Abstract
BACKGROUND
An ectopic pregnancy is a pregnancy which occurs outside of the uterine cavity, and over 98% implant in the Fallopian tube. Tubal ectopic pregnancy remains the most common cause of maternal mortality in the first trimester of pregnancy. The epidemiological risk factors for tubal ectopic pregnancy are well established and include: tubal damage as a result of surgery or infection (particularly Chlamydia trachomatis), smoking and in vitro fertilization. This review appraises the data to date researching the aetiology of tubal ectopic pregnancy.
METHODS
Scientific literature was searched for studies investigating the underlying aetiology of tubal ectopic pregnancy.
RESULTS
Existing data addressing the underlying cause of tubal ectopic pregnancy are mostly descriptive. There are currently few good animal models of tubal ectopic pregnancy. There are limited data explaining the link between risk factors and tubal implantation.
CONCLUSIONS
Current evidence supports the hypothesis that tubal ectopic pregnancy is caused by a combination of retention of the embryo within the Fallopian tube due to impaired embryo-tubal transport and alterations in the tubal environment allowing early implantation to occur. Future studies are needed that address the functional consequences of infection and smoking on Fallopian tube physiology. A greater understanding of the aetiology of tubal ectopic pregnancy is critical for the development of improved preventative measures, the advancement of diagnostic screening methods and the development of novel treatments.
Keywords: Fallopian tube, tubal ectopic pregnancy, Chlamydia trachomatis, smoking, embryo implantation
Introduction
An ectopic, or extra-uterine, pregnancy is defined as a pregnancy implanted outside of the uterine cavity with over 98% implanting in the Fallopian tube (Walker, 2007; Varma and Gupta, 2009). Approximately 1–2% of all pregnancies in Europe and the USA are ectopic and in the Western world tubal ectopic pregnancy remains the most common cause of maternal mortality in the first trimester of pregnancy (Farquhar, 2005; Varma and Gupta, 2009). In the developing world, the incidence is much higher and 1 in 10 women admitted with a diagnosis of tubal ectopic pregnancy ultimately die from the condition (Leke et al., 2004). The major risk factors for tubal ectopic pregnancy include: tubal damage as a result of surgery or infection (particularly Chlamydia trachomatis), smoking and in vitro fertilization (IVF; Pisarska et al., 1998; Tay et al., 2000; Bouyer et al., 2003; Varma and Gupta, 2009). Current data addressing the underlying cause of tubal ectopic pregnancy are mostly descriptive. However, it supports the hypothesis that it is caused by a combination of (i) retention of the embryo within the Fallopian tube due to impaired embryo-tubal transport and (ii) alterations in the tubal environment allowing early implantation to occur. Herein, we discuss these data, including the contribution of the above risk factors and the limitations of the studies to date.
Methods
Pubmed was searched using the terms ‘Fallopian tube’ and ‘ectopic pregnancy’ for studies published between 1999 and 2009. For a study to be included, it needed to be primarily focused on elucidating a functional mechanism behind one of the known risk factors for tubal ectopic pregnancy, such as cigarette smoking, C. trachomatis infection or IVF. Studies which were solely epidemiological in nature were not included.
Embryo-tubal transport
Tubal smooth muscle contractility and ciliary beat activity
Transport of the embryo through the Fallopian tube is controlled by smooth muscle contraction and ciliary beating (Halbert et al., 1976; Lyons et al., 2006b).
Muscle contraction produces oscillating movements in the isthmus of the tube that can modulate (speed or slow) transport through the Fallopian tube (Perez et al., 2000). Stimulation of alpha adrenergic receptors promotes contraction of the oviductal muscles, although stimulation of beta receptors inhibits contractions (Samuelson and Sjostrand, 1986). Adrenergic neurons may not be the primary means for controlling embryo transport since experimental depletion or inhibition of these neurons does not prevent transport nor decrease fertility (Eddy and Pauerstein, 1980). The sex steroid hormones and other factors produced by the oviduct itself, such as prostaglandins (Ziganshin et al., 2004; Wanggren et al., 2006; Wanggren et al., 2008), nitric oxide (NO; Ekerhovd et al., 1997; Ekerhovd and Norstrom, 2004), prostacyclin (Arbab et al., 2002) and cAMP (Lindblom et al., 1980) may also modulate muscle contraction and play a lead role in embryo transport (Lindblom et al., 1978; Arbab et al., 2002).
In addition to tubal smooth muscle contractility, embryo-tubal transport is influenced by ciliary activity (Jansen, 1984), which is controlled by factors such as sex steroid hormones (Paltieli et al., 2000) and IL-6 (Papathanasiou et al., 2008). Factors found within ovarian follicular fluid, which enter the Fallopian tube at the time of ovulation, have also been shown to affect tubal ciliary beat frequency (Lyons et al., 2006a). Although the relative importance of each of these mechanisms is unclear, there is evidence that ciliary action plays a dominant role in the movement of the embryo. When tubal smooth muscle activity is inhibited by isoproterenol, a β-adrenergic agonist, there is no difference in total embryo transit times through the Fallopian tube, suggesting that the cilia alone are capable of transporting the embryo within a suitable time frame to allow successful intrauterine implantation to occur (Halbert et al., 1976, 1989). Furthermore a Fallopian tube containing a tubal ectopic pregnancy demonstrates a marked reduction in the number of ciliated cells in comparison with an intrauterine pregnancy of the same gestational stage (Vasquez et al., 1983). Marked de-ciliation is also sometimes seen subsequent to a tubal ectopic pregnancy and in biopsies from women undergoing tubal surgery who later develop a tubal ectopic pregnancy (Vasquez et al., 1983).
Nitric oxide and nitric oxide synthase
NO is reported to play a role in the regulation of smooth muscle cell tone, platelet aggregation, cell growth, apoptosis, neurotransmission and infection-induced immune reactions (Afanas'ev, 2007). NO is produced from L-arginine in a reaction that is catalyzed by nitric oxide synthase (NOS), of which there are three isoforms: constitutively expressed endothelial (eNOS), neural (nNOS) and inducible (iNOS; Moncada and Higgs, 1993). NO is expressed by the Fallopian tube and has been shown to have a relaxing effect on tubal smooth muscle. Administration of iNOS inhibitors has been shown to increase tubal smooth muscle contractility in the rat (Ekerhovd et al., 1997; Perez et al., 2000). NO synthesized by iNOS has also been shown to increase ciliary beat frequency in epithelial cells of the airway (Jain et al., 1995), suggesting that NO may play a similar role in controlling ciliary beat in the Fallopian tube. In a recent study, iNOS mRNA and protein levels were shown to be greater in the Fallopian tube of women with tubal ectopic pregnancy compared with pseudo-pregnant women (Al-Azemi et al., 2009a). Together these results suggest that, in tubal ectopic pregnancy, increased iNOS levels contribute to increased expression of NO which may result in altered Fallopian tube smooth muscle contractility and/or ciliary beat frequency, which may lead to embryo retention.
Interstitial cells of Cajal
Interstitial cells of Cajals (ICCs) have recently been shown to be expressed in the human Fallopian tube (Popescu et al., 2005; Shafik et al., 2005). In the gut, ICCs play a role in muscle contractility by mediating neurotransmission between enteric nerves and the smooth muscle (Sanders, 1996). In mice, chlamydial infection was found to cause a loss of ICCs along the length of the oviduct and resulted in an absence of spontaneous contractions of the oviduct (Dixon et al., 2009). Cyclooxygenase-2 (COX-2) and iNOS were found to be up-regulated in the oviducts of these Chlamydia-infected mice and blockage of NOS prevented the loss of spontaneous contractions. These results suggest that the inflammatory response due to chlamydial infection results in up-regulation of iNOS causing a disruption in pacemaker activity of the ICCs. It is possible that this loss of pacemaker activity results in a disruption of smooth muscle contractility which may slow transport of the embryo through the oviduct.
Prokineticins
The prokineticins, PROK1 and PROK2, have angiogenic actions but are primarily known for their function as regulators of specific and potent contractions of smooth muscle (Maldonado-Perez et al., 2007). They are the cognate ligands for two closely homologous G protein-coupled receptors, PROK receptor (PROKR) 1 and PROKR2, through which either PROK can signal (Maldonado-Perez et al., 2007). PROKs were initially reported to be expressed in the gastrointestinal tract, where they were shown to directly stimulate contraction of the ileum longitudinal muscle of guinea pigs (Li et al., 2001). The opposing effect of PROKs on relaxation through a NO-mediated mechanism has also been reported recently in the proximal colon in mice (Hoogerwerf, 2006). PROKR mRNA levels are lower in Fallopian tube from tubal ectopic pregnancies than in mid-luteal phase Fallopian tube collected from non-pregnant women. The PROKs and their receptor proteins are localized to the smooth muscle of the Fallopian tube (Shaw et al., 2010). PROK1 has been shown to up-regulate COX-2 levels in the endometrium (Evans et al., 2008). COX-2 is a member of the cyclooxygenase family of enzymes responsible for the conversion of arachidonic acid into prostaglandin, and has been shown to play a role in implantation in mice (Lim et al., 1997). The human Fallopian tube is known to express COX-1 and COX-2 (Arbab et al., 2002) and some prostaglandins have recently been reported to increase the contractility of smooth muscle in the Fallopian tube (Wanggren et al., 2008). It is possible that reduced PROKR expression in Fallopian tube from women with tubal ectopic pregnancy contributes to impaired smooth muscle contractility and dysregulated embryo-tubal transport through the cyclooxygenases, COX-1/COX-2 and subsequent production of prostaglandins. Alternatively, the reduced PROK1 levels observed in Fallopian tube from women with ectopic pregnancies may simply be the result of lower maternal serum beta-human chorionic gonadotrophin (hCG) levels observed in tubal ectopic pregnancy. hCG increases PROK1 expression in the endometrium (Evans et al., 2009), and generally, tubal ectopic pregnancies have lower hCG levels than normal intrauterine pregnancies (Pittaway et al., 1985; Kadar et al., 1993). The signalling downstream of PROKR1 in the Fallopian tube warrants further investigation.
Endocannabinoids
In mice, genetic and pharmacological silencing of the endocannabinoid receptor, CB1, has been shown to cause embryo retention within the Fallopian tube, resulting in pregnancy failure (Wang et al., 2004). Embryo retention was reversed by the addition of a β-adrenergic receptor agonist which suggested that CB1 may play a role in controlling Fallopian tube contractility (Wang et al., 2004) by regulating the release of noradrenaline. In humans, CB1 has been shown to be localized to the epithelial and smooth muscle layers of the Fallopian tube and CB1 expression was found to be lower in Fallopian tube from women with tubal ectopic pregnancy compared with Fallopian tube tissue from non-pregnant women (Horne et al., 2008). Furthermore, in this study a possible association between polymorphism genotypes of the gene encoding for CB1, CNR1 and tubal ectopic pregnancy was demonstrated. The demonstration of a potential role for CB1 in the aetiology of human tubal ectopic pregnancy is important. Cigarette smoking is a major risk factor for tubal ectopic pregnancy and there is evidence of altered oviductal transport in rats exposed to nicotine (Yoshinaga et al., 1979; Farquhar, 2005). Chronic exposure of rats to nicotine affects brain endocannabinoid levels in a region-specific manner (Gonzalez et al., 2002). This crosstalk may also exist in the human female reproductive tract in humans and explain (at least in part) the association between cigarette smoking, Fallopian tube dysfunction and tubal ectopic pregnancy.
The tubal environment
Sex steroid hormone receptors
Knowledge of Fallopian tube sex steroid hormone receptor dynamics is important for furthering understanding of normal human Fallopian tube physiology, gene expression changes in the Fallopian tube in response to progesterone and disorders of Fallopian tube function, such as tubal ectopic pregnancy. Levels of progesterone receptor (PR) protein and its isoform PR-B (at an mRNA level) have been shown to be significantly reduced in Fallopian tubes from women with tubal ectopic pregnancy (Land and Arends, 1992; Horne et al., 2009). This may offer an explanation for the absence of adequate tubal decidualization observed with tubal ectopic pregnancy (Randall et al., 1987). Mice deficient in PR fail to mount a decidual response and a recent study in humans has shown that PRs regulate distinct gene networks and cellular functions in decidualizing endometrium (Lydon et al., 1995; Cloke et al., 2008). In addition, estrogen receptor alpha (ERα) protein has been shown to be absent in the Fallopian tube of women with tubal ectopic pregnancy (Horne et al., 2009). Although this may reflect the limitations of the immunohistochemical approach used, receptor protein is readily detected in Fallopian tubes obtained from women undergoing hysterectomy and who report normal menstrual cycles. ERα gene polymorphisms have been associated with female infertility (Corbo et al., 2007), and ERα has been shown to serve as a dominant regulator in Fallopian tube development in the rat (Mowa and Iwanaga, 2000). Furthermore, a recent study in mice has identified a molecular mechanism for ERα-mediated tubal protein synthesis and secretion that appears to be important for successful embryonic development (Shao et al., 2007). Further studies are required to determine whether ERα plays a critical role in Fallopian tube physiology and to establish whether reduced translation or enhanced degradation may contribute to reduced expression of protein.
Uteroglobin
Uteroglobin is a low molecular weight peptide which was initially identified in uterine secretions (Beier, 1968). It belongs to the secretoglobin family of molecules that are secreted from epithelial cells and have anti-inflammatory properties (Klug et al., 2000). Uteroglobin expression has been shown to be modulated by estrogen and progesterone in the female reproductive tract of rabbits (Shen et al., 1983). The secretory epithelial cells of the human Fallopian tube have been shown to express uteroglobin (Quintar et al., 2008) and uteroglobin levels have been shown to be increased in Fallopian tubes from women with tubal ectopic pregnancy and in women with pelvic inflammatory disease (PID; Quintar et al., 2008). Interestingly, decreased expression of uteroglobin was observed at the implantation sites in Fallopian tubes from women with tubal ectopic pregnancy compared with adjacent sites within the tube (Quintar et al., 2008). These results suggested that uteroglobin expression is regulated by the presence of the embryo in tubal ectopic pregnancy. Increased expression of uteroglobin in Fallopian tubes from women with tubal ectopic pregnancy and PID may result in an abnormally increased anti-inflammatory phenotype which may result in the increased expression of factors associated with implantation and a subsequent increase in receptivity within the Fallopian tube epithelium.
Interleukin-1 and interleukin-8
Interleukin-1 (IL-1) is a pro-inflammatory cytokine produced by epithelial cells in response to infection and is comprised of two agonists, IL-1 alpha and IL-1 beta (Hvid et al., 2007). Binding of IL-1 to its receptor results in initiation of downstream signalling pathways including mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK), which stimulate expression of interleukin-8 (IL-8). IL-8 is an inflammatory chemokine which is associated with immune response-mediated tissue damage through the recruitment of neutrophils (Mukaida et al., 1998). Expression of IL-8 has been found to be induced in the human Fallopian tube in response to chlamydial infection (Mukaida et al., 1998) and this increase in IL-8 expression in response to C. trachomatis was found to be ERK dependent (Buchholz and Stephens, 2007). Treatment of chlamydial-infected Fallopian tube explants with an IL-1 inhibitor has been shown to inhibit tissue damage caused by infection (Hvid et al., 2007). Implantation is known to induce a local proinflammatory response within the uterus which works to control migration, invasion and differentiation of the implanting embryo (Garlanda et al., 2008). Through the activation of signalling pathways, including cytokine, chemokine and integrin pathways, the embryo is guided towards the implantation site in a similar manner as leukocytes are guided towards an inflammatory site (Dominguez et al., 2005). It is likely that inflammation associated with tissue damage, in the Fallopian tube, may provide signals to the embryo that promote tubal implantation.
Leukemia inhibitory factor
Leukemia inhibitory factor (LIF) is a cytokine which has been demonstrated to be essential for murine implantation in a LIF knock-out mouse model (Stewart et al., 1992). Furthermore, hCG produced by the blastocyst has been shown to regulate LIF expression in the development of a receptive endometrium (Perrier et al., 2004; Evans et al., 2009). Increased LIF expression has been found in Fallopian tube from women with tubal ectopic pregnancy compared with Fallopian tubes from non-pregnant women (Guney et al., 2008), which was proposed to be the result of increased serum hCG levels present during pregnancy. However, more recently, LIF expression was found to be higher at the implantation site of tubal ectopic pregnancy compared with sites elsewhere in the Fallopian tube suggesting that increased serum hCG was an unlikely cause (Ji et al., 2009). It has been proposed that locally produced high levels of LIF arise from an exposed stromal surface in the Fallopian tube, resulting from epithelial shedding due to chronic salpingitis and that LIF facilitates implantation of the arrested embryo. Thus, these data provide a potential mechanism linking chronic pelvic inflammation to tubal ectopic pregnancy.
Homeobox protein A10
Homeobox protein A10 (HOXA10) is a transcription factor which acts as a regulator of cell identity during development, and in mice has been shown to be necessary for implantation (Bagot et al., 2000). Reduced expression of HOXA10 in the endometrium of mice has been found to result in fewer implantation sites and mice with a disruption in the HOXA10 gene were unable to support implantation and pregnancy (Bagot et al., 2000). In Fallopian tubes from women with tubal ectopic pregnancy, HOXA10 expression has been found to be up-regulated at the site of implantation compared with elsewhere in the tube and expression levels approached that of HOXA10 expression reported in the pregnant endometrium (Salih and Taylor, 2004). Up-regulation of HOXA10, specifically at the implantation site, in the Fallopian tube suggests that HOXA10 expression is controlled by the embryo, or may be expressed by the embryo, and indicates that dysregulated expression of HOXA10 may promote tubal implantation.
Integrins
Integrins are cell adhesion molecules that act as receptors for the extra-cellular matrix resulting in the activation of signal transduction pathways, leading to cell attachment (Lessey et al., 1992). The integrins, α1β1, α4β1 and αvβ3, have been largely accepted as markers of receptivity to the presenting embryo in the uterus, and functional blockout of αvβ3 results in reduced implantation in the mouse (Lessey, 1998; Illera et al., 2000). Furthermore, anandamide, the endogenous ligand for CB1, has been shown to regulate focal adhesion kinase, a tyrosine kinase involved in the regulation of integrin expression, linking these two groups of molecules in the control of tubal implantation (Derkinderen et al., 1996). Knowledge of integrin expression in the Fallopian tube is limited, however, to one immunohistochemical study and it is not known whether α1β1, α4β1 and αvβ3 have a similar expression in the Fallopian tube as in the uterus or are aberrantly expressed in tubal ectopic pregnancy (Inan et al., 2004).
Vascular endothelial growth factor
Vascular endothelial growth factor (VEGF) is a pro-angiogenic factor which has been reported to be present at higher levels in serum from women with tubal ectopic pregnancy compared with serum from women with intrauterine pregnancies (Felemban et al., 2002). VEGF and its receptors, KDR and flt-1, have also been shown to be up-regulated at the implantation site in Fallopian tubes from women with tubal ectopic pregnancy compared with elsewhere in the Fallopian tube (Lam et al., 2004). In addition, VEGF and VEGF receptor levels were found to correlate with serum hCG levels, indicating that VEGF and VEGF receptor expression could be influenced by signals from the embryo (Lam et al., 2004). Hypoxia at the tubal implantation site may trigger increased VEGF expression by the embryo (Ikeda et al., 1995; Shore et al., 1997). It is thought that increased VEGF promotes local angiogenesis increasing the supply of oxygen to the embryo thus favouring tubal implantation (Daniel et al., 1999).
Mucin 1
Mucin 1 (MUC1) is an anti-adhesive, high molecular weight glycoprotein found on the surface of most epithelial cells, including those of the female reproductive tract (Gipson et al., 1997). MUC1 is up-regulated in luminal and glandular endometrial epithelial cells during the implantation phase (Hey et al., 1994; Meseguer et al., 1998) and in vitro studies suggest that the human blastocyst produces factors that induce local removal of MUC1 to facilitate implantation (Meseguer et al., 1998). Two recent studies have shown that MUC1 expression is reduced, and its glycosylation pattern altered, in Fallopian tube of women with tubal ectopic pregnancy (Savaris et al., 2008; Al-Azemi et al., 2009b). The decrease in MUC1 expression and altered glycosylation in tubal epithelium from tubal ectopic pregnancy may reflect an increase in receptivity.
Trophinin
Trophinin is a membrane protein which has been shown to mediate adhesion between trophoblasts and the endometrial epithelium, when in complex with two cytoplasmic proteins, tastin and bystin (Aoki and Fukuda, 2000). In the endometrium, trophinin expression is tightly regulated and it is predominantly expressed during the window of implantation (Kimber and Spanswick, 2000). The Fallopian tube expresses very little trophinin when a pregnancy is intrauterine but Fallopian tubes from women with tubal ectopic pregnancy have been shown to strongly express trophinin, tastin and bystin (Nakayama et al., 2003). In addition, Fallopian tube explants cultured with hCG have been found to have increased levels of trophinin transcript, suggesting that trophinin expression in the Fallopian tube is controlled by the presence of the embryo (Nakayama et al., 2003). It has been postulated that signals from an arrested embryo in the Fallopian tube could cause increased expression of trophinin, tastin and bystin, contributing to a more receptive phenotype.
Activins
Activins are members of the TGF-β family of proteins and were initially identified for their activating effect on FSH in the rat pituitary gland (Ling et al., 1986). Activins are secreted proteins formed through the dimerization of two inhibin β subunits. Three potential activin proteins exist: activin-A (βA–βA), activin-B (βB–βB) and activin-AB (βA–βB; Refaat et al., 2004). The activins signal through serine/threonine kinase activin receptors of which there are two subgroups, I and II (Refaat et al., 2004). Activins are usually co-expressed with a protein called follistatin which controls their activity through tight and irreversible binding to activin dimers (Shimonaka et al., 1991). Using immunohistochemistry, activin and activin receptor protein levels have been shown to be increased in Fallopian tube from women with tubal ectopic pregnancy compared with Fallopian tubes from pseudo-pregnant women (see section Risk Factors; Refaat et al., 2008). Conversely, mRNA transcript levels of activins and activin receptors were decreased (Refaat et al., 2008). The authors suggest that this may indicate pathological secretion of activins and their receptors as causal in the development of tubal ectopic pregnancy. More recently, the activin βA subunit, activin receptors and follistatin have been reported to be increased in Fallopian tube from women with tubal ectopic pregnancy who were serologically positive for chlamydial infection or for the chlamydial HSP60 (CHSP60) protein compared with Fallopian tubes from women with tubal ectopic pregnancy who were C. trachomatis- or CHSP60-negative (Refaat et al., 2009). Activins were shown to play a role in endometrial decidualization and are likely to be important for implantation through their up-regulation of MMP expression (Dimitriadis et al., 2005). Increased activin expression, as a result of chlamydial infection, could cause dysregulated tissue remodelling within the Fallopian tube as a result of increased MMP expression. Activins increase NOS production (Nusing and Barsig, 1999), suggesting that their increased expression in Fallopian tubes from tubal ectopic pregnancy aberrantly control tubal motility by affecting smooth muscle contractility and/or ciliary beat activity.
The innate immune system
Intra-epithelial lymphocytes
Within the epithelium of the Fallopian tube, intra-epithelial lymphocytes are thought to be responsible for allowing the embryo to pass through the Fallopian tube without activation of an immune response (Kutteh et al., 1990). ER-β has been found to be co-expressed by CD8+ intraepithelial lymphocytes within the Fallopian tube and ER-β expressing lymphocytes may be regulated by progesterone levels over the menstrual cycle, with increased numbers of these cells present in the progesterone dominant mid-luteal phase of the cycle (Ulziibat et al., 2006). An increased number of ER-β positive intraepithelial lymphocytes are present in Fallopian tubes from women with tubal ectopic pregnancy suggesting that these cells may play a role in regulating the inflammatory response associated with tubal ectopic pregnancy.
Macrophages
Mounting of an immune response to infection can result in permanent scarring and damage to tissue, which is associated with the infiltration of macrophages. Macrophages are mononuclear phagocytes which are recruited to the site of tissue damage from the venous system by chemotactic signals (Osusky et al., 1997). In Fallopian tube tissue from tubal ectopic pregnancy, the leukocyte population is predominantly macrophages and T-lymphocytes (Vassiliadou and Bulmer, 1998). The population of macrophages found at the implantation site of Fallopian tubes from women with tubal ectopic pregnancy was found to be doubled compared with sites distant from the implantation site in the same Fallopian tube (Von Rango et al., 2001). Macrophages play immuno-regulatory roles in the reproductive system and also produce growth factors and cytokines which function to repair tissue damage (Coussens and Werb, 2002). Macrophages are also known to produce prostaglandins, which can alter tubal smooth muscle contractility, positively or negatively (Wanggren et al., 2008). Increased numbers of macrophages in the Fallopian tube, as a result of infection, could lead to an increase in growth factors responsible for mediating tissue remodelling required for blastocyst implantation. Increased prostaglandin production by macrophages present in the Fallopian tube may also contribute to altered tubal motility, leading to retention of the embryo within the tube (Tonello and Poli, 2007).
Natural anti-microbial peptides
The anti-protease and anti-microbial molecules, secretory leukocyte protease inhibitor (SLPI) and elafin are expressed in the human Fallopian tube and are up-regulated in tubal ectopic pregnancy and in response to chlamydial infection in a cell culture model (King et al., 2009). This suggests that SLPI and elafin are involved in the innate immune protection of the Fallopian tube during the normal menstrual cycle and may be important in the event of pathological conditions, such as infection and ectopic implantation. Although histological studies suggest there is little evidence for acute inflammatory changes at the site of a tubal ectopic pregnancy (Kutluay et al., 1994), recent studies suggest that there are inflammatory changes at the molecular level. Elafin and, to a lesser extent, SLPI are known to be up-regulated by inflammatory stimuli including IL-1 (Sallenave et al., 1994; King et al., 2003; Williams et al., 2006), and a shift towards a pro-inflammatory environment following chlamydial infection may result in their increased expression in tubal ectopic pregnancy.
Risk factors
Chlamydia trachomatis
Two recent systematic reviews of studies examining the risk of tubal ectopic pregnancy in women after genital C. trachomatis infection have demonstrated the absence of valid evidence of the attributable risk (Risser and Risser, 2007; Wallace et al., 2008). In the latter review, only one study satisfied the authors’ inclusion (Wallace et al., 2008). This study was a longitudinal investigation measuring pregnancy rates in adolescent women with, and without, current chlamydial infection at baseline, and reported no significant difference in subsequent pregnancy rates (Katz et al., 1994).
Difficulties in determining the effect of female genital chlamydial infection on reproductive outcome arise from flaws in specific study design and the lack of a reliable method for measuring a history of pelvic infection. Current assumptions on the risks of subsequent pregnancy problems, following pelvic infection, are based on retrospective case–control studies (Walters et al., 1988; Chrysostomou et al., 1992; Odland et al., 1993; van et al., 2004; Low et al., 2006; Bjartling et al., 2007; Machado et al., 2007; Bakken, 2008). Many of these studies were performed on populations where tubal ectopic pregnancy is extremely common (or rare), or used data that do not account for misdiagnoses, and thus there is considerable error in the estimates of risk ratios (van et al., 2004; Bakken, 2008; Garnett, 2008). Both retrospective and prospective case control studies on tubal ectopic pregnancy are prone to confounding variables which have not always been accounted for, such as the effect of other sexually transmitted infections (e.g. Neisseria gonorrhoea and syphilis) and smoking. Furthermore, in prospective studies, chlamydial infection can be reliably measured by nucleic acid amplification tests. In retrospective studies, a history of chlamydial infection is measured by the presence of a specific immune response (serum antibodies) using tests that can lead to misclassification due to a lack of sensitivity (Carder et al., 2006).
In addition, the exact mechanism by which C. trachomatis infection leads to tubal ectopic pregnancy remains relatively unknown. There are experimental animal models (mainly in rodent species) of genital chlamydial infection that provide clues to disease pathogenesis. However, these experimental infections are usually conducted using defined infectious doses under highly controlled conditions for relatively short periods of time and in animals that have limited genetic variability. Consequently, care needs to be taken when interpreting the data for the pathogenesis of human chlamydial infections where all of the above factors vary greatly. Lower genital tract chlamydial infection may ascend to the upper reproductive tract and result in salpingitis. It has been proposed that an antibody response to the chlamydial heat shock protein (hsp-60) may cause a tubal inflammatory response leading to tubal blockage or a predisposition to tubal implantation (Ault et al., 1998; Bjartling et al., 2007). Repeated infections with C. trachomatis are thought to increase tubal damage (Rank et al., 1995). Review of the literature highlights the paucity of solid evidence base for the link between C. trachomatis infection and tubal ectopic pregnancy.
Cigarette smoking
A recent meta-analysis of clinical outcomes from assisted reproduction has shown that cigarette smoking significantly increases the risk of tubal ectopic pregnancy (Waylen et al., 2009). However, the reason why smoking causes tubal ectopic pregnancy is not understood. Certainly, there is evidence that the Fallopian tube is a target for cigarette smoke. Embryo-tubal transport has been shown to be altered in both animals (rabbits and hamsters) and humans by the inhalation of cigarette smoke (Talbot and Riveles, 2005). Study of the specific affect of nicotine on tubal transport is difficult as nicotine has a very short half-life (Benowitz, 1983). Most studies investigating the effect of smoke on the Fallopian tube have been performed in rodents and relate to cigarette smoke's effect on ciliary beat frequency and smooth muscle contraction (Knoll et al., 1995; Riveles et al., 2004; Talbot and Riveles, 2005). Other parameters of Fallopian tube functioning, such as the synthesis and secretion of Fallopian tube proteins, would provide further insight into how cigarette smoke affects Fallopian tube function.
In vitro fertilization
The first IVF treatment in 1976 resulted in a tubal ectopic pregnancy (Steptoe and Edwards, 1976). The rate of tubal ectopic pregnancy following IVF still remains higher (approximately 2–5%) than the rate of tubal ectopic pregnancy with spontaneous pregnancy (1–2%; Strandell et al., 1999; Farquhar, 2005). The reason for the increased incidence of tubal ectopic pregnancy following IVF is unclear. The technique of embryo transfer is a potential cause but there is little evidence to support this. In addition, the risk of tubal ectopic pregnancy has also been reported to increase with the number of embryos that are transferred during IVF treatment (Weigert et al., 2009). The altered endocrine environment due to controlled ovarian hyperstimulation may affect tubal function and embryo-tubal transport (Fernandez et al., 1991; Strandell et al., 1999). Women who receive IVF treatment because of tubal factor infertility (usually the result of previous tubal damage due to surgery, infection or ectopic pregnancy) are at higher risk of developing a tubal ectopic pregnancy compared with women who undergo IVF because of male factor infertility (Strandell et al., 1999; Weigert et al., 2009). However, this does not account for the overall increased risk of tubal ectopic pregnancy that is associated with IVF indicating that there are other contributing factors.
A recent study has proposed an ‘embryo factor’. The cell adhesion protein, E-cadherin has been shown to be essential for blastocyst formation prior to implantation in the mouse where homozygous E-cadherin null embryos fail to implant (Larue et al., 1994; Riethmacher et al., 1995). Further support derives from a recent study where the expression of E-cadherin at the tubal implantation sites of spontaneous tubal pregnancies and of tubal pregnancies following IVF were compared using immunohistochemistry (Revel et al., 2008). E-cadherin protein was found to be more strongly expressed at the tubal implantation sites in the women with an IVF pregnancy compared with spontaneous pregnancy and was localized to the embryonic cells rather than the Fallopian tube epithelium (Revel et al., 2008). This difference in localization of E-cadherin was attributed to the fact that ‘IVF embryos’ are exposed to a different growth factor and cytokine milieu during in vitro culture compared with naturally conceived embryos. As a result, it is proposed that such embryos are unable to implant within the uterus during its receptive period and instead migrate into the Fallopian tube and attach to the tubal epithelium.
Limitations of the current studies and ideas for future research
Human in vivo models
It is difficult, for ethical reasons, to collect Fallopian tube from women with healthy intrauterine pregnancies for comparison with Fallopian tube from women with tubal ectopic pregnancy. However, tubal biopsies taken from women undergoing surgery for tubal ectopic pregnancy compared with biopsies taken from non-pregnant women at hysterectomy during the presumed time of implantation (mid-luteal phase of the menstrual cycle when progesterone levels are elevated) have allowed for the systematic study of changes in the expression pattern of genes and proteins in Fallopian tubes from tubal ectopic pregnancy (Horne et al., 2008, 2009; King et al., 2009). Information has also been derived from studies where tubal biopsies were collected from women injected with hCG in the days leading up to a hysterectomy, and pseudo-pregnancy confirmed by the presence of high serum progesterone levels and the decidualization of the endometrium (Al-Azemi et al., 2009a, b). Unfortunately the doses of hCG required to mimic the changes that would be found physiologically 15 days beyond the luteinizing hormone surge (at the time of implantation) require too great a volume of injection to be safe or practical and it is debatable whether this has any more validity as a model for tubal ectopic pregnancy than a mid-luteal phase Fallopian tube biopsy.
Animal models
Several differences between primates (human and non-human) and animals suffering from tubal ectopic pregnancy exist primarily with regard to the site of ectopic implantation (Corpa, 2006). In animals, the abdominal cavity is the most frequent extra-uterine implantation site and only three cases of tubal pregnancy in primates have been reported to date (Lapin and Yakovleva, 1963; Jerome and Hendrickx, 1982). These differences may be due to varying placentation mechanisms occurring in different species. In some species, such as horse, pig and sheep, attachment is delayed whilst the embryo develops (Johnson and Everitt, 1995). Attachment then occurs via multiple sites within the uterus and is followed by a non-invasive form of implantation. In humans, as well as in rodents and rabbits, placentation is achieved via a short period of attachment followed by invasive implantation of the uterus (Johnson and Everitt, 1995; Corpa, 2006).
Nevertheless, important information pertaining to the aetiology of tubal ectopic pregnancy has been derived from rodent models (Wang et al., 2004). Future studies should be aimed at inducing ectopic implantation in mice to generate useful animal models for tubal ectopic pregnancy. For example, mouse models employing conditional knock-in of specific genes important for implantation in the oviduct (i.e. COX-2, HOXA10 and LIF) may result in mice in which blastocysts (fertilized in vivo or in vitro) implant within the oviduct. Treatment of mice with agents aimed at directly disrupting tubal smooth muscle contractility and/or ciliary beat activity, such as PROK, CB1 or NO antagonists may also prove useful in developing an animal model for tubal ectopic pregnancy.
In vitro models
There are numerous studies which describe human co-culture methods using human embryos and endometrium for the study of endometrial biology (Gallery et al., 2001; Babawale et al., 2002; Carver et al., 2003; Li et al., 2003; Hoegh et al., 2006; Popovici et al., 2006; Hsu et al., 2008; Fafet et al., 2008; Helige et al., 2008; Grewal et al., 2008; Cohen and Bischof, 2009; Teklenburg and Macklon, 2009). Similar studies using Fallopian tube, designed for further understanding of the aetiology of tubal ectopic pregnancy are lacking. This is primarily due to the problems associated with the use of cells from human Fallopian tubes for co-culture: human Fallopian tube tissues are not always available; isolation of the epithelial cells from the tubal tissues is technically difficult; the number of cells prepared for co-culture is variable at each opportunity; and tubal epithelial cells, grown in monolayer culture, lose morphological features associated with the epithelium in situ, such as cilia. To our knowledge, only two immortalized oviductal epithelial cell lines have been established (Ando et al., 2000; Lee et al., 2001). Nevertheless, ex vivo culture and exposure of primary Fallopian tube explant tissue and/or Fallopian tube epithelial cells to factors known to increase the risk of tubal ectopic pregnancy (i.e. C. trachomatis, metabolites of cigarette smoke and inflammatory cues) may prove useful in delineating gene expression changes and signalling pathways important in Fallopian tube physiology and pathobiology. Co-culture studies with the above treated Fallopian tube explants and trophoblast tissue or embryos fertilized in vitro, similar to those by Landgren et al. (1996) where endometrial explants were co-cultured with embryos, would be useful in analysing gene expression changes induced by implantation in the Fallopian tube. Coupled with microarray and proteomic technologies, these studies would be invaluable in delineating similarities and differences between tubal and intrauterine implantation.
Conclusion
Current evidence suggests that tubal ectopic pregnancy results from Fallopian tube dysfunction causing embryo arrest and changes in the tubal environment (see summary of current data in Fig. 1, and Tables I and II). Inflammation within the Fallopian tube, resulting from infection or smoking, appears to affect embryo-tubal transport by disrupting smooth muscle contractility and ciliary beat activity. This inflammatory environment also provides many of the pro-implantation signals recognized by the arrested embryo, such as increased IL-8 expression and decreased MUC1 expression. Furthermore, an arrested embryo may facilitate the expression of pro-implantation factors in the uterus, such as LIF, uteroglobin, trophinin, HOXA10 and VEGF, which in turn signal the establishment of a tubal environment both suitable for and capable of implantation.
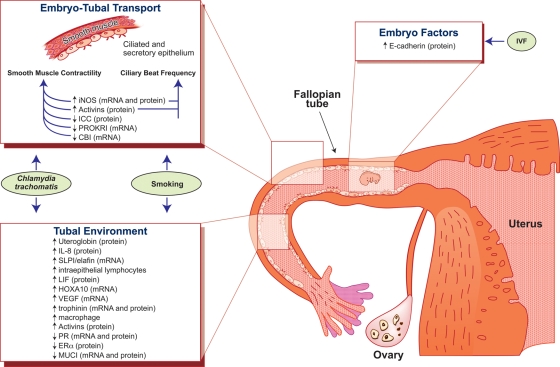
Figure 1.
Schematic diagram summarizing the potential factors contributing to the development of tubal ectopic pregnancy.
Tubal ectopic pregnancy is caused by a combination of factors within the Fallopian tube which affect embryo-tubal transport and the tubal environment. Impaired Fallopian tube motility is caused by alterations in smooth muscle contractility and ciliary beat frequency. Changes to the tubal environment are thought to result in a setting fit for early embryo implantation. Factors inherent to the embryo itself, particularly during IVF, may also be predisposing to early implantation and tubal ectopic pregnancy.
Table I.
Summary of maternally controlled factors: expression in tubal ectopic pregnancy and potential causal effect in the development of tubal ectopic pregnancy.
| Factor | Expression in tubal ectopic pregnancy | Potential effects |
|---|---|---|
| Inducible nitric oxide synthase (iNOS) | Reduced | Relaxed tubal smooth muscle contractility and/or ciliary beat resulting in reduced tubal motility |
| Interstitial cells of Cajal (ICC) | Reduced | Loss of pacemaker activity within the Fallopian tube resulting in reduced smooth muscle contractility |
| Endocannabinoid receptor (CB1) | Reduced | Aberrant tubal smooth muscle contractility |
| Prokineticin receptors (PROKRs) | Reduced | Aberrant tubal smooth muscle contractility |
| Activated macrophages | Increased | Up-regulation of prostaglandins resulting in aberrant tubal smooth muscle contractility |
| Interleukin-8 (IL-8) | Increased | Tubal tissue damage resulting from infection |
| Mucin 1 (MUC1) | Reduced | Allows implantation of abnormal embryos |
| Activins | Increased | Dysregulation of tissue remodelling allowing for tubal implantation |
| Increased NOS production contributing to aberrant tubal motility |
Table II.
Summary of embryo controlled factors: expression in Tubal ectopic and potential causal effect in the development of tubal ectopic pregnancy.
| Factor | Expression in tubal ectopic pregnancy | Potential effects |
|---|---|---|
| Uteroglobin | Increased | Increased inflammation |
| Increased receptivity | ||
| Leukemia inhibitory factor (LIF) | Increased | Increased receptivity |
| Homeobox protein A10 (HOXA10) | Increased | Increased receptivity |
| Vascular endothelial growth factor (VEGF) | Increased | Increased angiogenesis at implantation site |
| Trophinin | Increased | Increased adhesion leading to increased receptivity |
| Integrins | Increased | Increased adhesion leading to increased receptivity |
Studies on the aetiology of tubal ectopic pregnancy to date have however been largely descriptive and focused on dysregulated gene or protein expression, comparing Fallopian tube collected from women with tubal ectopic pregnancy and Fallopian tubes collected from non-pregnant women or women with a ‘pseudo-pregnancy’. There has been very limited analysis of the functional consequences of the observed changes in gene or protein expression reported in these studies.
This is largely due to the fact that the aetiology of tubal ectopic pregnancy is difficult to study. There are no suitable animal models because ectopic gestation is it rare in animals. Ethical constraints inhibit the collection of Fallopian tube biopsies from women with healthy pregnancies, making it difficult to obtain the ideal control for comparison to Fallopian tubes from women with tubal ectopic pregnancy. In addition, researchers have to rely on information derived from Fallopian tube biopsies taken from women with tubal ectopic pregnancy rather than prior to the event. It is difficult to ascertain whether the molecular changes observed predispose to tubal ectopic pregnancy, or whether they are simply the result of tubal implantation and/or the presence of the embryo.
Furthermore, although the epidemiological risk factors for tubal ectopic pregnancy have been well documented, the exact mechanism by which infection, or smoking, leads to tubal implantation remains unexplained. Data thus far have been largely descriptive and mechanistically speculative. Studies focusing on investigating the functional consequences of smoking and infection on Fallopian tube physiology and pathobiology are urgently required. A greater understanding of the aetiology of tubal ectopic pregnancy is critical for the development of improved preventative measures, the advancement of diagnostic screening methods and the development of novel treatments.
Authors’ Roles
J.L.V.S.: literature searches, manuscript preparation. S.K.D.: manuscript preparation. H.O.D.C.: manuscript preparation. A.W.H.: literature searches, manuscript preparation.
Funding
A part of the work described herein was supported by an NIH/NIDA grant (DA DA06668 to S.K.D.) and a Wellbeing of Women Project Grant (R40608 to A.W.H./H.O.D.C.). Andrew Horne is supported by an MRC Clinician Scientist Fellowship.
References
- Afanas'ev IB. Signaling functions of free radicals superoxide & nitric oxide under physiological & pathological conditions. Mol Biotechnol. 2007;37:2–4. doi: 10.1007/s12033-007-0056-7. [DOI] [PubMed] [Google Scholar]
- Al-Azemi M, Refaat B, Amer S, Ola B, Chapman N, Ledger W. The expression of inducible nitric oxide synthase in the human fallopian tube during the menstrual cycle and in ectopic pregnancy. Fertil Steril. 2009a doi: 10.1016/j.fertnstert.2009.04.020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Al-Azemi M, Refaat B, Aplin J, Ledger W. The expression of MUC1 in human Fallopian tube during the menstrual cycle and in ectopic pregnancy. Hum Reprod. 2009b;24:2582–2587. doi: 10.1093/humrep/dep233. [DOI] [PubMed] [Google Scholar]
- Ando H, Kobayashi M, Toda S, Kikkawa F, Masahashi T, Mizutani S. Establishment of a ciliated epithelial cell line from human Fallopian tube. Hum Reprod. 2000;15:1597–1603. doi: 10.1093/humrep/15.7.1597. [DOI] [PubMed] [Google Scholar]
- Aoki R, Fukuda MN. Recent molecular approaches to elucidate the mechanism of embryo implantation: trophinin, bystin, and tastin as molecules involved in the initial attachment of blastocysts to the uterus in humans. Semin Reprod Med. 2000;18:265–271. doi: 10.1055/s-2000-12564. [DOI] [PubMed] [Google Scholar]
- Arbab F, Goldsby J, Matijevic-Aleksic N, Huang G, Ruan KH, Huang JC. Prostacyclin is an autocrine regulator in the contraction of oviductal smooth muscle. Hum Reprod. 2002;17:3053–3059. doi: 10.1093/humrep/17.12.3053. [DOI] [PubMed] [Google Scholar]
- Ault KA, Statland BD, King MM, Dozier DI, Joachims ML, Gunter J. Antibodies to the chlamydial 60 kilodalton heat shock protein in women with tubal factor infertility. Infect Dis Obstet Gynecol. 1998;6:163–167. doi: 10.1002/(SICI)1098-0997(1998)6:4<163::AID-IDOG5>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babawale MO, Mobberley MA, Ryder TA, Elder MG, Sullivan MH. Ultrastructure of the early human feto-maternal interface co-cultured in vitro. Hum Reprod. 2002;17:1351–1357. doi: 10.1093/humrep/17.5.1351. [DOI] [PubMed] [Google Scholar]
- Bagot CN, Troy PJ, Taylor HS. Alteration of maternal Hoxa10 expression by in vivo gene transfection affects implantation. Gene Ther. 2000;7:1378–1384. doi: 10.1038/sj.gt.3301245. [DOI] [PubMed] [Google Scholar]
- Bakken IJ. Chlamydia trachomatis and ectopic pregnancy: recent epidemiological findings. Curr Opin Infect Dis. 2008;21:77–82. doi: 10.1097/QCO.0b013e3282f3d972. [DOI] [PubMed] [Google Scholar]
- Beier HM. Uteroglobin: a hormone-sensitive endometrial protein involved in blastocyst development. Biochim Biophys Acta. 1968;160:289–291. doi: 10.1016/0005-2795(68)90108-6. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. The use of biologic fluid samples in assessing tobacco smoke consumption. NIDA Res Monogr. 1983;48:6–26. [PubMed] [Google Scholar]
- Bjartling C, Osser S, Persson K. Deoxyribonucleic acid of Chlamydia trachomatis in fresh tissue from the Fallopian tubes of patients with ectopic pregnancy. Eur J Obstet Gynecol Reprod Biol. 2007;134:95–100. doi: 10.1016/j.ejogrb.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Bouyer J, Coste J, Shojaei T, Pouly JL, Fernandez H, Gerbaud L, Job-Spira N. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case–control, population-based study in France. Am J Epidemiol. 2003;157:185–194. doi: 10.1093/aje/kwf190. [DOI] [PubMed] [Google Scholar]
- Buchholz KR, Stephens RS. The extracellular signal-regulated kinase/mitogen-activated protein kinase pathway induces the inflammatory factor interleukin-8 following Chlamydia trachomatis infection. Infect Immun. 2007;75:5924–5929. doi: 10.1128/IAI.01029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carder C, Mercey D, Benn P. Chlamydia trachomatis. Sex Transm Infect. 2006;82:iv10–iv12. doi: 10.1136/sti.2006.023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver J, Martin K, Spyropoulou I, Barlow D, Sargent I, Mardon H. An in vitro model for stromal invasion during implantation of the human blastocyst. Hum Reprod. 2003;18:283–290. doi: 10.1093/humrep/deg072. [DOI] [PubMed] [Google Scholar]
- Chrysostomou M, Karafyllidi P, Papadimitriou V, Bassiotou V, Mayakos G. Serum antibodies to Chlamydia trachomatis in women with ectopic pregnancy, normal pregnancy or salpingitis. Eur J Obstet Gynecol Reprod Biol. 1992;44:101–105. doi: 10.1016/0028-2243(92)90053-2. [DOI] [PubMed] [Google Scholar]
- Cloke B, Huhtinen K, Fusi L, Kajihara T, Yliheikkila M, Ho KK, Teklenburg G, Lavery S, Jones MC, Trew G, et al. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology. 2008;149:4462–4474. doi: 10.1210/en.2008-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Bischof P. Coculture of decidua and trophoblast to study proliferation and invasion. Methods Mol Biol. 2009;550:63–72. doi: 10.1007/978-1-60327-009-0_3. [DOI] [PubMed] [Google Scholar]
- Corbo RM, Ulizzi L, Piombo L, Martinez-Labarga C, De Stefano GF, Scacchi R. Estrogen receptor alpha polymorphisms and fertility in populations with different reproductive patterns. Mol Hum Reprod. 2007;13:537–540. doi: 10.1093/molehr/gam041. [DOI] [PubMed] [Google Scholar]
- Corpa JM. Ectopic pregnancy in animals and humans. Reproduction. 2006;131:631–640. doi: 10.1530/rep.1.00606. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel Y, Geva E, Lerner-Geva L, Eshed-Englender T, Gamzu R, Lessing JB, Bar-Am A, Amit A. Levels of vascular endothelial growth factor are elevated in patients with ectopic pregnancy: is this a novel marker? Fertil Steril. 1999;72:1013–1017. doi: 10.1016/s0015-0282(99)00417-3. [DOI] [PubMed] [Google Scholar]
- Derkinderen P, Toutant M, Burgaya F, Le BM, Siciliano JC, de F V, Gelman M, Girault JA. Regulation of a neuronal form of focal adhesion kinase by anandamide. Science. 1996;273:1719–1722. doi: 10.1126/science.273.5282.1719. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update. 2005;11:613–630. doi: 10.1093/humupd/dmi023. [DOI] [PubMed] [Google Scholar]
- Dixon RE, Hwang SJ, Hennig GW, Ramsey KH, Schripsema JH, Sanders KM, Ward SM. Chlamydia infection causes loss of pacemaker cells and inhibits oocyte transport in the mouse oviduct. Biol Reprod. 2009;80:665–673. doi: 10.1095/biolreprod.108.073833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez F, Yanez-Mo M, Sanchez-Madrid F, Simon C. Embryonic implantation and leukocyte transendothelial migration: different processes with similar players? FASEB J. 2005;19:1056–1060. doi: 10.1096/fj.05-3781hyp. [DOI] [PubMed] [Google Scholar]
- Eddy CA, Pauerstein CJ. Anatomy and physiology of the fallopian tube. Clin Obstet Gynecol. 1980;23:1177–1193. doi: 10.1097/00003081-198012000-00023. [DOI] [PubMed] [Google Scholar]
- Ekerhovd E, Norstrom A. Involvement of a nitric oxide-cyclic guanosine monophosphate pathway in control of fallopian tube contractility. Gynecol Endocrinol. 2004;19:239–246. doi: 10.1080/09513590400019296. [DOI] [PubMed] [Google Scholar]
- Ekerhovd E, Brannstrom M, Alexandersson M, Norstrom A. Evidence for nitric oxide mediation of contractile activity in isolated strips of the human Fallopian tube. Hum Reprod. 1997;12:301–305. doi: 10.1093/humrep/12.2.301. [DOI] [PubMed] [Google Scholar]
- Evans J, Catalano RD, Morgan K, Critchley HO, Millar RP, Jabbour HN. Prokineticin 1 signaling and gene regulation in early human pregnancy. Endocrinology. 2008;149:2877–2887. doi: 10.1210/en.2007-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Catalano RD, Brown P, Sherwin R, Critchley HOD, Fazleabas AT, Jabbour HN. Prokineticin 1 mediates fetal-maternal dialogue regulating endometrial leukemia inhibitory factor. FASEB J. 2009;23:2165–2175. doi: 10.1096/fj.08-124495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fafet P, Rebouissou C, Maudelonde T, Vignais ML. Opposite effects of transforming growth factor-beta activation and rho-associated kinase inhibition on human trophoblast migration in a reconstituted placental-endometrial coculture system. Endocrinology. 2008;149:4475–4485. doi: 10.1210/en.2008-0253. [DOI] [PubMed] [Google Scholar]
- Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583–591. doi: 10.1016/S0140-6736(05)67103-6. [DOI] [PubMed] [Google Scholar]
- Felemban A, Sammour A, Tulandi T. Serum vascular endothelial growth factor as a possible marker for early ectopic pregnancy. Hum Reprod. 2002;17:490–492. doi: 10.1093/humrep/17.2.490. [DOI] [PubMed] [Google Scholar]
- Fernandez H, Coste J, Job-Spira N. Controlled ovarian hyperstimulation as a risk factor for ectopic pregnancy. Obstet Gynecol. 1991;78:656–659. [PubMed] [Google Scholar]
- Gallery ED, Campbell S, Ilkovski B, Sinosich MJ, Jackson C. A novel in vitro co-culture system for the study of maternal decidual endothelial cell-trophoblast interactions in human pregnancy. BJOG. 2001;108:651–653. doi: 10.1111/j.1471-0528.2001.00127.x. [DOI] [PubMed] [Google Scholar]
- Garlanda C, Maina V, Martinez de la TY, Nebuloni M, Locati M. Inflammatory reaction and implantation: the new entries PTX3 and D6. Placenta. 2008;29:129–134. doi: 10.1016/j.placenta.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Garnett GP. How much infertility does chlamydia cause? Sex Transm Infect. 2008;84:157–158. doi: 10.1136/sti.2008.031989. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Ho SB, Spurr-Michaud SJ, Tisdale AS, Zhan Q, Torlakovic E, Pudney J, Anderson DJ, Toribara NW, Hill JA., III Mucin genes expressed by human female reproductive tract epithelia. Biol Reprod. 1997;56:999–1011. doi: 10.1095/biolreprod56.4.999. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Di M V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- Grewal S, Carver JG, Ridley AJ, Mardon HJ. Implantation of the human embryo requires Rac1-dependent endometrial stromal cell migration. Proc Natl Acad Sci USA. 2008;105:16189–16194. doi: 10.1073/pnas.0806219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guney M, Erdemoglu E, Oral B, Karahan N, Mungan T. Leukemia inhibitory factor (LIF) is immunohistochemically localized in tubal ectopic pregnancy. Acta Histochem. 2008;110:319–323. doi: 10.1016/j.acthis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Halbert SA, Tam PY, Blandau RJ. Egg transport in the rabbit oviduct: the roles of cilia and muscle. Science. 1976;191:1052–1053. doi: 10.1126/science.1251215. [DOI] [PubMed] [Google Scholar]
- Halbert SA, Becker DR, Szal SE. Ovum transport in the rat oviductal ampulla in the absence of muscle contractility. Biol Reprod. 1989;40:1131–1136. doi: 10.1095/biolreprod40.6.1131. [DOI] [PubMed] [Google Scholar]
- Helige C, Ahammer H, Hammer A, Huppertz B, Frank HG, Dohr G. Trophoblastic invasion in vitro and in vivo: similarities and differences. Hum Reprod. 2008;23:2282–2291. doi: 10.1093/humrep/den198. [DOI] [PubMed] [Google Scholar]
- Hey NA, Graham RA, Seif MW, Aplin JD. The polymorphic epithelial mucin MUC1 in human endometrium is regulated with maximal expression in the implantation phase. J Clin Endocrinol Metab. 1994;78:337–342. doi: 10.1210/jcem.78.2.8106621. [DOI] [PubMed] [Google Scholar]
- Hoegh AM, Islin H, Moller C, Sorensen S, Hviid TV. Identification of differences in gene expression in primary cell cultures of human endometrial epithelial cells and trophoblast cells following their interaction. J Reprod Immunol. 2006;70:1–19. doi: 10.1016/j.jri.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Hoogerwerf WA. Prokineticin 1 inhibits spontaneous giant contractions in the murine proximal colon through nitric oxide release. Neurogastroenterol Motil. 2006;18:455–463. doi: 10.1111/j.1365-2982.2006.00776.x. [DOI] [PubMed] [Google Scholar]
- Horne AW, Phillips JA, III, Kane N, Lourenco PC, McDonald SE, Williams AR, Simon C, Dey SK, Critchley HO. CB1 expression is attenuated in Fallopian tube and decidua of women with ectopic pregnancy. PLoS ONE. 2008;3:e3969. doi: 10.1371/journal.pone.0003969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne AW, King AE, Shaw E, McDonald SE, Williams AR, Saunders PT, Critchley HO. Attenuated sex steroid receptor expression in Fallopian tube of women with ectopic pregnancy. J Clin Endocrinol Metab. 2009;94:5146–5154. doi: 10.1210/jc.2009-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu WL, Chen YH, Chao KC, Chang SP, Tsui KH, Li HY, Sung YJ. Anti-fas activating antibody enhances trophoblast outgrowth on endometrial epithelial cells by induction of P38 MAPK/JNK-mediated apoptosis. Placenta. 2008;29:338–346. doi: 10.1016/j.placenta.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Hvid M, Baczynska A, Deleuran B, Fedder J, Knudsen HJ, Christiansen G, Birkelund S. Interleukin-1 is the initiator of Fallopian tube destruction during Chlamydia trachomatis infection. Cell Microbiol. 2007;9:2795–2803. doi: 10.1111/j.1462-5822.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- Ikeda E, Achen MG, Breier G, Risau W. Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cells. J Biol Chem. 1995;270:19761–19766. doi: 10.1074/jbc.270.34.19761. [DOI] [PubMed] [Google Scholar]
- Illera MJ, Cullinan E, Gui Y, Yuan L, Beyler SA, Lessey BA. Blockade of the alpha(v)beta(3) integrin adversely affects implantation in the mouse. Biol Reprod. 2000;62:1285–1290. doi: 10.1095/biolreprod62.5.1285. [DOI] [PubMed] [Google Scholar]
- Inan S, Giray G, Vatansever HS, Ozbilgin K, Kuscu NK, Sayhan S. Immunolocalization of integrins and fibronectin in tubal pregnancy. Acta Histochem. 2004;106:235–243. doi: 10.1016/j.acthis.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Jain B, Rubinstein I, Robbins RA, Sisson JH. TNF-alpha and IL-1 beta upregulate nitric oxide-dependent ciliary motility in bovine airway epithelium. Am J Physiol. 1995;268:L911–L917. doi: 10.1152/ajplung.1995.268.6.L911. [DOI] [PubMed] [Google Scholar]
- Jansen RP. Endocrine response in the fallopian tube. Endocr Rev. 1984;5:525–551. doi: 10.1210/edrv-5-4-525. [DOI] [PubMed] [Google Scholar]
- Jerome CP, Hendrickx AG. A tubal pregnancy in a rhesus monkey (Macaca mulatta) Vet Pathol. 1982;19:239–245. doi: 10.1177/030098588201900303. [DOI] [PubMed] [Google Scholar]
- Ji YF, Chen LY, Xu KH, Yao JF, Shi YF. Locally elevated leukemia inhibitory factor in the inflamed fallopian tube resembles that found in tubal pregnancy. Fertil Steril. 2009;91:2308–2314. doi: 10.1016/j.fertnstert.2008.01.110. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Everitt BJ. Essential Reproduction. Oxford: Blackwell Science Ltd; 1995. Implantation and the establishment of the placenta; pp. 162–179. [Google Scholar]
- Kadar N, Bohrer M, Kemman E, Shelden R. A prospective, randomized study of the chorionic gonadotropin-time relationship in early gestation: clinical implications. Fertil Steril. 1993;60:409–412. doi: 10.1016/s0015-0282(16)56151-2. [DOI] [PubMed] [Google Scholar]
- Katz BP, Thom S, Blythe MJ, Arno JN, Caine VM, Jones RB. Fertility in adolescent women previously treated for genitourinary chlamydial infection. Adolesc Pediatr Gynecol. 1994;7:147–152. [Google Scholar]
- Kimber SJ, Spanswick C. Blastocyst implantation: the adhesion cascade. Semin Cell Dev Biol. 2000;11:77–92. doi: 10.1006/scdb.2000.0154. [DOI] [PubMed] [Google Scholar]
- King AE, Critchley HO, Kelly RW. Innate immune defences in the human endometrium. Reprod Biol Endocrinol. 2003;1:116. doi: 10.1186/1477-7827-1-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AE, Wheelhouse N, Cameron S, McDonald SE, Lee KF, Entrican G, Critchley HO, Horne AW. Expression of secretory leukocyte protease inhibitor and elafin in human fallopian tube and in an in vitro model of Chlamydia trachomatis infection. Hum Reprod. 2009;24:679–686. doi: 10.1093/humrep/den452. [DOI] [PubMed] [Google Scholar]
- Klug J, Beier HM, Bernard A, Chilton BS, Fleming TP, Lehrer RI, Miele L, Pattabiraman N, Singh G. Uteroglobin/Clara cell 10-kDa family of proteins: nomenclature committee report. Ann N Y Acad Sci. 2000;923:348–354. doi: 10.1111/j.1749-6632.2000.tb05549.x. [DOI] [PubMed] [Google Scholar]
- Knoll M, Shaoulian R, Magers T, Talbot P. Ciliary beat frequency of hamster oviducts is decreased in vitro by exposure to solutions of mainstream and sidestream cigarette smoke. Biol Reprod. 1995;53:29–37. doi: 10.1095/biolreprod53.1.29. [DOI] [PubMed] [Google Scholar]
- Kutluay L, Vicdan K, Turan C, Batioglu S, Oguz S, Gokmen O. Tubal histopathology in ectopic pregnancies. Eur J Obstet Gynecol Reprod Biol. 1994;57:91–94. doi: 10.1016/0028-2243(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Kutteh WH, Blackwell RE, Gore H, Kutteh CC, Carr BR, Mestecky J. Secretory immune system of the female reproductive tract. II. Local immune system in normal and infected fallopian tube. Fertil Steril. 1990;54:51–55. [PubMed] [Google Scholar]
- Lam PM, Briton-Jones C, Cheung CK, Leung SW, Cheung LP, Haines C. Increased messenger RNA expression of vascular endothelial growth factor and its receptors in the implantation site of the human oviduct with ectopic gestation. Fertil Steril. 2004;82:686–690. doi: 10.1016/j.fertnstert.2003.12.052. [DOI] [PubMed] [Google Scholar]
- Land JA, Arends JW. Immunohistochemical analysis of estrogen and progesterone receptors in fallopian tubes during ectopic pregnancy. Fertil Steril. 1992;58:335–337. doi: 10.1016/s0015-0282(16)55208-x. [DOI] [PubMed] [Google Scholar]
- Landgren BM, Johannisson E, Stavreus-Evers A, Hamberger L, Eriksson H. A new method to study the process of implantation of a human blastocyst in vitro. Fertil Steril. 1996;65:1067–1070. doi: 10.1016/s0015-0282(16)58291-0. [DOI] [PubMed] [Google Scholar]
- Lapin BA, Yakovleva LA. Comparative Pathology in Monkeys. Illinois: Charles C. Thomas, Springfield; 1963. [Google Scholar]
- Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci USA. 1994;91:8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YL, Lee KF, Xu JS, Wang YL, Tsao SW, Yeung WS. Establishment and characterization of an immortalized human oviductal cell line. Mol Reprod Dev. 2001;59:400–409. doi: 10.1002/mrd.1046. [DOI] [PubMed] [Google Scholar]
- Leke RJ, Goyaux N, Matsuda T, Thonneau PF. Ectopic pregnancy in Africa: a population-based study. Obstet Gynecol. 2004;103:692–697. doi: 10.1097/01.AOG.0000120146.48098.f2. [DOI] [PubMed] [Google Scholar]
- Lessey BA. Endometrial integrins and the establishment of uterine receptivity. Hum Reprod. 1998;13:247–258. doi: 10.1093/humrep/13.suppl_3.247. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Damjanovich L, Coutifaris C, Castelbaum A, Albelda SM, Buck CA. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest. 1992;90:188–195. doi: 10.1172/JCI115835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- Li HY, Chang SP, Yuan CC, Chao HT, Ng HT, Sung YJ. Induction of p38 mitogen-activated protein kinase-mediated apoptosis is involved in outgrowth of trophoblast cells on endometrial epithelial cells in a model of human trophoblast-endometrial interactions. Biol Reprod. 2003;69:1515–1524. doi: 10.1095/biolreprod.103.015669. [DOI] [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- Lindblom B, Hamberger L, Wiqvist N. Differentiated contractile effects of prostaglandins E and F on the isolated circular and longitudinal smooth muscle of the human oviduct. Fertil Steril. 1978;30:553–559. doi: 10.1016/s0015-0282(16)43637-x. [DOI] [PubMed] [Google Scholar]
- Lindblom B, Hamberger L, Ljung B. Contractile patterns of isolated oviductal smooth muscle under different hormonal conditions. Fertil Steril. 1980;33:283–287. doi: 10.1016/s0015-0282(16)44595-4. [DOI] [PubMed] [Google Scholar]
- Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta M, Guillemin R. Pituitary FSH is released by a heterodimer of the beta-subunits from the two forms of inhibin. Nature. 1986;321:779–782. doi: 10.1038/321779a0. [DOI] [PubMed] [Google Scholar]
- Low N, Egger M, Sterne JA, Harbord RM, Ibrahim F, Lindblom B, Herrmann B. Incidence of severe reproductive tract complications associated with diagnosed genital chlamydial infection: the Uppsala Women's Cohort Study. Sex Transm Infect. 2006;82:212–218. doi: 10.1136/sti.2005.017186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Lyons RA, Saridogan E, Djahanbakhch O. The effect of ovarian follicular fluid and peritoneal fluid on Fallopian tube ciliary beat frequency. Hum Reprod. 2006a;21:52–56. doi: 10.1093/humrep/dei306. [DOI] [PubMed] [Google Scholar]
- Lyons RA, Saridogan E, Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Hum Reprod Update. 2006b;12:363–372. doi: 10.1093/humupd/dml012. [DOI] [PubMed] [Google Scholar]
- Machado AC, Guimaraes EM, Sakurai E, Fioravante FC, Amaral WN, Alves MF. High titers of Chlamydia trachomatis antibodies in Brazilian women with tubal occlusion or previous ectopic pregnancy. Infect Dis Obstet Gynecol. 2007;2007:24816. doi: 10.1155/2007/24816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Perez D, Evans J, Denison F, Millar RP, Jabbour HN. Potential roles of the prokineticins in reproduction. Trends Endocrinol Metab. 2007;18:66–72. doi: 10.1016/j.tem.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meseguer M, Pellicer A, Simon C. MUC1 and endometrial receptivity. Mol Hum Reprod. 1998;4:1089–1098. doi: 10.1093/molehr/4.12.1089. [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Mowa CN, Iwanaga T. Differential distribution of oestrogen receptor-alpha and -beta mRNAs in the female reproductive organ of rats as revealed by in situ hybridization. J Endocrinol. 2000;165:59–66. doi: 10.1677/joe.0.1650059. [DOI] [PubMed] [Google Scholar]
- Mukaida N, Matsumoto T, Yokoi K, Harada A, Matsushima K. Inhibition of neutrophil-mediated acute inflammation injury by an antibody against interleukin-8 (IL-8) Inflamm Res. 1998;47:S151–S157. doi: 10.1007/s000110050308. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Bennett CJ, Hicks JL, Epstein JI, Platz EA, Nelson WG, De Marzo AM. Hypermethylation of the human glutathione S-transferase-pi gene (GSTP1) CpG island is present in a subset of proliferative inflammatory atrophy lesions but not in normal or hyperplastic epithelium of the prostate: a detailed study using laser-capture microdissection. Am J Pathol. 2003;163:923–933. doi: 10.1016/s0002-9440(10)63452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusing RM, Barsig J. Induction of prostanoid, nitric oxide, and cytokine formation in rat bone marrow derived macrophages by activin A. Br J Pharmacol. 1999;127:919–926. doi: 10.1038/sj.bjp.0702626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odland JO, Anestad G, Rasmussen S, Lundgren R, Dalaker K. Ectopic pregnancy and chlamydial serology. Int J Gynaecol Obstet. 1993;43:271–275. doi: 10.1016/0020-7292(93)90515-x. [DOI] [PubMed] [Google Scholar]
- Osusky R, Malik P, Aurora Y, Ryan SJ. Monocyte-macrophage differentiation induced by coculture of retinal pigment epithelium cells with monocytes. Ophthalmic Res. 1997;29:124–129. doi: 10.1159/000268006. [DOI] [PubMed] [Google Scholar]
- Paltieli Y, Eibschitz I, Ziskind G, Ohel G, Silbermann M, Weichselbaum A. High progesterone levels and ciliary dysfunction—a possible cause of ectopic pregnancy. J Assist Reprod Genet. 2000;17:103–106. doi: 10.1023/A:1009465900824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papathanasiou A, Djahanbakhch O, Saridogan E, Lyons RA. The effect of interleukin-6 on ciliary beat frequency in the human fallopian tube. Fertil Steril. 2008;90:391–394. doi: 10.1016/j.fertnstert.2007.07.1379. [DOI] [PubMed] [Google Scholar]
- Perez MS, Viggiano M, Franchi AM, Herrero MB, Ortiz ME, Gimeno MF, Villalon M. Effect of nitric oxide synthase inhibitors on ovum transport and oviductal smooth muscle activity in the rat oviduct. J Reprod Fertil. 2000;118:111–117. doi: 10.1530/jrf.0.1180111. [DOI] [PubMed] [Google Scholar]
- Perrier dS, Charlet-Renard C, Berndt S, Dubois M, Munaut C, Goffin F, Hagelstein MT, Noel A, Hazout A, Foidart JM, et al. Human chorionic gonadotropin and growth factors at the embryonic-endometrial interface control leukemia inhibitory factor (LIF) and interleukin 6 (IL-6) secretion by human endometrial epithelium. Hum Reprod. 2004;19:2633–2643. doi: 10.1093/humrep/deh450. [DOI] [PubMed] [Google Scholar]
- Pisarska MD, Carson SA, Buster JE. Ectopic pregnancy. Lancet. 1998;351:1115–1120. doi: 10.1016/S0140-6736(97)11476-3. [DOI] [PubMed] [Google Scholar]
- Pittaway DE, Reish RL, Wentz AC. Doubling times of human chorionic gonadotropin increase in early viable intrauterine pregnancies. Am J Obstet Gynecol. 1985;152:299–302. doi: 10.1016/s0002-9378(85)80215-5. [DOI] [PubMed] [Google Scholar]
- Popescu LM, Ciontea SM, Cretoiu D, Hinescu ME, Radu E, Ionescu N, Ceausu M, Gherghiceanu M, Braga RI, Vasilescu F, et al. Novel type of interstitial cell (Cajal-like) in human fallopian tube. J Cell Mol Med. 2005;9:479–523. doi: 10.1111/j.1582-4934.2005.tb00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovici RM, Betzler NK, Krause MS, Luo M, Jauckus J, Germeyer A, Bloethner S, Schlotterer A, Kumar R, Strowitzki T, et al. Gene expression profiling of human endometrial-trophoblast interaction in a coculture model. Endocrinology. 2006;147:5662–5675. doi: 10.1210/en.2006-0916. [DOI] [PubMed] [Google Scholar]
- Quintar AA, Mukdsi JH, del Valle BM, Aoki A, Maldonado CA, Perez AJ. Increased expression of uteroglobin associated with tubal inflammation and ectopic pregnancy. Fertil Steril. 2008;89:1613–1617. doi: 10.1016/j.fertnstert.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Randall S, Buckley CH, Fox H. Placentation in the fallopian tube. Int J Gynecol Pathol. 1987;6:132–139. doi: 10.1097/00004347-198706000-00005. [DOI] [PubMed] [Google Scholar]
- Rank RG, Dascher C, Bowlin AK, Bavoil PM. Systemic immunization with Hsp60 alters the development of chlamydial ocular disease. Invest Ophthalmol Vis Sci. 1995;36:1344–1351. [PubMed] [Google Scholar]
- Refaat BA, Bahathiq AO, Sockanathan S, Stewart RL, Wells M, Ledger WL. Production and localization of activins and activin type IIA and IIB receptors by the human endosalpinx. Reproduction. 2004;128:249–255. doi: 10.1530/rep.1.00156. [DOI] [PubMed] [Google Scholar]
- Refaat B, Amer S, Ola B, Chapman N, Ledger W. The expression of activin-betaA- and -betaB-subunits, follistatin, and activin type II receptors in fallopian tubes bearing an ectopic pregnancy. J Clin Endocrinol Metab. 2008;93:293–299. doi: 10.1210/jc.2007-1654. [DOI] [PubMed] [Google Scholar]
- Refaat B, Al-Azemi M, Geary I, Eley A, Ledger W. The role of activins and inducible nitric oxide in the pathogenesis of ectopic pregnancy in patients with or without Chlamydia trachomatis infection. Clin Vaccine Immunol. 2009;16:1493–1503. doi: 10.1128/CVI.00221-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel A, Ophir I, Koler M, Achache H, Prus D. Changing etiology of tubal pregnancy following IVF. Hum Reprod. 2008;23:1372–1376. doi: 10.1093/humrep/den018. [DOI] [PubMed] [Google Scholar]
- Riethmacher D, Brinkmann V, Birchmeier C. A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc Natl Acad Sci USA. 1995;92:855–859. doi: 10.1073/pnas.92.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser WL, Risser JM. The incidence of pelvic inflammatory disease in untreated women infected with Chlamydia trachomatis: a structured review. Int J STD AIDS. 2007;18:727–731. doi: 10.1258/095646207782212351. [DOI] [PubMed] [Google Scholar]
- Riveles K, Roza R, Arey J, Talbot P. Pyrazine derivatives in cigarette smoke inhibit hamster oviductal functioning. Reprod Biol Endocrinol. 2004;2:23. doi: 10.1186/1477-7827-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih SM, Taylor HS. HOXA10 gene expression in human fallopian tube and ectopic pregnancy. Am J Obstet Gynecol. 2004;190:1404–1406. doi: 10.1016/j.ajog.2004.01.066. [DOI] [PubMed] [Google Scholar]
- Sallenave JM, Shulmann J, Crossley J, Jordana M, Gauldie J. Regulation of secretory leukocyte proteinase inhibitor (SLPI) and elastase-specific inhibitor (ESI/elafin) in human airway epithelial cells by cytokines and neutrophilic enzymes. Am J Respir Cell Mol Biol. 1994;11:733–741. doi: 10.1165/ajrcmb.11.6.7946401. [DOI] [PubMed] [Google Scholar]
- Samuelson UE, Sjostrand NO. Myogenic and neurogenic control of electrical and mechanical activity in human oviductal smooth muscle. Acta Physiol Scand. 1986;126:355–363. doi: 10.1111/j.1748-1716.1986.tb07827.x. [DOI] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Savaris RF, da Silva LC, Moraes GS, Edelweiss MI. Expression of MUC1 in tubal pregnancy. Fertil Steril. 2008;89:1015–1017. doi: 10.1016/j.fertnstert.2007.04.036. [DOI] [PubMed] [Google Scholar]
- Shafik A, Shafik AA, El SO, Shafik IA. Specialized pacemaking cells in the human Fallopian tube. Mol Hum Reprod. 2005;11:503–505. doi: 10.1093/molehr/gah192. [DOI] [PubMed] [Google Scholar]
- Shao R, Egecioglu E, Weijdegard B, Kopchick JJ, Fernandez-Rodriguez J, Andersson N, Billig H. Dynamic regulation of estrogen receptor-alpha isoform expression in the mouse fallopian tube: mechanistic insight into estrogen-dependent production and secretion of insulin-like growth factors. Am J Physiol Endocrinol Metab. 2007;293:E1430–E1442. doi: 10.1152/ajpendo.00384.2007. [DOI] [PubMed] [Google Scholar]
- Shaw JL, Denison FC, Evans J, Durno K, Williams AR, Entrican G, Critchley HO, Jabbour HN, Horne AW. Evidence of prokineticin dysregulation in Fallopian tube from women with ectopic pregnancy. Fertil Steril. 2010 doi: 10.1016/j.fertnstert.2009.10.061. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XZ, Tsai MJ, Bullock DW, Woo SL. Hormonal regulation of rabbit uteroglobin gene transcription. Endocrinology. 1983;112:871–876. doi: 10.1210/endo-112-3-871. [DOI] [PubMed] [Google Scholar]
- Shimonaka M, Inouye S, Shimasaki S, Ling N. Follistatin binds to both activin and inhibin through the common subunit. Endocrinology. 1991;128:3313–3315. doi: 10.1210/endo-128-6-3313. [DOI] [PubMed] [Google Scholar]
- Shore VH, Wang TH, Wang CL, Torry RJ, Caudle MR, Torry DS. Vascular endothelial growth factor, placenta growth factor and their receptors in isolated human trophoblast. Placenta. 1997;18:657–665. doi: 10.1016/s0143-4004(97)90007-2. [DOI] [PubMed] [Google Scholar]
- Steptoe PC, Edwards RG. Reimplantation of a human embryo with subsequent tubal pregnancy. Lancet. 1976;1:880–882. doi: 10.1016/s0140-6736(76)92096-1. [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- Strandell A, Thorburn J, Hamberger L. Risk factors for ectopic pregnancy in assisted reproduction. Fertil Steril. 1999;71:282–286. doi: 10.1016/s0015-0282(98)00441-5. [DOI] [PubMed] [Google Scholar]
- Talbot P, Riveles K. Smoking and reproduction: the oviduct as a target of cigarette smoke. Reprod Biol Endocrinol. 2005;3:52. doi: 10.1186/1477-7827-3-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay JI, Moore J, Walker JJ. Ectopic pregnancy. Br Med J. 2000;320:916–919. doi: 10.1136/bmj.320.7239.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teklenburg G, Macklon NS. In vitro models for the study of early human embryo-endometrium interactions. Reprod Sci. 2009 doi: 10.1177/1933719109334966. [DOI] [PubMed] [Google Scholar]
- Tonello A, Poli G. Tubal ectopic pregnancy: macrophages under the microscope. Hum Reprod. 2007;22:2577–2584. doi: 10.1093/humrep/dem246. [DOI] [PubMed] [Google Scholar]
- Ulziibat S, Ejima K, Shibata Y, Hishikawa Y, Kitajima M, Fujishita A, Ishimaru T, Koji T. Identification of estrogen receptor beta-positive intraepithelial lymphocytes and their possible roles in normal and tubal pregnancy oviducts. Hum Reprod. 2006;21:2281–2289. doi: 10.1093/humrep/del176. [DOI] [PubMed] [Google Scholar]
- van VI, Morre SA, van den Brule AJ, Meijer CJ, Bouter LM, Boeke AJ. Overestimation of complication rates in evaluations of Chlamydia trachomatis screening programmes—implications for cost-effectiveness analyses. Int J Epidemiol. 2004;33:416–425. doi: 10.1093/ije/dyh029. [DOI] [PubMed] [Google Scholar]
- Varma R, Gupta J. Tubal ectopic pregnancy. Clin Evid (Online) 2009;Pii:1406. [PMC free article] [PubMed] [Google Scholar]
- Vasquez G, Winston RM, Brosens IA. Tubal mucosa and ectopic pregnancy. Br J Obstet Gynaecol. 1983;90:468–474. doi: 10.1111/j.1471-0528.1983.tb08946.x. [DOI] [PubMed] [Google Scholar]
- Vassiliadou N, Bulmer JN. Characterization of tubal and decidual leukocyte populations in ectopic pregnancy: evidence that endometrial granulated lymphocytes are absent from the tubal implantation site. Fertil Steril. 1998;69:760–767. doi: 10.1016/s0015-0282(98)00005-3. [DOI] [PubMed] [Google Scholar]
- Von Rango RU, Classen-Linke I, Kertschanska S, Kemp B, Beier HM. Effects of trophoblast invasion on the distribution of leukocytes in uterine and tubal implantation sites. Fertil Steril. 2001;76:116–124. doi: 10.1016/s0015-0282(01)01859-3. [DOI] [PubMed] [Google Scholar]
- Walker JJ. Ectopic pregnancy. Clin Obstet Gynecol. 2007;50:89–99. doi: 10.1097/GRF.0b013e31802f4f79. [DOI] [PubMed] [Google Scholar]
- Wallace LA, Scoular A, Hart G, Reid M, Wilson P, Goldberg DJ. What is the excess risk of infertility in women after genital chlamydia infection? A systematic review of the evidence. Sex Transm Infect. 2008;84:171–175. doi: 10.1136/sti.2007.026047. [DOI] [PubMed] [Google Scholar]
- Walters MD, Eddy CA, Gibbs RS, Schachter J, Holden AE, Pauerstein CJ. Antibodies to Chlamydia trachomatis and risk for tubal pregnancy. Am J Obstet Gynecol. 1988;159:942–946. doi: 10.1016/s0002-9378(88)80177-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Guo Y, Wang D, Kingsley PJ, Marnett LJ, Das SK, DuBois RN, Dey SK. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nat Med. 2004;10:1074–1080. doi: 10.1038/nm1104. [DOI] [PubMed] [Google Scholar]
- Wanggren K, Lalitkumar PG, Stavreus-Evers A, Stabi B, Gemzell-Danielsson K. Prostaglandin E2 and F2alpha receptors in the human Fallopian tube before and after mifepristone treatment. Mol Hum Reprod. 2006;12:577–585. doi: 10.1093/molehr/gal058. [DOI] [PubMed] [Google Scholar]
- Wanggren K, Stavreus-Evers A, Olsson C, Andersson E, Gemzell-Danielsson K. Regulation of muscular contractions in the human Fallopian tube through prostaglandins and progestagens. Hum Reprod. 2008;23:2359–2368. doi: 10.1093/humrep/den260. [DOI] [PubMed] [Google Scholar]
- Waylen AL, Metwally M, Jones GL, Wilkinson AJ, Ledger WL. Effects of cigarette smoking upon clinical outcomes of assisted reproduction: a meta-analysis. Hum Reprod Update. 2009;15:31–44. doi: 10.1093/humupd/dmn046. [DOI] [PubMed] [Google Scholar]
- Weigert M, Gruber D, Pernicka E, Bauer P, Feichtinger W. Previous tubal ectopic pregnancy raises the incidence of repeated ectopic pregnancies in in vitro fertilization-embryo transfer patients. J Assist Reprod Genet. 2009;26:13–17. doi: 10.1007/s10815-008-9278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Brown TI, Roghanian A, Sallenave JM. SLPI and elafin: one glove, many fingers. Clin Sci (Lond) 2006;110:21–35. doi: 10.1042/CS20050115. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Rice C, Krenn J, Pilot RL. Effects of nicotine on early pregnancy in the rat. Biol Reprod. 1979;20:294–303. doi: 10.1095/biolreprod20.2.294. [DOI] [PubMed] [Google Scholar]
- Ziganshin AU, Vafina ZR, Fatkullin IF. Pharmacological characterization of P2-receptors in human fallopian tubes. Bull Exp Biol Med. 2004;137:242–245. doi: 10.1023/b:bebm.0000031559.93848.07. [DOI] [PubMed] [Google Scholar]