Abstract
Rationale:
Nerve growth factor (NGF) promotes angiogenesis and cardiomyocyte survival, which are both desirable for postinfarction myocardial healing. Nonetheless, the NGF potential for cardiac repair has never been investigated.
Objective:
To define expression and localization of NGF and its high-affinity receptor TrkA (tropomyosin-related receptor A) in the human infarcted heart and to investigate the cardiac roles of both endogenous and engineered NGF using a mouse model of myocardial infarction (MI).
Methods and Results:
Immunostaining for NGF and TrkA was performed on heart samples from humans deceased of MI or unrelated pathologies. To study the post-MI functions of endogenous NGF, a NGF-neutralizing antibody (Ab-NGF) or nonimmune IgG (control) was given to MI mice. To investigate the NGF therapeutic potential, human NGF gene or control (empty vector) was delivered to the murine periinfarct myocardium. Results indicate that NGF is present in the infarcted human heart. Both cardiomyocytes and endothelial cells (ECs) possess TrkA, which suggests NGF cardiovascular actions in humans. In MI mice, Ab-NGF abrogated native reparative angiogenesis, increased EC and cardiomyocyte apoptosis and worsened cardiac function. Conversely, NGF gene transfer ameliorated EC and cardiomyocyte survival, promoted neovascularization and improved myocardial blood flow and cardiac function. The prosurvival/proangiogenic Akt/Foxo pathway mediated the therapeutic benefits of NGF transfer. Moreover, NGF overexpression increased stem cell factor (the c-kit receptor ligand) expression, which translated in higher myocardial abundance of c-kitpos progenitor cells in NGF-engineered hearts.
Conclusions:
NGF elicits pleiotropic beneficial actions in the post-MI heart. NGF should be considered as a candidate for therapeutic cardiac regeneration.
Keywords: myocardial infarction, angiogenesis, gene therapy, apoptosis
Myocardial infarction (MI) remains a major cause of morbidity and mortality and it is responsible for about one third of heart failure cases worldwide.1 Sudden occlusion of a major coronary artery and acute myocardial ischemia causes rapid death of cardiomyocytes and vascular cells in the area of interest.2 Loss of cardiomyocytes and vasculature leads to progressive fibrous replacement of myocardium, hypertrophic growth of the spared myocardium, and left ventricular (LV) dilatation. Maladaptive ventricular remodeling contributes to post-MI heart failure,3 and prognosis of heart failure patients is still poor.4 Myocardial ischemia triggers a spontaneous angiogenic response aimed at reestablishing myocardial blood flow. Nonetheless, this protective response is usually not sufficient.5
Nerve growth factor (NGF) is a secreted glycoprotein of the neurotrophin family. NGF elicits its biological effects mainly by binding the high-affinity TrkA receptor (tropomyosin-related receptor A, which is a tyrosine kinase). We and others previously demonstrated that NGF, via TrkA, promotes angiogenesis and endothelial cells (ECs) survival through a mechanism involving the serine/threonine kinase Akt (also known as protein kinase B).6,7 Akt regulates a variety of cellular functions and it is strongly implicated in angiogenesis and cell survival.8 We also demonstrated that cultured cardiomyocytes express TrkA and release NGF.9 Moreover, NGF is an autocrine prosurvival factor for the cardiomyocyte through the Akt/Forkhead box-O transcription factors (Foxo) pathway.9 Foxo factors stimulate cell death and are downstream targets of Akt.10 Akt-mediated phosphorylation regulates Foxo-3a subcellular localization and activity: unphosphorylated Foxo-3a resides in the nucleus, whereas Foxo-3a phosphorylation leads to its nuclear exclusion and inactivation.11 Moreover, Foxo-1 and -3a represses angiogenesis by downregulating endothelial nitric oxide synthase.12
Experimental evidences suggest that myocardial repair is, at least in part, dependent on c-kit receptor–expressing (c-kitpos) progenitor cell populations.13-15 Lineage negative (Linneg) c-kitpos cells are increased in the infarcted heart and heart-derived Linnegc-kitpos cells can generate new vascular cells and myocytes both in vivo and in vitro.13 Activation of the c-kit receptor is mediated by its ligand stem cell factor (SCF), a cytokine that exists in soluble or membrane-associated form.16 The membrane-bound SCF isoform, which is predominant in vivo, is able to induce more persistent c-kit activation.17 It was recently reported that transgenic mice with cardiomyocyte-specific membrane-associated SCF over-expression have improved post-MI cardiac function and angiogenesis in comparison to wild types.18
In the present study, we firstly investigated NGF and TrkA expression in the human infarcted heart. We then evaluated the functions of endogenous NGF and the therapeutic potential of its overexpression in the post-MI heart using mice. Finally, we studied the potential involvement of the Akt/Foxo-3a pathway and of cardiac progenitor cell populations, with particular emphasis on Linnegc-kitpos cells, in the NGF-mediated cardiac repair after MI.
Methods
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org.
Immunohistochemical Analysis of Human Cardiac Samples
Cardiac samples were collected at autopsy from human subjects (described in Online Table I) died after MI or causes other than cardiovascular diseases. NGF expression and TrkA expression and localization in cardiomyocytes and endothelial cells (ECs) were determined.
MI Protocol in Mice
In vivo experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and with the prior approval of the UK Home Office and the University of Bristol. MI was induced in anesthetized CD1 male mice, as described.19 To study the actions of endogenous NGF in the postinfarct myocardium, mice received IP a goat-raised NGF-neutralizing antibody (Ab-NGF) or control non immune goat-IgG.6,9 To investigate the therapeutic potential of local NGF overexpression, mice were injected in the periinfarct myocardium with an adenoviral vector carrying human NGF (V5-tagged) or Ad.Null (control).9 Sham-operated mice received Ad.Null. Survival curves of mice enrolled in the NGF neutralization and the hNGF gene therapy protocols were studied. To verify whether NGF-induced therapeutic effect depends on the Akt/Foxo-3a pathway activation, additional MI mice were injected in the periinfarct with a Foxo-3a mutant form which is resistant to Akt phosphorylation (Ad.AAAFoxo3a) or Ad.Null in combination with Ad.hNGF or Ad.Null. LV function and dimensions were measured by echocardiography at 14 and 30 days post-MI. LV function at 14 days was additionally measured with a miniaturized 1.4F transducer Millar tip-catheter. Myocardial blood flow (mL/min per gram of tissue) at 14 days postsurgery was measured using fluorescent microspheres.20 Myocardial capillary and arteriolar densities were measured at 14 and 30 days postsurgery. EC and cardiomyocyte apoptosis was evaluate at 14 days. Putative cardiomyocyte progenitor cells were identified in myocardial sections as those cells expressing both c-kit and the cardiomyocyte transcription factor GATA-4.21 The rate of c-kitpos cell proliferation was determined by double-staining of sections for c-kit and the cell cycle marker minichromosome maintenance protein (MCM)-2.22
The effect of SCF neutralization on NGF-induced improvement of cardiac function and angiogenesis was studied at 14 days postoperation.
Analyses of Cardiac Progenitor Cells
Cardiac flow cytometry analyses of LV-resident Linnegc-kitpos and Linnegsca-1pos progenitor cells were performed at 3 and 14 days (Linnegc-kitpos, only) postoperation. Expression of TrkA and p75NTR (low-affinity NGF receptor) by isolated c-kitpos cells was evaluated by RT-PCR. The effect of neutralizing the c-kit receptor ligand SCF on Ad.hNGF-induced Linnegc-kitpos expansion was assessed at 3 and 14 days postsurgery by flow cytometry.
Expressional Analyses
At 3 days postsurgery, mRNA levels of transgenic (human) NGF and murine NGF, TrkA and SCF were evaluated in the periinfarct zone. Western blot analyses for V5-tag, TrkA, total and phospho(Ser473)-Akt, total and phospho(Thr32)-Foxo-3a, total and phospho(Tyr730)-c-kit, SCF, and GAPDH (loading control) were performed. Human and murine NGF and SCF levels were measured by ELISA.
Results
NGF and TrkA Expression in the Human Heart
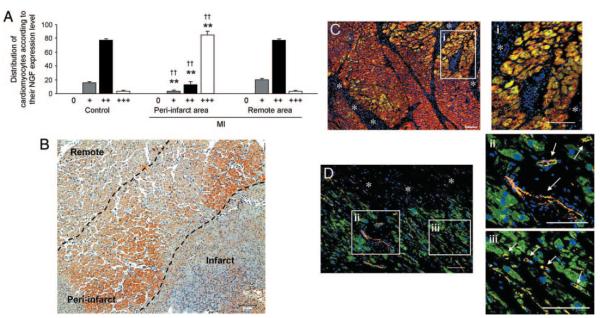
Immunohistochemical analyses detected NGF presence in the human myocardium. As shown in Figure 1A, according to a semiquantitative analysis, the majority of cardiomyocytes of both not infarcted hearts and the remote area of infarcted hearts expressed NGF weakly to moderately. By contrast, most cardiomyocytes in the periinfarct area express high NGF levels, thus suggesting that NGF is upregulated by ischemia. Figure 1B shows NGF expression in a human infarcted heart. Figure 1C and 1D shows TrkA expression by cardiomyocytes and ECs in an infarcted heart, respectively.
Figure 1. NGF protein is expressed in the human infarcted heart, with higher expression level in the periinfarct area.
The TrkA receptor of NGF is present in cardiomyocytes and ECs. A, Bar graph represents the distribution of cardiomyocytes subdivided for their amount of NGF expression. NGF expression by each cardiomyocyte was determined by a semiquantitative scoring system: no staining: 0; weak staining: +; weak to moderate staining: ++; strong staining: +++. Data are expressed as means±SEM. **P<0.01 vs controls without MI; ††P<0.01 vs MI remote area (n=8 hearts for each group). B, Immunohistochemical staining for NGF in human heart after acute MI shows an increased protein content (brown) of NGF in the MI border zone compared to the remote area. Scale bar: 100 μm. C and D, Immunohistochemical detection of TrkA in human acute MI. The coexpression of TrkA (green fluorescence) in either α-sarcomeric actin (α-sarc)–positive (red fluorescence) (C) cardiomyocytes or CD34-positive (red fluorescence) (D) ECs results in yellow fluorescence. A gradient in the TrkA expression from cardiomyocytes bordering the acute damage (asterisks) is apparent. Nuclei are recognized by the blue fluorescence of DAPI. Scale bars: 100 μm. TrkA expression in cardiomyocytes (i inset) and in vascular structures (indicated by white arrows in ii inset), including capillaries (indicated by white arrows in iii inset) is also shown at higher magnification. Scale bars: 100 μm.
Myocardial NGF and TrkA Levels Are Increased by MI in Mice
To confirm that MI increases NGF myocardial expression, we used a mouse MI model. At 3 days post-MI, relative NGF and TrkA mRNA expression in the periinfarct LV increased by 2.0-folds and 4.2-folds in comparison versus sham-operation (Online Figure I, A and B). Furthermore, protein expression of NGF and TrkA increased in the periinfarct myocardium, as assessed by ELISA and western blot, respectively (Online Figure I, C and D).
NGF Neutralization Increases Apoptosis, Abrogates Spontaneous Neovascularization, and Worsens LV Function After MI in Mice
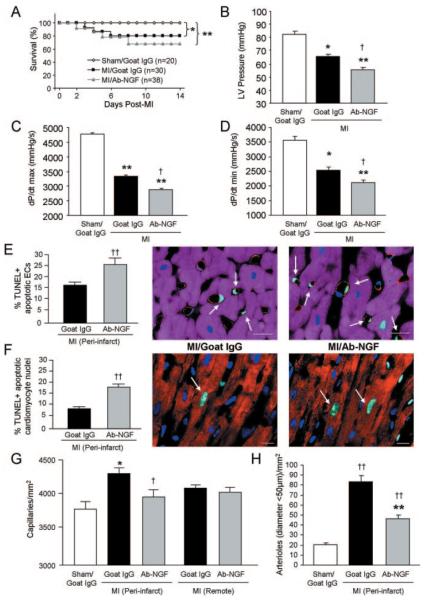
To investigate the role of endogenous NGF in the spontaneous responses to MI, we neutralized NGF. Postoperation survival of mice was monitored for 14 days. Whereas survival of sham-operated mice was 100%, mortality in MI mice was significantly increased, although no differences were observed between MI/Ab-NGF- and MI/goat-IgG-injected mice (Figure 2A). Importantly, NGF neutralization aggravated the MI-induced deterioration of cardiac function (Online Table II). In comparisons with non immune goat-IgG, Ab-NGF further decreased LV pressure (Figure 2B), dP/dtmax (Figure 2C), dP/dtmin (Figure 2D) and LV ejection fraction (LVEF) (Online Table II). Moreover, Ab-NGF increased LV chamber volume and LV internal diameter (Online Table II), which are features of maladaptive LV remodeling. Histological analyses of the periinfarct myocardium (14 days post-MI) indicated that, in comparison with nonimmune goat-IgG, Ab-NGF increased apoptosis of ECs (Figure 2E) and cardiomyocyte (Figure 2F), abrogated the spontaneous capillary growth (Figure 2G), and reduced the density of small (diameter <50 μm) arterioles (Figure 2H).
Figure 2. NGF neutralization (by Ab-NGF) reduces LV function, increases apoptosis, and abrogates spontaneous neovascularization at 14 days post-MI.
A, Survival of mice after MI or sham operation was monitored for 14 days. B through D, Bar graphs represent LV pressure (B), dP/dtmax (C), and dP/dtmin (D). E, Bar graph shows the increased percentage of TUNEL-positive apoptotic ECs in the LV periinfarct area. Apoptotic ECs (indicated by arrows in the microphotograph) were revealed by TUNEL (green fluorescence) in combination with the EC marker von Willebrand factor (red fluorescence). Cardiomyocytes are recognized by α-sarc staining (purple fluorescence). Scale bars: 10 μm. F, Bar graph shows the increased percentage of TUNEL-positive apoptotic cardiomyocyte nuclei after Ab-NGF. Apoptotic cardiomyocyte nuclei (indicated by arrows in the adjacent microphotographs) were revealed by TUNEL-positive (green fluorescence) in α-sarc-positive (red fluorescence) cardiomyocytes. Nuclei are stained by DAPI (blue fluorescence). Scale bars: 10 μm. G and H, Bar graphs show the reduced number of capillaries (G) and arterioles (H) in the LV periinfarct zone. All data are expressed as means±SEM. *P<0.05 and **P<0.01 vs sham/goat IgG; †P<0.05, ††P<0.01 vs MI/goat IgG (n=5 mice/group for histological analyses; n=at least 10 mice/group for hemodynamic analyses).
NGF Overexpression in the Infarcted Myocardium Improves LV Function After MI
To test the therapeutic potential of NGF, we delivered Ad.hNGF or Ad.Null in the mouse periinfarct myocardium. As expected, at 3 days from Ad.hNGF, human NGF mRNA was expressed in the mouse LV, with higher expression in the periinfarct zone (Online Figure II, A). Moreover, the V5-tag was detected in the periinfarct zone of Ad.hNGF-injected mice (Online Figure II, B). Furthermore, ELISA documented the presence of transgenic NGF in the plasma of Ad.hNGF-injected MI mice, only (Online Figure II, C).
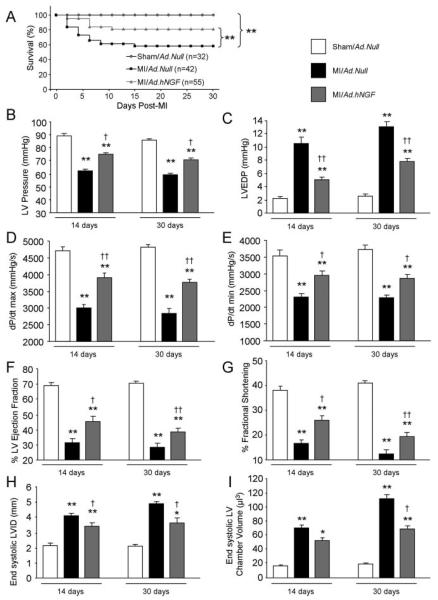
Importantly, Ad.hNGF reduced mortality at 30 days post-MI (P<0.01 versus MI/Ad.Null; Figure 3A) and improved LV function at both 14 and 30 days post-MI. In fact, in comparisons to MI/Ad.Null, at both time points, NGF overexpression increased LV pressure (Figure 3B), decreased LV end-diastolic pressure (Figure 3C), and improved dP/dtmax and dP/dtmin (Figure 3D and 3E), as well as LVEF and LV fractional shortening (LVFS) (Figure 3F and 3G). Furthermore, Ad.hNGF reduced systolic LV internal diameter and LV chamber volume (Figure 3H and 3I), indicating the preventive effect of NGF on post-MI LV chamber dilatation. All measured functional and dimensional data are reported in Online Table III.
Figure 3. NGF overexpression improves post-MI survival and ameliorates LV function at 14 and 30 days post-MI.
A, Survival curve shows reduced post-MI mortality of Ad.hNGF-injected mice. B through E, Bar graphs illustrate the improved LV pressure (B), LV end-diastolic pressure (C), dP/dtmax (D), and dP/dtmin (E) after Ad.hNGF (analyzed by Millar Tip-catheter). F through I, Bar graphs show increased LVEF (F) and LVFS (G) and reduced LV internal diameter (LVID) (H) and LV chamber volume (I) after Ad.hNGF (measured by echocardiography). Data are expressed as means±SEM. *P<0.05, **P<0.01 vs sham/Ad.Null; †P<0.05, ††P<0.01 vs MI/Ad.Null (n=at least 10 mice/group).
NGF Overexpression Reduces Apoptosis and Improves Angiogenesis and Cardiac Perfusion
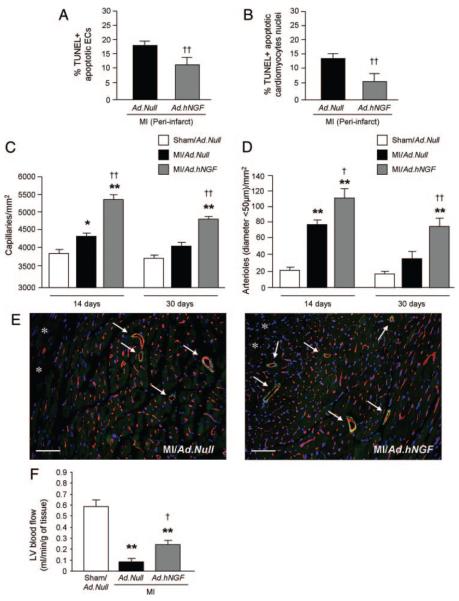
At 14 days post-MI, in comparison with Ad.Null, Ad.hNGF reduced the percentage of TUNEL-positive apoptotic ECs and cardiomyocytes in the periinfarct area (Figure 4A and 4B). Moreover, Ad.hNGF increased the densities of both capillaries and small (diameter, <50 μm) arterioles at both 14 and 30 days post-MI and gene transfer (Figure 4C and 4D). Figure 4E shows mouse myocardial sections were capillaries are stained by isolectin-B4 (red fluorescence) and arterioles (indicated by arrows) are identified by both isolectin-B4 and α-smooth muscle actin staining (green fluorescence). The proangiogenic effect of Ad.hNGF was associated with improved LV perfusion, as myocardial blood flow was increased to 152% versus MI/Ad.Null (Figure 4F).
Figure 4. NGF overexpression inhibits post-MI EC and cardiomyocyte apoptosis and improves neovascularization and cardiac perfusion.
A and B, Bar graphs show reduced percentage of TUNEL-positive apoptotic ECs (A) and cardiomyocytes (B) in the LV periinfarct zone at 14 days post-MI. C and D, Bar graphs show increased densities of capillaries (C) and arterioles (D) in the periinfarct area at 14 and 30 days post-MI. E, Representative microphotographs at 14 days post-MI showing capillaries (stained by isolectin-B4) (red fluorescence) and arterioles (stained by both isolectin-B4 and α-smooth muscle actin staining [green] and indicated by arrows). Asterisks indicate the infarct area (scale bar: 50 μm). F, Bar graph shows LV absolute blood flow at 14 days postsurgery. Data are expressed as means±SEM. *P<0.05, **P<0.01 vs sham/ Ad.Null; †P<0.05, ††P<0.01 vs MI/Ad.Null (n=5 mice/group for histological analyses; n=10 mice/group for blood flow analyses).
NGF Therapeutic Effects Are Regulated by the Akt/Foxo-3a Pathway
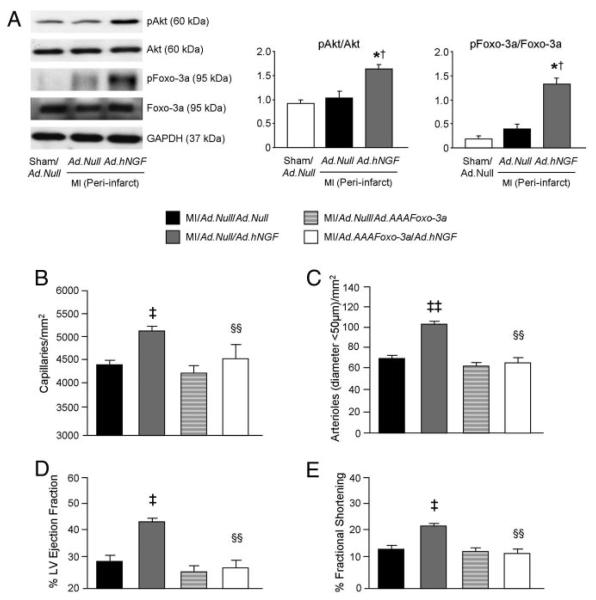
Ad.hNGF increased phospho-Akt and phospho–Foxo-3a levels in the periinfarct myocardium (Figure 5A). Moreover, Ad.hNGF-induced neovascularization (Figure 5B and 5C) and LV function improvement (Figure 5D and 5E) at 14 days post-MI were abolished by coinjection with Ad.AAAFoxo-3a. All functional and LV dimensional data of this experiment are reported in the Online Table IV.
Figure 5. NGF-induced neovascularization and cardiac function improvement depends on the Akt/Foxo-3a pathway.
A, Western blot analyses show that NGF overexpression induces Akt and Foxo-3a phosphorylation in the periinfarct myocardium at 3 days postinfarction. GAPDH was used for loading control. B through E, Bar graphs show that expression of an Akt phosphorylation-resistant mutant form of Foxo-3a abolished Ad.hNGF-induced neovascularization (B and C) and prevented Ad.hNGF-induced improvement in LVEF (D) and LVFS (E) at 14 days post-MI. Data are expressed as means±SEM. *P<0.05 vs sham/Ad.Null; †P<0.05 vs MI/Ad.Null; ‡P<0.05, ‡‡P<0.01 vs MI/Ad.Null/Ad.Null; §§P<0.01 vs MI/Ad.Null/Ad.hNGF (n=5 mice/group for Western blot and 6 mice/group for histological analyses; n=at least 10 mice/group for cardiac function measurements).
NGF Increases the Abundance of c-kitpos the Infarcted Heart
To investigate whether NGF overexpression may increase the myocardial abundance of c-kitposGATA-4pos, putative cardiomyocyte progenitor cells were counted at 3 days postsurgery.13,23 Results show that hNGF increases the abundance of c-kitposGATA-4pos cells in the periinfarct area (Figure 6A), but not in the infarct area (Online Figure III, A). In addition, Ad.hNGF increased the number of c-kitpos cells in both the periinfarct and the infarct area at 3 days post-MI (Online Figure III, B). To address whether NGF overexpression induced proliferation of c-kitpos cells, a double-staining for c-kit and the proliferation marker MCM-2 was performed. As shown by Figure 6B, proliferating c-kitpos cells were higher in NGF-engineered hearts. Heart-resident Linnegc-kitpos cells have been proposed to represent cardiac stem cells.24 To investigate the hypothesis that NGF overexpression increases the abundance of Linnegc-kitpos cells, flow cytometric analysis of cardiomyocyte-depleted cardiac cell populations was performed. As shown in Figure 6C, MI increased the number of Linnegc-kitpos cells. The abundance of Linnegc-kitpos cells was further enhanced after NGF overexpression. We also evaluated whether Ad.hNGF selectively enriches for Linnegc-kitpos cells without affecting other putative progenitor cell populations. To this aim, flow cytometric analysis for Linnegsca-1pos cells was performed at 3 days postsurgery and gene transfer. The percentage of heart-resident Linnegsca-1pos cells was increased by MI, without being affected by Ad.hNGF (Online Figure III, C), thus suggesting that NGF activates an enhancement mechanism, which selectively impacts on c-kitpos cells. To determine whether the increase of Linnegc-kitpos cells was attributable to a direct effect of NGF on these cells, we evaluated whether heart-resident c-kitpos cells possess NGF receptors, but RT-PCR showed that neither TrkA nor the NGF low-affinity p75NTR receptor were expressed (Online Figure IV). As the activation of c-kit receptor occurs by SCF binding,13,21 we next analyzed whether Ad.hNGF increases SCF levels in the myocardium and in cultured cardiomyocytes. Real-time RT-PCR showed that Ad.hNGF increased SCF mRNA in both the mouse infarcted heart (Figure 7A) and rat neonatal cardiomyocytes (RNCMs) (Online Figure V). Moreover, Western blot analyses showed that Ad.hNGF increased the myocardial level of the 30-kDa membrane-associated SCF isoform (Figure 7B).18 ELISA confirmed increased SCF and increased c-kit receptor phosphorylation in NGF-overexpressing myocardium (Figure 7C and 7D). These findings suggest that NGF-induced increase in c-kitpos progenitor cells may be dependent on the enhancement of the SCF ligand/c-kit receptor signaling. To further investigate this hypothesis, we neutralized SCF in mice with MI or sham-operation and gene transfer. We verified the efficacy of our SCF neutralization approach by measuring (by ELISA) the LV amount of immunoreactive SCF, which resulted decreased by Ab-SCF at 3 days post-MI (Figure 7C). Next, we observed that SCF neutralization prevented the Ad.hNGF-induced increase in Linnegc-kitpos cells (Figure 7E) and diminished Ad.hNGF-mediated increase in c-kit phosphorylation (Figure 7D) at 3 days post-MI. These data provide further evidence that SCF mediates NGF overexpression-induced increase in heart-resident Linnegc-kitpos cells at 3 days post-MI. The MI- and Ad.hNGF-induced increase in heart-resident Linneg c-kitpos cells was not maintained up to 14 days from MI and gene transfer, and it was also not affected by long-term SCF neutralization (Online Figure VI).
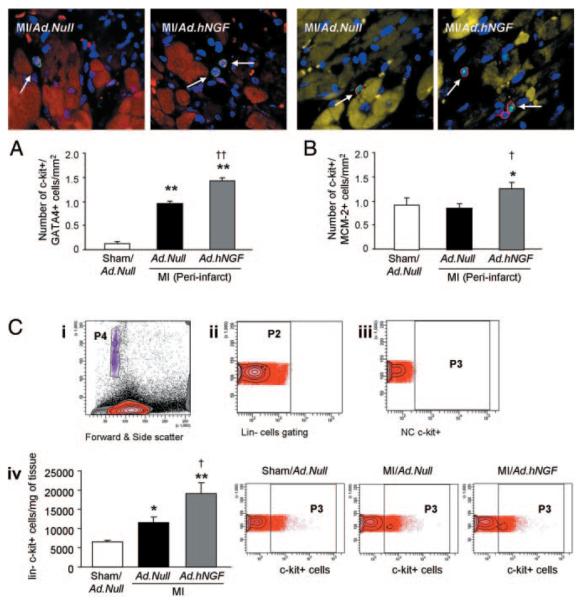
Figure 6. NGF overexpression increases the number of putative c-kitpos progenitor cells in the mouse infarcted heart.
A, Immunostaining for c-kit and GATA-4 in heart sections (c-kit, green fluorescence; GATA-4, purple dots; α-sarc–positive cardiomyocytes, red; nuclei, blue). c-kitposGATA-4pos cells are indicated by white arrows. Bar graph represents the number of c-kitposGATA-4pos cells per millimeter squared of periinfarct myocardium. B, Immunostaining for proliferating c-kitpos cells in heart sections evaluated by double staining for c-kit (red fluorescence) and MCM-2 (green). Cardiomyocytes are visualized in yellow after staining for α-sarc; nuclei are pictured in blue. c-kitposMCM-2pos cells are indicated by white arrows. Bar graph represents the number of c-kitposMCM-2pos cells per millimeter squared in the periinfarct area. C, Identification and quantification of cardiac Linnegc-kitpos cells by flow cytometry. Forward and side scatter (i) shows the total population (in red) analyzed after LV digestion and cardiomyocyte depletion. For data analysis, counting beads (purple) were identified by their size (in P4 in the forward and side scatter). ii, Linneg cells (in P2) were gated from the total population of extracted cells. iii, Negative control (NC) for c-kitpos cells. iv, Bar graphs and representative microphotographs show the number of Linnegc-kitpos cells per milligram of LV tissue. The number of c-kitpos cells (in P3) was analyzed within the Linneg cell population. Data are expressed as means±SEM. *P<0.05, **P<0.01 vs sham/Ad.Null; †P<0.05, ††P<0.05 vs MI/Ad.Null. All analyses were performed at 3 days postoperation (n=5 mice/group for histological analyses and 6 mice/group for flow cytometry).
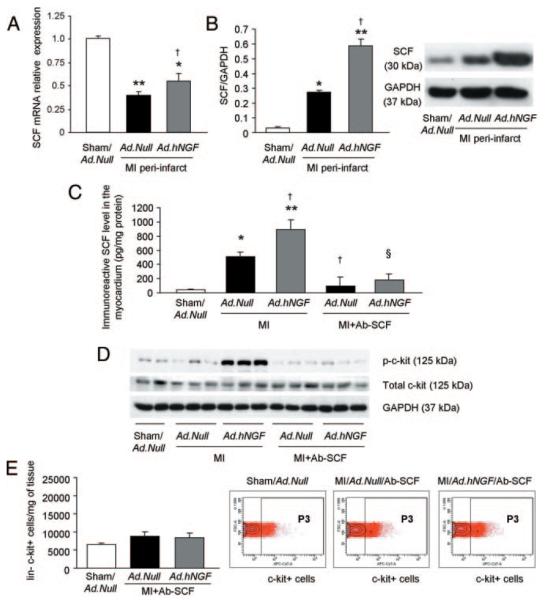
Figure 7. NGF-induced increase of c-kitpos progenitor cells in the infarcted heart is mediated by the SCF/c-kit signaling.
All analyses were performed at 3 days postsurgery. A, Bar graph shows increased SCF mRNA expression in the periinfarct myocardium following NGF gene transfer. B, Western blot analysis shows the NGF-induced increase in membrane-bound (30-kDa) SCF protein. GAPDH was used for normalization. C, ELISA performed on LV tissue shows increased level of immunoreactive SCF after MI and Ad.hNGF. These responses were prevented by SCF neutralization (by Ab-SCF). D, Representative western blot shows increased c-kit phosphorylation in Ad.hNGF-injected LV, which is prevented by Ab-SCF. E, Identification and quantification of cardiac Linnegc-kitpos cells by flow cytometry after SCF neutralization. In the presence of SCF blockade, the number of Linnegc-kitpos cells per milligram of LV was not increased by either MI or hNGF gene transfer. Data are expressed as means±SEM. *P<0.05, **P<0.01 vs sham/Ad.Null; †P<0.05 vs MI/Ad.Null; §P<0.05 vs MI/Ad.hNGF (n=5 mice/group for PCR and Western blot, 4 mice/group for ELISA and at least 6 mice/group for flow cytometry).
Finally, NGF neutralization did not change the abundance of Linnegc-kitpos cells at 3 or 14 days post-MI (Online Figure VII, A and B), thus suggesting that endogenous factors other than NGF may support Linnegc-kitpos homeostasis in the infarcted heart.
Blocking SCF-Mediated Increase in Linnegc-kitpos Cells Early After MI Does Not Impair NGF Therapeutic Effects in MI Mice
To investigate whether the rise in Linnegc-kitpos cells at 3 days post-MI plays a role in NGF gene therapy-induced therapeutic benefits, we treated mice with the short-term SCF neutralization protocol, earlier (Figure 7E) shown to prevent NGF-induced increase in Linnegc-kitpos cells. We did not prolong SCF neutralization because we knew that MI- and Ad.hNGF-induced initial increase in Linnegc-kitpos cells are not maintained at later time points (Online Figure VI). Short-term SCF neutralization did not affect the Ad.hNGF-induced improvement of cardiac function (for LVEF, Online Figure VIII, A; for LVFS, Online Figure VIII, B; other parameters are not shown) and myocardial angiogenesis (Online Figure VIII, C) at 14 days post-MI. These results suggest that although NGF overexpression, via SCF, is able to increase c-kitpos progenitor cells in the infarcted heart, this response is dispensable for the full therapeutic potential of NGF- based therapy.
Discussion
NGF was initially considered with respect of its neural functions, but we and others have provided seminal evidence of cardiovascular actions of NGF (for review, see elsewhere25). The present study has demonstrated a series of novel and important cardiovascular features of NGF and unveiled the therapeutic potential of this neurotrophin for ischemic heart disease.
NGF neutralization reduced the native angiogenic response to MI. Spontaneous neovascularization is one of the compensatory responses through which the infarcted heart attempts to salvage the spared myocardium.26 In fact, reduced blood flow (ischemia) is cause of progressive cardiomyocyte and EC depletion by apoptotic death, which contributes to cardiac dysfunction.2,27 We found apoptosis of both cardiomyocytes and ECs increased by NGF blockade and this may account for impaired LV function and LV chamber dilatation observed at 14 days post-MI in Ab-NGF mice.
In consideration of the worsened outcome caused by NGF neutralization, we hypothesized that intramyocardial NGF gene therapy could preserve cardiomyocyte/ECs viability and promote reparative processes in the infarcted heart, thereby sustaining cardiac function. Angiogenesis gene therapy has received severe criticisms, but it still remains a promising approach to resolve or at least improve ischemic cardiovascular disease.28,29 In our study, adenovirus-mediated NGF overexpression stimulated angiogenesis at both capillary and arteriolar levels and reduced apoptosis of ECs and cardiomyocytes in the periinfarct myocardium. The significant angiogenic effect of NGF was also emphasized by increased myocardial perfusion in NGF-engineered hearts. The proangiogenic effect of NGF might be explained by its capacity to directly induce survival, proliferation and migration/invasion of ECs, all essentials for the angiogenic process. In fact, the capacity of NGF to induce EC survival and proliferation has been already reported.7,30,31 NGF capacity to promote EC migration and invasion was also described.32,33
We previously demonstrated that NGF-induced angiogenesis in ischemic limb muscles is mediated by Akt6 and that NGF promotes in vitro cardiomyocyte survival via the Akt/Foxo-3a pathway.9 In line with these previous findings, we show here that overexpressing NGF increases Akt and Foxo-3a phosphorylation in the myocardium. Importantly, cotransduction of the MI heart with a mutant form of Foxo-3a which cannot be phosphorylated by Akt prevented NGF gene therapy-induced improvements in myocardial angiogenesis and cardiac function. The impact of NGF on the Akt/Foxo-3a pathway may explain both the proangiogenic and antiapoptotic effects of NGF gene therapy.9,12 In addition, we have newly identified that NGF increases the expression level of the c-kit receptor ligand SCF and promotes c-kit phosphorylation in the infarcted heart. SCF reportedly induces survival, migration, and tube-like structure formation of human fetal umbilical vein ECs, which express c-kit.34 SCF was also shown to induce neovascularization in the adult myocardium.18 However, we did not observe c-kit receptor expression by adult myocardial capillary ECs.35 A possible explanation for this discrepancy is that SCF-induced neovascularization in adult hearts is exclusively dependent on vasculogenesis.18 In our study, SCF resulted fundamental for NGF-induced expansion of heart-resident c-kitpos progenitor cells. Solid experimental evidence originating from the Anversa laboratory, and confirmed by others, suggests that c-kitpos stem and progenitor cells participate in the native myocardial repair and regeneration after MI.13,14,36 Few agents able to modulate in vivo expansion of cardiac c-kitpos cells have been identified to date14,37; thus, the understanding that NGF is one of such factors represents a novel and relevant finding in the perspective of therapeutic cardiac regeneration. Heart-derived c-kitpos cells do not express the mRNA for NGF receptors, thus pinpointing SCF as the mediator of NGF indirect actions on these cells. In agreement with this theory, SCF promotes proliferation of c-kitpos cells38 and proliferating c-kitpos cells were increased in the NGF-engineered infarcted hearts. Of note, SCF neutralization blunted NGF-induced increase in heart-resident Linnegc-kitpos cells at 3 days post-MI. Furthermore, NGF appears to act selectively or preferentially on the Linnegc-kitpos cells, because it did not affect the cardiac abundance of Linnegsca-1pos cells, a different population of putative progenitor cells. We then tried to rule out the contribution of Linnegc-kitpos cells in the overall therapeutic benefit induced by NGF gene therapy. To this aim, we used a short-term SCF neutralization protocol, which proved able to prevent NGF-induced increase in Linnegc-kitpos. Under these experimental conditions, NGF overexpression maintained its capacity to improve cardiac function and to promote angiogenesis at 14 days post-MI. This suggests that the increase in Linnegc-kitpos early after MI is not important for the therapeutic benefit induced by NGF overexpression. However, we cannot exclude that NGF-induced increase in SCF expression level and thus in c-kitpos progenitor cells could be utilitarian under different experimental and clinical circumstances.
In conclusion, this study provides strong evidence for the therapeutic potential of NGF in the post-MI heart and furthermore reinforces the concept that neurotrophins have important cardiovascular actions.
Novelty and Significance.
What Is Known?
Nerve growth factor (NGF) is important for the development, homeostasis and regeneration of the nervous system.
The TrkA receptor for NGF is present in endothelial cells and cardiomyocytes.
NGF promotes angiogenesis and cardiomyocyte survival.
What New Information Does This Article Contribute?
NGF expression is increased in the human and murine infarcted heart.
Endogenous NGF promotes angiogenesis and inhibits cardiovascular apoptosis in the mouse infarcted heart.
Intramyocardial NGF gene delivery increases post-MI survival and improves post-MI left ventricular function and remodeling.
Intramyocardial NGF gene delivery upregulates the expression of stem cell factor, which is the c-kit receptor ligand, and by this mechanism it enriches the infarcted heart with Linnegckitpos cardiac progenitor cells.
NGF has historically been implicated in several functions of the nervous system. More recently, we and others have introduced the new concept that NGF can also elicit cardiovascular actions, including angiogenesis and cardiomyocyte survival. However, whether NGF plays a role in the healing of the infarcted heart is unknown. Here we show that NGF expression is upregulated by myocardial infarction (MI) in both humans and mice and that endogenous NGF protects the murine infarcted heart by inhibiting cardiovascular apoptosis and increasing coronary microvasculature. Enhancing this endogenous mechanism by intramyocardial NGF gene therapy reduces post-MI mortality and improves left ventricular perfusion, function, and remodeling. This study also uncovers the previously unknown capacity of NGF to promote expansion of Linnegckitpos cardiac progenitor cells through an indirect mechanism involving the c-kit receptor ligand, stem cell factor (SCF). These results reveal novel pleiotropic properties of NGF and suggest that NGF-based therapy may be a useful approach to alleviate post-MI remodeling.
Supplementary Material
Acknowledgments
We thank Drs Nicolle Kraenkel, Atsuhiko Oikawa, Mauro Siragusa (all from the University of Bristol) and Beatrice Testa (University of Parma) for technical assistance.
Sources of Funding
The study was supported by British Heart Foundation grants BS/05/ 001 and PG/06/146/21946 (to C.E.).
Non-standard Abbreviations and Acronyms
- α-sarc
α-sarcomeric actin
- Akt
serine/threonine-specific protein kinase
- EC
endothelial cell
- Foxo
forkhead box-O transcription factors
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- LVFS
left ventricular fractional shortening
- MCM
minichromosome maintenance protein
- MI
myocardial infarction
- NGF
nerve growth factor
- p75NTR
p75 neurotrophin receptor
- SCF
stem cell factor
- TrkA
tropomyosin-related receptor A
Footnotes
Disclosures
None.
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Olivetti G, Quaini F, Sala R, Lagrasta C, Corradi D, Bonacina E, Gambert SR, Cigola E, Anversa P. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol. 1996;28:2005–2016. doi: 10.1006/jmcc.1996.0193. [DOI] [PubMed] [Google Scholar]
- 3.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 4.Thomas S, Rich MW. Epidemiology, pathophysiology, and prognosis of heart failure in the elderly. Heart Fail Clin. 3:381–387. doi: 10.1016/j.hfc.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasayama S, Fujita M. Recent insights into coronary collateral circulation. Circulation. 85:1197–1204. doi: 10.1161/01.cir.85.3.1197. [DOI] [PubMed] [Google Scholar]
- 6.Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation. 2002;106:2257–2262. doi: 10.1161/01.cir.0000033971.56802.c5. [DOI] [PubMed] [Google Scholar]
- 7.Cantarella G, Lempereur L, Presta M, Ribatti D, Lombardo G, Lazarovici P, Zappala G, Pafumi C, Bernardini R. Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 2002;16:1307–1309. doi: 10.1096/fj.01-1000fje. [DOI] [PubMed] [Google Scholar]
- 8.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 9.Caporali A, Sala-Newby GB, Meloni M, Graiani G, Pani E, Cristofaro B, Newby AC, Madeddu P, Emanueli C. Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell Death Differ. 2008;15:299–311. doi: 10.1038/sj.cdd.4402263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 12.Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 14.Limana F, Germani A, Zacheo A, Kajstura J, Di Carlo A, Borsellino G, Leoni O, Palumbo R, Battistini L, Rastaldo R, Muller S, Pompilio G, Anversa P, Bianchi ME, Capogrossi MC. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ Res. 2005;97:e73–e83. doi: 10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- 15.Cimini M, Fazel S, Zhuo S, Xaymardan M, Fujii H, Weisel RD, Li RK. c-kit dysfunction impairs myocardial healing after infarction. Circulation. 2007;116:I77–I82. doi: 10.1161/CIRCULATIONAHA.107.708107. [DOI] [PubMed] [Google Scholar]
- 16.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- 17.Miyazawa K, Williams DA, Gotoh A, Nishimaki J, Broxmeyer HE, Toyama K. Membrane-bound Steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood. 1995;85:641–649. [PubMed] [Google Scholar]
- 18.Xiang FL, Lu X, Hammoud L, Zhu P, Chidiac P, Robbins J, Feng Q. Cardiomyocyte-specific overexpression of human stem cell factor improves cardiac function and survival after myocardial infarction in mice. Circulation. 2009;120:1065–1074. doi: 10.1161/CIRCULATIONAHA.108.839068. [DOI] [PubMed] [Google Scholar]
- 19.Siragusa M, Katare R, Meloni M, Damilano F, Hirsch E, Emanueli C, Madeddu P. Involvement of phosphoinositide 3-kinase γ in angiogenesis and healing of experimental myocardial infarction in mice. Circ Res. 2010:106. doi: 10.1161/CIRCRESAHA.109.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katare RG, Caporali A, Oikawa A, Meloni M, Emanueli C, Madeddu P. Vitamin B1 analogue benfotiamine prevents diabetes-induced diastolic dysfunction and heart failure through Akt/Pim-1 mediated survival pathway. Circ Heart Fail. doi: 10.1161/CIRCHEARTFAILURE.109.903450. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 22.Scott IS, Morris LS, Bird K, Davies RJ, Vowler SL, Rushbrook SM, Marshall AE, Laskey RA, Miller R, Arends MJ, Coleman N. A novel immunohistochemical method to estimate cell-cycle phase distribution in archival tissue: implications for the prediction of outcome in colorectal cancer. J Pathol. 2003;201:187–197. doi: 10.1002/path.1444. [DOI] [PubMed] [Google Scholar]
- 23.Anversa P, Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature. 2002;415:240–243. doi: 10.1038/415240a. [DOI] [PubMed] [Google Scholar]
- 24.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caporali A, Emanueli C. Cardiovascular actions of neurotrophins. Physiol Rev. 2009;89:279–308. doi: 10.1152/physrev.00007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anversa P, Ricci R, Olivetti G. Coronary capillaries during normal and pathological growth. Can J Cardiol. 1986;2:104–113. [PubMed] [Google Scholar]
- 27.Lancel S, Joulin O, Favory R, Goossens JF, Kluza J, Chopin C, Formstecher P, Marchetti P, Neviere R. Ventricular myocyte caspases are directly responsible for endotoxin-induced cardiac dysfunction. Circulation. 2005;111:2596–2604. doi: 10.1161/CIRCULATIONAHA.104.490979. [DOI] [PubMed] [Google Scholar]
- 28.Tongers J, Roncalli JG, Losordo DW. Therapeutic angiogenesis for critical limb ischemia: microvascular therapies coming of age. Circulation. 2008;118:9–16. doi: 10.1161/CIRCULATIONAHA.108.784371. [DOI] [PubMed] [Google Scholar]
- 29.Yla-Herttuala S, Markkanen JE, Rissanen TT. Gene therapy for ischemic cardiovascular diseases: some lessons learned from the first clinical trials. Trends Cardiovasc Med. 2004;14:295–300. doi: 10.1016/j.tcm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Salis MB, Graiani G, Desortes E, Caldwell RB, Madeddu P, Emanueli C. Nerve growth factor supplementation reverses the impairment, induced by Type 1 diabetes, of hindlimb post-ischaemic recovery in mice. Diabetologia. 2004;47:1055–1063. doi: 10.1007/s00125-004-1424-5. [DOI] [PubMed] [Google Scholar]
- 31.Lecht S, Arien-Zakay H, Marcinkiewicz C, Lelkes PI, Lazarovici P. Nerve growth factor-induced protection of brain capillary endothelial cells exposed to oxygen-glucose deprivation involves attenuation of Erk phosphorylation. J Mol Neurosci. doi: 10.1007/s12031-009-9318-0. In press. [DOI] [PubMed] [Google Scholar]
- 32.Dolle JP, Rezvan A, Allen FD, Lazarovici P, Lelkes PI. Nerve growth factor-induced migration of endothelial cells. J Pharmacol Exp Ther. 2005;315:1220–1227. doi: 10.1124/jpet.105.093252. [DOI] [PubMed] [Google Scholar]
- 33.Park MJ, Kwak HJ, Lee HC, Yoo DH, Park IC, Kim MS, Lee SH, Rhee CH, Hong SI. Nerve growth factor induces endothelial cell invasion and cord formation by promoting matrix metalloproteinase-2 expression through the phosphatidylinositol 3-kinase/Akt signaling pathway and AP-2 transcription factor. J Biol Chem. 2007;282:30485–30496. doi: 10.1074/jbc.M701081200. [DOI] [PubMed] [Google Scholar]
- 34.Matsui J, Wakabayashi T, Asada M, Yoshimatsu K, Okada M. Stem cell factor/c-kit signaling promotes the survival, migration, and capillary tube formation of human umbilical vein endothelial cells. J Biol Chem. 2004;279:18600–18607. doi: 10.1074/jbc.M311643200. [DOI] [PubMed] [Google Scholar]
- 35.Graiani G, Emanueli C, Desortes E, Van Linthout S, Pinna A, Figueroa CD, Manni L, Madeddu P. Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of type 1 diabetic mice. Diabetologia. 2004;47:1047–1054. doi: 10.1007/s00125-004-1414-7. [DOI] [PubMed] [Google Scholar]
- 36.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 38.Serve H, Yee NS, Stella G, Sepp-Lorenzino L, Tan JC, Besmer P. Differential roles of PI3-kinase and Kit tyrosine 821 in Kit receptor-mediated proliferation, survival and cell adhesion in mast cells. EMBO J. 1995;14:473–483. doi: 10.1002/j.1460-2075.1995.tb07023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.