Abstract
The central nervous system (CNS) is a closed system guarded by the blood brain barrier (BBB), with a complicated network of microvascular endothelial cells, astrocytes, and neurons engaged in selective neurophysiological mechanisms. Exploration for a molecule such as a cofactor, hormone, enzyme, signaling molecule, or second messenger (collectively addressed as CHESS as we proceed), which has the ability to cross the BBB will be the goal of this hypothesis. The ratio of amino acids (AA) to neurotransmitters (NT) is over one-to-one thousand in the CNS, with the ultimate effect at the end receptor level. Diagnostic modalities utilizing oxygen and glucose for identifying organic brain diseases via functional properties have become popular. Delineation from the background signal, however, poses an enormous challenge. Targeting neurotransmitter metabolism with little or possibly no background signal using a cofactor able to cross the BBB is hypothesized.
Keywords: blood brain barrier, cofactors, amino acids, neurotransmitters
Introduction
Amino acid to neurotransmitter ratio plays a vital role in the CNS.1 Alteration in dopamine (DA) metabolism is a well recognized pathophysiological mechanism for schizophrenia, serotonergic (5-HT) effects for depression, noradrenergic (NE) activity for anxiety, and gamma amino butyric acid (GABA) stimulation for central inhibition. Multiple receptor involvement in a single disorder involving dopamine, serotonin, and norepinephrine has been reported.2 Although the final effects are attributed to neurotransmitters in major psychiatric illnesses, actual release via exocytosis of these neurotransmitters is 1000 times less than their precursor amino acids. Thus, any alterations, associated with production of neurotransmitters, such as deficiencies in cofactors, the development of antibodies to key enzymes,3 or deficiency of a particular amino acid4 can shift the balance from a normal to a transiently dysfunctional state. Figure 1 demonstrates the steps preceding neurotransmitter release and the relationship between amino acids and neurotransmitters, i.e., normal brain metabolism.
Figures 1.
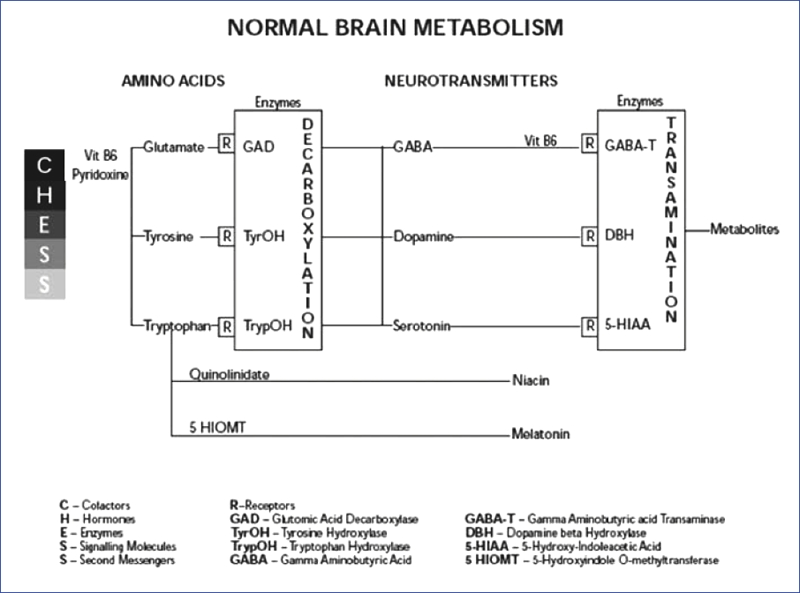
The relationship between amino acids and neurotransmitters
The pathophysiology of schizophrenia, described previously and tested subsequently, focused on the second messenger calcium as a common factor linking early developmental, late maturational, and degenerative etiologies.5,6,7 The role of an antibody to the enzyme glutamic acid decarboxylase (GAD) was recently reported as another possible cause of chronic psychotic disorders.8 The cofactor pyridoxal-5- phosphate (PLP) a phosphorylated form of vitamin B6, common to all neurotransmitter metabolism, will be discussed in detail as its precursors could potentially cross the BBB.
Basics of Pyridoxine
While other molecules in the cascade have site, age, and gender i.e., hormone specific variations, vitamin B6 is less specific and its deficiency is hypothesized to play a key role in the development of chronic psychotic disorders. The requirement of pyridoxine in the CNS is a 100-fold greater than in the peripheral organs. In humans, an exogenous source of vitamin B6 is required for amino acid metabolism. It is stored primarily in the liver and to a lesser extent in the muscle and brain. Additionally, vitamin B6 is involved in more than 100 reactions peripherally.9
In the periphery, vitamin B6 exists in three forms: pyridoxine, pyridoxamine, and pyridoxal. It is converted to its active form pyridoxal-5-phosphate (PLP) prior to neurotransmitter production. It is unclear at this time which of the forms crosses the blood brain barrier (BBB) and what biochemical reactions centrally activate the vitamin. Considering the selective permeability of the BBB (a phosphorylated molecule cannot cross the BBB), PLP levels in the brain could substantially differ from the periphery. Imaging of brain vitamin B6 with radioactive tracers becomes possible only when they are available in the phosphorylated form (essentially by being trapped intracellularly), hence, the significance of a detailed pathway exploration of this vital supplement. Serious concerns related to sub-optimal vitamin levels in chronic disease states have been reported. Therefore, the need to develop a reference range for optimal levels of this cofactor, especially in the CNS, is important.
Discussion
Efforts to quantify brain metabolism face enormous challenges secondary to the transient nature of the reactions, i.e., exocytosis. Currently, no marker exists for the measurement of cerebral metabolism with SPECT imaging.10 Identification of specific markers may have diagnostic applications leading to a better understanding of disease states and current treatments. Most of the psychopharmacotherapy available today is directed toward reuptake inhibition or blockade at specific receptor sites. It is becoming increasingly clear, however, that metabolism improves as multiple receptor sites are targeted instead of single receptor types. The benefits of some atypical antipsychotics are attributed to stabilizing effects in the CNS, i.e., improved neuroplasticity,11 possibly by regulating the CHESS factors. Although it may be true with some agents, it may not be with others, as they block end products at the postsynaptic receptor level resulting in altered precursor Amino acid feedback mechanisms. Since amino acids are building blocks for proteins and peptides, the process may result in new receptor recruitment or susceptibility to altered gene expression with time. Therefore, more specific markers targeting neurotransmitter metabolism exclusively need to be explored.
Relevance of pyridoxine
Levels of B6 outside the normal range may explain the various manifestations of psychiatric symptoms discussed earlier. Early interventions to maintain adequate levels of essential cofactors may prevent, delay, or mitigate the onset or severity of psychiatric symptoms. Different mental illnesses vary in course and outcome. The reactions and clinical examples described lead us to believe that the severity of psychiatric symptoms reflect fluctuations on a spectrum rather than a focal deficit. The overlap of symptoms and disease states requires detailed CNS metabolism studies if differentiation is to be made possible. An example is the acute deficiency of GABA, providing a substrate for seizures with multiple potential triggers, such as benzodiazepine withdrawal, alcohol withdrawal, fever in infants, and use of medications that lower the seizure threshold. These could be acute manifestations on the spectrum of transient yet severe deficiency in GABA production with a potential for reversal/prevention by the administration of GABAergic agents. The established treatment of choice for infantile seizures, also termed pyridoxine-dependant seizures and isoniazid (INH)-induced seizures, is vitamin B6. Depletion of B6 is proportional to the dose of INH administered in case of toxicity-induced seizures, and supplemental doses of B6 appear to control seizures via increased production of GABA.12 On the other hand, it remains unclear if vitamin B6 can effectively control seizures resulting from alcohol, benzodiazepines, or neuroleptic withdrawal. Similarly, if acute deficits of vitamin B6 result in infantile seizures and INH toxicity by compromising the blood brain barrier,13 an argument could be made that milder deficits along the spectrum could manifest themselves in behavioral changes associated with other neuropsychiatric disorders.
An example would be the role of vitamin B3 (niacin), a byproduct of pyridoxine, in supplying energy for transport of oxidative metabolites from neurons. This effect is decreased with age presenting with memory loss, and concentration and attention problems prior to the onset of dementia, suggesting a possible need for vitamin B supplements. Evidence for increases in advanced age-related seizures have also been reported in a recent study,14 supporting the increased demand for these supplements.
Conclusion
A thorough understanding of the role of B vitamins, particularly B6, in neurotransmitter metabolism and cytoprotective mechanisms may help develop specific diagnostic and treatment approaches to benefit individuals with neuropsychiatric disorders. In conclusion, the synthesis of supplements like pyridoxine, as radioactive tracers in expanding newer diagnostic modalities, such as fMRI, deserves a closer look. From a practicing psychiatrist's point of view, the addition of vitamin supplements, such as B6, in doses equivalent or higher to the recommended daily allowance, may be beneficial in improving the overall metabolism and preserving the integrity of the blood brain barrier by reducing cytotoxicity related microvascular damage.
Acknowledgments
The authors wish to thank Dr. Gerald Brown for editorial assistance.
Contributor Information
Atmaram Yarlagadda, Dr. Yarlagadda is from McDonald Army Health Center, Fort Eustis, Virginia, and from Clinical Associates of Tidewater, Newport News, Virginia.
Anita H. Clayton, Dr. Clayton is Professor, Department of Psychiatry & Neurobehavioral Sciences, University of Virginia, Charlottesville, Virginia.
References
- 1.Roskoski R., Jr. Neurochemistry. In: Frolich ED, Rypin H, editors. Rypins' Basic Sciences Review. Philadelphia, PA: Lippincott,Williams, & Wilkins; 1993. pp. 387–91. [Google Scholar]
- 2.Stahl SM. Essential Psychopharmacology. New York, NY: Cambridge University Press; 2000. [Google Scholar]
- 3.Vincent A, Grimaldi LME, Martino G, et al. Antibodies to 125I-glutamic acid decarboxylase in patients with stiff man syndrome. J Neurol Neurosurg Psychiatry. 1997;62:395–7. doi: 10.1136/jnnp.62.4.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riedel WJ, Klassen T, Schmit JAJ. Tryptophan, mood, and cognitive function. Brain Behavior Immunity. 2002;16(5):581–9. doi: 10.1016/s0889-1591(02)00013-2. [DOI] [PubMed] [Google Scholar]
- 5.Yarlagadda A. Role of calcium regulation in pathophysiology model of schizophrenia and possible interventions. Medical Hypothesis. 2002;58(2):182–6. doi: 10.1054/mehy.2001.1511. [DOI] [PubMed] [Google Scholar]
- 6.Koh PO, Undie AS, Kabbani N, et al. Up-regulation of neuronal calcium sensor-1 in the prefrontal cortex of schizophrenic and bipolar patients. Proc Natl Acad Sci USA. 2003;100(1):313–17. doi: 10.1073/pnas.232693499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlecker C, Boehmerle W, Jeromin A, et al. Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of lithium. J Clin Invest. 2006;116(6):1668–74. doi: 10.1172/JCI22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarlagadda A, Helvink B, Chou C. Clayton A. Blood brain barrier: The role of GAD antibodies in psychiatry. Psychiatry. 2007;4(6):57–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson GE, Blass JP. Nutrition and brain function. In: Seigel GJ, Agranoff BW, Albers RW, et al., editors. Basic Neurochemistry, Molecular, Cellular, and Medical Aspects. Philadelphia, PA: Lippincott, Williams & Wilkins; 1999. pp. 691–704. [Google Scholar]
- 10.Devous MD., Sr. Instrumentation, radiopharmaceuticals, and technical factors. In: Heertum Van RL, Tikofsky RS, editors. Cerebral SPECT Imaging. New York, NY: Raven Press Ltd; 1995. pp. 3–19. [Google Scholar]
- 11.Coyle JT, Manji HK. Getting balance: Drugs for bipolar disorder share target. Nat Med. 2002;8(6):557–8. doi: 10.1038/nm0602-557. [DOI] [PubMed] [Google Scholar]
- 12.Temmerman W, Dhondt A, Vandewoude K. Acute isoniazid intoxication: Seizures, acidosis, and coma. Acta Clin Belg. 1999;54(4):211–6. [PubMed] [Google Scholar]
- 13.Chang SJ, Vitamin B6. Vitamin B6 antagonists alter the function and ultrastructure of mice endothelial cells. J Nutr Sci Vitaminol (Tokyo) 2000;46(4):149–53. doi: 10.3177/jnsv.46.149. [DOI] [PubMed] [Google Scholar]
- 14.Velez L, Selwa LM. Seizure disorder in the elderly. Am Fam Physician. 2003;67:325–32. [PubMed] [Google Scholar]


