Abstract
Background
Sooty (Puffinus griseus) and short-tailed (P. tenuirostris) shearwaters are abundant seabirds that range widely across global oceans. Understanding the foraging ecology of these species in the Southern Ocean is important for monitoring and ecosystem conservation and management.
Methodology/Principal Findings
Tracking data from sooty and short-tailed shearwaters from three regions of New Zealand and Australia were combined with at-sea observations of shearwaters in the Southern Ocean, physical oceanography, near-surface copepod distributions, pelagic trawl data, and synoptic near-surface winds. Shearwaters from all three regions foraged in the Polar Front zone, and showed particular overlap in the region around 140°E. Short-tailed shearwaters from South Australia also foraged in Antarctic waters south of the Polar Front. The spatial distribution of shearwater foraging effort in the Polar Front zone was matched by patterns in large-scale upwelling, primary production, and abundances of copepods and myctophid fish. Oceanic winds were found to be broad determinants of foraging distribution, and of the flight paths taken by the birds on long foraging trips to Antarctic waters.
Conclusions/Significance
The shearwaters displayed foraging site fidelity and overlap of foraging habitat between species and populations that may enhance their utility as indicators of Southern Ocean ecosystems. The results highlight the importance of upwellings due to interactions of the Antarctic Circumpolar Current with large-scale bottom topography, and the corresponding localised increases in the productivity of the Polar Front ecosystem.
Introduction
Sooty (Puffinus griseus) and short-tailed shearwaters (P. tenuirostris) are abundant seabirds that range widely across global oceans [1], breeding principally in New Zealand, south-eastern Australia, and southern South America [2], [3]. They are of cultural and economic importance for Māori and Tasmanian aboriginal societies [4], [5], important as predators within their marine ecosystems, and as engineers within their island ecosystems due to their burrowing and deposition of biological material [6], [7], [8]. Understanding the foraging ecology of these birds is important for continued monitoring of their populations [9], conservation and management of their island ecosystems [10], and facilitating their use as indicators of Southern Ocean prey stocks [9], [11].
Breeding adult sooty and short-tailed shearwaters forage locally to the colony in order to provision their chicks at a maximal rate, but periodically undertake long foraging trips to Antarctic waters [12], [13], [14], [15]. During long trips the adults forage to replenish their own body mass as well as to provision their chicks [12]. This pattern has also been reported for other species [e.g. 16]. New Zealand sooty shearwaters make long foraging trips in a spatially bimodal pattern, utilising foraging grounds around the Polar Front, to the south-east and south-west of New Zealand [1], [13]. The foraging patterns of these birds were recently examined in relation to bathymetry, sea surface temperature, primary productivity, and surface wind speeds [13]. However, questions remain regarding the foraging behaviour of these shearwaters in the high latitude regions of the Southern Ocean, including their apparent lack of use of the Polar Front zone directly south of New Zealand, and the overlap of their foraging ranges with those of conspecifics such as short-tailed shearwaters [2].
Many studies have shown that oceanic winds can influence the flight of seabirds during foraging trips [e.g. 17], [18], [19], [20], [21]. The weather systems and wind patterns south of New Zealand and Australia generally comprise polar easterlies near the Antarctic continent, and westerly winds around and to the south of the Subantarctic and Polar Fronts (∼50–55°S). North of the Subantarctic Front, alternating low- and high-pressure systems result in variable winds [22]. It seems likely that oceanic winds should have an effect on the foraging strategies of these birds, and plausible that a spatially bimodal foraging pattern might be due in part to wind considerations.
The study of wide-ranging marine predators such as these is a challenging field. Electronic tagging technologies typically play an vital role in such studies [23], but greatest insight can potentially be obtained by combining tag data with other information such as ship-based observations of predators and prey, hydrodynamic model data, and ocean parameters [11], [23], [24], [25]. Here, we integrate tracking data from sooty and short-tailed shearwaters, a long-term data set of at-sea observations of seabirds, pelagic trawl data, records of near-surface zooplankton, and remotely-sensed ocean surface wind data to provide a synoptic view of the foraging behaviour of these seabirds. Our aims were to determine the extent to which oceanic winds influenced foraging behaviour, the overlap in foraging habitat between species and populations, and to examine the relationships between foraging and potential prey distributions and oceanographic features.
Methods
Ethics statement
All protocols employed in the sooty shearwater tracking study were approved by the University of California at Santa Cruz Institutional Animal Care and Use Committees, the Wellington Conservancies of the Department of Conservation, New Zealand, and the Kia Mau Te Tītī Mo Ake Tōnu Atu (Keep the Tītī Forever) research programme. The South Australian short-tailed shearwater study was approved by the Department of Primary Industries and Resources of South Australia (ethics number 16/03), and the Department of Environment and Heritage (permit number A24684). The Tasmanian short-tailed shearwater study was approved by the University of Tasmania Ethics Committee (A0008138) and the Nature Conservation Branch of Tasmanian Parks and Wildlife Service (permit FA 05151). The SO-CPR survey, shipboard surveys of seabirds, and pelagic trawls were approved by the Australian Antarctic Science Advisory Committee (AAS Projects 472, 2208, and 2070), with AMLR Act permits 96/1 (trawls) and 05-10_047 (SO-CPR).
Data collection – sooty shearwater foraging trips
Tagging was conducted on breeding adult birds on Whenua Hou (Codfish Island), New Zealand (167°39′E 46°46′S) and Mana Island, New Zealand (174°50′E 41°06′S). Details are given by Shaffer et al. [13]. The tags used were Lotek LTD 2400 archival data loggers (Lotek Wireless, St. John's, Newfoundland). These tags yielded one location fix per day, and also recorded light intensity, temperature, and pressure at a user-programmable sampling rate. The pressure data were used to infer diving activity, and the temperature data to refine position estimates by matching against sea surface temperature data [13], [26]. Eight birds were tagged for a single foraging trip with tags set to record at either 24 or 32 seconds (referred to as fast sampling rate tags). This sampling rate was chosen to provide reasonably fine-scale information on diving activity. A subsequent deployment of 19 birds was made using a slower sampling rate (432 seconds), designed to record behaviour throughout the remainder of breeding and the six month migration that followed [1]. The slower sampling rate reduces the amount of data collected each day, and so allows for longer times between recovery and download of tags.
Data collection – short-tailed shearwater foraging trips
Tagging was conducted on breeding adult birds in two separate studies: one from two South Australian offshore islands (Althorpe Island, 136°52′E 35°22′S, and St Peter Island, 133°35′E 32°17′S) and the other from an offshore Tasmanian island (Wedge Island, 140°40′E 43°08′S). Details of the South Australian studies are given in [27]. Briefly, these birds were tagged with KiwiSat 202 satellite transmitters (Sirtrack Ltd, North Havelock, New Zealand), with a transmission interval of 60s. In this study we use data from long foraging trips, comprising a single long trip from each of 11 individual birds, spread over three breeding seasons (Feb/Mar 2005, n = 4; Feb 2006, n = 1; Feb/Mar 2007 n = 6). Due to transmitter loss and battery failure, none of these 11 trips was completely recorded. However, all but one trip were recorded to at least 50°S, and three trips were recorded to south of 60°S [27]. Thus, these records give a reasonable indication of the outward leg of long foraging trips by these birds. The Tasmanian birds were tagged with light-based geolocation tags (Lotek LTD 2400 and BAS Mk3, British Antarctic Survey). Long foraging trip data from February and March of 2006 (n = 2 birds, 5 trips total) and 2008 (n = 2 birds, 5 trips total) were used here.
Data collection – at-sea observations of shearwaters
The at-sea observations were taken from data collected by the Australian Antarctic programme over the 1980/81 to 2005/06 austral summer seasons [28], [29]. The details of the methodologies used to collect observations and physical oceanographic and environmental data are described elsewhere [30], [31]. Briefly, observations of the numbers and behaviours of all species of seabirds present within a 300m forward quadrant of the ship were recorded continuously while the vessel was underway during daylight hours. Ship-followers were excluded from all analyses, following the BIOMASS Working Party on Bird Ecology protocols [32]. Ship-followers typically associate with the vessel for extended periods, either following the vessel at the stern or circling the vessel, or both. Neither sooty nor short-tailed shearwaters are generally regarded as ship-followers, typically flying in straight lines and only detouring around the vessel if required to avoid collision.
Data collection – fish, squid, and zooplankton distributions
Mesopelagic fish and squid distributions in the Polar Front region around 140°E were obtained from an Australian voyage conducted during September 1996. Fish and squid sampling was undertaken using an International Young Gadoid Pelagic Trawl with a multiple-opening pelagic net (MIDOC). Four depth strata were sampled at each station, at nominal depths of 0–250m, 250–500m, 500–750m, and 750–1000m [33]. 37 trawls were made from 5–20 September, on a northward transect from approximately 140°E, 60°S to 146°E, 46°S (Figure 1).
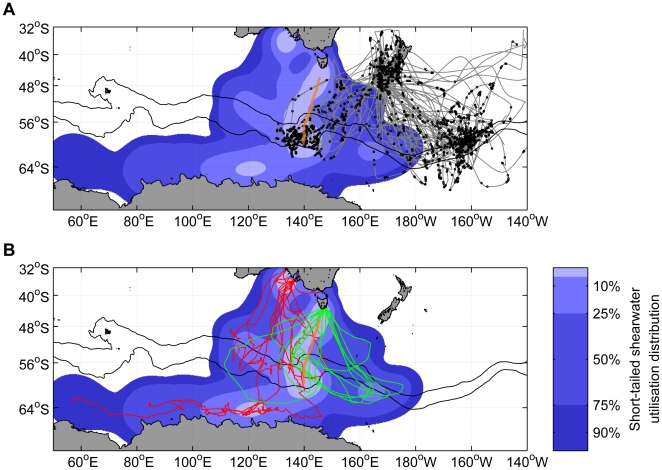
Figure 1. Sooty and short-tailed shearwater foraging in the Southern Ocean.
A. Sooty shearwater tracks (grey lines) and dive locations (black dots), with the short-tailed shearwater habitat utilisation from panel B included for reference. Note the overlapping use of the Polar Front zone around 140°E. The northern and southern branches of the Polar Front (black) and the trawl transect (dotted orange) are shown. B. Short-tailed shearwater tracks from two South Australian islands (red lines) and from Wedge Island, Tasmania (green lines), and their corresponding combined habitat utilisation (background colours).
Near-surface zooplankton data were obtained from the Southern Ocean Continuous Plankton Recorder (SO-CPR) survey [34]. In this survey, the CPR instrument is towed at a constant depth of ∼10m, at ∼100m behind the vessel. A 270 µm mesh is used to filter the water flowing through the instrument and the samples preserved in formalin. In subsequent laboratory analysis, the silks are cut into segments representing 5 nautical miles (referred to as “tow segments”) and all zooplankton on the silk identified [34], [35]. The SO-CPR survey has been operating since 1991, and due to the relative ease of deployment of the instrument, the survey has broad coverage of the waters south of Australia, and to a lesser extent, New Zealand. We used data from 48 voyages that surveyed areas of the Polar Front zone between 130°E and 200°E during the months of February–April. These data comprised a total of 1288 tow segments within the Polar Front zone — that is, within the northern and southern branches of the Polar Front, as defined by Sokolov & Rintoul [36]. The continuous plankton recorder survey effort in the Polar Front zone was mostly concentrated between 130°E and 155°E, with particularly heavy coverage at 140°E and 150°E resulting from repeat sampling along the World Ocean Circulation Experiment SR3 transect (∼140°E) and by the Japanese vessel Shirase along 150°E. We examined the pooled abundances of all copepod taxa, since copepods are a common prey of myctophids [37], [38], [39]. We note that the patterns of total zooplankton abundance were very similar to those of copepods.
Physical oceanography
Mean positions of Southern Ocean fronts were obtained using the technique of Sokolov & Rintoul [36]. The Polar Front zone is defined here as the zone between the northern and southern branches of the Polar Front. Estimates of vertical water velocity at 1095m depth were obtained from the CSIRO Mk 3.5 model [40]. Surface chlorophyll concentrations derived from satellite ocean color measurements were based on eight day mean level 3 standard mapped images of chlorophyll on a global 9 km equidistant cylindrical grid from SeaWiFS and were obtained from the Goddard Space Flight Center [41]. Except where otherwise specified in the text, we use surface chlorophyll to refer to chlorophyll-a detected by satellite and use this quantity as a proxy for phytoplankton biomass.
Analyses
All analyses were conducted in Matlab (Mathworks, Natick MA, 2009) and R (R Foundation for Statistical Computing, Vienna 2009).
At-sea observations from all years were pooled. The continuous observations were binned into records representing 10-minute surveys. There were a total of 13097 10-minute at-sea surveys during February–April in the region 60°E–200°E, 40°S to the Antarctic continent. Data for short-tailed and sooty shearwaters were pooled, as these species are difficult to separate at sea. Shearwaters were sighted in 3266 of these surveys, with an estimated total of approximately 110000 individuals. The at-sea data were collected on Australian Antarctic resupply and scientific voyages, and so the survey effort is generally concentrated to the south and west of Tasmania, extending to the Antarctic continent. There was also survey effort in between Tasmania and Macquarie Island (154°52′E, 54°37′S), but only very limited survey effort in the region south of Macquarie Island. At-sea observations of shearwaters with the behavioural category of ‘feeding’ were extremely sparse: of the 3266 surveys in which shearwaters were sighted, only 38 contained a record of feeding shearwaters. We did not examine these records in detail. To assist in visual interpretation, the spatial distribution of observed densities was smoothed by local scatterplot (Lowess) smoothing applied to log10(x+1)-transformed densities [42].
The processing steps applied to the sooty shearwater geolocation tag data in order to obtain daily position and diving activity estimates have been described [1], [43]. The Tasmanian short-tailed shearwater geolocation data were processed similarly, although these data were not processed to determine dive locations. The South Australian satellite-tagged short-tailed shearwater positions were filtered using the method of McConnell et al. [44].
The archival tags deployed on the sooty shearwaters allow diving behaviour to be estimated. However, the tags using slow sampling rates are unlikely to correctly represent behaviours that occur on relatively short time scales. Thus, we examined dive depths only from fast-sampling archival tags. The times of local sunrise and sunset were calculated for each dive [45]. Sooty shearwater diving activity recorded with fast sampling tags was divided into two sets, such that dives south of 50°S (i.e. associated with foraging in Antarctic waters) were in one set, and the remainder in the other. The distributions of dive times and maximum dive depths were compared between the two sets.
Diving information was not available for the short-tailed shearwaters, and so we used an estimate of habitat utilisation distribution to infer broad patterns in foraging effort. The habitat usage was computed using a kernel density estimator, with a cross-validation selection of smoothing parameter [46], [47].
To gain a perspective on the overall effect of wind on potential foraging flights, grids covering the birds' potential foraging grounds were constructed, with 2.5° longitude by 1.25° latitude bins. The wind cost of visiting each grid cell was calculated using method based on that of Felicísimo et al. [48]. Each simulated foraging flight was broken into 6-hour segments. For a given simulated flight segment, the associated wind was used to calculate the energetic cost of that segment. The speed of the bird over the ground (ground speed; 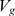 ) can be written as a function of the speed of the bird through the air (airspeed;
) can be written as a function of the speed of the bird through the air (airspeed; 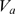 ) and wind speed and direction [49]:
) and wind speed and direction [49]:
where 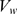 is the wind speed and
is the wind speed and 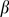 is the direction of the wind with respect to the flight track. The theoretical power required for a bird in level flapping flight is given by [50], [51]:
is the direction of the wind with respect to the flight track. The theoretical power required for a bird in level flapping flight is given by [50], [51]:
where q, r, and s are constants that incorporate aerodynamical flight parameters such as wing span and body mass [50]. We assumed that the birds fly at their maximum range speed (that is, the airspeed that yields maximum distance over the ground per unit energy expended). For a given wind speed and direction, 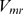 , and the corresponding
, and the corresponding 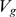 and P, can be found by minimising
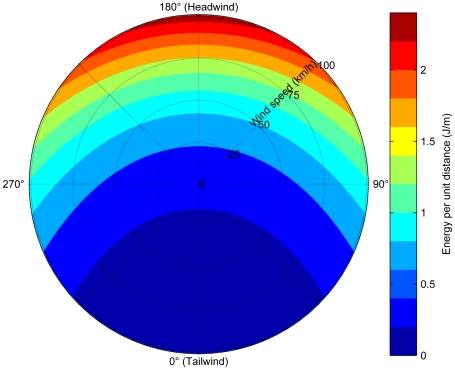
and P, can be found by minimising 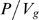 [49]. An example of the resulting cost function is shown in Figure 2, and is minimal for tailwinds, moderate for cross-winds, and heaviest for head winds.
[49]. An example of the resulting cost function is shown in Figure 2, and is minimal for tailwinds, moderate for cross-winds, and heaviest for head winds.
Figure 2. An example of the wind cost function used for the cost simulations.
Costs are shown for a range of wind speeds and angles. This example is for a sooty shearwater carrying no food payload. Angles are relative to the bird flight direction: an angle of 0° corresponds to a tailwind, 90° and 270° to cross-winds, and 180° a headwind.
For the flight parameters (q, r, and s above), we used a wing span of 1.03m and wing area of 0.0893m2 for sooty shearwaters and 0.93m and 0.076m2 for short-tailed shearwaters [52]. Sooty shearwaters embarking on a long foraging trip were assumed to have a body mass of 790g and carrying no food payload [12]. Adult birds gain body mass on long foraging trips, and on their return trips we assumed a body mass of 870g and a food payload of 193g [12]. For short-tailed shearwaters the corresponding values were 570g (outward body mass), 590g (return body mass), and 156g (return food payload) [14].
The cost of an individual simulated foraging trip was calculated as the sum of the costs of its 6-hour segments. We used near-surface wind data from the NCEP/DOE Reanalysis 2 data set (http://www.esrl.noaa.gov/psd/). These are model-based estimates of the 10m wind speed and direction, at 6-hour intervals and on a spatial grid of approximately 2° resolution. Outward flight legs were simulated as flights from the colony to the centre of the destination grid cell, and in the other direction for return flights. The intervening foraging time was not considered for cost calculations. 10000 foraging trips were simulated to each grid cell. The departure date of each simulated foraging trip was randomly sampled from the departure dates of the actual (recorded) foraging trips.
In the absence of wind effects, the energy expended during flight at constant speed is proportional to the distance travelled. Including wind effects introduces variations about this relationship. Thus, to best illustrate the spatial variability in flight costs due to wind effects, the costs are presented as residuals (percentages with respect to a smooth fit of cost against distance, fitted using natural cubic splines in the R splines library). In order to make the residuals relevant to potential Southern Ocean foraging locations, only grid points south of the colony and within 3500km (sooty shearwaters and Tasmanian short-tailed shearwaters) or 5000km (South Australian short-tailed shearwaters) of the colony were included in the fit.
Results
Foraging flights and spatial distributions of shearwaters
Shearwaters from all three tracking studies foraged in the vicinity of the Polar Front (Figure 1). Sooty shearwaters made use of two principal foraging areas: one to the south-west of New Zealand at approximately 140°E, and the other to the south-east at approximately 160°W (Figure 1a). Of the 31 long foraging trips made by sooty shearwaters, 21 were directed to the south-eastern foraging zone (by ten individual birds), and ten to the south-west (by four birds). Individual birds almost exclusively made long foraging trips in only one direction: only one bird made trips to both the south-western and south-eastern zones. The South Australian short-tailed shearwaters foraged from ∼110°E–150°E, with particularly high usage of the region around 140°E (Figure 1b). Foraging flights were generally made with an approximately southward outward leg followed by east-to-west foraging. The South Australian short-tailed shearwaters also foraged south of the Polar Front, as far west as 55°E in waters at or south of the southern boundary of the Antarctic Circumpolar Current (∼64°S). The foraging flights of the Tasmanian short-tailed shearwaters were consistently made in a clockwise direction, heading to the Polar Front zone at ∼165°E and then foraging east-to-west, with an approximately north-east return route to their colonies (Figure 1b).
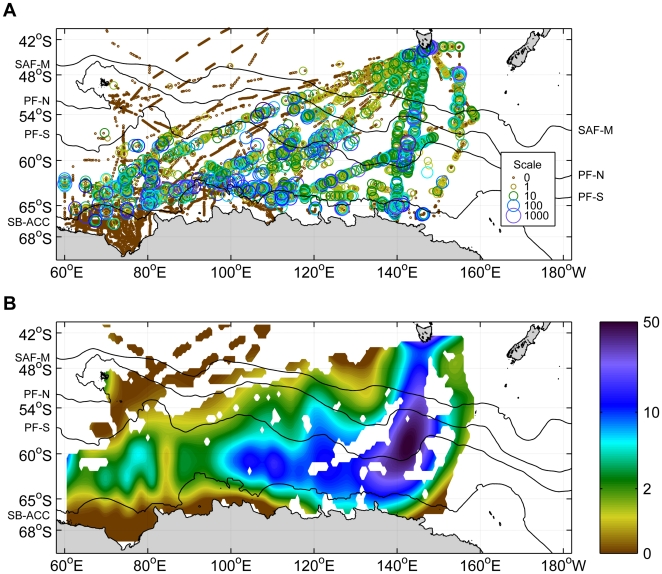
At-sea observations of shearwaters were generally distributed over offshore waters around and to the south of the Polar Front, from approximately 60°E to 150°E (Figure 3). The highest densities were observed in the vicinity of the Polar Front around approximately 140°E, and also close to the coast of Tasmania. The high densities of at-sea observations of shearwaters in the Polar Front zone at ∼140°E were reasonably consistent across years. Six voyages intersected this zone (1993, 1994, 1998, 2000 (2), and 2006). On four of these voyages the observed densities of shearwaters were higher in this zone than the remainder of the voyage (Wilcoxon rank sum test, p<0.001 in each case). In the remaining two voyages the densities were not significantly different in this zone compared to the remainder of the voyage.
Figure 3. Observed at-sea densities of sooty and short-tailed shearwaters in the Southern Ocean.
A. Individual survey records (number of birds per 10-minute survey). B. Smoothed density surface fit with local scatterplot smoothing (see text). Locations of Southern Ocean fronts from north to south are shown in black: SAF-M, middle branch of the Subantarctic Front; PF-N, northern branch of the Polar Front; PF-S, southern branch of the Polar Front; SB-ACC, southern boundary of the Antarctic Circumpolar Current.
Diving behaviour of sooty shearwaters
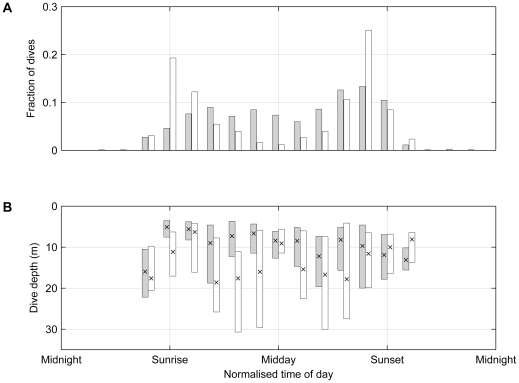
Sooty shearwater dives recorded by fast sampling archival tags north of 50°S (n = 801 dives by seven individual birds) occurred from just before sunrise through to sunset, with broad peaks of dive activity centred on mid-morning and late afternoon (grey bars in Figure 4a). Dive activity south of 50°S (n = 933 dives by two individual birds) also occurred throughout the day, but showed distinct peaks just after sunrise and just before sunset (white bars in Figure 4a). The diel distributions of dive activity north and south of 50°S were significantly different (p<0.001, χ2 12 = 245.1).
Figure 4. Diel distributions of sooty shearwater diving activity.
A. Distributions of sooty shearwater dives with respect to time of day. B. Dive depths with respect to time of day. The median (cross) and interquartile range (bars) are shown. Grey bars indicate dives made from 30°S–50°S, and white bars indicate dives made south of 50°S.
The median maximum dive depth (fast tags; all dives combined, n = 1734) was 10.1m (interquartile range IQR 12.8m). The dives south of 50°S (median maximum depth 11.9m, IQR 14.5m) were deeper than those north of 50°S (8.5m, IQR 10.2 m; Wilcoxon p<0.001). The dive depths tended to be shallowest around sunrise and sunset, becoming deeper during mid-morning and mid-afternoon, and shallow again around midday (Figure 4b).
Simulations of wind costs during long foraging flights
The simulated wind costs for the three tracking studies showed similar broad patterns, with low-cost regions directly south of the colonies, and higher-cost regions extending in corridors roughly south-east and south-west from the colonies (Figure 5). The Polar Front region directly south of New Zealand, sparsely utilised by the sooty shearwaters, was both closer to the colonies and more energetically favourable (i.e. lower cost) than their observed foraging zones to the south-east and south-west of New Zealand.
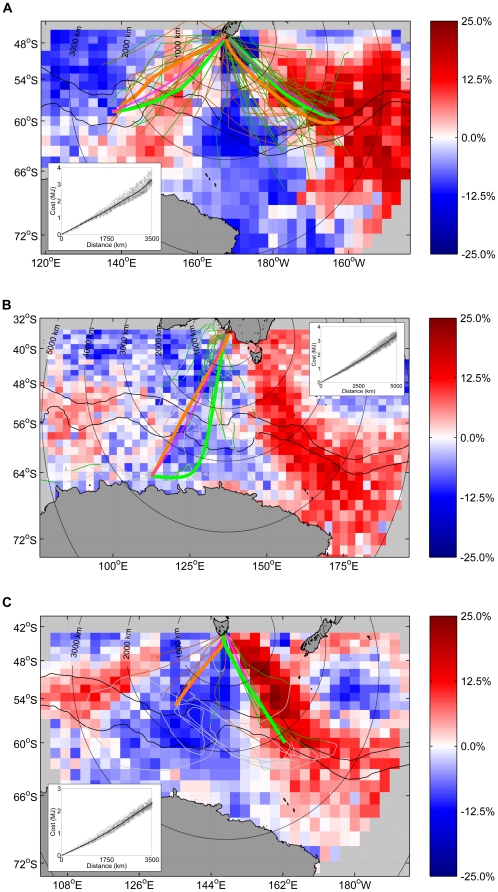
Figure 5. Simulated wind costs of long foraging trips by sooty and short-tailed shearwaters.
A. Simulated wind costs (background colours) for sooty shearwaters. B. For South Australian short-tailed shearwaters. C. For Tasmanian short-tailed shearwaters. Costs are shown as percentage residuals from smooth regression of cost against distance. For example, a value of 25% indicates that the cost to visit the area in question is 25% higher than the average cost for potential foraging locations at the same geographical distance from the colony. Insets show regressions of cost against distance. The thin green lines show outward flights from the colony; the thick green lines show the simulated minimum-cost paths from the colony to representative points on the birds' foraging grounds. The orange lines show the same information for the return trips. Grey lines show the foraging components of flights (not shown in panel A for clarity). The purple lines show the direct (geodesic) routes. The northern and southern branches of the Polar Front are also shown (black).
The observed South Australian short-tailed shearwater foraging activity corresponded to energetically-favourable areas (Figure 5b), whereas both the Tasmanian short-tailed shearwaters (Figure 5c) and the sooty shearwaters (Figure 5a) made outward flights to the south-east of their colonies, into apparently energetically-costly areas. Although variable, the prevailing wind over much of the Southern Ocean south of Australia and New Zealand was approximately north-westerly (i.e. blowing towards the south-east). South-easterly flight from the colonies was therefore accompanied by favourable (near-tailwinds) on the outward leg, but entailed return flights against headwinds. Because the wind cost function is asymmetric (that is, the penalties associated with head winds are proportionally higher than the benefits provided by tailwinds), such trips were more energetically costly overall.
For short-tailed shearwaters the minimum-cost flight paths from the simulations generally showed good agreement with the actual flight paths recorded from the tags. The simulated minimum-cost flight paths for South Australian short-tailed shearwaters foraging in regions south of the Polar Front zone involved a roughly southerly flight to near-Antarctic waters, followed by westerly flight using the polar easterly winds near the Antarctic coast as tailwinds (thick green line in Figure 5b), in good agreement with the observed flight paths of the tagged birds. These birds did not tend to forage to the east of their colonies south of the Polar Front, possibly because this would entail return flights against unfavourable winds. The minimum-cost return flight path from Antarctic waters was close to the most direct route (purple line in Figure 5b). Although none of the tags survived long enough to capture a return flight path, the at-sea densities (Figure 3) are consistent with reasonably direct return flights, as are previously published tracks from short-tailed shearwaters [53].
Physical oceanography of the foraging zones
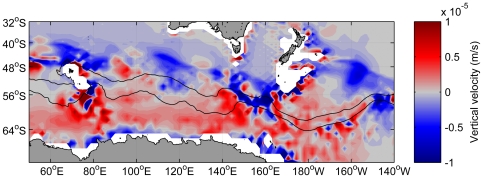
Strong, vertically-coherent, topographically-driven upwelling is present in several sectors of the Polar Front zone in the region of interest, notably around 140°E and 165°E, and east of 170°E (Figures 6 and 7a). These zones of strong upwelling extend over several hundred kilometres and are driven by the interaction of the eastward-flowing Antarctic Circumpolar Current with large-scale bathymetric features: the South-East Indian Ridge (140°E), and the Pacific-Antarctic Ridge (east of 165°E). These regions of upwelling correspond to the regions of high surface chlorophyll concentrations (Figure 7b), and with shearwater foraging effort in the Polar Front zone (Figure 7d).
Figure 6. Vertical water velocity at 1095m in the Southern Ocean.
Twenty-year mean vertical velocity from the CSIRO Mk3.5 climate system model. The northern and southern branches of the Polar Front are shown.
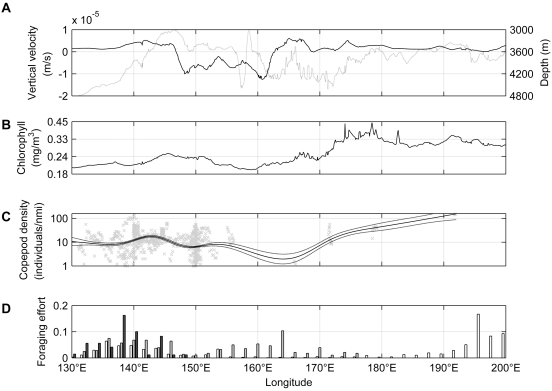
Figure 7. Shearwater foraging in the Polar Frontal zone, in relation to oceanographic processes and copepod abundances.
A. Longitudinal distributions in the Polar Frontal zone of water vertical velocity at 1095m depth (solid line, positive = upward) and water depth (dotted). B. Mean summer surface chlorophyll-a from SeaWiFS satellite estimates. C. Near-surface copepod density from continuous plankton recorder surveys (points = individual tow segments, black lines = mean±SE fit by generalised additive model). D. Shearwater relative foraging effort within 3° of latitude of the Polar Frontal zone (white = sooty shearwaters, black = South Australian short-tailed shearwaters, grey = Tasmanian short-tailed shearwaters).
Productivity is generally higher south of the Polar Front, due to a range of factors including higher upwelling rates, iron input from melting sea ice, and shallower mixed layers [54], [55]. The at-sea observations and the tracks of the South Australian short-tailed shearwaters extend into this zone, and the observed at-sea shearwater densities (Figure 3) tend to coincide with the changes in the upwelling rates and primary production (e.g. the higher densities of shearwaters observed west of approximately 85°E correspond to the region of strong upwelling and intensive chlorophyll bloom near the southern Kerguelen Plateau [54]).
Potential prey abundances
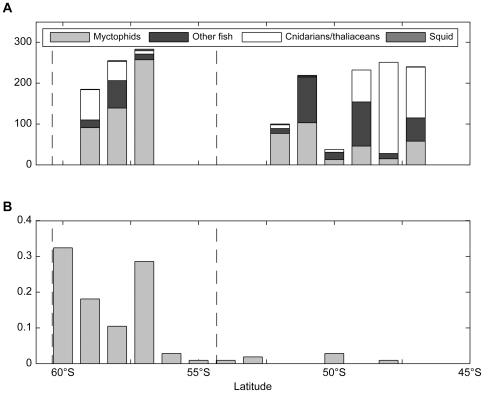
The trawl transect was made in the longitude band 140°E–146°E, and gives an indication of latitudinal variation in pelagic prey availability. North of 50°S, the trawls from the top 500m of the water column were dominated by pyrosomes (65% by number) and salps (11%), and the fish Photichthys argenteus (8%; Figure 8). From 50°S–54°S, myctophids overwhelmingly dominated (90%), but these numbers were strongly skewed by one particularly large haul of Electrona carlsbergi, which contributed 61% of the total myctophids caught in this latitude band. Temporarily excluding this haul of E. carlsbergi (Figure 8 is presented with this haul of E. carlsbergi excluded), the total myctophid component in the 54°S–50°S band was 54% (mainly Gymnoscopelus microlampas, 15%; G. piabilis, 8%; and unidentifiable Protomyctophum, 8%). Other fish (41%; mainly unidentifiable fish, 17%; and Persparsia kopua, 13%) and small numbers of cnidarians and salps (3%) made up the remainder. South of 54°S the hauls were again dominated by myctophids (67%; mainly G. braueri, 22%; Krefftichthys anderssoni, 15%; and E. antarctica, 9%), with smaller numbers of other fish (14%; mainly Bathylagus species, 6%; and unidentifiable fish, 4%), and the coronate scyphomedusae Atolla wyvillei (11%) and Periphylla periphylla (6%).
Figure 8. Latitudinal distributions of pelagic trawl abundances and sooty shearwater diving activity.
A. Pelagic prey abundances (individuals/trawl) in the 60°S to 45°S latitude band, 0–500m depth, at approximately 140°E. B. Sooty shearwater relative dive distribution in the 135°E–145°E sector for the same latitude range. Dotted lines show the latitudes of the southern and northern branches of the Polar Front.
Figure 7c shows the longitudinal distribution of near-surface copepod abundances in the Polar Front zone. Abundances were highest around 145°E and to the east of 170°E, although survey effort in the latter sector was sparse. The regions of higher copepod abundance generally coincided with the regions of persistent upwelling (Figure 7a), high surface chlorophyll concentrations (Figure 7b), and with shearwater foraging effort (Figure 7d).
Discussion
Likely prey of shearwaters on long foraging trips
The diet of short-tailed shearwaters has been relatively well described [14], [56], [57], and on long foraging trips they predominantly consume myctophids (lantern fish), with a small euphausiid component (likely Euphausia vallentini) [56], [57]. Some myctophids, including the relatively common E. antarctica, G. braueri and K. anderssoni are rich in wax esters [58], [59], which act as long-term energy reserve molecules rather than immediate energy supply [60] and they form an important component of stomach oil that is fed to chicks [61]. A number of studies show that sooty shearwaters consume a wide range of crustaceans, fish, cephalopods, and salps [e.g. 62], [63], [64], [65]. The specific prey of sooty shearwaters on long foraging trips has not to our knowledge been studied, although it has been suggested that in Antarctic waters sooty shearwaters also principally forage for myctophids [12]. The results from our study support this suggestion.
The observed foraging zones of the sooty shearwaters in this study, like those of the short-tailed shearwaters, are consistent with the general patterns of myctophid distribution in the Southern Ocean. Myctophids are widely distributed and abundant, particularly from the Polar Front zone south to the Antarctic continental shelf [37], [38], [66]. Around and to the south of the Polar Front zone, myctophids are commonly recorded within 200m of the surface [66], [67], and rise to the surface waters at night [68], [69]. Some myctophid species are also found north of the Polar Front zone (e.g. E. carlsbergi, G. microlampas, G. piabilis), but inhabit deeper waters (250–600m) in these areas [67], [70], which may limit their availability to surface-diving seabirds. These general distribution patterns were observed in the trawl data, with increased myctophid densities south of 50°S.
The observed timing of sooty shearwater dive activity in Antarctic waters is also consistent with the proposition that the sooty shearwaters were foraging for myctophids, or other prey that migrate to near-surface waters during night-time hours. Sooty shearwaters use visual prey detection methods, scanning for underwater prey by immersing their heads before diving [71], [72]. The observed distribution of dive activity in Polar Front waters (peaking just after sunrise and just before sunset, with no night-time diving) might represent a compromise between increased prey availability (proximity to surface) and decreased visual prey detectability during the hours of darkness. Sooty shearwaters have been observed to feed on myctophids during the day in the presence of other marine predators that drive the fish towards the surface waters [65], and shearwaters are commonly observed in association with cetaceans in Antarctic waters [e.g. 73]. The dive depths reported here for sooty shearwaters are underestimates of the true maximum dive depths, due to the low sampling rate (one sample per 24 or 32s) of the tags relative to the time spent by the birds at the deepest part of their dives. Capillary tube measurements show that sooty shearwaters are capable of diving to depths exceeding 60m [74], [75].
The relatively low sampling rate of the tags also means that the probability of failing to detect a dive is greater for dives of shorter duration. It is possible that our finding of dawn and dusk diving in high latitudes has been confounded by this bias. If, for example, dive activity was in reality constant throughout the day, but the dive durations were shorter around midday and longer around dawn and dusk, then the distribution of detected dives would peak around dawn and dusk, as we found here. However, we compared the diel distribution of dive activity from fast sampling tags (sampling rate 24s or 32s) to the distribution obtained from slow sampling tags (432s; n = 3301 dives total by 18 individual birds; results not shown), and found the distributions to be very similar. Missed detections of short duration dives should be more pronounced with the slow sampling tags, and since the distributions match, we do not believe that the diel distribution of dive activity is an artefact of sampling bias in this case.
Cherel et al. [57] reported that Euphausia vallentini were the main crustacean prey from long-trip samples in short-tailed shearwaters from Tasmanian (Bruny Island) colonies. E. vallentini is distributed to the north of the Polar Front [14], [76], [77]. The short-tailed shearwaters in this study, particularly the South Australian birds, were observed to forage in this region. Although E. vallentini have been reported in sooty shearwater diet samples [63], it seems unlikely that sooty shearwaters principally target this species on long foraging trips, since their primary foraging zones lie around and to the south of the Polar Front zone (Figure 1). The South Australian short-tailed shearwaters also foraged well south of the Polar Front, in waters where E. superba (Antarctic krill) is the most abundant euphausiid. Birds tracked from Montague Island, NSW [53] were observed to travel to Antarctic waters where krill is common, as did a post-breeding bird from French Island, Victoria [78]. Connan et al. [56], [79] reported that lipid concentrations from 14 short-tailed shearwaters after long foraging trips did not match those of E. superba, and concluded that this euphausiid might not be a major component of their diet. This result appeared to contradict the above tracking studies as well as earlier stomach samples and observed predation of shearwaters on E. superba [80], [81]. However, the results of Connan et al. [56], [79] were obtained from birds breeding on Bruny Island, Tasmania. The small sample of Tasmanian (Wedge Island) birds tracked in this study did not forage in waters where E. superba might be expected. Thus, this apparent contradiction might be a result of site-specific differences in foraging area preferences of birds from different regions and colonies. E. superba might still be an important dietary component for some populations of short-tailed shearwaters, such as those from South Australian islands.
Wind cost analyses
One of the objectives for the current study was to evaluate whether the longitudinal distribution of sooty shearwater foraging effort in the Polar Front zone was related to patterns of oceanic winds. There is little in our results to suggest that the region directly south of New Zealand was sparsely utilized by the birds because of wind cost factors. However, wind patterns do appear to explain other broad-scale patterns in foraging, such as the lack of use of near-Antarctic waters east of about 150°E by South Australian short-tailed shearwaters. This use of wind on long foraging trips has previously been noted [e.g. 53] but not formally investigated.
Both the sooty shearwaters and the Tasmanian short-tailed shearwaters made long foraging trips to the south-east of their colonies, into apparently energetically-costly areas. Shaffer et al. [13] noted the bias of the sooty shearwaters toward the south-eastern sector, and provided several possible explanations, including potentially higher prey abundances in that zone. Our wind cost analyses suggest two further factors. First, while such trips were costly overall, the outward flight legs were generally accompanied by favourable tail and hind-quarter winds. Long foraging trips are generally initiated when the adults have poor body condition [12], [14] and so a foraging trip with minimal initial energy cost might be preferable to one that has a lower total cost (i.e. when the return leg also considered). Second, shearwaters breeding in south-eastern Australia do not appear to forage in this zone, possibly because of the high energy costs that they would incur in doing so. The south-eastern zone might therefore represent an area of reduced competition for the sooty shearwaters.
There are a number of potential concerns with the wind cost analyses. Shearwaters generally fly close to the water, where wind speeds will be lower than the estimates provided by the NCEP2 data (which are for winds at 10m above the ocean surface, where shearwaters rarely fly). Flight close to the sea surface will reduce the induced drag and therefore the energy cost of flight [82], but perhaps more importantly, means that ocean waves become a potentially important consideration. Shearwaters are known to use wave troughs and wind gradients during flight [e.g. 83], [84]. Dynamic flight techniques that exploit the interaction of winds and waves, such as gust soaring [50], [51], have the potential to dramatically reduce the energy expenditure of seabirds in flight over the open ocean. The cost function used here does not account for such techniques. The decision not to incorporate these factors into the wind cost analyses was in part a reflection of the lack of fine-scale wind and wave data over the regions of interest (which would be required to model dynamic flight costs), and also the relatively coarse spatial and temporal resolution of the seabird locations (particularly those from the geolocation tags, which provide only one position fix per day and with relatively large spatial uncertainty). The wind cost analyses must therefore be interpreted in the context of relatively large-scale behaviours (of the order of days, or hundreds of kilometres).
Further caution should be exercised in interpreting the insights into adult foraging behaviour obtained from tagged birds, since it is known that the handling of birds and presence of tags can reduce colony attendance and provisioning of chicks by the adult birds [43], [85], [ but see also 86].
Relationship with physical oceanography in the Polar Front zone
The foraging effort of the shearwaters within the Polar Front zone was not uniform in longitude, but was focused on areas of persistent upwelling. The association of shearwater foraging activity with these areas reflects elevated prey availability (as measured by near-surface copepod abundances), resulting either from elevated production in these areas, and/or the aggregation of prey in near-surface waters by upwelling and convergence [e.g. 87].
The persistent upwellings in the Polar Front zone are largely coincident with the surface productivity, as measured by satellite estimates of surface chlorophyll (Figure 7; see also Figure 8 in [54]). In the open ocean away from shallow regions, blooms along Southern Ocean fronts are generally initiated by upwellings due to the interactions of the Antarctic Circumpolar Current with large-scale bottom topography [54]. A large and intense topography-induced upwelling is found where the Polar Front interacts with Pacific-Antarctic Ridge, from ∼170°E–200°E, and this zone corresponds to the strongest chlorophyll bloom found in the Pacific sector of the Southern Ocean [54], and also to the sooty shearwaters' foraging zone south-east of New Zealand. The upwellings in the vicinity of the South-East Indian and Macquarie Ridges (140°E and 165°E; also foraging zones) have relatively weak signatures in surface chlorophyll distribution (Figure 7a). However, in-situ observations along the World Ocean Circulation Experiment SR3 section (140°E line) show a persistent subsurface chlorophyll maximum at depths of 50–100m [88], [89]. This subsurface maximum does not extend northward into the subantarctic zone. Parslow et al. [88] concluded that vertical processes (rather than horizontal advection) were the dominant controllers of this feature, consistent with the vertical velocity distributions shown here (Figure 6).
Consistency and overlap of foraging areas
The overlap of foraging zones between the two shearwater species, and with commercial fisheries, is an issue of ongoing interest [2]. The Polar Front region around 140°E was utilised by shearwaters from all three tracking studies incorporated here. The range of years covered by those studies, combined with the consistency of high at-sea densities in this region, strongly suggests that this region is used repeatedly across years. This is also consistent with previously published tracks of short-tailed shearwaters from south-eastern Australia [53], [78], and with land-based observations of southward-, but not northward-flying shearwaters at Macquarie Island [90]. The south-eastern sooty shearwater foraging zone matches that recorded in 2000 (Figure 1 in [2]), suggestive of inter-annual consistency in the use of other foraging locations. The sooty shearwaters also showed foraging zone fidelity at an individual level, with only one bird foraging in both the south-eastern and south-western zones. Fidelity to mesoscale features such as fronts and upwelling zones is extremely common in pelagic seabirds [91]. Central-place foragers, such as breeding seabirds, tend to repeatedly utilise foraging zones where prey are consistent [92], [93]. The strategy of returning to learned foraging zones provides a means of counteracting the natural patchiness of marine prey [94].
The lack of foraging activity by the sooty shearwaters in the Polar Front zone sector from ∼145°E–180°E does not appear to be a result of wind-cost factors, but likely prey availability and other factors. This sector encompasses a sector of persistent downwelling (∼148°E–162°E, Figure 6). While the sooty shearwaters did not utilise the region around 165°E, this is a region of upwelling and was utilised by the Tasmanian short-tailed shearwaters. The lack of use of this particular area by the sooty shearwaters might therefore reflect differences in preferred (learned) foraging areas of the birds.
Tracking studies on other species have shown foraging distributions in similar areas to the shearwaters. Southern elephant seals Mirounga leonina from Macquarie Island, like the sooty shearwaters, showed a bimodal south-east/south-west foraging pattern (Figure 1b in [95]), although these seals foraged farther south than did the sooty shearwaters. Grey-headed albatrosses Thalassarche chrysostoma from Macquarie Island foraged just west of the sooty shearwaters' south-western foraging zone, around 180°E–190°E (Figure 5 in [96]), as did grey-headed and black-browed T. melanophrys albatrosses from Campbell Island [97]. Light-mantled sooty albatrosses Phoebetria palpebrata, also from Macquarie Island, foraged just south of the south-western zone (approximately 130–140°E, 64°S) [98]. King penguins Aptenodytes patagonicus from Macquarie Island foraged east and south of the island [99], including the Polar Front zone at ∼165°E where the Tasmanian short-tailed shearwaters foraged. Stomach samples from these penguins consisted of fish (primarily the myctophids K. anderssoni and E. carlsbergi) and squid (Moroteuthis ingens and Mastigoteuthis sp.). M. ingens is a predator of myctophids, particularly K. anderssoni [100]. The dives made by these penguins in the Polar Front zone were similar in pattern to those of the sooty shearwaters reported here, occurring from sunrise until mid-morning, and again from mid-afternoon until sunset, with little dive activity around midday (B. Wienecke, unpublished). The dive depths were also similar: shallowest at sunrise and sunset, increasing in depth closer to mid-day; consistent with the diurnal vertical migration of myctophids and their predators. The penguins' deepest dives (up to 170m) were well beyond the maximum diving depths of shearwaters, suggesting that such prey would be accessible to the shearwaters for a much shorter period of each day. However, they can cover a much greater distance over this shorter time period.
Upper-level trophic predators, including seabirds, are commonly used as indicators for ecosystem monitoring [e.g. 101], [102], [103] and fish stock assessment [e.g. 104]. Clearly, their value in this role is determined by the extent to which variations in indices derived from these predators reflect changes occurring at lower trophic levels of the ecosystem [e.g. 105]. The use of sooty and short-tailed shearwaters as indicators of Southern Ocean ecosystems is compromised to some extent by their annual migrations and corresponding exposure to factors external to the Southern Ocean [e.g. 106], [107]. However, our results suggest that during the breeding season, these birds are consistent in their foraging habitats. The foraging overlap between the two species, and between populations, and the similarities in their foraging areas to those of other marine predators, suggests that these birds might be useful as indicators of aspects of the Southern Ocean ecosystem, particularly of the Polar Front zone. Combining indices from multiple colonies and/or different species might allow variations in breeding success due to localised factors (e.g. density dependence, or predation from introduced mammals) to be disentangled from those due to changes in the ecosystem of the Polar Front zone. Combining indicator information from populations at multiple sites, and from multiple species, can assist in reducing single-site effects, and potentially make indicators based on seabird breeding performance more robust [108]. An index of the abundance of Australian krill (Nyctiphanes australis) based on a combination of indices of the growth of short-tailed shearwater chicks in Tasmania and mortality occurrences in Japan has previously been proposed [109].
Summary and conclusions
Electronic tagging technologies can provide detailed insights into the foraging and migratory behaviours of wide-ranging marine predators. In this study we combined tracking data from two shearwater species that originate from colonies separated by thousands of kilometres in New Zealand and Australia, along with ship-based and remote-sensed information to evaluate and describe the predators' foraging behaviour within the wider context of their marine ecosystem.
The birds displayed foraging site fidelities at individual and population levels, with overlap in foraging habitat between species and populations. Wind cost modelling yielded new information about the putative mechanisms that influence foraging destination. The distributions of foraging effort at broad spatial scales were consistent with patterns in oceanic winds. Prey availability modulated these patterns, and influenced foraging effort at smaller spatial scales. The spatial distribution of shearwater foraging effort in the Polar Front zone was matched by patterns in large-scale upwelling, primary production, and abundances of copepods and myctophid fish. These results offer great promise for modelling and projecting possible changes in foraging behaviour and distribution related to environmental factors.
Acknowledgments
We thank D. Pinaud, C. Bragg, R. Mules, B. Newton, S. McKecknie, and J. Adams for assistance in the field with sooty shearwater deployments, R. Williams for providing the trawl data, B. Wienecke the king penguin foraging data, and V. Wadley for support and coordination. H. Moller and J. Adams provided valuable comments on an earlier version of this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was conducted as part of the Census of Antarctic Marine Life (CAML) and Tagging of Pacific Pelagics (TOPP) projects, and was supported in part by the Alfred P. Sloan, Gordon and Betty Moore, and David and Lucile Packard Foundations, and the Australian Antarctic Program (Projects 472, 681, 2208, and 3227). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Shaffer SA, Tremblay Y, Weimerskirch H, Scott D, Thompson DR, et al. Migratory shearwaters integrate oceanic resources across the Pacific Ocean in an endless summer. Proceedings of the National Academy of Sciences. 2006;103:12799–12802. doi: 10.1073/pnas.0603715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Söhle IS, Robertson CJR, Nicholls DG, Mouritsen H, Frost B, et al. Satellite tracking of sooty shearwaters (Puffinus griseus) during their pre-laying “exodus” and incubation. Notornis. 2007;54:180–188. [Google Scholar]
- 3.Serventy DL, Serventy V, Warham J. The Handbook of Australian Sea-Birds. Sydney: Reed; 1971. 254 [Google Scholar]
- 4.Moller H, Lyver POB, Bragg C, Newman J, Clucas R, et al. Guidelines for cross-cultural participatory action research partnerships: a case study of a customary seabird harvest in New Zealand. New Zealand Journal of Zoology. 2009;36:211–241. [Google Scholar]
- 5.Skira IJ, Wapstra JE, Towney GN, Naarding JA. Conservation of the short-tailed shearwater Puffinus tenuirostris in Tasmania, Australia. Biological Conservation. 1985;37:225–236. [Google Scholar]
- 6.McKechnie S. Biopedturbation by an island ecosystem engineer: burrowing volumes and litter deposition by sooty shearwaters (Puffinus griseus). New Zealand Journal of Zoology. 2006;33:259–265. [Google Scholar]
- 7.Croll DA, Maron JL, Estes JA, Danner EM, Byrd GV. Introduced predators transform subarctic islands from grassland to tundra. Science. 2005;307:1959–1961. doi: 10.1126/science.1108485. [DOI] [PubMed] [Google Scholar]
- 8.Fukami T, Wardle DA, Bellingham PJ, Mulder CPH, Towns DR, et al. Above- and below-ground impacts of introduced predators in seabird-dominated island ecosystems. Ecology Letters. 2006;9:1299–1307. doi: 10.1111/j.1461-0248.2006.00983.x. [DOI] [PubMed] [Google Scholar]
- 9.Moller H, Fletcher D, Johnson PN, Bell BD, Flack D, et al. Changes in sooty shearwater (Puffinus griseus) abundance and harvesting on the Rakiura Tītī Islands. New Zealand Journal of Zoology. 2009;36:325–341. [Google Scholar]
- 10.Moller H, Frampton C, Hocken AG, McLean IG, Saffer V, et al. The importance of seabird research for New Zealand. New Zealand Journal of Zoology. 2000;27:255–260. [Google Scholar]
- 11.Bost CA, Jaeger A, Huin W, Koubbi P, Halsey LG, et al. Monitoring prey availability via data loggers deployed on seabirds: advances and present limitations. In: Tsukamoto K, Kawamura T, Takeuchi T, Beard TD Jr, Kaiser MJ, editors. Fisheries for Global Welfare and Environment Memorial book of the 5th World Fisheries Congress 2008. Yokohama: Terrapub; 2008. pp. 121–137. [Google Scholar]
- 12.Weimerskirch H. How can a pelagic seabird provision its chick when relying on a distant food resource? Cyclic attendance at the colony, foraging decision and body condition in sooty shearwaters. Journal of Animal Ecology. 1998;67:99–109. [Google Scholar]
- 13.Shaffer SA, Weimerskirch H, Scott D, Pinaud D, Thompson DR, et al. Spatiotemporal habitat use by breeding sooty shearwaters Puffinus griseus. Marine Ecology Progress Series. 2009;391:209–220. [Google Scholar]
- 14.Weimerskirch H, Cherel Y. Feeding ecology of short-tailed shearwaters: breeding in Tasmania and foraging in the Antarctic? Marine Ecology Progress Series. 1998;167:261–274. [Google Scholar]
- 15.Schultz MA, Klomp NI. Does the foraging strategy of adult short-tailed shearwaters cause obesity in their chicks? Journal of Avian Biology. 2000;31:287–294. [Google Scholar]
- 16.Ropert-Coudert Y, Wilson RP, Daunt F, Kato A. Patterns of energy acquisition by a central place forager: benefits of alternating short and long foraging trips. Behavioral Ecology. 2004;15:824–830. [Google Scholar]
- 17.Weimerskirch H, Guionnet T, Martin J, Shaffer SA, Costa DP. Fast and fuel-efficient? Optimal use of wind by flying albatrosses. Proceedings of the Royal Society of London B. 2000;267:1869–1874. doi: 10.1098/rspb.2000.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catry P, Phillips RA, Croxall JP. Sustained fast travel by a gray-headed albatross (Thalassarche chrysostoma) riding an Antarctic storm. Auk. 2004;121:1208–1213. [Google Scholar]
- 19.Lawton K, Kirkwood R, Robertson G, Raymond B. Preferred foraging areas of Heard Island albatrosses during chick raising and implications for the management of incidental mortality in fisheries. Aquatic Conservation: Marine and Freshwater Ecosystems. 2008;18:309–320. [Google Scholar]
- 20.Adams J, Flora S. Correlating seabird movements with ocean winds: linking satellite telemetry with ocean scatterometry. Marine Biology. 2009 doi: 10.1007/s00227-009-1367-y. [Google Scholar]
- 21.Wakefield ED, Phillips RA, Matthiopoulos J, Fukuda A, Higuchi H, et al. Wind field and sex constrain flight speeds of central-place foraging albatrosses. Ecological Monographs. 2009;79:663–679. [Google Scholar]
- 22.Nicholls DG, Murray MD, Butcher E, Moors P. Weather systems determine the non-breeding distribution of wandering albatrosses over southern oceans. Emu. 1997;97:240–244. [Google Scholar]
- 23.Burger AE, Shaffer SA. Application of tracking and data-logging technology in research and conservation of seabirds. The Auk. 2008;125:253–264. [Google Scholar]
- 24.Tremblay Y, Bertrand S, Henry RW, Kappes MA, Costa DP, et al. Analytical approaches to investigating seabird–environment interactions: a review. Marine Ecology Progress Series. 2009;391:153–163. [Google Scholar]
- 25.Rutz C, Hays GC. New frontiers in biologging science. Biology Letters. 2009;5:289–292. doi: 10.1098/rsbl.2009.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaffer SA, Tremblay Y, Awkerman JA, Henry WR, Teo SLH, et al. Comparison of light- and SST-based geolocation with satellite telemetry in free-ranging albatrosses. Marine Biology. 2005;147:833–843. [Google Scholar]
- 27.Einoder LD, Goldsworthy SD. Foraging flights of short-tailed shearwaters (Puffinus tenuirostris) from Althorpe Island: assessing their use of neritic waters. Transactions of the Royal Society of South Australia. 2005;129:209–216. [Google Scholar]
- 28.Woehler EJ. 1999, updated 2009. Distribution and abundance of seabirds in the Southern Indian Ocean, 1980/81+, Australian Antarctic Data Centre - CAASM Metadata ( http://data.aad.gov.au/aadc/metadata/)
- 29.Woehler EJ, Raymond B, Watts DJ. Convergence or divergence: where do short-tailed shearwaters forage in the Southern Ocean? Marine Ecology Progress Series. 2006;324:261–270. [Google Scholar]
- 30.Woehler EJ. 1995. Variability in foraging ecology and food consumption by seabirds at high latitudes: Doctoral thesis, University of California Irvine.
- 31.Woehler EJ. Seabird abundance, biomass and prey consumption within Prydz Bay, Antarctica, 1980/81 to 1992/93. Polar Biology. 1997;17:371–383. [Google Scholar]
- 32.BIOMASS Working Party on Bird Ecology. Recording observations of birds at sea. BIOMASS Handbook. 1982;18 [Google Scholar]
- 33.Williams R. Survey of pelagic fish. In: Wright S, Potter S, editors. Voyage Report, Voyage 1 1996/97. Hobart: Australian Antarctic Division; 1997. [Google Scholar]
- 34.Hosie GW, Fukuchi M, Kawaguchi S. Development of the southern ocean continuous plankton recorder survey. Progress in Oceanography. 2003;58:263–283. [Google Scholar]
- 35.Reid PC, Colebrook JM, Matthews JBL, Aiken J, Team CPR. The Continuous Plankton Recorder: concepts and history, from the Plankton Indicator to undulating recorders. Progress in Oceanography. 2003;58:117–173. [Google Scholar]
- 36.Sokolov S, Rintoul SR. Circumpolar structure and distribution of the Antarctic Circumpolar Current fronts: 1. Mean circumpolar paths. Journal of Geophysical Research. 2009;114:C11018. doi: 11010.11029/12008JC005108. [Google Scholar]
- 37.Kozlov AN. A review of the trophic role of mesopelagic fish of the family Myctophidae in the Southern Ocean ecosystem. CCAMLR Science. 1995;2:71–77. [Google Scholar]
- 38.Pakhomov EA, Perissinotto R, McQuaid CD. Prey composition and daily rations of myctophid fishes in the Southern Ocean. Marine Ecology Progress Series. 1996;134:1–14. [Google Scholar]
- 39.Pusch C, Hulley PA, Kock K-H. Community structure and feeding ecology of mesopelagic fishes in the slope waters of King George Island (South Shetland Islands, Antarctica). Deep-Sea Research I. 2004;51:1685–1708. [Google Scholar]
- 40.Gordon HB, Rotstayn LD, McGregor JL, Dix MR, Kowalczyk EA, et al. The CSIRO Mk3 Climate System Model. Aspendale; 2002. 130 [Google Scholar]
- 41.Feldman GC, McClain CR. Ocean Color Web, SeaWiFS Reprocessing, NASA Goddard Space Flight Center. 2010. Eds. Kuring, N., Bailey, S.W. http://oceancolor.gsfc.nasa.gov/
- 42.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. Journal of the American Statistical Association. 1979;74:829–836. [Google Scholar]
- 43.Adams J, Scott D, McKechnie S, Blackwell G, Shaffer SA, et al. Effects of geolocation archival tags on reproduction and adult body mass of sooty shearwaters (Puffinus griseus). New Zealand Journal of Zoology. 2009;36:355–366. [Google Scholar]
- 44.McConnell BJ, Chambers C, Fedak MA. Foraging ecology of southern elephant seals in relation to the bathymetry and productivity of the Southern Ocean. Antarctic Science. 1992;4:393–398. [Google Scholar]
- 45.Reda I, Andreas A. Solar position algorithm for solar radiation application. National Renewable Energy Laboratory (NREL) Technical report NREL/TP-560-34302. 2003. http://www.osti.gov/bridge.
- 46.Worton BJ. Kernel methods for estimating the utilization distribution in home-range studies. Ecology. 1989;70:164–168. [Google Scholar]
- 47.Brodtkorb PA, Johannesson P, Lindgren G, Rychlik I, Rydén J, et al. WAFO — a Matlab toolbox for analysis of random waves and loads. 2000. pp. 343–350. Proceedings of the 10th International Offshore and Polar Engineering conference. Seattle, USA.
- 48.Felicísimo Á, Muñoz J, González-Solis J. Ocean surface winds drive dynamics of transoceanic aerial movements. PLoS ONE. 2008;3:e2928. doi: 10.1371/journal.pone.0002928. doi: 2910.1371/journal.pone.0002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liechti F, Hedenström A, Alerstam T. Effects of sidewinds on optimal flight speed of birds. Journal of Theoretical Biology. 1994;170:219–225. [Google Scholar]
- 50.Pennycuick CJ. Bird Flight Performance: A Practical Calculation Manual. Oxford: Oxford University Press; 1989. [Google Scholar]
- 51.Pennycuick CJ. 2008. Modelling the Flying Bird: Elsevier.
- 52.Spear LB, Ainley DG. Flight behaviour of seabirds in relation to wind direction and wing morphology. Ibis. 1997;139:221–233. [Google Scholar]
- 53.Klomp NI, Schultz MA. Short-tailed shearwaters breeding in Australia forage in Antarctic waters. Marine Ecology Progress Series. 2000;194:307–310. [Google Scholar]
- 54.Sokolov S, Rintoul SR. On the relationship between fronts of the Antarctic Circumpolar Current and surface chlorophyll concentrations in the Southern Ocean. Journal of Geophysical Research. 2007;112:C07030. doi: 07010.01029/02006JC004072. [Google Scholar]
- 55.Sokolov S. Chlorophyll blooms in the Antarctic Zone south of Australia and New Zealand in reference to the Antarctic Circumpolar Current fronts and sea ice forcing. Journal of Geophysical Research. 2008;113:C03022. doi: 03010.01029/02007JC004329. [Google Scholar]
- 56.Connan M, Mayzaud P, Boutoute M, Weimerskirch H, Cherel Y. Lipid composition of stomach oil in a procellariiform seabird Puffinus tenuirostris: implications for food web studies. Marine Ecology Progress Series. 2005;290:277–290. [Google Scholar]
- 57.Cherel Y, Hobson KA, Weimerskirch H. Using stable isotopes to study resource acquisition and allocation in procellariiform seabirds. Oecologia. 2005;145:533–540. doi: 10.1007/s00442-005-0156-7. [DOI] [PubMed] [Google Scholar]
- 58.Phleger CF, Nichols PD, Virtue P. The lipid, fatty acid and fatty alcohol composition of the myctophid fish Electrona antarctica: high level of wax esters and foodchain implications. Antarctic Science. 1997;9:258–265. [Google Scholar]
- 59.Phleger CF, Nelson MM, Mooney BD, Nichols PD. Wax esters versus triacylglycerols in myctophid fishes from the Southern Ocean. Antarctic Science. 1999;11:436–444. [Google Scholar]
- 60.Lee RF, Patton JS. Alcohol and waxes. In: Ackman RG, editor. Marine biogenic lipids, fats, and oils. Boca Raton, Florida: CRC Press; 1989. pp. 73–102. [Google Scholar]
- 61.Warham J. The Incidence, functions and ecological significance of petrel stomach oils. Proceedings of the New Zealand Ecological Society. 1977;24:84–93. [Google Scholar]
- 62.Kitson JC, Cruz JB, Lalas C, Jillett JB, Newman J, et al. Interannual variations in the diet of breeding sooty shearwaters (Puffinus griseus). New Zealand Journal of Zoology. 2000;27:347–355. [Google Scholar]
- 63.Cruz JB, Lalas C, Jillett JB, Kitson JC, Lyver POB, et al. Prey spectrum of breeding sooty shearwaters (Puffinus griseus) in New Zealand. New Zealand Journal of Marine and Freshwater Research. 2001;35:817–829. [Google Scholar]
- 64.Briggs KT, Chu EW. Sooty shearwaters off California: Distribution, abundance and habitat use. The Condor. 1986;88:355–364. [Google Scholar]
- 65.Jackson S. Diets of the white-chinned petrel and sooty shearwater in the Southern Benguela region, South Africa. The Condor. 1988;90:20–28. [Google Scholar]
- 66.Williams R, McEldowney A. A guide to the fish otoliths from waters off the Australian Antarctic Territory, Heard and Macquarie Islands. ANARE Research Notes. 1990;75:173pp. [Google Scholar]
- 67.Hulley PA. Family Myctophidae (lanternfishes). In: Gon O, Heemstra PS, editors. Fishes of the Southern Ocean. Grahamstown, South Africa: J.L.B. Smith Institute of Ichthyology; 1990. pp. 146–178. [Google Scholar]
- 68.Duhamel G, Koubbi P, Ravier C. Day and night mesopelagic assemblages off the Kerguelen Islands (Southern Ocean). Polar Biology. 2000;23:106–112. [Google Scholar]
- 69.Robison BH. What drives the diel vertical migrations of Antarctic midwater fish? Journal of the Marine Biological Association of the United Kingdom. 2003;83:639–642. [Google Scholar]
- 70.Williams R. The nearshore fishes of Macquarie Island. Papers and Proceedings of the Royal Society of Tasmania. 1988;122:233–245. [Google Scholar]
- 71.Brown RGB, Bourne WRP, Wahl TR. Diving by shearwaters. The Condor. 1978;80:123–125. [Google Scholar]
- 72.Harper PC. Feeding behaviour and other notes on 20 species of procellariiformes at sea. Notornis. 1987;34:169–192. [Google Scholar]
- 73.Hodges CL, Woehler EJ. Associations between seabirds and cetaceans in the Australian sector of the Southern Indian Ocean. Marine Ornithology. 1994;22:205–212. [Google Scholar]
- 74.Weimerskirch H, Sagar PM. Diving depths of Sooty Shearwaters Puffinus griseus. Ibis. 1996;138:786–788. [Google Scholar]
- 75.Taylor GA. Maximum dive depths of eight New Zealand procellariiformes, including Pterodroma species. Papers and Proceedings of the Royal Society of Tasmania. 2008;142:89–97. [Google Scholar]
- 76.Hunt BPV, Hosie GW. Zonal structure of zooplankton communities in the Southern Ocean south of Australia: results from a 2150km continuous plankton recorder transect. Deep-Sea Research I. 2005;52:1241–1271. [Google Scholar]
- 77.McLeod DJ, Hosie GW, Kitchener JA, Takahashi KT, Hunt BPV. in press. (in press) Zooplankton atlas of the Southern Ocean: The SCAR SO-CPR Survey (1991–2008) Polar Science. doi: 10.1016/j.polar.2010.03.004.
- 78.Nicholls DG, Stampton P, Klomp NI, Schultz M. Post-breeding flight to Antarctic waters by a short-tailed shearwater Puffinus tenuirostris. Emu. 1998;98:79–82. [Google Scholar]
- 79.Connan M, Cherel Y, Mayzaud P. Lipids from stomach oil of procellariiform seabirds document the importance of myctophid fish in the Southern Ocean. Limnology and Oceanography. 2007;52:2445–2455. [Google Scholar]
- 80.Kerry KR, Horne RSC, Dorward DF. Records of the short-tailed shearwater Puffinus tenuirostris in Antarctic waters. Emu. 1983;83:35–37. [Google Scholar]
- 81.Veit RR, Hunt GL. Broadscale density and aggregation of pelagic birds from a circumnavigational survey of the Antarctic Ocean. The Auk. 1991;108:790–800. [Google Scholar]
- 82.Rosén M, Hedenström A. Testing predictions from flight mechanical theory: a case study of Cory's shearwater and Audouin's gull. Acta Ethologica. 2001;3:135–140. [Google Scholar]
- 83.Robertson JS. Migrating shearwaters. Emu. 1957;57:191–197. [Google Scholar]
- 84.Blomqvist S, Peterz M. Cyclones and pelagic seabird movements. Marine Ecology Progress Series. 1984;20:85–92. [Google Scholar]
- 85.Söhle IS. Effects of satellite telemetry on sooty shearwater, Puffinus griseus, adults and chicks. Emu. 2003;103:373–379. [Google Scholar]
- 86.Igual JM, Forero MG, Tavecchia G, González-Solís J, Martínez-Abraín A, et al. Short-term effects of data-loggers on Cory's shearwater (Calonectris diomedea). Marine Biology. 2005;146:619–624. [Google Scholar]
- 87.Bost CA, Cotté C, Bailleul F, Cherel Y, Charrassin JB, et al. The importance of oceanographic fronts to marine birds and mammals of the southern oceans. Journal of Marine Systems. 2009;78:363–376. [Google Scholar]
- 88.Parslow JS, Boyd PW, Rintoul SR, Griffiths FB. A persistent subsurface chlorophyll maximum in the Interpolar Frontal Zone south of Australia: Seasonal progression and implications for phytoplankton-light-nutrient interactions. Journal of Geophysical Research. 2001;106:C31543–C31557. [Google Scholar]
- 89.Yamaguchi Y, Shibata Y. Standing stock and distribution of phytoplankton chlorophyll in the Southern Ocean south of Australia. Transactions of the Tokyo University of Fisheries. 1982;5:111–128. [Google Scholar]
- 90.Clarke RH, Schulz M. Land-based observations of seabirds off sub-Antarctic Macquarie Island during 2002 and 2003. Marine Ornithology. 2005;33:7–17. [Google Scholar]
- 91.Weimerskirch H. Are seabirds foraging for unpredictable resources? Deep-Sea Research Part II: Topical Studies in Oceanography. 2007;54:211–223. [Google Scholar]
- 92.Bradshaw CJA, Hindell MA, Sumner MD, Michael KJ. Loyalty pays: potential life history consequences of fidelity to marine foraging regions by southern elephant seals. Animal Behaviour. 2004;68:1349–1360. [Google Scholar]
- 93.Staniland IJ, Boyd IL, Reid K. An energy–distance trade-off in a central-place forager, the Antarctic fur seal (Arctocephalus gazella). Marine Biology. 2007;152:233–241. [Google Scholar]
- 94.Hamer KC, Phillips RA, Hill JK, Wanless S, Wood AG. Contrasting foraging strategies of gannets Morus bassanus at two North Atlantic colonies: foraging trip duration and foraging area fidelity. Marine Ecology Progress Series. 2001;224:283–290. [Google Scholar]
- 95.Charrassin J-B, Hindell M, Rintoul SR, Roquet F, Sokolov S, et al. Southern Ocean frontal structure and sea-ice formation rates revealed by elephant seals. Proceedings of the National Academy of Sciences. 2008;105:11634–11639. doi: 10.1073/pnas.0800790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Terauds A, Gales R, Baker GB, Alderman R. Foraging areas of black-browed and grey-headed albatrosses breeding on Macquarie Island in relation to marine protected areas. Aquatic Conservation: Marine and Freshwater Ecosystems. 2006;16:133–146. [Google Scholar]
- 97.Waugh SM, Weimerskirch H, Cherel Y, Shankar U, Prince PA, et al. Exploitation of the marine environment by two sympatric albatrosses in the Pacific Southern Ocean. Marine Ecology Progress Series. 1999;177:243–254. [Google Scholar]
- 98.Weimerskirch H, Robertson G. Satellite tracking of light-mantled sooty albatrosses. Polar Biology. 1994;14:123–126. [Google Scholar]
- 99.Wienecke B, Robertson G. Comparison of foraging strategies of incubating king penguins Aptenodytes patagonicus from Macquarie and Heard islands. Polar Biology. 2006;29:424–438. [Google Scholar]
- 100.Phillips KL, Jackson GD, Nichols PD. Predation on myctophids by the squid Moroteuthis ingens around Macquarie and Heard Islands: stomach contents and fatty acid analyses. Marine Ecology Progress Series. 2001;215:179–189. [Google Scholar]
- 101.Einoder LD. A review of the use of seabirds as indicators in fisheries and ecosystem management. Fisheries Research. 2009;95:6–13. [Google Scholar]
- 102.Diamond AW, Devlin CM. Seabirds as indicators of changes in marine ecosystems: ecological monitoring on Machias Seal Island. Environmental Monitoring and Assessment. 2003;88:153–175. doi: 10.1023/a:1025560805788. [DOI] [PubMed] [Google Scholar]
- 103.Agnew DJ. The CCAMLR ecosystem monitoring programme. Antarctic Science. 1997;9:235–242. [Google Scholar]
- 104.Cairns DK. Bridging the gap between ornithology and fisheries science: use of seabird data in stock assessment models. The Condor. 1992;94:811–824. [Google Scholar]
- 105.Reid K, Croxall JP, Briggs DR, Murphy EJ. Antarctic ecosystem monitoring: quantifying the response of ecosystem indicators to variability in Antarctic krill. ICES Journal of Marine Science. 2005;62:366–373. [Google Scholar]
- 106.Baduini CL, Hyrenbach KD, Coyle KO, Pinchuk A, Mendenhall V, et al. Mass mortality of short-tailed shearwaters in the south-eastern Bering Sea during summer 1997. Fisheries Oceanography. 2001;10:117–130. [Google Scholar]
- 107.Veit RR, McGowan JA, Ainley DG, Wahl TR, Pyle P. Apex marine predator declines ninety percent in association with changing oceanic climate. Global Change Biology. 1997;3:23–28. [Google Scholar]
- 108.Frederiksen M, Mavor RA, Wanless S. Seabirds as environmental indicators: the advantages of combining data sets. Marine Ecology Progress Series. 2007;352:205–211. [Google Scholar]
- 109.Oka N, Maruyama N, Skira I. Chick growth and mortality of short-tailed shearwaters in comparison with sooty shearwaters, as a possible index of fluctuations of Australian krill abundance. Proceedings of the National Institute of Polar Research Symposium on Polar Biology. 1987;1:166–174. [Google Scholar]