Abstract
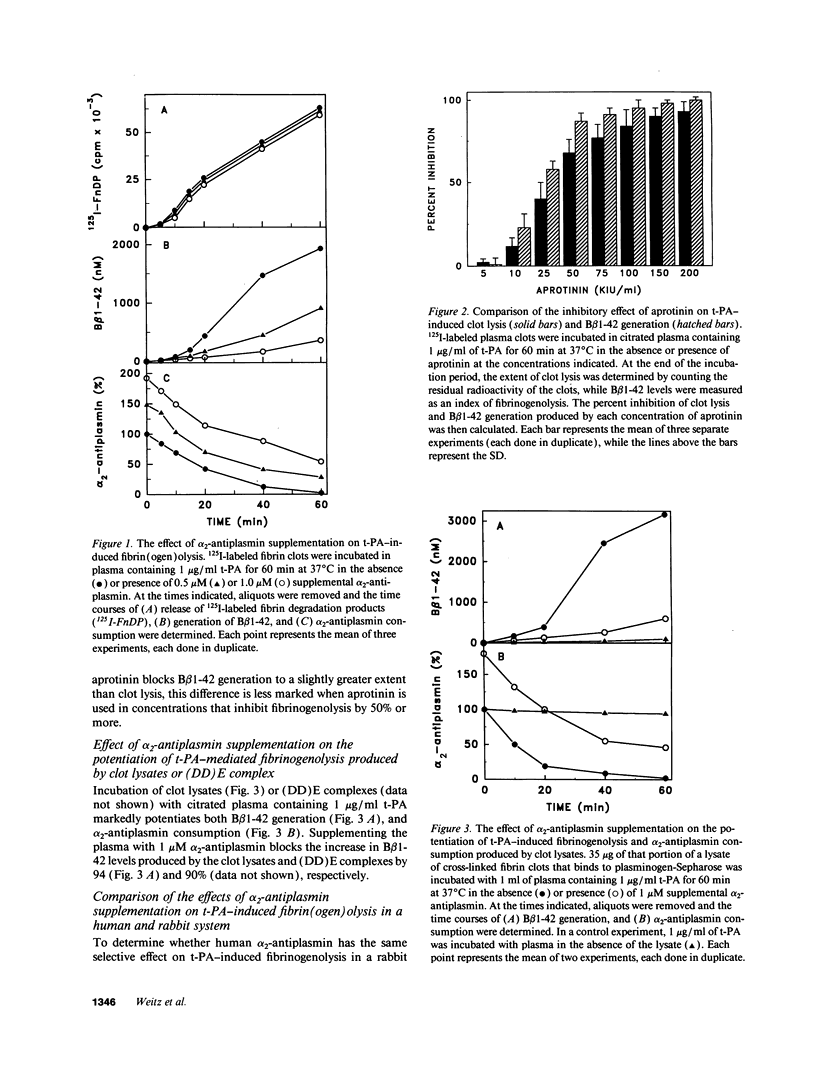
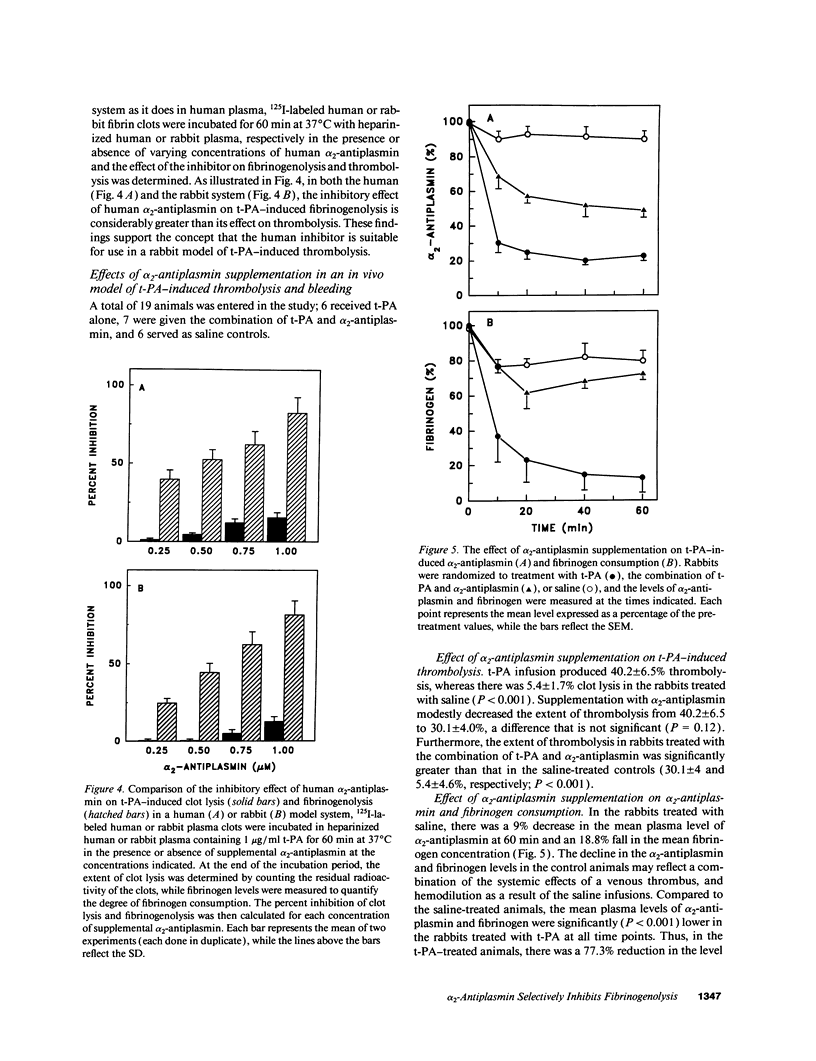
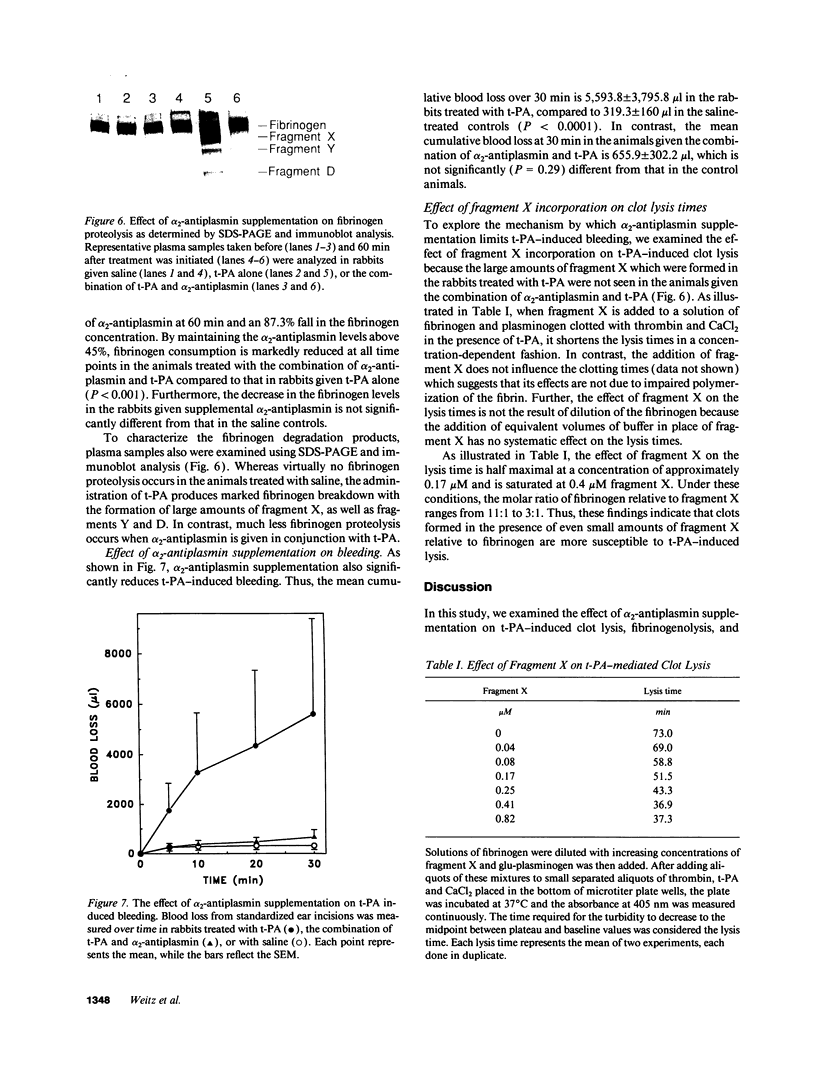
Tissue plasminogen activator (t-PA) causes fibrinogen proteolysis when alpha 2-antiplasmin levels fall, and this may contribute to t-PA-induced hemorrhage. Because clot-bound plasmin is protected from alpha 2-antiplasmin inhibition, we tested the possibility that alpha 2-antiplasmin supplementation would block t-PA-induced fibrinogenolysis and bleeding without affecting thrombolysis. When added to human or rabbit plasma, alpha 2-antiplasmin inhibits t-PA-induced fibrinogenolysis, but hat little effect on the lysis of 125I-fibrin clots. To examine its effect in vivo, rabbits with preformed 125I-labeled-jugular vein thrombi were randomized to receive t-PA, t-PA and alpha 2-antiplasmin, or saline. alpha 2-Antiplasmin infusion produced a modest decrease in t-PA-induced thrombolysis (from 40.2% to 30.1%, P = 0.12), but reduced fibrinogen consumption from 87% to 27% (P = 0.0001), and decreased blood loss from standardized ear incisions from 5,594 to 656 microliter (P < 0.0001). We hypothesize that alpha 2-antiplasmin limits t-PA-induced hemorrhage by inhibiting fibrinogenolysis and subsequent fragment X formation because (a) SDS-PAGE and immunoblot analysis indicate less fragment X formation in alpha 2-antiplasmin treated animals, and (b) when added to a solution of fibrinogen and plasminogen clotted with thrombin in the presence of t-PA, fragment X shortens the lysis time in a concentration-dependent fashion. These findings suggest that fragment X incorporation into hemostatic plugs contributes to t-PA-induced bleeding. By blocking t-PA-mediated fibrinogenolysis, alpha 2-antiplasmin supplementation may improve the safety of fibrin-specific plasminogen activators.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki N., Harpel P. C. Inhibitors of the fibrinolytic enzyme system. Semin Thromb Hemost. 1984 Jan;10(1):24–41. doi: 10.1055/s-2007-1004405. [DOI] [PubMed] [Google Scholar]
- CLAUSS A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. Acta Haematol. 1957 Apr;17(4):237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- Christensen U., Clemmensen I. Kinetic properties of the primary inhibitor of plasmin from human plasma. Biochem J. 1977 May 1;163(2):389–391. doi: 10.1042/bj1630389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen U., Clemmensen I. Purification and reaction mechanisms of the primary inhibitor of plasmin from human plasma. Biochem J. 1978 Nov 1;175(2):635–641. doi: 10.1042/bj1750635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C. W., Marder V. J., Martin S. E. Plasmic degradation of crosslinked fibrin. I. Structural analysis of the particulate clot and identification of new macromolecular-soluble complexes. Blood. 1980 Sep;56(3):456–464. [PubMed] [Google Scholar]
- Koehn J. A., Canfield R. E. Purification of human fibrinopeptides by high-performance liquid chromatography. Anal Biochem. 1981 Sep 15;116(2):349–356. doi: 10.1016/0003-2697(81)90370-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MCFARLANE A. S. Labelling of plasma proteins with radioactive iodine. Biochem J. 1956 Jan;62(1):135–143. doi: 10.1042/bj0620135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor I. R., Micklem L. R., James K., Pepper D. S. Characterisation of epitopes on human tissue plasminogen activator recognised by a group of monoclonal antibodies. Thromb Haemost. 1985 Feb 18;53(1):45–50. [PubMed] [Google Scholar]
- Marder V. J., Sherry S. Thrombolytic therapy: current status (1). N Engl J Med. 1988 Jun 9;318(23):1512–1520. doi: 10.1056/NEJM198806093182306. [DOI] [PubMed] [Google Scholar]
- Moroi M., Aoki N. Isolation and characterization of alpha2-plasmin inhibitor from human plasma. A novel proteinase inhibitor which inhibits activator-induced clot lysis. J Biol Chem. 1976 Oct 10;251(19):5956–5965. [PubMed] [Google Scholar]
- Mosesson M. W., Galanakis D. K., Finlayson J. S. Comparison of human plasma fibrinogen subfractions and early plasmic fibrinogen derivatives. J Biol Chem. 1974 Jul 25;249(14):4656–4664. [PubMed] [Google Scholar]
- Naito K., Aoki N. Assay of alpha2-plasmin inhibitor activity by means of a plasmin specific tripeptide substrate. Thromb Res. 1978 Jun;12(6):1147–1156. doi: 10.1016/0049-3848(78)90069-5. [DOI] [PubMed] [Google Scholar]
- Noe D. A., Bell W. R. A kinetic analysis of fibrinogenolysis during plasminogen activator therapy. Clin Pharmacol Ther. 1987 Mar;41(3):297–303. doi: 10.1038/clpt.1987.31. [DOI] [PubMed] [Google Scholar]
- Owen J., Friedman K. D., Grossman B. A., Wilkins C., Berke A. D., Powers E. R. Quantitation of fragment X formation during thrombolytic therapy with streptokinase and tissue plasminogen activator. J Clin Invest. 1987 Jun;79(6):1642–1647. doi: 10.1172/JCI113001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. K., Pratt C., Berke A., Jaffe A., Ockene I., Schreiber T. L., Bell W. R., Knatterud G., Robertson T. L., Terrin M. L. Thrombolysis in Myocardial Infarction (TIMI) Trial--phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988 Jan;11(1):1–11. doi: 10.1016/0735-1097(88)90158-1. [DOI] [PubMed] [Google Scholar]
- Shen L. L., McDonagh R. P., McDonagh J., Hermans J. Early events in the plasmin digestion of fibrinogen and fibrin. Effects of plasmin on fibrin polymerization. J Biol Chem. 1977 Sep 10;252(17):6184–6189. [PubMed] [Google Scholar]
- Sherman L. A., Mosesson M. W., Sherry S. Isolation and characterization of the clottable low molecular weight fibrinogen derived by limited plasmin hydrolysis of human fraction I-4. Biochemistry. 1969 Apr;8(4):1515–1523. doi: 10.1021/bi00832a030. [DOI] [PubMed] [Google Scholar]
- Sobel B. E., Gross R. W., Robison A. K. Thrombolysis, clot selectivity, and kinetics. Circulation. 1984 Aug;70(2):160–164. doi: 10.1161/01.cir.70.2.160. [DOI] [PubMed] [Google Scholar]
- Stump D. C., Califf R. M., Topol E. J., Sigmon K., Thornton D., Masek R., Anderson L., Collen D. Pharmacodynamics of thrombolysis with recombinant tissue-type plasminogen activator. Correlation with characteristics of and clinical outcomes in patients with acute myocardial infarction. The TAMI Study Group. Circulation. 1989 Nov;80(5):1222–1230. doi: 10.1161/01.cir.80.5.1222. [DOI] [PubMed] [Google Scholar]
- Tamaki T., Aoki N. Cross-linking of alpha 2-plasmin inhibitor to fibrin catalyzed by activated fibrin-stabilizing factor. J Biol Chem. 1982 Dec 25;257(24):14767–14772. [PubMed] [Google Scholar]
- Topol E. J., Bell W. R., Weisfeldt M. L. Coronary thrombolysis with recombinant tissue-type plasminogen activator. A hematologic and pharmacologic study. Ann Intern Med. 1985 Dec;103(6 ):837–843. doi: 10.7326/0003-4819-103-6-837. [DOI] [PubMed] [Google Scholar]
- Weitz J. I., Koehn J. A., Canfield R. E., Landman S. L., Friedman R. Development of a radioimmunoassay for the fibrinogen-derived peptide B beta 1-42. Blood. 1986 Apr;67(4):1014–1022. [PubMed] [Google Scholar]
- Weitz J. I., Leslie B., Ginsberg J. Soluble fibrin degradation products potentiate tissue plasminogen activator-induced fibrinogen proteolysis. J Clin Invest. 1991 Mar;87(3):1082–1090. doi: 10.1172/JCI115069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiman B., Collen D. Molecular mechanism of physiological fibrinolysis. Nature. 1978 Apr 6;272(5653):549–550. doi: 10.1038/272549a0. [DOI] [PubMed] [Google Scholar]
- Wiman B., Collen D. On the kinetics of the reaction between human antiplasmin and plasmin. Eur J Biochem. 1978 Mar 15;84(2):573–578. doi: 10.1111/j.1432-1033.1978.tb12200.x. [DOI] [PubMed] [Google Scholar]
- Wiman B., Collen D. On the mechanism of the reaction between human alpha 2-antiplasmin and plasmin. J Biol Chem. 1979 Sep 25;254(18):9291–9297. [PubMed] [Google Scholar]
- Wiman B., Collen D. Purification and characterization of human antiplasmin, the fast-acting plasmin inhibitor in plasma. Eur J Biochem. 1977 Aug 15;78(1):19–26. doi: 10.1111/j.1432-1033.1977.tb11709.x. [DOI] [PubMed] [Google Scholar]




