48–54. doi: 10.1093/humrep/17.1.48. [DOI] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin's disease. Oncologist. 2007;12:1437–1442. doi: 10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15:649–665. doi: 10.1093/humupd/dmp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hooghe TM, Kyama CM, Chai D, Fassbender A, Vodolazkaia A, Bokor A, Mwenda JM. Nonhuman primate models for translational research in endometriosis. Reprod Sci. 2009;16:152–161. doi: 10.1177/1933719108322430. [DOI] [PubMed] [Google Scholar]
- di Clemente N, Ghaffari S, Pepinsky RB, Pieau C, Josso N, Cate RL, Vigier B. A quantitative and interspecific test for biological activity of anti-Müllerian hormone: the fetal ovary aromatase assay. Development. 1992;114:721–727. doi: 10.1242/dev.114.3.721. [DOI] [PubMed] [Google Scholar]
- Dolmans MM, Demylle D, Martinez-Madrid B, Donnez J. Efficacy of in-vitro fertilization after chemotherapy. Fertil Steril. 2005;83:897–901. doi: 10.1016/j.fertnstert.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Dolmans MM, Michaux N, Camboni A, Martinez-Madrid B, Van Langendonckt A, Nottola S, Donnez J. Evaluation of Liberase, a purified enzyme blend, for the isolation of human primordial and primary ovarian follicles. Hum Reprod. 2006;21:413–420. doi: 10.1093/humrep/dei320. [DOI] [PubMed] [Google Scholar]
- Dolmans MM, Martinez-Madrid B, Gadisseux E, Guiot Y, Yuan WY, Torre A, Camboni A, Van Langendonckt A, Donnez J. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction. 2007;134:253–262. doi: 10.1530/REP-07-0131. [DOI] [PubMed] [Google Scholar]
- Dolmans MM, Yuan WY, Camboni A, Torre A, Van Langendonckt A, Martinez-Madrid B, Donnez J. Development of antral follicles after xenografting of isolated small human preantral follicles. Reprod Biomed Online. 2008;16:705–711. doi: 10.1016/s1472-6483(10)60485-3. [DOI] [PubMed] [Google Scholar]
- Dolmans MM, Donnez J, Camboni A, Demylle D, Amorim C, Van Langendonckt A, Pirard C. IVF outcome in patients with orthotopically transplanted ovarian tissue. Hum Reprod. 2009;24:2778–2787. doi: 10.1093/humrep/dep289. [DOI] [PubMed] [Google Scholar]
- Donnez J, Bassil S. Indications for cryopreservation of ovarian tissue. Hum Reprod Update. 1998;4:248–259. doi: 10.1093/humupd/4.3.248. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A. Live birth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, Van Langendonckt A. Restoration of ovarian function after orthotopic (intraovarian and periovarian) transplantation of cryopreserved ovarian tissue in a woman treated by bone marrow transplantation for sickle cell anaemia: case report. Hum Reprod. 2006a;21:183–188. doi: 10.1093/humrep/dei268. [DOI] [PubMed] [Google Scholar]
- Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12:519–535. doi: 10.1093/humupd/dml032. [DOI] [PubMed] [Google Scholar]
- Donnez J, Squifflet J, Van Eyck A-S, Demylle D, Van Langendonckt A, Dolmans MM. Restoration of ovarian function in orthotopically transplanted cryopreserved ovarian tissue: a pilot experience. Reprod Biomed Online. 2008;16:694–704. doi: 10.1016/s1472-6483(10)60484-1. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Further reflections on culture systems for the growth of oocytes in-vitro. Hum Reprod. 1994;9:974–976. doi: 10.1093/oxfordjournals.humrep.a138669. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien MJ. Development in-vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Cahill N, Richards JS. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol. 2008;22:2128–2140. doi: 10.1210/me.2008-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender M, Hildebrandt TB, Paris MC, Colenbrander B, Jewgenow K. High-resolution ultrasonography of xenografted domestic cat ovarian cortex. J Reprod Dev. 2007;53:1023–1034. doi: 10.1262/jrd.19021. [DOI] [PubMed] [Google Scholar]
- Figueiredo JR, Hulshof SC, Van den Hurk R, Ectors FJ, Fontes RS, Nusgens B, Bevers MM, Beckers JF. Development of a combined new mechanical and enzymatic method for the isolation of intact preantral follicles from fetal, calf and adult bovine ovaries. Theriogenology. 1993;40:789–799. doi: 10.1016/0093-691x(93)90214-p. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Kito S, Wandji S-A, Srsen V. Activation of bovine and baboon primordial follicles in-vitro. Theriogenology. 1998;49:441–449. doi: 10.1016/s0093-691x(97)00416-0. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Kito S, Byrd DD. Activation of primordial follicles in-vitro. J Reprod Fertil. 1999;54:439–448. [PubMed] [Google Scholar]
- Fortune JE, Cushman RA, Wahl CM, Kito S. The primordial to primary follicle transition. Mol Cell Endocrinol. 2000;163:53–60. doi: 10.1016/s0303-7207(99)00240-3. [DOI] [PubMed] [Google Scholar]
- Garor R, Abir R. Effects of basic fibroblast growth factor on in-vitro development of human ovarian primordial follicles. Fertil Steril. 2009;91:1967–1975. doi: 10.1016/j.fertnstert.2008.04.075. [DOI] [PubMed] [Google Scholar]
- Gigli I, Cushman RA, Wahl CM, Fortune JE. Evidence for a role for anti-Mullerian hormone in the suppression of follicle activation in mouse ovaries and bovine ovarian cortex grafted beneath the chick chorioallantoic membrane. Mol Reprod Dev. 2005;71:480–488. doi: 10.1002/mrd.20338. [DOI] [PubMed] [Google Scholar]
- Gigli I, Byrd DD, Fortune JE. Effects of oxygen tension and supplements to the culture medium on activation and development of bovine follicles in-vitro. Theriogenology. 2006;66:344–353. doi: 10.1016/j.theriogenology.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, T°hompson JG. Oocyte maturation: emerging concepts and technologies to improve developmental potential in-vitro Theriogenology. 2007;67:6–15. doi: 10.1016/j.theriogenology.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Gosden RG. Restitution of fertility in sterilized mice by transferring primordial ovarian follicles. Hum Reprod. 1990;5:117–122. doi: 10.1093/oxfordjournals.humrep.a137053. [DOI] [PubMed] [Google Scholar]
- Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomised sheep by ovarian autografts stored at −196°C. Hum Reprod. 1994a;9:597–603. doi: 10.1093/oxfordjournals.humrep.a138556. [DOI] [PubMed] [Google Scholar]
- Gosden RG, Boulton MI, Grant K, Webb R. Follicular development from ovarian xenografts in SCID mice. J Reprod Fertil. 1994b;101:619–623. doi: 10.1530/jrf.0.1010619. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1:81–87. doi: 10.1093/oxfordjournals.humrep.a136365. [DOI] [PubMed] [Google Scholar]
- Gougeon A, Busso D. Morphologic and functional determinants of primordial and primary follicles in the monkey ovary. Mol Cell Endocrinol. 2000;163:33–41. doi: 10.1016/s0303-7207(00)00220-3. [DOI] [PubMed] [Google Scholar]
- Greenwald GS, Moor RM. Isolation and preliminary characterization of pig primordial follicles. J Reprod Fertil. 1989;87:561–571. doi: 10.1530/jrf.0.0870561. [DOI] [PubMed] [Google Scholar]
- Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in-vitro. Biol Reprod. 2000;62:1322–1328. doi: 10.1095/biolreprod62.5.1322. [DOI] [PubMed] [Google Scholar]
- Harris SE, Picton HM. Metabolism of follicles and oocytes during growth and maturation. In: Tan SL, Chian RC, Buckett WM, editors. In-Vitro Maturation of Human Oocytes—Basic Science to Clinical Application. Oxon, UK: Informa Health; 2007. pp. 15–36. [Google Scholar]
- Harris SE, Adriens I, Leese HJ, Gosden RG, Picton HM. Carbohydrate metabolism by murine ovarian follicles and oocytes grown in-vitro. Reproduction. 2007;134:415–424. doi: 10.1530/REP-07-0061. [DOI] [PubMed] [Google Scholar]
- Harris SE, Leese HJ, Gosden RG, Picton HM. Pyruvate and oxygen consumption throughout the growth and development of murine oocytes. Mol Reprod Dev. 2009;76:231–238. doi: 10.1002/mrd.20945. [DOI] [PubMed] [Google Scholar]
- Heise M, Koepsel R, Russell AJ, McGee EA. Calcium alginate microencapsulation of ovarian follicles impacts FSH delivery and follicle morphology. Reprod Biol Endocrinol. 2005;3:47. doi: 10.1186/1477-7827-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovatta O, Silye R, Krausz T, Abir R, Winston RML. Extracellular matrix improves the survival of human ovarian fresh and frozen-thawed primordial and primary follicles in long-term culture. Hum Reprod. 1997;12:1032–1036. doi: 10.1093/humrep/12.5.1032. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Wright C, Krausz T, Hardy K, Winston RML. Human primordial, primary and secondary ovarian follicles in long-term culture: effect of partial isolation. Hum Reprod. 1999;14:2519–2524. doi: 10.1093/humrep/14.10.2519. [DOI] [PubMed] [Google Scholar]
- Hreinsson J, Scott J, Swahn ML, Rasmussen C, Hsueh A, Hovatta O. Growth development promoting growth factor 9 (GDF 9) promotes the growth and survival of human ovarian follicles in organ culture. J Clin Endocrinol Metab. 2002;87:316–321. doi: 10.1210/jcem.87.1.8185. [DOI] [PubMed] [Google Scholar]
- Hsueh AJ, McGee EA, Hayashi M, Hsu SY. Hormonal regulation of early follicle development in the rat ovary. Mol Cell Endocrinol. 2000;163:95–100. doi: 10.1016/s0303-7207(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Huntriss J, Picton HM. Epigenetic consequences of assisted reproduction and infertility on the human preimplantation embryo. Hum Fertil. 2008;11:85–94. doi: 10.1080/14647270802116250. [DOI] [PubMed] [Google Scholar]
- Hutson J, Ikawa H, Donahoe PK. The ontogeny of Mullerian inhibiting substance in the gonads of the chicken. J Ped Surg. 1981;16:822–827. doi: 10.1016/s0022-3468(81)80827-5. [DOI] [PubMed] [Google Scholar]
- Imhof M, Bergmeister H, Lipovac M, Rudas M, Hofstetter G, Huber J. Orthotopic microvascular reanastomosis of whole cryopreserved ovine ovaries resulting in pregnancy and live birth. Fertil Steril. 2006;85:1208–1215. doi: 10.1016/j.fertnstert.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Irving-Rodgers HF, Rodgers RJ. Extracellular matrix of the developing ovarian follicle. Semin Reprod Med. 2006;24:195–203. doi: 10.1055/s-2006-948549. [DOI] [PubMed] [Google Scholar]
- Irving-Rodgers HF, Morris S, Collett RA, Peura TT, Davy M, Thompson JG, Mason HD, Rodgers RJ. Phenotypes of the ovarian follicular basal lamina predict developmental competence of oocytes. Hum Reprod. 2009;24:936–944. doi: 10.1093/humrep/den447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnukainen K, Hou M, Petersen C, Setchell B, Söder O. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res. 2001;61:706–710. [PubMed] [Google Scholar]
- Jeremias E, Bedaiwy MA, Gurunluoglu R, Biscotti CV, Siemionow M, Falcone T. Heterotopic autotransplantation of the ovary with microvascular anastomosis: a novel surgical technique. Fertil Steril. 2002;77:1278–1282. doi: 10.1016/s0015-0282(02)03110-2. [DOI] [PubMed] [Google Scholar]
- Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewgenow K. Impact of peptide growth factors on the culture of small preantral follicles of domestic cats. Theriogenology. 1996;45:889–895. doi: 10.1016/0093-691x(96)00017-9. [DOI] [PubMed] [Google Scholar]
- Jewgenow K. Role of media, protein and energy supplements on maintenance of morphology and DNA-synthesis of small preantral domestic cat follicles during short-term culture. Theriogenology. 1998;49:1567–1577. doi: 10.1016/s0093-691x(98)00102-2. [DOI] [PubMed] [Google Scholar]
- Jewgenow K, Paris MC. Preservation of female germ cells from ovaries of cat species. Theriogenology. 2006;66:93–100. doi: 10.1016/j.theriogenology.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Jewgenow K, Pitra C. Hormone-controlled culture of secondary follicles of domestic cats. Theriogenology. 1993;39:527–535. doi: 10.1016/0093-691x(93)90394-k. [DOI] [PubMed] [Google Scholar]
- Jewgenow K, Blottner S, Lengwinat T, Meyer HH. New methods for gamete rescue from gonads of nondomestic felids. J Reprod Fertil Suppl. 1997;51:33–39. [PubMed] [Google Scholar]
- Johnson J, McLaughlin M, Yu J, Telfer EE. Inhibition of mTOR kinase in cultured bovine ovarian cortex results in altered follicle dynamics. Reprod Sci. 2009;16 Abs. No. 1031. [Google Scholar]
- Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod Biomed Online. 2009;18:568–577. doi: 10.1016/s1472-6483(10)60136-8. [DOI] [PubMed] [Google Scholar]
- Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, Hreinsson J, Hovatta O. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24:1670–1683. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- Kim SS, Radford J, Harris M, Varley J, Rutherford AJ, Lieberman B, Shalet S, Gosden R. Ovarian tissue harvested from lymphoma patients to preserve fertility may be safe for autotransplantation. Hum Reprod. 2001;16:2056–2060. doi: 10.1093/humrep/16.10.2056. [DOI] [PubMed] [Google Scholar]
- Kim SS, Lee WS, Chung MK, Lee HC, Lee HH, Hill D. Long-term ovarian function and fertility after heterotopic autotransplantation of cryobanked human ovarian tissue: 8-year experience in cancer patients. Fertil Steril. 2009;91:2349–2354. doi: 10.1016/j.fertnstert.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Kong HJ, Smith MK, Mooney DJ. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials. 2003;24:4023–4029. doi: 10.1016/s0142-9612(03)00295-3. [DOI] [PubMed] [Google Scholar]
- Kreeger PK, Woodruff TK, Shea LD. Murine granulosa cell morphology and function are regulated by a synthetic. Arg-Gly-Asp matrix Mol Cell Endocrinol. 2003;205:1–10. doi: 10.1016/s0303-7207(03)00209-0. [DOI] [PubMed] [Google Scholar]
- Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in-vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73:942–950. doi: 10.1095/biolreprod.105.042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in-vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714–723. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenie S, Cortvrindt R, Adriaenssens T, Smitz J. A reproducible two-step culture system for isolated primary mouse ovarian follicles as single functional units. Biol Reprod. 2004;71:1730–1738. doi: 10.1095/biolreprod.104.028415. [DOI] [PubMed] [Google Scholar]
- Lie Fong S, Lugtenburg PJ, Schipper I, Themmen AP, de Jong FH, Sonneveld P, Laven JS. Anti-Müllerian hormone as a marker of ovarian function in women after chemotherapy and radiotherapy for haematological malignancies. Hum Reprod. 2008;23:674–678. doi: 10.1093/humrep/dem392. [DOI] [PubMed] [Google Scholar]
- Lie Fong S, Laven JS, Hakvoort-Cammel FG, Schipper I, Visser JA, Themmen AP, de Jong FH, Van den Heuvel-Eibrink MM. Assessment of ovarian reserve in adult childhood cancer survivors using anti-Müllerian hormone. Hum Reprod. 2009;24:982–990. doi: 10.1093/humrep/den487. [DOI] [PubMed] [Google Scholar]
- Louhio H, Hovatta O, Sjöberg J, Tuuri T. The effects of insulin, insulin-like growth factor I (IGF-I) and insulin-like growth factor II (IGF-II) on human ovarian follicles in long term culture. Mol Hum Reprod. 2000;6:694–698. doi: 10.1093/molehr/6.8.694. [DOI] [PubMed] [Google Scholar]
- Lucci CM, Amorim CA, Rodrigues AP, Figueiredo JR, Báo SN, Silva JR, Gonçalves PB. Study of preantral follicle population in situ and after mechanical isolation from caprine ovaries at different reproductive stages. Anim Reprod Sci. 1999;56:223–236. doi: 10.1016/s0378-4320(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Martinez-Madrid B, Dolmans MM, Van Eyck AS, Van Langendonckt A, Defrère S, Donnez J. Ficoll density gradient method for recovery of isolated human ovarian primordial follicles. Fertil Steril. 2004;82:1648–1653. doi: 10.1016/j.fertnstert.2004.05.084. [DOI] [PubMed] [Google Scholar]
- Martinez-Madrid B, Camboni A, Dolmans MM, Nottola S, Van Langendonckt A, Donnez J. Apoptosis and ultrastructural assessment after cryopreservation of whole human ovaries with their vascular pedicle. Fertil Steril. 2007;87:1153–1165. doi: 10.1016/j.fertnstert.2006.11.019. [DOI] [PubMed] [Google Scholar]
- McCaffery FH, Leask R, Riley SC, Telfer EE. Culture of bovine preantral follicles in a serum-free system: markers for assessment of growth and development. Biol Reprod. 2000;63:267–273. doi: 10.1095/biolreprod63.1.267. [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- McLaughlin EA, McIver SC. Awakening the oocyte: controlling primordial follicle development. Reproduction. 2009;137:1–11. doi: 10.1530/REP-08-0118. [DOI] [PubMed] [Google Scholar]
- Meirow D, Ben Yehuda D, Prus D, Poliack A, Schenker J, Rachmilewitz E, Lewin A. Ovarian tissue banking in patients with Hodgkin's disease: is it safe? Fertil Steril. 1998;69:996–998. doi: 10.1016/s0015-0282(98)00093-4. [DOI] [PubMed] [Google Scholar]
- Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- Mizunuma H, Liu X, Andoh K, Abe Y, Kobayashi J, Yamada K, Yokota H, Ibuki Y, Hasegawa Y. Activin from secondary follicles causes small preantral follicles to remain dormant at the resting stage. Endocrinology. 1999;140:37–42. doi: 10.1210/endo.140.1.6409. [DOI] [PubMed] [Google Scholar]
- Muruvi W, Fortune JE. Kit ligand-KIT interaction is required for follicle activation and mediates insulin-stimulated activation of bovine primordial follicles in-vitro. Biol Reprod. 2009;81:200. [Google Scholar]
- Muruvi W, Picton HM, Rodway RG, Joyce IM. In-vitro growth of oocytes from primordial follicles isolated from frozen-thawed lamb ovaries. Theriogenology. 2005;64:1357–1370. doi: 10.1016/j.theriogenology.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Muruvi W, Picton HM, Rodway RG, Joyce IM. In-vitro growth and differentiation of primary follicles isolated from cryopreserved sheep ovarian tissue. Anim Reprod Sci. 2009;112:36–50. doi: 10.1016/j.anireprosci.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Nayudu PL, Wu J, Michelmann HW. In vitro development of marmoset monkey oocytes by pre-antral follicle culture. Reprod Domest Anim. 2003;38:90–96. doi: 10.1046/j.1439-0531.2003.00398.x. [DOI] [PubMed] [Google Scholar]
- Newton H, Picton HM, Gosden RG. In-vitro growth of oocyte–granulosa cell complexes isolated from cryopreserved ovine tissue. J Reprod Fertil. 1999;115:141–150. doi: 10.1530/jrf.0.1150141. [DOI] [PubMed] [Google Scholar]
- Nisolle M, Casanas-Roux F, Qu J, Motta P, Donnez J. Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. Fertil Steril. 2000;74:122–129. doi: 10.1016/s0015-0282(00)00548-3. [DOI] [PubMed] [Google Scholar]
- Nogueira D, Albano C, Adriaenssens T, Cortvrindt R, Bourgain C, Devroey P, Smitz J. Human oocytes reversibly arrested in prophase I by phosphodiesterase Type 3 inhibitor in-vitro. Biol Reprod. 2003;69:1042–1052. doi: 10.1095/biolreprod.103.015982. [DOI] [PubMed] [Google Scholar]
- Nogueira D, Ron-El R, Friedler S, Schachter M, Raziel A, Cortvrindt R, Smitz J. Meiotic arrest in-vitro by phosphodiesterase 3-inhibitor enhances maturation capacity of human oocytes and allows subsequent embryonic development. Biol Reprod. 2006;74:177–184. doi: 10.1095/biolreprod.105.040485. [DOI] [PubMed] [Google Scholar]
- Nogueira D, Romero S, Vanhoutte L, de Matos DG, Smitz J. Oocyte in-vitro maturation. In: Gardner DK, Weissman A, Howles CM, Shoham Z, editors. Textbook of Assisted Reproductive Technologies: Laboratory and Clinical Perspectives. 3rd edn. UK: Informa Health Care; 2008. pp. 111–154. Chapter 9. [Google Scholar]
- Nottola SA, Camboni A, Van Langendonckt A, Demylle D, Macchiarelli G, Dolmans MM, Martinez-Madrid B, Correr S, Donnez J. Cryopreservation and xenotransplantation of human ovarian tissue: an ultrastructural study. Fertil Steril. 2008;90:23–32. doi: 10.1016/j.fertnstert.2007.05.069. [DOI] [PubMed] [Google Scholar]
- O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in-vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- Oktay K, Nugent D, Newton H, Salha O, Chatterjee P, Gosden RG. Isolation and characterization of primordial follicles from fresh and cryopreserved human ovarian tissue. Fertil Steril. 1997;67:481–486. doi: 10.1016/s0015-0282(97)80073-8. [DOI] [PubMed] [Google Scholar]
- Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. J Am Med Assoc. 2001;286:1490–1493. doi: 10.1001/jama.286.12.1490. [DOI] [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Rosenwaks Z, Rucinski J. A technique for transplantation of ovarian cortical strips to the forearm. Fertil Steril. 2003;80:193–198. doi: 10.1016/s0015-0282(03)00568-5. [DOI] [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, Opsahl M, Rosenwaks Z. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363:837–840. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003;9:1013–1021. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, Rosenberg R, Przypyszny M, Rein A, Winer EP. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–4183. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- Pesty A, Miyara F, Debey P, Lefevre B, Poirot C. Multiparameter assessment of mouse oogenesis during follicular growth in-vitro. Mol Hum Reprod. 2007;13:3–9. doi: 10.1093/molehr/gal089. [DOI] [PubMed] [Google Scholar]
- Picton HM. Activation of follicle development: the primordial follicle. Theriogenology. 2001;55:1193–1210. doi: 10.1016/s0093-691x(01)00478-2. [DOI] [PubMed] [Google Scholar]
- Picton HM, Campbell BK, Hunter MG. Maintenance of oestradiol production and cytochrome P450 aromatase enzyme messenger ribonucleic acid expression in long-term serum-free cultures of porcine granulosa cells. J Reprod Fertil. 1999a;115:67–77. doi: 10.1530/jrf.0.1150067. [DOI] [PubMed] [Google Scholar]
- Picton HM, Mkandla A, Salha O, Wynn P, Gosden RG. Initiation of human primordial follicle growth in-vitro in ultra-thin slices of ovarian cortex. Hum Reprod. 1999b;14 Abstract Book 1, 11 (0-020) [Google Scholar]
- Picton HM, Harris SE, Muruvi W, Chambers EL. The in-vitro growth and maturation of follicles. Reproduction. 2008;136:703–715. doi: 10.1530/REP-08-0290. [DOI] [PubMed] [Google Scholar]
- Piver P, Amiot C, Agnani G, Pech JC, Rohrlich PS, Vidal E, Aubard Y, Roux C. Two pregnancies obtained after a new technique of autotransplantation of cryopreserved ovarian tissue. Hum Reprod. 2009;24:i14–i16. O-035. [Google Scholar]
- Pope CE, Gomez MC, Dresser BL. In-vitro production and transfer of cat embryos in the 21st century. Theriogenology. 2006;66:59–71. doi: 10.1016/j.theriogenology.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Romero S, Smitz J. Improvement of in-vitro culture of mouse cumulus-oocyte complexes using PDE3-inhibitor followed by meiosis induction with epiregulin. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2008.10.016. doi:10.1016/j.fertnstert.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Romero S, Smitz J. Epiregulin can effectively mature isolated COCs, but fails as a substitute for the hCG/EGF stimulus on cultured follicles. Reproduction. 2009;137:997–1005. doi: 10.1530/REP-08-0523. [DOI] [PubMed] [Google Scholar]
- Roy SK, Kole AR. Ovarian transforming growth factor-b (TGF-β) receptors: in-vitro effects of follicle stimulating hormone, epidermal growth factor and TGF-β on receptor expression in human preantral follicles. Mol Hum Reprod. 1998;4:207–214. doi: 10.1093/molehr/4.3.207. [DOI] [PubMed] [Google Scholar]
- Roy SK, Treacy BJ. Isolation and long-term culture of human preantral follicles. Fertil Steril. 1993;59:783–790. [PubMed] [Google Scholar]
- Sadeu JC, Smitz J. Growth differentiation factor-9 and anti-Mullerian hormone expression in cultured follicles from frozen-thawed ovarian tissue. Reprod Biomed Online. 2008;17:537–548. doi: 10.1016/s1472-6483(10)60242-8. [DOI] [PubMed] [Google Scholar]
- Sadeu JC, Cortvrindt R, Ron-El R, Kasterstein E, Smitz J. Morphological and ultrastructural evaluation of cultured frozen-thawed human fetal ovarian tissue. Fertil Steril. 2006;85:1130–1141. doi: 10.1016/j.fertnstert.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Sadeu JC, Mazoyer C, Smitz J. Human follicle culture in-vitro. In: Rizk B, Garcia-Velasco J, Sallam H, Makrigiannakis A, editors. Infertility and Assisted Reproduction. UK: Cambridge University Press; 2008. pp. 25–38. [Google Scholar]
- Salle B, Demirci B, Franck M, Rudigoz RC, Guerin JF, Lornage J. Normal pregnancies and live births after autograft of frozen-thawed hemi-ovaries into ewes. Fertil Steril. 2002;77:403–408. doi: 10.1016/s0015-0282(01)02960-0. [DOI] [PubMed] [Google Scholar]
- Schmidt KTL, Kryger-Baggesen N, Byskov AG, Yding Andersen C. Anti-Mullerian hormone initiates growth of human primordial follicles in-vitro. Mol Cell Endocrinol. 2005;234:87–93. doi: 10.1016/j.mce.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Schubert B, Canis M, Darcha C, Artonne C, Smitz J, Grizard G. Follicular growth and estradiol follow-up after subcutaneous xenografting of fresh and cryopreserved human ovarian tissue. Fertil Steril. 2008;89:1787–1794. doi: 10.1016/j.fertnstert.2007.03.101. [DOI] [PubMed] [Google Scholar]
- Scott J, Zhang P, Hreinsson J, Hovatta O. Cyclic guanosine monophosphate improves the survival of human ovarian follicles in culture. Reprod Biomed Online. 2004a;8:319–324. doi: 10.1016/s1472-6483(10)60912-1. [DOI] [PubMed] [Google Scholar]
- Scott J, Carlsson IB, Bavister B, Hovatta O. Human ovarian tissue cultures: extracellular matrix composition, coating density and tissue dimensions. Reprod Biomed Online. 2004b;9:287–293. doi: 10.1016/s1472-6483(10)62143-8. [DOI] [PubMed] [Google Scholar]
- Seshadri T, Gook D, Lade S, Spencer A, Grigg A, Tiedemann K, McKendrick J, Mitchell P, Stern C, Seymour JF. Lack of evidence of disease contamination in ovarian tissue harvested for cryopreservation from patients with Hodgkin lymphoma and analysis of factors predictive of oocyte yield. Br J Cancer. 2006;94:1007–1010. doi: 10.1038/sj.bjc.6603050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber SJ, Gosden RG. Ovarian transplantation in a series of monozygotic twins discordant for ovarian failure. N Engl J Med. 2007;356:1382–1384. doi: 10.1056/NEJMc066574. [DOI] [PubMed] [Google Scholar]
- Silber SJ, Lenahan KM, Levine DJ, Pineda JA, Gorman KS, Friez MJ, Crawford EC, Gosden RG. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N Engl J Med. 2005;353:58–63. doi: 10.1056/NEJMoa043157. [DOI] [PubMed] [Google Scholar]
- Silber SJ, Derosa M, Pineda J, Lenahan K, Grenia D, Gorman K, Gosden RG. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod. 2008a;23:1531–1537. doi: 10.1093/humrep/den032. [DOI] [PubMed] [Google Scholar]
- Silber SJ, Grudzinskas G, Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. N Engl J Med. 2008b;359:2617–2618. doi: 10.1056/NEJMc0804321. [DOI] [PubMed] [Google Scholar]
- Smitz J, Cortvrindt R. The earliest stages of folliculogenesis in-vitro. Reproduction. 2002;123:185–202. doi: 10.1530/rep.0.1230185. [DOI] [PubMed] [Google Scholar]
- Smitz J, Picton HM, Platteau P, Rutherford A, Cortvrindt R, Clyde J, Nogueira D, Devroey P, Lyby K, Grondahl C. Principal findings from a multicenter trial investigating the safety of follicular-fluid meiosis-activating sterol for in-vitro maturation of human cumulus-enclosed oocytes. Fertil Steril. 2007;87:949–964. doi: 10.1016/j.fertnstert.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Sutton ML, Gilchrist RB, Thompson JG. Effects of in-vivo and in-vitro environments on the metabolism of the cumulus-oocyte complex and its influence on oocyte developmental capacity. Hum Reprod Update. 2003;9:35–48. doi: 10.1093/humupd/dmg009. [DOI] [PubMed] [Google Scholar]
- Telfer EE, McLaughlin M. Natural history of the mammalian oocyte RBM online. 2007;15:288–295. doi: 10.1016/s1472-6483(10)60341-0. [DOI] [PubMed] [Google Scholar]
- Telfer EE, Binnie JP, McCaffery FH, Campbell BK. In-vitro development of oocytes from porcine and bovine primary follicles. Mol Cell Endocrinol. 2000;163:117–123. doi: 10.1016/s0303-7207(00)00216-1. [DOI] [PubMed] [Google Scholar]
- Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step, serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- Telfer EE, McLaughlin M, Kini S, Thong KJ, Anderson RA, Johnson J. Inhibition of mTOR kinase in cultured human ovarian cortex results in altered follicle dynamics. Hum Reprod. 2009;24:O-197. p i80. [Google Scholar]
- Teng CS. Quantification of Müllerian inhibiting substance in developing chick gonads by a competitive enzyme-linked immunosorbent assay. Dev Biol. 1987;123:255–263. doi: 10.1016/0012-1606(87)90447-7. [DOI] [PubMed] [Google Scholar]
- Thomas FH, Leask R, Srsen V, Riley SC, Spears N, Telfer EE. Effect of ascorbic acid on health and morphology of bovine preantral follicles during long-term culture. Reproduction. 2001;122:487–495. doi: 10.1530/rep.0.1220487. [DOI] [PubMed] [Google Scholar]
- Thomas FH, Walters KA, Telfer EE. How to make a good oocyte: An update on in-vitro models to study follicle regulation. Hum Reprod Update. 2003a;9:1–15. doi: 10.1093/humupd/dmg042. [DOI] [PubMed] [Google Scholar]
- Thomas FH, Armstrong DG, Telfer EE. Activin promotes oocyte development in ovine preantral follicles in-vitro. Reprod Biol Endocrinol. 2003b;1:76. doi: 10.1186/1477-7827-1-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas FH, Armstrong DG, Campbell BK, Telfer EE. Effects of insulin-like growth factor-1 bioavailability on bovine preantral follicular development in-vitro. Reproduction. 2007;133:1121–1128. doi: 10.1530/REP-06-0382. [DOI] [PubMed] [Google Scholar]
- van Beek RD, van den Heuvel-Eibrink MM, Laven JSE, de Jong FH, Themmen APN, Hakvoort-Cammel FG, van den Bos C, van den Berg H, Pieters R, de Muinck Keizer-Schrama SM. Anti-Müllerian hormone is a sensitive serum marker for gonadal function in women treated for Hodgkin's lymphoma during childhood. J Clin Endocrinol Metab. 2007;92:3869–3874. doi: 10.1210/jc.2006-2374. [DOI] [PubMed] [Google Scholar]
- Van den Broecke R, Van der Elst J, Liu J, Hovatta O, Dhont M. The female-to-male transsexual patient: a source for human ovarian cortical tissue for experimental use. Hum Reprod. 2001;16:145–147. doi: 10.1093/humrep/16.1.145. [DOI] [PubMed] [Google Scholar]
- Van den Hurk R, Zhao J. Formation of mammalian oocytes and their growth, differentiation and maturation within ovarian follicles. Theriogenology. 2005;63:1717–1751. doi: 10.1016/j.theriogenology.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Van Eyck AS, Jordan BF, Gallez B, Heilier JF, Van Langendonckt A, Donnez J. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009a;92:374–381. doi: 10.1016/j.fertnstert.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Van Eyck AS, Bouzin C, Feron O, Romeu L, Van Langendonckt A, Donnez J, Dolmans MM. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2009b doi: 10.1016/j.fertnstert.2009.04.048. doi:10.1016/j.fertnstert.2009.04.048. [DOI] [PubMed] [Google Scholar]
- Walters KA, Binnie JP, Campbell BK, Armstrong DG, Telfer EE. The effects of IGF-I on bovine follicle development and IGFBP-2 expression are dose and stage dependent. Reproduction. 2006;131:515–523. doi: 10.1530/rep.1.00682. [DOI] [PubMed] [Google Scholar]
- Wandji S-A, Eppig JJ, Fortune JE. FSH and growth factors affect the growth and endocrine function in-vitro of granulosa cells of bovine preantral follicles. Theriogenology. 1996a;45:817–832. doi: 10.1016/0093-691x(96)00011-8. [DOI] [PubMed] [Google Scholar]
- Wandji S-A, Srsen V, Voss AK, Eppig JJ, Fortune JE. Initiation in-vitro of growth of bovine primordial follicles. Biol Reprod. 1996b;55:942–948. doi: 10.1095/biolreprod55.5.942. [DOI] [PubMed] [Google Scholar]
- Wandji S-A, Srsen V, Nathanielsz PW, Eppig JJ, Fortune JE. Initiation of growth of baboon primordial follicles in-vitro. Hum Reprod. 1997;12:1993–2001. doi: 10.1093/humrep/12.9.1993. [DOI] [PubMed] [Google Scholar]
- West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in-vitro follicle development. Biomaterials. 2007;28:4439–4448. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Farrell ER, Xu M, Gomberg MA, Chow YH, Woodruff TK, Shea LD. The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol Reprod. 2009;80:432–439. doi: 10.1095/biolreprod.108.071142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildt DE, Monfort SL, Donoghue AM, Johnston LA, Howard J. Embryogenesis in conservation biology—or, how to make an endangered species embryo. Theriogenology. 1992;37:161–184. [Google Scholar]
- Wright C, Hovatta O, Margara R, Trew G, Winston RML, Franks S, Hardy K. Effects of FSH and serum substitution on the in-vitro growth and development of early human follicles. Hum Reprod. 1999;14:1555–1562. doi: 10.1093/humrep/14.6.1555. [DOI] [PubMed] [Google Scholar]
- Wu J, Emery BR, Carrell DT. In-vitro growth, maturation, fertilization, and embryonic development of oocytes from porcine preantral follicles. Biol Reprod. 2001;64:375–381. doi: 10.1095/biolreprod64.1.375. [DOI] [PubMed] [Google Scholar]
- Wynn P, Picton HM, Krapez JA, Rutherford AJ, Balen AH, Gosden RG. Pretreatment with follicle stimulating hormone promotes the numbers of human oocytes reaching metaphase II by in-vitro maturation. Hum Reprod. 1998;13:3132–3138. doi: 10.1093/humrep/13.11.3132. [DOI] [PubMed] [Google Scholar]
- Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in-vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006a;75:916–923. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Engineering. 2006b;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009a;81:587–594. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009b;103:378–386. doi: 10.1002/bit.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009c;24:2531–2540. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski MB, Bernuci M, Lawson M, Yeoman RR, Stouffer RL. Culture of preantral follicles from prepubertal and adult rhesus monkeys in a three-dimensional (3D) alginate matrix: FSH required for growth to early antral stage. Fertil Steril. 2008;90:S275. (P-497) [Google Scholar]