Abstract
Developmental inadequacy in hepatic antioxidant defenses may contribute to chemical toxicity and pediatric liver diseases. We measured a comprehensive panel of antioxidants in liver tissue from 27 normal pediatric donors. Glutathione reductase declined with age (P = 0.008, r = −0.54, Spearman) while microsomal glutathione-S-transferase increased (GST, P < 0.001, r = 0.81). Males had significantly lower superoxide dismutase and Vitamin E (P < 0.05) and may have lower glutathione reductase (P = 0.06), while females show less cytosolic GST (P = 0.07). Hepatic antioxidants are high in neonates, decline throughout childhood then increase in adolescence to adult levels.
Keywords: oxidative stress, pediatric liver disease, detoxification enzymes
Reactive species, including reactive oxygen species and chemical metabolites, are generally prevented from causing cell and organ damage through the actions of a coordinated set of antioxidant enzymes and compounds. This “antioxidant defense network” prevents hepatocellular damage and ultimately protects against many systemic toxicities (1, 2).
Since the development of pediatric liver detoxification is largely unknown, we measured a comprehensive panel of antioxidant enzymes and compounds in normal liver tissue from 27 pediatric donors aged 13 days – 20 years. These samples were derived from post-mortem donors with healthy livers (Xenotech, Lenexa, KS) and their use was approved by the University of Hawaii Institutional Review Board for Human Subjects. Because tissues were collected post mortem and ischemia causes high levels of oxidative stress, we avoided total antioxidant status or reactive species assays. However, ischemia has lesser effects on antioxidant enzymes and compounds. For example, hepatocytes can be extracted from liver up to 8 hrs post-mortem yet still possess enzyme activities that have a high degree of concordance with in vivo activities (3).
In this study, we investigated glutathione-dependent antioxidant enzymes: Glutathione Peroxidase (GPx), Gluthathione Reductase (GR, commercial kits from Cayman Chemical Company, Ann Arbor MI) and Glutathione-S-transferase (GST, cytosolic and microsomal forms), assayed as previously described with the substrate 1-chloro-2,4-dinitrobenzene (4). Additionally, non-glutathione antioxidant enzymes Catalase (CAT), Superoxide Dismutase (SOD) and Thioredoxin Reductase (TR) were analyzed using Cayman Kits and Quinone Oxido-reductase (NQO1) was measured with dichloroindophenol and dicumarol as previously described (5).
Quality control samples were prepared from pooled adult human livers (n = 50, Xenotech, Lenexa, KS) assessed in triplicate to gauge the precision of each method and also provide a comparison to average antioxidant activities in adults. This pooled sample comprised of Hispanic (8%), African American (2%) and Caucasian (90%) donors, 25 each men and women with an average age of 48.5 yrs (ranging: 21–69). The coefficients of variation (CV) for each assay were: CAT: 9.5%, GPx: 10.7%, GR: 7.5%, SOD: 9.0%, and TR 15.7% for kit assays. For biochemical assays, intra- and inter-day CV were 2.6% and 3.8% for cytosolic GST, 5.3% and 7.9% for microsomal GST and 10.6 and 11.2% for NQO1.
The activities of several hepatic glutathione enzymes differed with age and sex in pediatric liver samples. Although linear correlations with age were not significant for GPx and cytosolic GST, a consistent pattern showing highest activities in neonates and adults with lower activities between 1 and 10 years was observed (Figures 1A and 1E). The activity of GR was significantly negatively correlated with age, being highest under one year of age (P = 0.0077, r = −0.54 Spearman, Fig 1C). The most convincing and startling findings of this study were results from microsomal GST activities in pediatric liver. Microsomal GST activities were extremely low in neonatal liver and increased significantly as children aged, appearing to plateau some time in the teenage years (P < 0.0001, r = 0.81 Spearman, Figure 1G). Males had consistently lower GR (P = 0.06, Figure 1D) and microsomal GST (P = 0.10, Figure 1H) activities than females, which may become significant with a larger sample size. The opposite is true for cytosolic GST, where females had consistently less GST activity than males (P = 0.07, Figure 1F).
Figure 1. Changes in glutathione-related antioxidant enzymes are age- and gender-related in pediatric liver tissue.
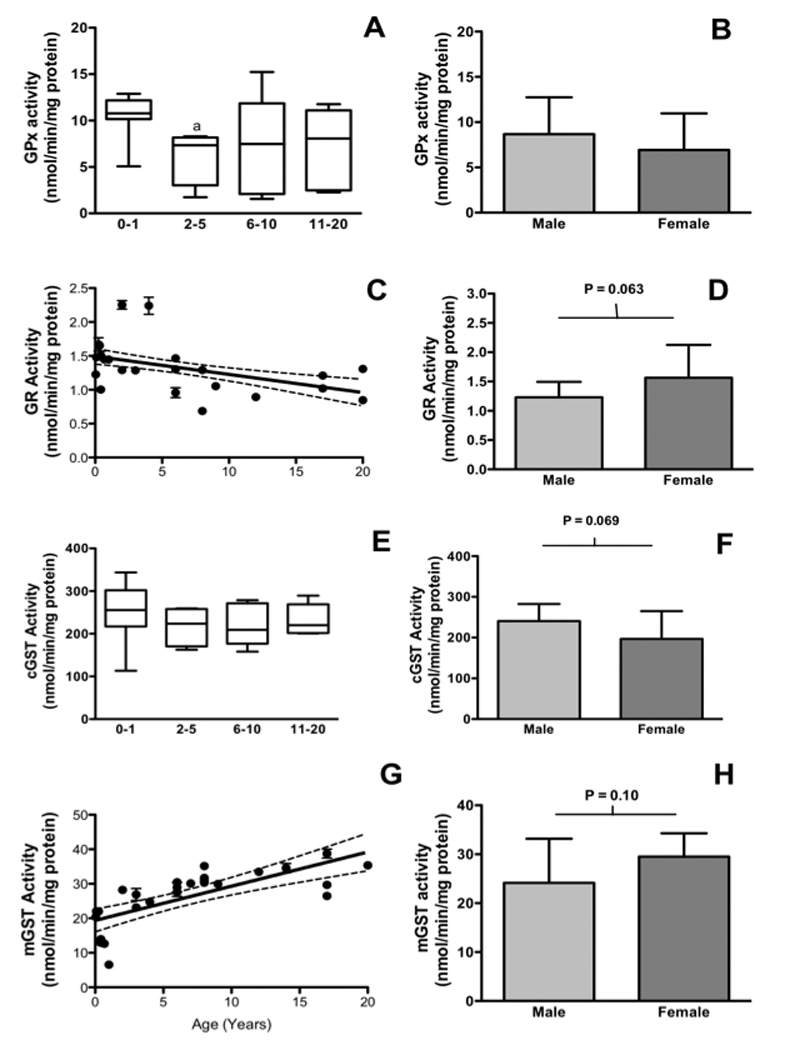
1A: The activity of GPx with age. 1B: The activity of GPx with sex. 1C: GR enzyme activity with age1D: GR enzyme activity with sex. 1E: Cytosolic GST activity with age. 1F: Cytosolic cGST activity with sex. 1G: Microsomal GST enzymes with age. 1H: Microsomal GST activities with sex. Categories for cytosolic enzymes correspond to n = 8 (0–1), n = 4 (2–5), n = 6 (6–10) and n = 5 (11–20) and for microsomal enzymes n = 8 (0–1), n = 4 (2–5), n = 9 (6–10) and n = 6 (11–20). a = P < 0.05 vs. 0–1 using Bonferroni’s multiple comparison test. Bars are means ± SD of triplicate determinations for each liver. * = P < 0.05, male vs. female, students t-test. Dashed line = 95% confidence interval.
For non-glutathione antioxidant enzymes, SOD enzymes differed significantly with age and gender. Children under 1 have significantly higher SOD activity than children aged 6–10 but rates of neonatal SOD activity are not different than hepatic SOD activities in those aged 11 and older (Figure 2A, Bonferroni’s multiple comparison test). SOD was also significantly lower in males than females (P < 0.05, Figure 2B). CAT, NQO1 and TR did not change with age or gender (Figure 2C–H).
Figure 2. The activities of SOD but not other non-glutathione antioxidant enzymes in the cytosol are significantly age- and gender-related in pediatric liver tissue.
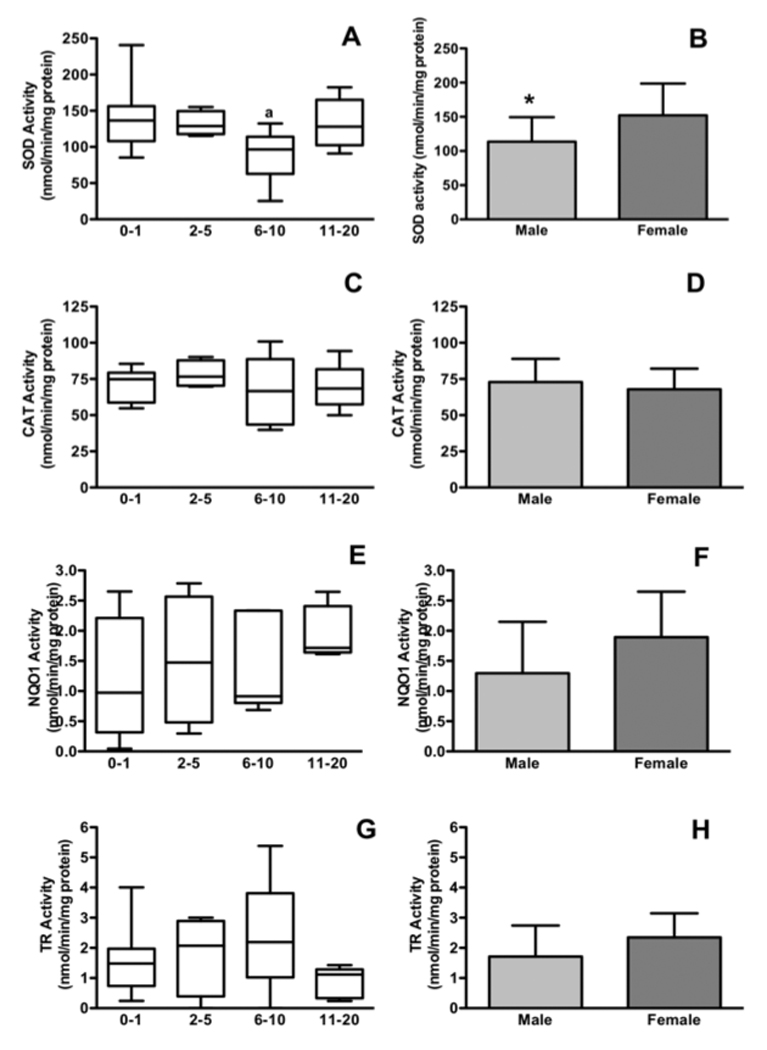
2A: Changes in SOD activity with age. 2B: SOD activities with sex. 2C: Cytosolic CAT activity with age. 2D: Cytosolic CAT activity with sex. 2E: Cytosolic NQO1 with age. 2F: Cytosolic NQO1 with sex. 2G: Cytosolic TR with age. 2H: Cytosolic TR with sex. a = P < 0.05 vs. 0–1 using Bonferroni’s multiple comparison test. * = P < 0.05, student’s t-tests. N values for each category are as listed in Figure 1.
There are few clinical investigations of hepatic antioxidant status in children and even fewer comparing antioxidant status to liver disease in humans. In one study, significantly lower SOD activity was reported in the blood of children with Wilson’s disease and levels of SOD activity were inversely proportional to disease severity (6). Additionally, children with cystic fibrosis who also have decreased activity in the GSTP isoform were eight-fold more likely to develop biliary disease (7). Finally, adult patients with non-alcoholic fatty liver disease have significantly lower hepatic glutathione content, as well as significantly lower SOD and CAT activity compared to normal liver tissue (8). Hence, these disease-specific studies strengthen our speculation that developmentally compromised antioxidant status may be related to the etiology of pediatric liver diseases.
In addition to antioxidant enzymes, reactive species can also be removed through binding and sequestration by antioxidant compounds such as glutathione, Vitamins C and E (2). In neonates, the accumulation of antioxidant compounds is highly complex and begins before birth. For example, the fat-soluble nutrient and an antioxidant compound Vitamin E is mainly present in the neonate through placental active transport that occurs in third trimester of pregnancy (8). In contrast, Vitamin E is primarily accumulated in children through diet (8). Pre-term infants can have very low Vitamin E status and high levels of oxidative stress which are both implicated in sepsis, respiratory distress and retinopathy (2, 8). Therefore, appropriate systemic levels of neonatal and pediatric antioxidant compounds are very important.
Because blood constantly circulates and may gain or lose compounds rapidly via distribution, metabolism and elimination, measuring antioxidant vitamins and minerals directly in liver tissue may present a more stable and accurate picture of hepatic detoxification capacity. Therefore, we measured total glutathione (Cayman Chemical Company Kit, Ann Arbor MI) as well as Vitamin E (9) and Vitamin C (10) in liver S9, which is a sub-cellular fraction that contains both microsomes and cytosol but not nuclei or mitochondria. The precision (quality control CVs) for these assays were 0.2 % glutathione, 4.8% Vitamin E and 0.8% for Vitamin C. Our results demonstrated that the levels of hepatic glutathione, Vitamins C and E were not significantly affected by age (Figures 3A–C). Vitamin E was significantly lower in males than females (P = 0.015, t-test, Figure 3B) but glutathione and Vitamin C were not (Figures 3A and D).
Figure 3. Differences in antioxidant compound levels are not age-dependent but some gender differences occur in pediatric liver tissue.
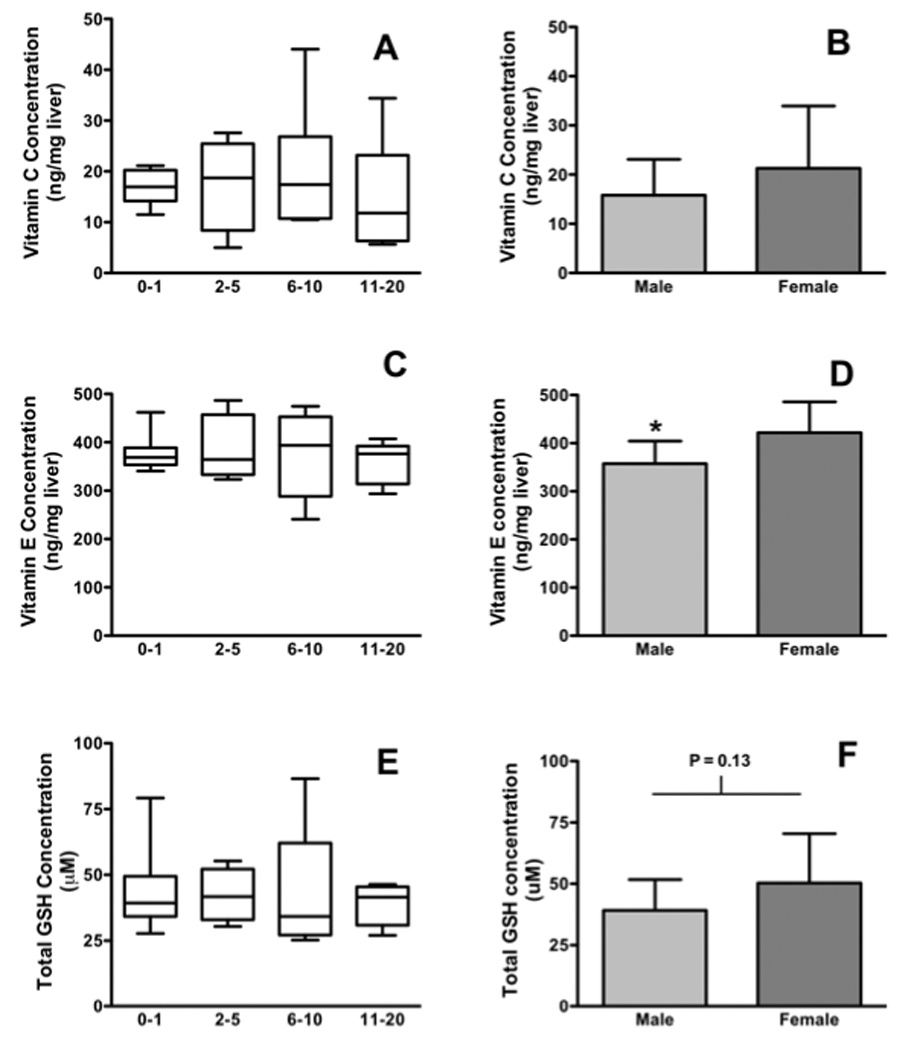
1A Hepatic Vitamin C levels with age. 1b: Hepatic vitamin C levels with sex. 1C: Hepatic Vitamin E activities with age. 1D: Hepatic Vitamin E activities with sex. 1D Total hepatic glutathione content with age. 1E: Total Hepatic glutathione with sex. Bars are means ± SD and n values for each category are as listed in Figure 1. * = P < 0.05, t-test.
The most striking findings of this study are the age-related changes occurring in antioxidant enzymes in the pediatric liver. All of the cytosolic antioxidant enzymes, both glutathione and non-glutathione related, showed high activity in the neonatal liver that declines during childhood with a nadir around 6–10 years and then increased to adulthood. For GPx and SOD, significant decreases were apparent and for the remaining enzymes a consistent trend was observed. In contrast, the microsomal GST enzymes that are anchored in the endoplasmic reticulum, show sparingly low activity in neonatal liver that increases with age, apparently peaking to adult levels in adolescence. Microsomal GST enzymes have a distinct physiological role in addition to detoxification: they are integrally involved in eicosanoid and leukotriene signaling and the production of pro-inflammatory stimuli (11–13). Since the inflammatory and immune systems are not fully developed until late childhood, it follows that inflammatory recruitment enzymes such as microsomal GST develop to the same time line.
Although small sample size is a limitation of this study, the study’s strength is that the tissues derive from normal livers. Most large studies present data from patients undergoing surgery for abnormalities and these studies cannot rule out confounding of their results secondary to disease (14). Thus, these data represent a valuable contribution to defining developmental dynamics in the normal pediatric population. Furthermore, the high level of significance despite the small sample size, argues strongly for the robustness of the data.
Oxidative stress is an initiating event for liver injury. Mechanistically, bile acids and fat accumulate in hepatocytes leading to pathological changes in liver architecture and function that cause clinical symptoms. Our results showing declining antioxidant capacity in childhood that is more pronounced in males, are compatible with this mechanism and also with the clinical incidence of pediatric fatty liver disease which is mainly diagnosed in males between the ages of 11 and 14 (15). The pro-inflammatory role of microsomal GST enzymes also fits this paradigm because when hepatic detoxification capacity is least effective, microsomal GST peaks, increasing inflammatory stimuli and the potential for hepatocyte damage (12,13).
The incidence of liver disease in children is increasing and fatty liver disease is becoming one of the most serious clinical concerns in childhood (15). This study demonstrates that antioxidant capacity in children differs with age and gender and while neonates may be well protected, children under ten have particularly low hepatic detoxification ability in comparison to either neonates or adults. We believe this provides insight into potential mechanisms for altered pediatric response to drugs and other xenobiotics and for the developmental basis of pediatric liver disease. Additional studies should focus on investigating differences in detoxification enzymes between normal and diseased liver, with and without obesity, to confirm and expand upon these findings. Furthermore, research into nuclear receptors and signaling pathways activated by hepatic oxidative stress and fat accumulation, will be useful to define the mechanisms behind these changes and provide targets for pharmacological and clinical intervention.
Acknowledgements
This study was supported by Grants #17442 from The Chun Foundation and NIH RR024206 to Abby C. Collier.
References
- 1.Aboutwerat A, Pemberton P, Smith A, Burrows P, McMahon R, Jain S, Warnes T. Oxidant stress is a significant feature of primary biliary cirrhosis. Biochim et Biophys Acta. 2003;1637(2):147–150. doi: 10.1016/s0925-4439(02)00225-9. [DOI] [PubMed] [Google Scholar]
- 2.Tsukahara H. Biomarkers of oxidative stress: clinical application in pediatric medicine. Curr Med Chem. 2007;14(3):339–351. doi: 10.2174/092986707779941177. [DOI] [PubMed] [Google Scholar]
- 3.Guillouzo A, Morel F, Fardel O, Meunier B. Use of human hepatocyte cultures for drug metabolism studies. Toxicology. 1993;82(1–3):209–219. doi: 10.1016/0300-483x(93)90065-z. [DOI] [PubMed] [Google Scholar]
- 4.Habig W, Pabst M, WJ Glutathione-S-Transferases. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 5.Ernster L, Lind C, Rase B. A study of the DT-diaphorase activity of warfarin-resistant rats. Eur J Biochem. 1972;25:198–206. doi: 10.1111/j.1432-1033.1972.tb01685.x. [DOI] [PubMed] [Google Scholar]
- 6.Nagasaka H, Inoue I, Inui A, Komatsu H, Sogo T, Murayama K, Murakami T, Yorifuji T, Asayama K, Katayama S, Uemoto S, Kobayashi K, Takayanagi M, Fujisawa T, Tsukahara H. Relationship between oxidative stress and antioxidant systems in the liver of patients with Wilson disease: hepatic manifestation in Wilson disease as a consequence of augmented oxidative stress. Ped Res. 2006;60(4):472–477. doi: 10.1203/01.pdr.0000238341.12229.d3. [DOI] [PubMed] [Google Scholar]
- 7.Henrion-Caude A, Flamant C, Roussey M, Housset C, Flahault A, Fryer A, Chadelat K, Strange R, Clement A. Liver disease in pediatric patients with cystic fibrosis is associated with glutathione S-transferase P1 polymorphism. Hepatology. 2002;36(4 Pt 1):913–917. doi: 10.1053/jhep.2002.35534. [DOI] [PubMed] [Google Scholar]
- 8.Debier C. Vitamin E during pre- and postnatal periods. Vitam Horm. 2007;76:357–373. doi: 10.1016/S0083-6729(07)76013-2. [DOI] [PubMed] [Google Scholar]
- 9.Tütem E, Apak R, Günaydi E, Sözgen K. Spectrophotometric determination of vitamin E (-tocopherol) using copper(II)-neocuproine reagent. Talanta. 1997;44(2):249–255. doi: 10.1016/s0039-9140(96)02041-3. [DOI] [PubMed] [Google Scholar]
- 10.Omaye S, Turnbull J, Sauberlich H. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Meth Enzymol. 1979;62:311. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- 11.Jakobsson PJTS, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc Nat Acad Sci (USA) 1999;96(13):7220–7225. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes J, Flanagan J, Jowsey I. Glutathione transferases. Ann Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 13.Dwivedi S, Sharma A, Patrick B, Sharma R, Awasthi Y. Role of 4-hydroxynonenal and its metabolites in signaling. Redox Rep. 2007;12(1):4–10. doi: 10.1179/135100007X162211. [DOI] [PubMed] [Google Scholar]
- 14.Strassburg C, Strassburg A, Kneip S, Barut A, Tukey R, Rodek B, Manns M. Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut. 2002;50:259–265. doi: 10.1136/gut.50.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bezerra J. The next challenge in pediatric cholestasis: deciphering the pathogenesis of biliary atresia. J Ped Gastroenterol Nutr. 2006;43 Suppl 1:S23–S29. doi: 10.1097/01.mpg.0000228197.28056.2f. [DOI] [PubMed] [Google Scholar]


