Abstract
Background
One pathological hallmark of Alzheimer's disease (AD) is amyloid plaques, composed primarily of amyloid-β peptide (Aβ). Over-production or diminished clearance of the 42 amino acid form of Aβ (Aβ42) in the brain leads to accumulation of soluble Aβ and plaque formation. Soluble oligomeric Aβ (oAβ) has recently emerged to be as a likely proximal cause of AD.
Results
Here we demonstrate that endocytosis is critical in mediating oAβ42-induced neurotoxicity and intraneuronal accumulation of Aβ. Inhibition of clathrin function either with a pharmacological inhibitor, knock-down of clathrin heavy chain expression, or expression of the dominant-negative mutant of clathrin-assembly protein AP180 did not block oAβ42-induced neurotoxicity or intraneuronal accumulation of Aβ. However, inhibition of dynamin and RhoA by expression of dominant negative mutants reduced neurotoxicity and intraneuronal Aβ accumulation. Pharmacologic inhibition of the dynamin-mediated endocytic pathway by genistein also reduced neurotoxicity.
Conclusions
These data suggest that dynamin-mediated and RhoA-regulated endocytosis are integral steps for oligomeric Aβ42-induced neurotoxicity and intraneuronal Aβ accumulation.
Background
Amyloid-β peptide (Aβ) is believed to be a causative agent underlying the pathological mechanism for Alzheimer's disease, the major form of dementia in the elderly [1]. The levels of soluble Aβ species appear to correlate with disease progression [2-12]. Evidence points to soluble oligomeric Aβ (oAβ) as the assembly form of the peptide that is likely the proximal cause in AD [13-24], leading to synaptic dysfunction and eventual neuron loss in the vulnerable regions of AD brains (for recent review [25]). Extracellular oAβ has been proposed to bind the cell surface, leading to functional disruption of NMDAR [26,27] and AMPAR [28,29], and activation of caspases [30].
In addition to extracellular Aβ, Aβ accumulates inside neurons. Intraneuronal Aβ accumulation has been identified in Down syndrome and AD patients, amyloid precursor protein (APP) and PS1 Presenilin 1 transgenic mice, and cultured cells [31-48]. In AD patients, intraneuronal Aβ42 accumulation appears in vulnerable brain regions prior to extracellular amyloid formation and accumulates with aging [31-37,39,44,45,49-52]. In addition, synaptic dysfunction occurs prior to, or in the absence of, amyloid plaques in both AD and APP transgenic mouse brains [9,53-56]. Studies using triple transgenic mice demonstrated that intraneuronal Aβ causes the onset of early AD-related cognitive deficits [43,57,58]. Intriguingly, clearance of intraneuronal Aβ by immunotherapy rescued early cognitive deficits, prior to changes in plaque density. Intraneuronal Aβ and cognitive deficits re-emerged with the subsequent withdrawal of immunotherapy [58,59]. These observations support the hypothesis that intraneuronal Aβ accumulation may be one of the initial steps in a cascade of events leading to AD [60,61]. Neurons internalize and accumulate exogenous Aβ [62-65]. Intraneuronal Aβ could be viewed as compromised clearance of extracellular soluble Aβ by neurons, and excessive accumulation of intraneuronal Aβ could lead to cellular organelle dysfunction and eventual neuron death. For example, intraneuronal Aβ was reported to activate caspase 6 leading to neuronal apoptosis [66]. We recently demonstrated that intracellular oAβ42 can activate casein kinase-2, causing inhibition of fast axonal transport [67].
Neurons, like many other cell types, have several major endocytic pathways, including clathrin-dependent, caveolae-dependent, and clathrin- and caveolar-independent pathways. However, the specific endocytic pathways involved in oAβ-uptake and neurotoxicity remain unclear. Using complementary approaches of pharmacological inhibition, genetic manipulation by over-expressing dominant-negative mutants and gene knock-down, we provide data that show that the endocytosis of oAβ42 is linked to neurotoxicity via a dynamin-dependent and RhoA-mediated endocytic pathway in vitro.
We previously established a homogenous preparation of oAβ42 [19] that causes neurotoxicity in co-cultures of primary neurons and glia, as well as Neuro-2A cells (N2A) [18,68,69]. This oAβ42 preparation also inhibits LTP [70], causes cognitive deficits [71], disrupts fast axonal transport [67], and induces neuroinflammation [72]. Here we focus mainly on endocytic pathways in relation to oAβ42 toxicity in N2A cells.
Results
Clathrin-dependent endocytic pathway is not involved in oAβ42 mediated toxicity
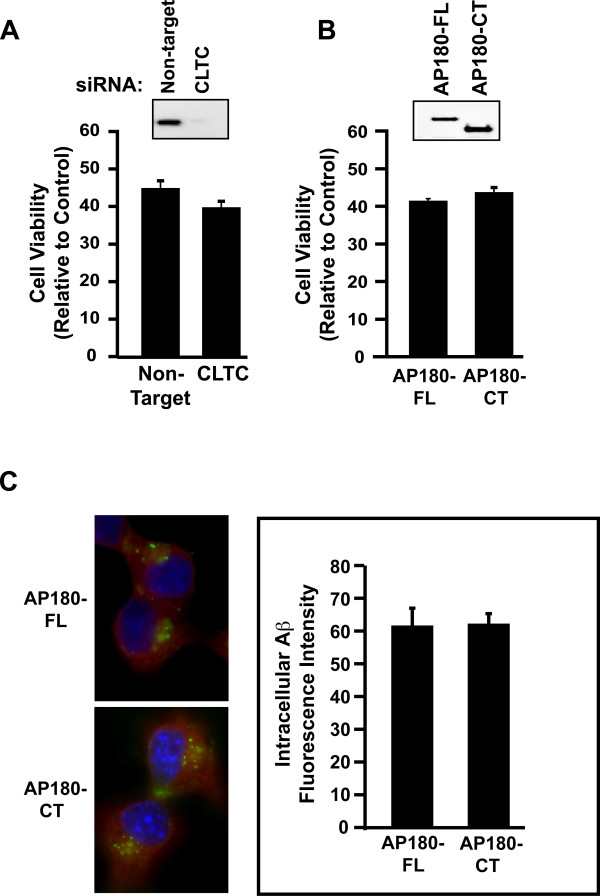
As accumulation of intracellular Aβ42 accompanies neurotoxicity, we wanted to determine whether blocking specific endocytic pathways would inhibit neurotoxicity. We used several approaches to examine clathrin-mediated endocytosis, a major endocytic pathway. First, to directly target clathrin, we transiently transfected N2A cells with siRNA specifically targeting mouse clathrin heavy chain. Western blot analysis showed that siRNA substantially reduced clathrin protein levels, in comparison to non-target siRNA control (Figure 1A inset). Further, the knock-down of clathrin inhibited transferrin uptake in these transfected cells (data not shown). However, clathrin siRNA failed to block oAβ42 toxicity, similar to the non-targeting siRNA control (Figure 1A). Second, we expressed a dominant-negative mutant of the neuron-specific clathrin-assembly protein AP180, AP180-CT. The construct contains the clathrin-binding domain at C-terminal region of AP180, and its expression is known to inhibit clathrin-mediated endocytosis [73,74]. We transiently transfected N2A cells with AP180 full-length wild type or a dominant-negative mutant AP180-CT. Transiently transfected N2A cells expressed AP180 and AP180-CT as detected by Western blot analysis (Figure 1B inset). However, the AP180-CT mutant did not inhibit cell toxicity induced by oAβ42, similar to the wild type control (Figure 1B). Furthermore, in both AP180 and AP180-CT mutant transfected cells, there were similar levels of intracellular Aβ accumulation as detected by immunofluorescence quantitation (Figure 1C). Third, chlorpromazine, a cationic amphiphilic drug that inhibits the formation of clathrin-coated pits [75], was tested. While this compound is toxic to N2A cells at high concentration, treatment with 2 mM chlorpromazine retained 90% cell viability. Again, this treatment failed to block oAβ42 neurotoxicity (data not shown). These combined results strongly suggest that under our experimental conditions, the clathrin-dependent endocytic pathway does not participate in oAβ42-induced neurotoxicity.
Figure 1.
The clathrin mediated endocytic pathway is not involved in oligomeric Aβ42-induced neurotoxicity. A. N2A cells were transiently transfected with siRNA for clathrin heavy chain, treated with 10 μM oAβ42 for 24 hours and assayed for neurotoxicity as detected with an ATP-based luminescence cell viability assay (CellTiter-Glo, Promega); no difference with treatment. Inset, clathrin heavy chain levels were characterized with clathrin heavy chain antibody (Sigma) by Western blot analysis with an equal amount of lysates from cells transfected with non-targeted siRNA and CLTC siRNA. B. N2A cells were transiently transfected with wild type AP180-FL or dominant-negative AP180-CT construct, treated with 10 μM oAβ42 for 24 hours, and assayed for neurotoxicity; no difference with treatment. Inset, AP180-CT was detected with Flag antibody (Sigma) by Western blot analysis with an equal amount of lysate from cells transfected with AP180-FL and AP180-CT. C. N2A cells were transiently transfected with AP180-FL or AP180-CT. 48 hours post-transfection, cells were treated with 10 μM oAβ42 for 30 minutes, fixed and stained for Aβ with Aβ42 specific antibody (Invitrogen, green). AP180-FL and AP180-CT mutant transfected cells were identified by anti-Flag antibody (red). Nuclei appear blue as detected by DAPI staining. Cells were individually outlined and mean fluorescence intensity of Aβ signals was quantified with NIH image software. There were similar levels of Aβ accumulation in these transfected cells.
Dynamin mediates oAβ42 neurotoxicity and intracellular accumulation
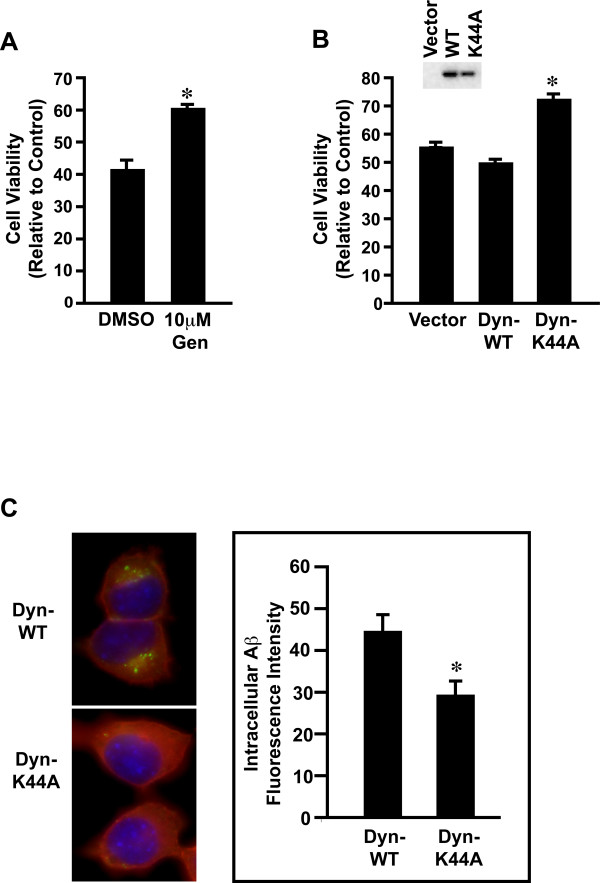
Dynamin mediates both clathrin-dependent and -independent endocytosis (for review, [76]). We first used two pharmacological inhibitors to block the dynamin-dependent endocytic pathway, genistein (a general tyrosine kinase inhibitor) and PP2 (a Src family tyrosine kinase inhibitor). As shown in Figure 2A, genistein significantly inhibited oAβ42-induced toxicity. The result was consistent with previous reports that this agent protected from Aβ induced toxicity in cultured hippocampal neurons [77] and SH-SY5Y cells [78]. PP2 pre-treatment also decreased Aβ42 toxicity though to a lesser extent than genistein (data not shown). In addition, we used a dominant-negative dynamin mutant K44A, an established reagent to specifically abolish dynamin function [79]. Both dynamin K44A mutant and wild type proteins were expressed in the transfected cells, as determined by Western blot analysis (Figure 2B inset). With oAβ42 treatment, the K44A mutant inhibited neurotoxicity compared to wild type dynamin (Figure 2B). Thus, the prediction would be that blocking dynamin mediated oAβ42 endocytosis would decrease intracellular Aβ accumulation. N2A cells were transfected with dynamin wild type or mutant K44A. Treatment of these transfected cells with oAβ42 resulted in significantly less intracellular Aβ in dynamin mutant cells compared to dynamin wild type cells (Figure 2C). These data collectively support a role for dynamin in oAβ42 endocytosis and neurotoxicity.
Figure 2.
Dynamin mediates oligomeric Aβ42-induced neurotoxicity. A. N2A cells were pre-treated ± 10 mM genistein for 1 hour, treated with 10 μM oAβ42 for 24 hours ± genistein, and assayed for neurotoxicity as described in Figure Legend 1. Significant difference (p < 0.01) between cells ± genistein is indicated by an asterisk (*). B. N2A cells were transiently transfected with dynamin wild type, dominant-negative K44A mutant expression plasmids, or vector control; treated with 10 μM oAβ42 for 24 hours and assayed for neurotoxicity. Significant difference (p < 0.01) between cells ± dominant-negative K44A mutant are indicated by an asterisk (*). Inset, expression levels of c-myc tagged dynamin were characterized by anti-myc antibody with Western blot analysis with an equal amount of lysate from cells transfected with vector, dynamin wild type, or dynamin dominant-negative K44A mutant. C. N2A cells were transiently transfected with dynamin wild type or dominant-negative K44A mutant expression plasmids. 48 hours post-transfection, cells were treated with 10 μM oAβ42 for 30 minutes, and stained for Aβ with Aβ42 specific antibody (Invitrogen, green). Transfected cells were identified by anti-myc antibody (Abcam, red). Nuclei appear blue as detected by DAPI staining. Cells were individually outlined and mean fluorescence intensity of Aβ signals were quantified with NIH image software. Significant difference in Aβ levels (p < 0.01) between cells with dynamin wild type and K44A mutant is indicated by an asterisk (*).
RhoA regulates oAβ42 neurotoxicity and intracellular accumulation
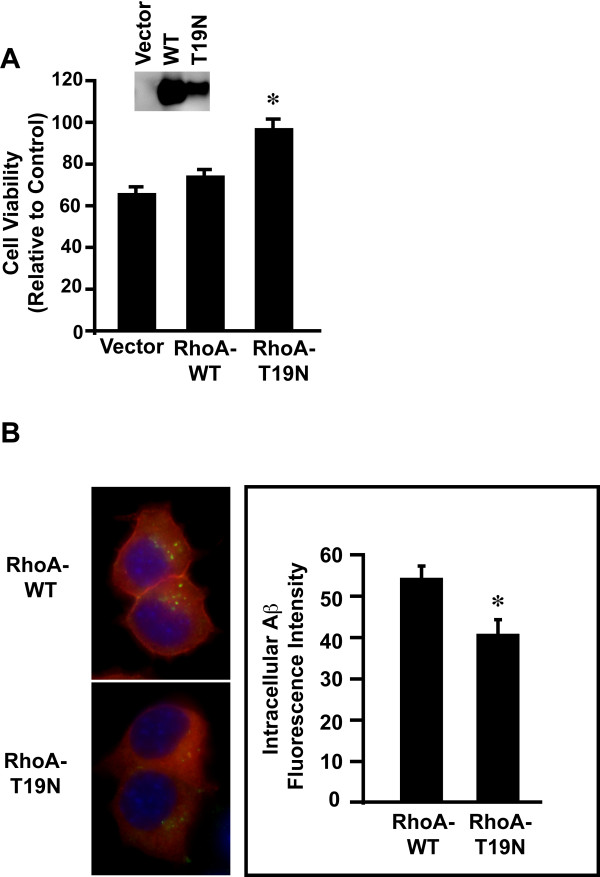
We next determined the role of RhoA in oAβ42 neurotoxicity and endocytosis. The small GTPase RhoA regulates the clathrin-independent endocytic pathways [76]. For these experiments, we transiently transfected N2A cells with vector alone, RhoA wild type or a dominant-negative RhoA mutant T19N [80]. Western blot analysis confirmed expression of RhoA and T19N proteins (Figure 3A inset). In cells treated with oAβ42, the T19N RhoA mutant significantly protected cells from oAβ42-induced neurotoxicity compare to RhoA wild type (Figure 3A). To confirm that RhoA is involved in oAβ42 endocytosis, we assessed accumulation of intracellular Aβ by treating these transfected cells oAβ42. Significantly less Aβ was detected in RhoA mutant positive cells compared to RhoA wild type cells (Figure 3B). These results suggest that RhoA is involved in oAβ42 endocytosis and neurotoxicity.
Figure 3.
RhoA mediates oligomeric Aβ42-induced neurotoxicity. A. N2A cells were transiently transfected with RhoA wild type, dominant-negative T19N mutant, or vector control; treated with oAβ42 for 24 hours; and assayed for neurotoxicity as described in Figure Legend 1. Significant difference (p < 0.01) between cells ± RhoA dominant-negative T19N mutant are indicated by an asterisk (*). Inset, expression levels of HA-taged RhoA were characterized by anti-HA antibody (Roche) with Western blot analysis with an equal amount of lysate from cells transfected vector, RhoA wild type or RhoA dominant-negative T19N mutant. B. N2A cells were transiently trasfected with RhoA wild type or dominant-negative T19N mutant expression plasmids. 48 hours post-transfection, cells were treated with 10 μM oAβ42 for 30 minutes, fixed and stained for Aβ with Aβ42 specific antibody (Invitrogen, green). RhoA wild type or T19N mutant transfected cells were identified by anti-HA antibody (red). Nuclei appear blue as detected by DAPI staining. Cells were individually outlined and mean fluorescence intensity of Aβ signals were quantified with NIH image software. Significant difference (p < 0.01) between cells transfected with RhoA wild type and T19N mutant is indicated by an asterisk (*).
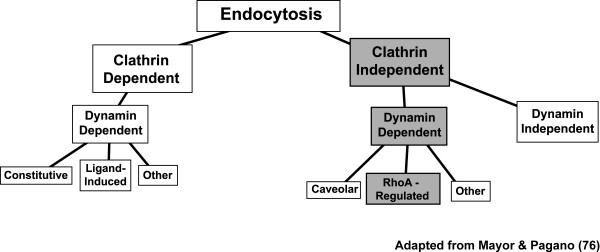
Taken together, these data strongly suggest that endocytosis is critical for oAβ42-induced neurotoxicity. This process is dependent on dynamin, but not clathrin, and further regulated by RhoA (Figure 4).
Figure 4.
Model for endocytic pathways mediating oligomeric Aβ42-induced neurotoxicity. The endocytic pathways for oAβ neurotoxicity and intracellular accumulation is clathrin independent, but dynamin dependent. The pathways are further regulated by small GTPase RhoA. This figure is adapted from Mayor and Pagano [76].
Discussion
In recent years, it has become increasingly clear that soluble oAβ plays an essential role in the neuronal loss characteristic of AD pathology. Soluble oAβ could mediate neuronal dysfunction extracellularly by binding to cell surface receptors and disturbing downstream signaling pathways, leading to disruption of LTP and LTD, and eventual neuronal death. Alternatively, soluble oAβ toxicity could arise from intraneuronal accumulation as a result of impaired exocytosis or failed clearance following endocytosis. The importance of endocytosis in AD is underscored by a recent report identifying genetic variances in phosphatidylinositol-binding clathrin assembly protein (PICALM) associated with late onset AD [81]. PICALM facilitates endocytosis in hippocampal neurons and thus could play a role in Aβ clearance in the brain [82]. However, the mechanisms underlying binding and subsequent signalling pathways or endocytosis leading to Aβ intracellular accumulation remain poorly understood.
Although a major endocytic pathway in neurons is clathrin-dependent [83], we show by three complementary approaches that inhibition of this pathway did not inhibit oAβ42 neurotoxicity (Figure 1). A reduced level of AP180 has been reported in AD patient brains [84]. Our data that AP180 did not mediate Aβ toxicity suggests that AP180 could potentially regulate trafficking of proteins/enzymes involved in Aβ production [85].
There is increasing evidence of clathrin- and caveolin-independent pathways mediating ligand-induced endocytosis [76,86]. The large GTPase dynamin is involved in both clathrin-dependent and -independent pathways [76]. Our results suggest an important role for dynamin in oAβ42-induced neurotoxicity and intraneuronal Aβ accumulation. Interestingly, clathrin-independent but dynamin-dependent endocytosis was required for Aβ internalization in sympathetic neurons in vitro [87]. The small monomeric GTPase RhoA regulates other clathrin-independent pathways, such as IL2-receptor endocytosis, [88]. Our data show RhoA regulates oAβ42 endocytosis and neurotoxicity. The role of RhoA in oAβ42-induced neurotoxicity is further supported by recent reports of potential roles for this GTPase in AD. For example, RhoA had an altered subcellular localization in both AD and APP transgenic Tg2576 mouse brains [89]. Further, RhoA levels increased specifically around amyloid plaques in these models [90].
As neurons do not express caveolin-1, the principal structural protein in caveolae, and do not have caveolae structure [91], we did not pursue this pathway. Another possible route for Aβ uptake is pinocytosis. Aβ40 directly conjugated with fluorescein was taken up by neurons via diffusion in a non-saturable, energy-independent process [92]. In our experiments, ATP levels were used as a measurement of neurotoxicity, precluding results based on energy independence. More importantly, while we have been able to consistently label oligomers with Alexa-488 after formation and maintain conformational stability, we are unable to prepare consistent oligomeric conformations using pre-labeled fluorescein-Aβ42 [65]. In addition, comparison between Aβ40 and 42 is problematic.
Our data (summarized in the schematic shown in Figure 4) show RhoA and dynamin-dependent steps involved in oAβ42 neurotoxicity and intracellular Aβ accumulation.
Conclusions
Our experiments identify the initial steps of endocytosis required for oAβ42-induced neurotoxicity and intracellular Aβ accumulation. Specifically, Aβ-induced neurotoxicity is dynamin-dependent and RhoA-regulated, but clathrin-independent. Further studies will be needed to identify potential steps in the endocytic pathways as therapeutic targets in AD.
Methods
Materials
Recombinant Aβ42 was purchased from rPeptide (Bogart, GA). Hexafluoroisopropanol (HFIP) and anhydrous dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich. Phenol-red free Ham's F12 media was obtained from Promocell (Heidelberg, Germany) and supplemented with L-glutamine (146 mg/L) prior to use. Genistein and chlorpromazine were purchased from Sigma. PP2 was purchased from EMD Biosciences.
Oligomer formation conditions
Oligomer preparations of Aβ42 were formed according to our previously established protocols [18,19]. Briefly, following evaporation of HFIP in a fume hood overnight, the resulting Aβ42 peptide film was stored desiccated at -20°C. Immediately prior to use, the films were allowed to come to room temperature, solubilized to 5 mM in anhydrous DMSO, sonicated in a bath sonicator (Branson) for 10 minutes, diluted to 100 μM in phenol-red free Hams F12, and stored at 4°C for 24 hours. Oligomeric Aβ42 morphology was routinely confirmed by atomic force microscope [19].
Cell culture and cell viability assay
Mouse neuroblastoma, N2A cells (ATCC) were maintained in MEM (ATCC) supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml of penicillin, 100 ug/ml of streptomycin, as previously described [18,69]. 5000 cells per well were plated on to 96-well plates 24 hours prior to treatment to allow attachment. Cells were then treated 10 mM oAβ42 in DMEM medium without phenol red and with 1% N2 supplement (Invitrogen). At the end of the experiment (24 hours post-treatment), cell viability was assessed by relative cellular ATP levels using CellTiter-Glo assay kit (Promega) according to the manufacture's instruction. Statistical significance was established at p < 0.01 by One-way ANOVA with Tukey test for comparison in different groups.
Pharmacological inhibition of endocytosis
N2A cells were treated with pharmacological inhibitors that block specific steps during endocytosis. Pilot experiments were performed to find inhibitor concentrations that did not significantly compromise cell viability as inhibitors for cellular endocytosis could adversely affect cell viability. For example, as has been reported, chlorpromazine at higher concentration (10-4-10-3 M) killed cells, while at lower concentration (10-6-10-5 M) inhibited Ca++-mediated toxicity in a neuroblastoma cell line [93]. Genistein substantially inhibited the growth of N2A cells in a dose-dependent manner with an IC50 value of 18 mM, and PP2 at 3 mM was lethal to the N2A cells [94]. Chlorpromazine (2 mM), genistein (10 mM), PP2 (1 mM) were added to cell cultures at indicated concentrations 1 hr before oAβ42 treatment in DMEM with 1% N2 supplement. The final concentration of vehicle (DMSO) was 0.05% in all cultures.
Genetic manipulation of selected endocytic pathway proteins
To block specific routes in the endocytic pathways, we blocked the function of key proteins in the endocytic pathway by either expressing dominant-negative proteins, or knock-down of endogenous proteins. N2A cells were transiently transfected with expression plasmids or siRNA using LipofeactAmine 2000 (Invitrogen). The following endocytic proteins were transfected for expression: Rat wild type (WT) Dynamin and the dominant-negative dynamin mutant K44A (myc-tag), the dominant-negative AP180 mutant AP180-CT (Flag-tag), and RhoA WT and dominant-negative mutant T19N (HA-tag). These plasmids were kindly provided by Dr. R. Minshall (UIC, dynamin) and Dr. L. Greene (NIH, AP180), or purchased from Missouri S&T (RhoA). To achieve highest possible transfection efficiency, we tested several transfection reagents (such as LipofectAmine and PLUS reagent, GenJet, and LipofectAmine 2000) and transfection conditions (cell density, pH of the medium and transfection incubation duration) with EGFP expression plasmid. We obtained the highest transfection efficiency with LipofectAmine 2000 at cell density of 90-100% confluence.
Small interfering RNA (siRNA) for the clathrin heavy chain (CLTC, SMARTpool L-004001-00-0005) and control Non-Targeting siRNA were purchased from Dharmacon. Cells were transfected at 20 pmol siRNA in 24-well culture plates using LipofectAmine 2000 according to vendor's recommended transfection protocol. A second transfection was done the next day. These transfected cells were then split and seeded on to 96-well plate in MEM with 10% FBS. 48 hours post-transfection, these cells were treated with oAβ42 for 24 hours.
SDS-PAGE/Western blot characterization of targeted endocytic proteins
Transfected cells treated in parallel to those used for cell viability assays were lysed by 15-minute incubation in RIPA buffer (50 mM Tris-HCl, pH 8.0, with 150 mM sodium chloride, 1.0% Igepal CA-630 (NP-40), 0.5% sodium deoxycholate, and 0.1% SDS, Sigma-Aldrich) containing protease inhibitors (Protease Inhibitor Cocktail Set I, Calbiochem), followed by centrifugation. Equal amounts of total protein were analyzed for levels of indicated proteins by Western blot analysis following SDS-PAGE using 4-12% Bis-Tris 1.5 mm NuPAGE precast gels (Invitrogen). Supernatants were mixed with LDS sample buffer (Invitrogen) and electrophoresed at 90-100 V for 80-90 minutes. Proteins were transferred to 0.2 μm polyvinylidene difluoride membranes. Membranes were blocked for 1 hour in a solution of 5% nonfat dry milk in Tris-buffered saline containing 0.0625% Tween-20 prior to incubation with primary antibody solutions. Molecular mass was estimated using pre-stained molecular weight markers (Invitrogen). CLTC was detected using a mouse anti-CLTC monoclonal antibody (C1860, Sigma; 1:1,000), myc tagged dynamin with anti-myc antibody 9E10 (Sigma; 1:5000), Flag-tagged AP180-CT with Flag antibody M2 (Sigma, 1:5000), HA tagged RhoA with rat HA antibody 3F10 (Roche Applied Science; 1:2500), and appropriate horse radish peroxidase conjugated secondary antibody. Actin, as detected with rabbit anti-actin antibody (Sigma; 1:5000), was used as total lysate loading control. Proteins were visualized with enhanced chemiluminescence Western blotting substrate (Pierce) on the Kodak 4000R imaging system.
Cellular uptake of oligomeric Aβ42
Intracellular Aβ was detected by immunofluorescence analysis using a rabbit polyclonal anti-Aβ42 specific antibody (Invitrogen). N2A cells or transiently transfected N2A cells (24 hours post transfection) were seeded at 20,000 cells/well on poly-D-lysine glass coverslips in phenol-red free DMEM + 10% FBS overnight. Recombinant oAβ42 was added to cells in DMEM medium and incubated for 30 min at 37°C. At the end of the treatment, cells were washed with PBS. Cell surface bound oAβ was striped off in a solution of 0.2 M acetic acid and 0.5 M NaCl. Cells were fixed in 4% paraformaldehyde for 20 minutes at room temperature. Cells were permeablized with 0.3% Triton X-100 in 1×PBS for 5 minutes, and blocked for 15 minutes with 3% BSA, incubated overnight with rabbit anti-Aβ42 (1:100) at 4°C, followed by 1 hour incubation at room temperature with Alexa488-labeled donkey-anti-rabbit IgG (1:500, Invitrogen). Transfected cells were identified by co-staining with anti-Flag antibody (M2, 1:250, Sigma) for AP180, rat anti-HA (3F10, Roche) for RhoA, or mouse anti-myc antibody 9E10 (1:200, Abcam) for dynamin, and appropriate 2nd antibody conjugated with Alexa594 (all from Invitrogen). Coverslips were mounted with Prolong Gold antifade reagent with DAPI (Invitrogen) fluorescence mounting medium on glass slides. Confocal laser scanning microscopy images were acquired on a Zeiss LSM 510 META, Axiovert 200 M laser scanning confocal microscope using a Plan-Apochromate Zeiss 40×/1.3 oil immersion objective. Mean brightness of Aβ signals were quantified with NIH image software.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CY designed and performed the experiments, collected and analyzed the data, and wrote the manuscript. EN and KL collected and analyzed the data. MJL contributed to experimental design and data analyses, and preparation of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Chunjiang Yu, Email: cjyu@uic.edu.
Evelyn Nwabuisi-Heath, Email: enwabu1@uic.edu.
Kevin Laxton, Email: klaxto2@uic.edu.
Mary Jo LaDu, Email: mladu@uic.edu.
Acknowledgements
We thank Lisa Jungbauer, Katie Younmans, and Jennifer Graham for technique assistance and manuscript preparation. This work was supported by grants from the Alzheimer's Association NIRG-06-26957 (CY); and ZEN-08-899000 and NIH/NIA PO1AG021184 (MJL). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Emmerling MR, Vigo-Pelfrey C, Kasunic TC, Kirkpatrick JB, Murdoch GH, Ball MJ, Roher AE. Water-soluble Abeta (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem. 1996;271(8):4077–4081. doi: 10.1074/jbc.271.8.4077. [DOI] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46(6):860–866. doi: 10.1002/1531-8249(199912)46:6<860::AID-ANA8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Roher AE, Chaney MO, Kuo YM, Webster SD, Stine WB, Haverkamp LJ, Woods AS, Cotter RJ, Tuohy JM, Krafft GA, Bonnell BS, Emmerling MR. Morphology and toxicity of Abeta-(1-42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer's disease. J Biol Chem. 1996;271(34):20631–20635. doi: 10.1074/jbc.271.34.20631. [DOI] [PubMed] [Google Scholar]
- Funato H, Enya M, Yoshimura M, Morishima-Kawashima M, Ihara Y. Presence of sodium dodecyl sulfate-stable amyloid beta-protein dimers in the hippocampus CA1 not exhibiting neurofibrillary tangle formation. Am J Pathol. 1999;155(1):23–28. doi: 10.1016/s0002-9440(10)65094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440(7082):352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. American Journal Of Pathology. 1999;155(3):853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20(11):4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. Jama. 2000;283(12):1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25(31):7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolman DL, Vitolo OV, Vonsattel JP, Shelanski ML. Dendrite and dendritic spine alterations in Alzheimer models. J Neurocytol. 2004;33(3):377–387. doi: 10.1023/B:NEUR.0000044197.83514.64. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ. The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry. 2000;39(35):10831–10839. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- Podlisny MB, Walsh DM, Amarante P, Ostaszewski BL, Stimson ER, Maggio JE, Teplow DB, Selkoe DJ. Oligomerization of endogenous and synthetic amyloid beta-protein at nanomolar levels in cell culture and stabilization of monomer by Congo red. Biochemistry. 1998;37(11):3602–3611. doi: 10.1021/bi972029u. [DOI] [PubMed] [Google Scholar]
- Harper JD, Wong SS, Lieber CM, Lansbury PT. Observation of metastable Abeta amyloid protofibrils by atomic force microscopy. Chem Biol. 1997;4(2):119–125. doi: 10.1016/S1074-5521(97)90255-6. [DOI] [PubMed] [Google Scholar]
- Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999;19(20):8876–8884. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95(11):6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren KN, Manelli AM, Stine WB Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277(35):32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- Stine WB Jr, Dahlgren KN, Krafft GK, LaDu MJ. In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. J Biol Chem. 2003;278:11612–11622. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300(5618):486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, Margol L, Wu J, Breydo L, Thompson JL, Rasool S, Gurlo T, Butler P, Glabe CG. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener. 2007;2:18. doi: 10.1186/1750-1326-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB. Amyloid beta-protein fibrillogenesis. Detection of a protofibrillar intermediate. J Biol Chem. 1997;272(35):22364–22372. doi: 10.1074/jbc.272.35.22364. [DOI] [PubMed] [Google Scholar]
- Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, Barlow E, Ebert U, Hillen H. Globular amyloid beta-peptide oligomer - a homogenous and stable neuropathological protein in Alzheimer's disease. Journal of neurochemistry. 2005;95(3):834–847. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- Ono K, Condron MM, Teplow DB. Structure-neurotoxicity relationships of amyloid beta-protein oligomers. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(35):14745–14750. doi: 10.1073/pnas.0905127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20(2):187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8(8):1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Chang EH, Savage MJ, Flood DG, Thomas JM, Levy RB, Mahadomrongkul V, Shirao T, Aoki C, Huerta PT. AMPA receptor downscaling at the onset of Alzheimer's disease pathology in double knockin mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(9):3410–3415. doi: 10.1073/pnas.0507313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52(5):831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guilloz Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: Linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci USA. 2003. [DOI] [PMC free article] [PubMed]
- D'Andrea MR, Reiser PA, Polkovitch DA, Gumula NA, Branchide B, Hertzog BM, Schmidheiser D, Belkowski S, Gastard MC, Andrade-Gordon P. The use of formic acid to embellish amyloid plaque detection in Alzheimer's disease tissues misguides key observations. Neurosci Lett. 2003;342(1-2):114–118. doi: 10.1016/S0304-3940(03)00252-0. [DOI] [PubMed] [Google Scholar]
- D'Andrea MR, Nagele RG, Wang HY, Lee DH. Consistent immunohistochemical detection of intracellular beta-amyloid42 in pyramidal neurons of Alzheimer's disease entorhinal cortex. Neurosci Lett. 2002;333(3):163–166. doi: 10.1016/S0304-3940(02)00875-3. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, George L, Tung YC, Kim KS, Wisniewski HM. Amyloid protein and neurofibrillary tangles coexist in the same neuron in Alzheimer disease. Proc Natl Acad Sci USA. 1989;86(8):2853–2857. doi: 10.1073/pnas.86.8.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM, Troncoso JC, Strickland DK, Kawas CH, Jay G. Neuronal cell death in Alzheimer's disease correlates with apoE uptake and intracellular Abeta stabilization. J Clin Invest. 1997;100(2):310–320. doi: 10.1172/JCI119536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 2002;161(5):1869–1879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori C, Spooner ET, Wisniewsk KE, Wisniewski TM, Yamaguch H, Saido TC, Tolan DR, Selkoe DJ, Lemere CA. Intraneuronal Abeta42 accumulation in Down syndrome brain. Amyloid. 2002;9(2):88–102. [PubMed] [Google Scholar]
- Gyure KA, Durham R, Stewart WF, Smialek JE, Troncoso JC. Intraneuronal abeta-amyloid precedes development of amyloid plaques in Down syndrome. Arch Pathol Lab Med. 2001;125(4):489–492. doi: 10.5858/2001-125-0489-IAAPDO. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Pelsman A, Wong C, Pigino G, Yuan M, Mori H, Yankner BA. Altered metabolism of the amyloid beta precursor protein is associated with mitochondrial dysfunction in Down's syndrome. Neuron. 2002;33(5):677–688. doi: 10.1016/S0896-6273(02)00604-9. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Abeta42 accumulation in human brain. Am J Pathol. 2000;156(1):15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langui D, Girardot N, El Hachimi KH, Allinquant B, Blanchard V, Pradier L, Duyckaerts C. Subcellular topography of neuronal Abeta peptide in APPxPS1 transgenic mice. Am J Pathol. 2004;165(5):1465–1477. doi: 10.1016/s0002-9440(10)63405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DH. Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer's disease. Histopathology. 2001;38(2):120–134. doi: 10.1046/j.1365-2559.2001.01082.x. [DOI] [PubMed] [Google Scholar]
- Wirths O, Multhaup G, Czech C, Blanchard V, Moussaoui S, Tremp G, Pradier L, Beyreuther K, Bayer TA. Intraneuronal Abeta accumulation precedes plaque formation in beta-amyloid precursor protein and presenilin-1 double-transgenic mice. Neurosci Lett. 2001;306(1-2):116–120. doi: 10.1016/S0304-3940(01)01876-6. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/S0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Shie FS, LeBoeuf RC, Jin LW. Early intraneuronal Abeta deposition in the hippocampus of APP transgenic mice. Neuroreport. 2003;14(1):123–129. doi: 10.1097/00001756-200301200-00023. [DOI] [PubMed] [Google Scholar]
- Takahashi RH, Almeida CG, Kearney PF, Yu F, Lin MT, Milner TA, Gouras GK. Oligomerization of Alzheimer's beta-amyloid within processes and synapses of cultured neurons and brain. J Neurosci. 2004;24(14):3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovronsky DM, Doms RW, Lee VM. Detection of a novel intraneuronal pool of insoluble amyloid beta protein that accumulates with time in culture. J Cell Biol. 1998;141(4):1031–1039. doi: 10.1083/jcb.141.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur-Kolecka B, Frackowiak J, Carroll RT, Wisniewski HM. Accumulation of Alzheimer amyloid-beta peptide in cultured myocytes is enhanced by serum and reduced by cerebrospinal fluid. J Neuropathol Exp Neurol. 1997;56(3):263–272. doi: 10.1097/00005072-199703000-00005. [DOI] [PubMed] [Google Scholar]
- Casas C, Sergeant N, Itier JM, Blanchard V, Wirths O, Kolk N van der, Vingtdeux V, Steeg E van de, Ret G, Canton T, Drobecq H, Clark A, Bonici B, Delacourte A, Benavides J, Schmitz C, Tremp G, Bayer TA, Benoit P, Pradier L. Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Abeta42 accumulation in a novel Alzheimer transgenic model. Am J Pathol. 2004;165(4):1289–1300. doi: 10.1016/s0002-9440(10)63388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki A, Tamaoka A, Shimohata A, Komatsuzaki Y, Shoji S. Abeta42-positive non-pyramidal neurons around amyloid plaques in Alzheimer's disease. Lancet. 2000;355(9197):42–43. doi: 10.1016/S0140-6736(99)04937-5. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Tran L, Lambert MP, Glabe CG, Klein WL, Laferla FM. Temporal profile of Abeta oligomerization in an in vivo model of Alzheimer's disease: A link between Abeta and tau pathology. J Biol Chem. 2005. [DOI] [PubMed]
- Kawarabayashi T, Shoji M, Younkin LH, Wen-Lang L, Dickson DW, Murakami T, Matsubara E, Abe K, Ashe KH, Younkin SG. Dimeric amyloid beta protein rapidly accumulates in lipid rafts followed by apolipoprotein E and phosphorylated tau accumulation in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2004;24(15):3801–3809. doi: 10.1523/JNEUROSCI.5543-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima N, Morishima-Kawashima M, Yamaguchi H, Yoshimura M, Sugihara S, Khan K, Games D, Schenk D, Ihara Y. Accumulation of amyloid beta-protein in the low-density membrane domain accurately reflects the extent of beta-amyloid deposition in the brain. Am J Pathol. 2001;158(6):2209–2218. doi: 10.1016/s0002-9440(10)64693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Haute CV, Checler F, Godaux E, Cordell B, Van Leuven F. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem. 1999;274(10):6483–6492. doi: 10.1074/jbc.274.10.6483. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci USA. 1999;96(6):3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TVP, Hyman BT, Younkin SG, Hsiao KK. Impaired synpatic plasticity and learning in aged amyloid precursor protein transgenic mice. Nature Genet. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Zerbinatti CV, Wahrle SE, Kim H, Cam JA, Bales K, Paul SM, Holtzman DM, Bu G. Apolipoprotein E and low density lipoprotein receptor-related protein facilitate intraneuronal Abeta42 accumulation in amyloid model mice. The Journal of biological chemistry. 2006;281(47):36180–36186. doi: 10.1074/jbc.M604436200. [DOI] [PubMed] [Google Scholar]
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43(3):321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, Laferla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45(5):675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort L, Blazquez G, Canete T, Johansson B, Oddo S, Tobena A, Laferla FM, Fernandez-Teruel A. Modeling behavioral and neuronal symptoms of Alzheimer's disease in mice: A role for intraneuronal amyloid. Neurosci Biobehav Rev. 2006;31(1):125–147. doi: 10.1016/j.neubiorev.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Shie FS, LeBoeuf RC, Jin LW. Early intraneuronal Abeta deposition in the hippocampus of APP transgenic mice.[erratum appears in Neuroreport. 2004 Aug 26;15(12):1993 Note: LeBoeur, Renee C [corrected to LeBoeuf, Renee C]] Neuroreport. 2003;14(1):123–129. doi: 10.1097/00001756-200301200-00023. [DOI] [PubMed] [Google Scholar]
- Glabe C. Intracellular mechanisms of amyloid accumulation and pathogenesis in Alzheimer's disease. J Mol Neurosci. 2001;17(2):137–145. doi: 10.1385/JMN:17:2:137. [DOI] [PubMed] [Google Scholar]
- Knauer MF, Soreghan B, Burdick D, Kosmoski J, Glabe CG. Intracellular accumulation and resistance to degradation of the Alzheimer amyloid A4/beta protein. Proc Natl Acad Sci USA. 1992;89(16):7437–7441. doi: 10.1073/pnas.89.16.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick D, Kosmoski J, Knauer MF, Glabe CG. Preferential adsorption, internalization and resistance to degradation of the major isoform of the Alzheimer's amyloid peptide, A beta 1-42, in differentiated PC12 cells. Brain Res. 1997;746(1-2):275–284. doi: 10.1016/S0006-8993(96)01262-0. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Hoffman KB, Yang AJ, Hess US, Glabe CG, Lynch G. Amyloid beta protein is internalized selectively by hippocampal field CA1 and causes neurons to accumulate amyloidogenic carboxyterminal fragments of the amyloid precursor protein. J Comp Neurol. 1998;397(1):139–147. doi: 10.1002/(SICI)1096-9861(19980720)397:1<139::AID-CNE10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Jungbauer LM, Yu C, Laxton KJ, LaDu MJ. Preparation of fluorescently-labeled amyloid-beta peptide assemblies: the effect of fluorophore conjugation on structure and function. J Mol Recognit. 2009;22(5):403–413. doi: 10.1002/jmr.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McLaughlin R, Goodyer C, LeBlanc A. Selective cytotoxicity of intracellular amyloid beta peptide1-42 through p53 and Bax in cultured primary human neurons. J Cell Biol. 2002;156(3):519–529. doi: 10.1083/jcb.200110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Atagi Y, Deshpande A, Yu C, Jungbauer L, LaDu M, Busciglio J, Brady S. Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid beta. Proc Natl Acad Sci USA. 2009;106(14):5907–5912. doi: 10.1073/pnas.0901229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manelli AM, Bulfinch LC, Sullivan PM, LaDu MJ. Abeta42 neurotoxicity in primary co-cultures: effect of apoE isoform and Abeta conformation. Neurobiol Aging. 2007;28(8):1139–1147. doi: 10.1016/j.neurobiolaging.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manelli AM, Stine WB, Van Eldik LJ, LaDu MJ. ApoE and Abeta1-42 interactions: effects of isoform and conformation on structure and function. J Mol Neurosci. 2004;23(3):235–246. doi: 10.1385/JMN:23:3:235. [DOI] [PubMed] [Google Scholar]
- Trommer BL, Shah C, Yun SH, Gamkrelidze G, Pasternak ES, Stine WB, Manelli A, Sullivan P, Pasternak JF, LaDu MJ. ApoE isoform-specific effects on LTP: blockade by oligomeric amyloid-beta1-42. Neurobiology of disease. 2005;18(1):75–82. doi: 10.1016/j.nbd.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Reed MN, Hofmeister JJ, Jungbauer L, Welzel AT, Yu C, Sherman MA, Lesne S, Ladu MJ, Walsh DM, Ashe KH, Cleary JP. Cognitive effects of cell-derived and synthetically derived Abeta oligomers. Neurobiology of aging. 2009. [DOI] [PMC free article] [PubMed]
- White JA, Manelli AM, Holmberg KH, Van Eldik LJ, LaDu MJ. Differential effects of oligomeric and fibrillar amyloid-beta1-42 on astrocyte-mediated inflammation. Neurobiol Dis. 2005;18(3):459–465. doi: 10.1016/j.nbd.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Zhao X, Greener T, Al-Hasani H, Cushman SW, Eisenberg E, Greene LE. Expression of auxilin or AP180 inhibits endocytosis by mislocalizing clathrin: evidence for formation of nascent pits containing AP1 or AP2 but not clathrin. J Cell Sci. 2001;114(Pt 2):353–365. doi: 10.1242/jcs.114.2.353. [DOI] [PubMed] [Google Scholar]
- Ford MG, Pearse BM, Higgins MK, Vallis Y, Owen DJ, Gibson A, Hopkins CR, Evans PR, McMahon HT. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science (New York, NY) 2001;291(5506):1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123(5):1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8(8):603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- Zeng H, Chen Q, Zhao B. Genistein ameliorates beta-amyloid peptide (25-35)-induced hippocampal neuronal apoptosis. Free Radic Biol Med. 2004;36(2):180–188. doi: 10.1016/j.freeradbiomed.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Bang OY, Hong HS, Kim DH, Kim H, Boo JH, Huh K, Mook-Jung I. Neuroprotective effect of genistein against beta amyloid-induced neurotoxicity. Neurobiol Dis. 2004;16(1):21–28. doi: 10.1016/j.nbd.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127(4):915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZK, Ye RD, Christiansen SC, Jagels MA, Bokoch GM, Zuraw BL. Role of the Rho GTPase in bradykinin-stimulated nuclear factor-kappaB activation and IL-1beta gene expression in cultured human epithelial cells. Journal of immunology (Baltimore, Md) 1998;160(6):3038–3045. [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41(10):1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushlin I, Petralia RS, Wu F, Harel A, Mughal MR, Mattson MP, Yao PJ. Clathrin assembly protein AP180 and CALM differentially control axogenesis and dendrite outgrowth in embryonic hippocampal neurons. J Neurosci. 2008;28(41):10257–10271. doi: 10.1523/JNEUROSCI.2471-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SA, Malerod L, Berg T, Kjeken R. Clathrin-dependent endocytosis. Biochem J. 2004;377(Pt 1):1–16. doi: 10.1042/BJ20031000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PJ, Morsch R, Callahan LM, Coleman PD. Changes in synaptic expression of clathrin assembly protein AP180 in Alzheimer's disease analysed by immunohistochemistry. Neuroscience. 1999;94(2):389–394. doi: 10.1016/S0306-4522(99)00360-7. [DOI] [PubMed] [Google Scholar]
- Small SA, Gandy S. Sorting through the cell biology of Alzheimer's disease: intracellular pathways to pathogenesis. Neuron. 2006;52(1):15–31. doi: 10.1016/j.neuron.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham M, Parton RG. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta. 2005;1745(3):273–286. doi: 10.1016/j.bbamcr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Saavedra L, Mohamed A, Ma V, Kar S, de Chaves EP. Internalization of beta-amyloid peptide by primary neurons in the absence of apolipoprotein E. The Journal of biological chemistry. 2007;282(49):35722–35732. doi: 10.1074/jbc.M701823200. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell. 2001;7(3):661–671. doi: 10.1016/S1097-2765(01)00212-X. [DOI] [PubMed] [Google Scholar]
- Huesa G, Baltrons MA, Gomez-Ramos P, Moran A, Garcia A, Hidalgo J, Frances S, Santpere G, Ferrer I, Galea E. Altered Distribution of RhoA in Alzheimer's Disease and AbetaPP Overexpressing Mice. J Alzheimers Dis. 2009;19(1):37–56. doi: 10.3233/JAD-2010-1203. [DOI] [PubMed] [Google Scholar]
- Petratos S, Li QX, George AJ, Hou X, Kerr ML, Unabia SE, Hatzinisiriou I, Maksel D, Aguilar MI, Small DH. The beta-amyloid protein of Alzheimer's disease increases neuronal CRMP-2 phosphorylation by a Rho-GTP mechanism. Brain. 2008;131(Pt 1):90–108. doi: 10.1093/brain/awm260. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Heuser JE, Harris DA. A glycolipid-anchored prion protein is endocytosed via clathrin-coated pits. J Cell Biol. 1994;125(6):1239–1250. doi: 10.1083/jcb.125.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandimalla KK, Scott OG, Fulzele S, Davidson MW, Poduslo JF. Mechanism of neuronal versus endothelial cell uptake of Alzheimer's disease amyloid beta protein. PLoS One. 2009;4(2):e4627. doi: 10.1371/journal.pone.0004627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe K, Sekizawa T, Kogure K. Biphasic effects of chlorpromazine on cell viability in a neuroblastoma cell line. Neurosci Lett. 1986;71(3):335–339. doi: 10.1016/0304-3940(86)90643-9. [DOI] [PubMed] [Google Scholar]
- Gyllberg H, Lofgren K, Lindegren H, Bedecs K. Increased Src kinase level results in increased protein tyrosine phosphorylation in scrapie-infected neuronal cell lines. FEBS Lett. 2006;580(11):2603–2608. doi: 10.1016/j.febslet.2006.03.092. [DOI] [PubMed] [Google Scholar]