Abstract
The components of receptor tyrosine kinase signaling complexes help to define the specificity of the effects of their activation. The Caenorhabditis elegans fibroblast growth factor receptor (FGFR), EGL-15, regulates a number of processes, including sex myoblast (SM) migration guidance and fluid homeostasis, both of which require a Grb2/Sos/Ras cassette of signaling components. Here we show that SEM-5/Grb2 can bind directly to EGL-15 to mediate SM chemoattraction. A yeast two-hybrid screen identified SEM-5 as able to interact with the carboxy-terminal domain (CTD) of EGL-15, a domain that is specifically required for SM chemoattraction. This interaction requires the SEM-5 SH2-binding motifs present in the CTD (Y1009 and Y1087), and these sites are required for the CTD role of EGL-15 in SM chemoattraction. SEM-5, but not the SEM-5 binding sites located in the CTD, is required for the fluid homeostasis function of EGL-15, indicating that SEM-5 can link to EGL-15 through an alternative mechanism. The multi-substrate adaptor protein FRS2 serves to link vertebrate FGFRs to Grb2. In C. elegans, an FRS2-like gene, rog-1, functions upstream of a Ras/MAPK pathway for oocyte maturation but is not required for EGL-15 function. Thus, unlike the vertebrate FGFRs, which require the multi-substrate adaptor FRS2 to recruit Grb2, EGL-15 can recruit SEM-5/Grb2 directly.
FIBROBLAST growth factors (FGFs) play important roles in many developmental and physiological processes, including cell migration, angiogenesis, proliferation, differentiation, and survival (Ornitz and Itoh 2001; Polanska et al. 2009). Mammals have a battery of both FGF ligands and high-affinity receptors to carry out this diverse set of important functions. These ligands and their receptors are generated from a set of 18 genes encoding FGFs and 4 genes encoding their receptors (Eswarakumar et al. 2005). Upon ligand binding, fibroblast growth factor receptors (FGFRs) dimerize, activating their intrinsic tyrosine kinase activity, which causes both autophosphorylation on intracellular tyrosine residues and phosphorylation of additional substrates (Eswarakumar et al. 2005). These phosphorylation events lead to the assembly of a signaling complex around the activated receptor, ultimately promoting various downstream signaling pathways (Eswarakumar et al. 2005).
A large portion of mammalian FGFR signaling is mediated by the multi-substrate adaptor protein FRS2/snt-1 (Kouhara et al. 1997; Hadari et al. 1998, 2001; Lax et al. 2002; Gotoh et al. 2005). FRS2 constitutively associates with the juxtamembrane region of the FGFR via its amino-terminal PTB domain (Xu et al. 1998; Ong et al. 2000). Upon FGFR activation, FRS2 becomes heavily phosphorylated, allowing it to recruit Grb2 and Shp2 via their SH2 domains (Kouhara et al. 1997; Eswarakumar et al. 2005). Since these components cannot associate with the receptor in the absence of FRS2 (Hadari et al. 2001), FRS2 serves as an essential link between the activated receptor and many downstream signal transduction pathways.
The understanding of FGF-stimulated signal transduction pathways has been aided by the study of FGF signaling in model organisms. Powerful genetic screens and the reduced complexity of the set of FGFs and their receptors in both Drosophila melanogaster and Caenorhabditis elegans have helped promote an understanding of the conserved aspects of FGF signaling pathways (Huang and Stern 2005; Polanska et al. 2009). In C. elegans, FGF signaling is mediated by two FGF ligands, EGL-17 and LET-756, and a single FGF receptor, EGL-15 (DeVore et al. 1995; Burdine et al. 1997; Roubin et al. 1999). The EGL-15 FGFR is structurally very similar to mammalian FGF receptors, with the highest level of sequence conservation found within the intracellular tyrosine kinase domain and the three extracellular immunoglobulin (IG) domains.
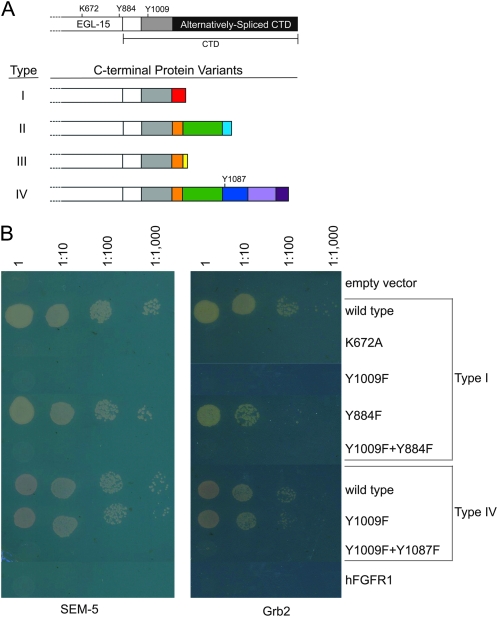
Similar to mammalian FGFRs, alternative splicing also generates functionally distinct EGL-15 isoforms. A major structural difference between EGL-15 and other FGFRs lies in an additional domain located between the first IG domain and the acid box of EGL-15. This EGL-15-specific insert is encoded by a pair of mutually exclusive fifth exons, generating two EGL-15 isoforms, 5A and 5B, with different functions (Goodman et al. 2003). Alternative splicing also affects the sequence at the very end of the carboxy-terminal domain (CTD) of EGL-15 (Goodman et al. 2003), giving rise to four distinct C-terminal isoform types, referred to as types I–IV (see Figure 1A).
Figure 1.—
The SEM-5∷EGL-15 interaction is dependent upon the SEM-5 SH2 binding sites in the CTD of EGL-15. (A) Important residues in the EGL-15 CTD isoforms. The C-terminal portion of the kinase domain and the CTD are shown. The gray box is common to all C-terminal isoforms. Sequences of the CTD isoforms can be found in Figure S5. (B) SEM-5 binds directly to EGL-15 Y1009 and Y1087. A full-length SEM-5 prey was mated to eight EGL-15 baits: empty vector control (pBTM116) and derivatives containing variants of either the type I or the type IV EGL-15(Intra). Growth is an indication of an interaction between SEM-5 and the EGL-15 bait. Dilutions of the culture mixture are indicated above. hFGFR1, a bait containing the corresponding portion of the intracellular domain of the human FGFR1. Similar results were obtained using the human SEM-5 ortholog, Grb2.
EGL-15, like its mammalian orthologs, is also involved in a large variety of functions, including cell migration guidance affecting the sex myoblast (SM) (Stern and Horvitz 1991; DeVore et al. 1995; Goodman et al. 2003) and CAN cells (Fleming et al. 2005), muscle arm extension (Dixon et al. 2006), a number of processes controlling terminal axon morphology (Bulow et al. 2004), muscle protein degradation (Szewczyk and Jacobson 2003), and fluid homeostasis (Huang and Stern 2004). A conserved FGFR signaling pathway in C. elegans was established by identifying the genes necessary for the role of EGL-15 in fluid homeostasis, and much of this same pathway is utilized in other functions of EGL-15 (DeVore et al. 1995; Borland et al. 2001; Huang and Stern 2004, 2005). The identification of these genes was facilitated by a temperature-sensitive mutation affecting a receptor tyrosine phosphatase, CLR-1 (CLeaR), which functions to negatively regulate EGL-15 signaling (Kokel et al. 1998). Mutations in clr-1 abolish this regulatory constraint on EGL-15, resulting in fluid accumulation in the pseudocoelomic cavity due to hyperactive EGL-15 signaling. The buildup of this clear fluid, resulting in the Clr phenotype, is easily scored and can be used to identify suppressors (soc, suppressor of Clr) that reduce EGL-15 signaling efficiency. These suppressors define an EGL-15 signaling pathway necessary for fluid homeostasis, which involves the activation of the Ras/MAPK cascade via the SEM-5/Grb2 adaptor protein, the let-341/sos-1 Sos-like guanine nucleotide exchange factor, and a PTP-2-SOC-1/Shp2-Gab1 cassette (Borland et al. 2001).
Mutations in egl-15 have been identified on the basis of their effects on either fluid homeostasis or the guidance of the migrating SMs (DeVore et al. 1995; Goodman et al. 2003). Most of these alleles fall into an allelic series and affect the general aspects of EGL-15 signaling (Goodman et al. 2003). The strongest of these alleles confers an early larval arrest phenotype, whereas weaker alleles confer either a scrawny body morphology (Scr) or just the ability to suppress the Clr phenotype (Soc). While the weakest of these alleles does not affect egg laying, more highly compromised mutants show an egg-laying defect due to the mispositioning of the SMs. Four egl-15 alleles specifically affect SM migration; when homozygous, these mutations cause dramatic mispositioning of the SMs, but do not cause a Soc phenotype (Goodman et al. 2003). Three of these are nonsense mutations in exon 5A and eliminate the 5A EGL-15 isoform. The phenotype of these mutants highlights the specific requirement of the 5A isoform for SM migration guidance. The fourth mutation in this class, egl-15(n1457), is a nonsense mutation that truncates the carboxy-terminal domain, specifically implicating the CTD in SM migration guidance.
Immediately following their birth at the end of the first larval stage (L1), the two bilaterally symmetric SMs undergo anteriorly directed migrations to final positions that flank the precise center of the gonad (Sulston and Horvitz 1977). In the middle of the third larval stage, the SMs divide to generate 16 cells that differentiate into the egg-laying muscles. Multiple mechanisms help guide the migrations of the SMs (Chen and Stern 1998; Branda and Stern 2000), including a chemoattraction mediated by EGL-15 that guides the SMs to their precise final positions (Burdine et al. 1998). The EGL-17 FGF serves as the chemoattractive cue, emanating from central gonadal cells (Branda and Stern 2000). In the absence of this chemoattraction, SMs are posteriorly displaced (Stern and Horvitz 1991). While mispositioned SMs still generate sex muscles, these muscles end up too far posterior to attach properly, causing the animal to be defective in egg laying (Egl).
The signal transduction pathway downstream of EGL-15 that mediates SM chemoattraction is not well established. Several lines of evidence implicate SEM-5/Grb2, LET-341/Sos, and LET-60/Ras in SM chemoattraction. The roles of components in the Ras-MAPK cascade in this event are less clear (Sundaram et al. 1996; Chen et al. 1997; Chen and Stern 1998). A crucial gap in our understanding lies in the link between activated EGL-15 and the downstream signaling components. Here we show that SEM-5, the C. elegans GRB2 ortholog, appears to bind directly to SH2 binding sites within the carboxy terminal tail of EGL-15. These interactions are required for SM chemoattraction, but not for the essential function of EGL-15.
MATERIALS AND METHODS
Genetic manipulations:
All strains were derived from C. elegans var. Bristol, strain N2, using standard genetic protocols (Brenner 1974) and standard genetic manipulations (Herman 1988). Nematode strains were grown and maintained on NGM agar plates and raised at 20° unless otherwise indicated. Transgenic assays were conducted as previously described for the genomic and cassette assays (Lo et al. 2008) and for the deletion and truncation assays (Goodman et al. 2003), using egl-15(n1456) as the null allele. The tm1031 deletion in rog-1 was isolated by the knockout consortium at the National Bioresource Project for C. elegans (Tokyo). This allele deletes a critical portion of the PTB domain (supporting information, Figure S1), thereby destroying the sole structure on which the putative interaction with EGL-15 is based. tm1031 mutants were maintained as balanced heterozygotes using the semidominant sup-9(n1550sd) allele, which lies 3 map units from rog-1 on chromosome II (Levin and Horvitz 1993). sup-9(n1550sd) causes heterozygotes to display a characteristic suite of phenotypes, including Lon (Long), Unc (Uncoordinated), Rbr (Rubberband), and Egl (Egg-laying defective); n1550 homozygotes are inviable. Mutants were identified as non-Rbr animals, and recombinants were identified by non-Rbr non-Unc animals that had broods of wild-type size. PCR identification of tm1031 mutants was by duplex PCR, which can differentiate wild-type, mutant, and heterozygote animals by the presence or absence of both alleles. RNA interference (RNAi) was carried out according to standard protocols (Fire et al. 1998).
Determination and representation of sex myoblast position:
SM final positions were determined with respect to the underlying hypodermal Pn.p cells (Pn.p, the posterior daughters of cells P1–P11) as previously described (Thomas et al. 1990) and are depicted using box-and-whisker plots (Moore and McCabe 1993) aligned to a schematic representation of the Pn.p cell positions (see Figure 2, Figure 3, and Figure S4). In brief (Goodman et al. 2003), each set of SMs is ordered according to anteroposterior position and divided into quartiles. The “box” includes the positions of SMs within the two central quartiles. An additional vertical line within the box indicates the median SM position at the boundary between the second and third quartiles. Overlap of the line representing the median position with a right or left border of the box is depicted as a thickening of that line. A quartile length (1Q) is determined on the basis of the range of positions covered by the 2Q box length. Bars (“whiskers”) of up to 1.5Q length extend from the edges of the box to additional data points. As whisker length does not extend beyond the range of data points, these bars may be shorter than a 1.5Q length or even absent. Data points beyond the edge of the bars (“outliers”) are indicated by individual hatch marks. This representation of SMs therefore depicts the overall range of SMs as well as their general distribution and median position. To score SMs of tm1031 sterile mutant homozygotes, a large population of tm1031/n1550 heterozygotes was grown to adulthood and then cloned to individual NGM agar plates. The mixed progeny of these heterozygotes were grown to mid-L3 stage at 20° and individual non-Rbr animals were numbered and mounted, and their SMs were scored using Nomarski optics. To eliminate nonmutant recombinant progeny, these individually scored animals were then recovered to PCR tubes and individually genotyped for the tm1031 deletion via duplex PCR, and recombinants were excluded from the data set.
Figure 2.—
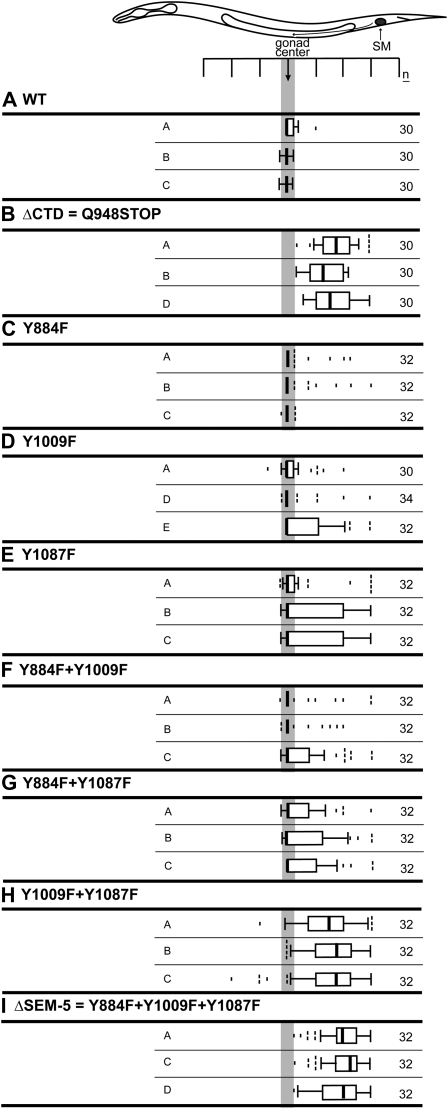
Genomic assay results of the EGL-15 CTD role in SM chemoattraction. EGL-15 Y1009 and Y1087 are functionally redundant. Sex myoblast distributions for three lines for each construct are shown. The indicated transgenes were all tested in an egl-15(null) background. (A) Wild-type EGL-15 (NH#112). (B) A truncated version of EGL-15 that correlates to the egl-15(n1457) mutant (NH#838). (C) The single Y884F mutation fails to disrupt sex myoblast migration (NH#1379). (D and E) The single Y1009F (NH#818) or Y1087F (NH#1382) mutations do not account for the posterior SM positions of ΔCTD. (F and G) Double mutations with Y884 (Y884F/Y1009F, NH#841; Y884F/Y1087F, NH#1380) are not more affected than the respective single mutations. (H and I) The Y1009F mutation in conjunction with either the Y1087F mutation (NH#1370) or with both Y1087F and Y884F (NH#1356) (ΔSEM-5) abolishes SM chemoattraction. n, number of sex myoblasts scored.
Figure 3.—
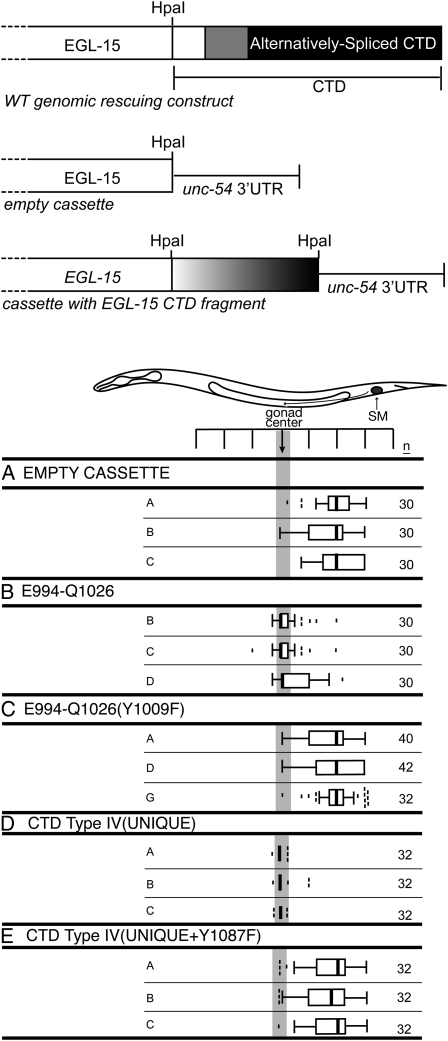
The cassette assay (“sufficiency”) results. (Top) Schematic of the cassette constructs in comparison with the wild-type genomic rescuing construct. (Bottom) Cassette constructs with the indicated CTD fragments were all tested in an egl-15(null) background. (A) Empty cassette (NH#1321, negative control). (B) E994-Q1026 (the gray fragment in Figure 1A) is sufficient for mediating sex myoblast migration (NH#1322). (C) Y1009 is required within the gray (E994-Q1026) fragment to mediate sex myoblast migration (NH#1325). (D and E) Sex myoblast distribution for the wild-type (D) unique portion of the type IV isoform (NH#1348) or the same unique portion with the Y1087F mutation (E; NH#1349).
Plasmid construction:
All plasmids constructed using PCR were confirmed by sequencing. Details of plasmid construction are available upon request. NH#112 is the wild-type egl-15 genomic rescuing fragment (DeVore et al. 1995). NH#838 egl-15(Q948STOP) was generated by introducing the nonsense mutation in the EGL-15 CTD that corresponds to the egl-15(n1457) mutation, thereby truncating the CTD from all EGL-15 isoforms expressed from this construct. The genomic constructs used for the experiments the results of which are summarized in Figure 2 are derivatives of NH#112. NH#934, the EGL-15(Intra I) yeast two-hybrid bait, was created by PCR amplification of the intracellular domain of EGL-15 from the EGL-15 type I cDNA (NH#324). The resulting PCR product was cloned into the HpaI sites of the yeast two-hybrid “bait” vector pBTM116. The following constructs are all derivatives of NH#934: NH#726, egl-15(K672A); NH#1285, egl-15(Y1009F); NH#1286, egl-15(Y884F); and NH#1287, egl-15(Y1009F, Y884F). NH#1354, the EGL-15(Intra IV) yeast two-hybrid bait, was created by two-round PCR amplification, using NH#934 for the common upstream portion of EGL-15(Intra IV) and the EGL-15 type IV cDNA for the unique downstream portion of EGL-15(Intra IV). The resulting PCR product was cloned into the PstI site of the yeast two-hybrid “bait” vector pBTM116. The following constructs are derivatives of NH#1354: NH#1355, egl-15(Intra IV, Y1009F); NH#1360, egl-15(Intra IV, Y1009F, Y1087F). NH#1321, the backbone vector for our cassette assays, was generated from the NH#112 genomic rescuing construct as follows. The C-terminal domain of EGL-15, downstream of the unique HpaI site at amino acid L932, was replaced with the unc-54 3′ UTR from pPD136.61. Various fragments were cloned into this HpaI site to generate transgenes that correspond to specific portions of the alternatively spliced CTD isoforms. Mutations in these constructs were generated using standard PCR methods. Full-length ced-2 and ptp-2 were cloned into the XhoI site in pACT2 to generate CED-2 and PTP-2 yeast two-hybrid constructs.
Yeast two-hybrid screen selection procedure:
The constructs in the yeast two-hybrid vectors, pBTM116 and pACT or pACTII, were used to transform the Saccharomyces cerevisiae L40 (MATa, trp1, leu2, his3, LYS∷lexA-HIS3, URA3∷lexA-LacZ) and AMR70 (MATα, trp1, leu2, his3, URA3∷lexA-LacZ) yeast strains, respectively. L40 yeast was sequentially transformed with the EGL-15(Intra I) plasmid, NH#934, and with a C. elegans cDNA library using the lithium acetate method and treated as described (Rose et al. 1990). Transformations were plated to synthetic medium lacking histidine, leucine, and tryptophan. The plates were incubated at 30° for 3 days. Colonies that grew were patched on selective plates to test for regrowth. Colonies that regrew were assayed for β-galactosidase activity by a filter assay (Dalton and Treisman 1992), and colonies that turned blue in 24 hr at 30° were retained. Library plasmid DNA was recovered via yeast miniprep and retested via a mating assay and filter-lift β-galactosidase assays. Library plasmids that passed both retests were sequenced for identification.
Yeast two-hybrid interaction assays:
Dilution series for yeast matings were set up in the following manner. Individual cultures for dilution interaction assays were grown in the appropriate selective liquid medium cultures overnight at 30°. Samples were diluted to OD600 = 0.2, 5 μl was dotted on plates 1 cm apart undiluted and at dilutions of 1:100, 1:1000, and 1:10,000, and the plate was incubated for 3 days at 30°.
Quantitative β-galactosidase assays:
Mated yeast cells were grown overnight in TRP−LEU− media at 30°. An OD600 was taken, and the pellet of 1.0 ml of the overnight culture was resuspended in 1.0 ml of Z buffer (8.52 g anhydrous Na2HPO4, 4.8 g anhydrous NaH2PO4, 0.12 g anhydrous MgSO4, 0.74 g KCl dissolved in 1.0 liter water, and pH was adjusted to 7.0 with HCl), pelleted, and resuspended in 150 μl Z buffer supplemented with 2-mercaptoethanol (27 μl of 2-mercaptoethanol/10 ml Z buffer); 50 μl of chloroform and 20 μl of 0.1% SDS were added, and the sample was vortexed for 15 sec. A total of 700 μl of prewarmed (30°) ONPG (ortho-nitrophenyl-B(beta)-galactoside; 1 mg/ml in Z buffer with 2-mercaptoethanol; Sigma) was added, and the reactions were incubated at 30° until the solutions turned a yellow color. The reactions were checked at 1 and 5 min and then every 5 min until 15 min. Reactions were stopped by adding 0.5 ml 1 m Na2CO3 and microcentrifuged for 10 min to pellet debris. OD420 was taken for each of the reactions. Miller units were calculated using the following equation: (OD420 × 1000)/OD600 × time (in min) × volume (in ml). All assays were repeated at least three times.
Genetic assays for putative interactors:
To determine if the putative EGL-15 interactors played a role in EGL-15 signaling, we examined the available mutants for the Egl phenotype. L4 animals were picked and observed 48 hr later for the presence of accumulated eggs in the uterus. For interactors in which a genetic mutant was not available, we utilized standard RNAi techniques (Fire et al. 1998). To determine if the interactors played a role in the essential function pathway, we screened the mutants for the ability to suppress the Clr phenotype. We used RNAi (against the interactors) in a clr-1(e1745ts) background. L4 animals were placed on RNAi feeding clones, and their progeny were shifted to 25° (the restrictive temperature) and examined for the presence of Soc animals. egl-15 and L4440 RNAi were used as a positive and negative control, respectively.
RESULTS
A yeast two-hybrid screen reveals an important interaction between the C. elegans Grb2 homolog, SEM-5, and EGL-15:
To identify potential signaling components that might bind directly to EGL-15, we performed a yeast two-hybrid screen using the intracellular domain of EGL-15. Since the identification of interactors might be dependent on tyrosine autophosphorylation, the entire intracellular domain, including the juxtamembrane domain, the full kinase domain, and the CTD, was inserted in a two-hybrid bait vector. This EGL-15 “bait” construct (NH#934) contains the type I CTD [EGL-15(Intra I)], since it is the most prevalent C-terminal isoform (Goodman et al. 2003), and preliminary analysis indicated that this CTD isoform could mediate SM chemoattraction (Branda 2001). This bait is tyrosine-phosphorylated in yeast (Figure S2). We screened 2.0 × 108 transformants and identified 104 clones that specifically interact with EGL-15(Intra I). These clones define 10 putative interactors (Table 1). Six of the putative interactors have an SH2 domain, including the C. elegans homologs of Grb2 (SEM-5), Vav (VAV-1), Abl (ABL-1), and Csk (CSK-1) and the p85 subunit of PI3K (AAP-1). The sixth SH2 domain-containing interactor is a protein of unknown function (F13B12.6); clones of this gene represented the majority of isolates from this screen (76/104).
TABLE 1.
EGL-15 interactors
| Genea | Function/structure | Strength of interaction (MU)b | Mutant phenotype |
|---|---|---|---|
| SH2-containing interactors | |||
| F13B12.6 (76) | SH2 domain protein | 3300 ± 1118 | Wild type |
| sem-5 (2) | Grb2 homolog | 2100 ± 1050 | Vul, Let, Mig |
| vav-1 (3) | Vav proto-oncogene | 1900 ± 398 | Let |
| abl-1 (13) | Abl homolog | 1200 ± 724 | Wild type |
| aap-1 (1) | AGE-1 adapter protein | 38 ± 11 | Wild type |
| csk-1 (3) | C-terminal Src kinase | 46 ± 52 | Let, Ste |
| Non-SH2-containing interactors | |||
| tag-153 (1) | NOT2/NOT3/NOT5 domain protein | 1300 ± 360 | Wild type |
| tdc-1 (1) | Tyrosine decarboxylase | 1000 ± 547 | Wild type |
| T05E11.3 (3)c | Endoplasmic reticulum protein | 33 ± 13 | Wild type |
| Y48G8AL.9 (1)c | C2H2-type zinc-finger protein | 32 ± 12 | Wild type |
The number of screen isolates of each gene is shown in parentheses.
Strength of interaction is measured by a quantitative β-gal assay and reported in Miller units (MU) as the mean ± SD.
For T05E11.3 and Y48G8AL.9, the RNAi phenotype is listed because mutant alleles were not available.
A number of phenotypic tests were conducted to determine whether any of the genes corresponding to the identified interactors shared functions with EGL-15. These tests (see materials and methods) measured effects on either fluid homeostasis or SM chemoattraction when the interactor genes were compromised using RNA interference or existing mutant alleles. The only assays that revealed egl-15-like phenotypes were those conducted with sem-5 (data not shown); these assays confirmed the common functions shared between SEM-5 and EGL-15 (DeVore et al. 1995).
An alternative way to authenticate the yeast two-hybrid interactors was to show the specificity of the observed interaction. Because SH2 domains have a well-defined interaction mechanism, it was possible to test the SH2 domain-containing interactors further for the mechanism by which they bound EGL-15. Phosphorylated tyrosines, like those present in the EGL-15 intracellular domain, often serve as high-affinity binding sites for SH2 domain-containing proteins (Pawson 2004). We tested whether EGL-15-interactor binding activity was dependent on the kinase activity of EGL-15(Intra I). Mutating the ATP-binding site (K672A) of the kinase domain within the EGL-15(Intra I) bait construct abolishes EGL-15 autophosphorylation in yeast (Figure S2). This mutation is also known to abolish the phenotypic effects of otherwise hyperactivated egl-15 constructs (Kokel et al. 1998). When tested with this EGL-15(K672A Intra I) bait (NH#726), all 10 interactors failed to show yeast two-hybrid binding activity (Figure 1B and data not shown), indicating that all 10 require an active kinase domain to interact with EGL-15(Intra I).
To test the specificity of these interactions further, we investigated whether the six SH2-containing proteins identified in our screen [of 61 predicted from the C. elegans genome sequence (http://www.wormbase.org)] indicated binding specificity in these assays. Two additional SH2-containing constructs, which encode CED-2/CrkII and PTP-2/Shp2, were built and tested for their ability to interact with EGL-15(Intra I). Both CED-2 and PTP-2 fail to interact with EGL-15(Intra I), despite being expressed and able to interact with another EGL-15 isoform (Figure S3; see below). Taken together, these data indicate that the mode of these interactions is specific and suggest that they may be mechanistically relevant.
The migration-specific phenotype of egl-15(n1457) suggests that the CTD might be an important site for protein–protein interactions required for SM chemoattraction. Thus, we tested whether deletion of the C-terminal domain of the EGL-15(Intra I) bait [EGL-15(ΔCTD); NH#1278] would abolish the binding of any of the 10 interactors. Deletion of the C-terminal domain had a variable effect on the strength of the interactions between EGL-15 and all of the interactors (Table S1). However, 2 of the 10 interactors, SEM-5 and CSK-1, showed the most dramatic reductions, consistent with their interactions requiring the C-terminal domain of EGL-15(Intra I). Of these 2 interactors, only SEM-5 has been observed to exhibit egl-15-like phenotypes, with established roles in both the essential and the SM migration functions of EGL-15 (Clark et al. 1992; Stern et al. 1993; Chen et al. 1997).
Within the C-terminal domain of EGL-15, there are two consensus binding sites (YXNX) for the SH2 domain of SEM-5 (see Figure 1A; Songyang et al. 1993). Y1009 (YCND) lies in the amino-terminal portion of the CTD within a fragment that is common to all EGL-15 isoforms, including the type I isoform that was used for the yeast two-hybrid screen. Y1087 (YYNT) lies within a fragment that is unique to the type IV isoform. An additional consensus SEM-5 binding site sequence is found at Y884 (YANL) within the kinase domain of EGL-15. We tested the relevance of each of these potential binding sites by mutating these tyrosines to phenylalanines, both individually and in combination, and by testing the constructs in yeast two-hybrid assays (Figure 1B, Table S1). Mutating Y1009 to phenylalanine in the type I construct abolishes the EGL-15∷SEM-5 interaction. By contrast, the Y884F mutation does not, which is consistent with the CTD being necessary for this interaction. The type IV isoform contains both Y1009 and Y1087 (Figure 1A). Mutating both of these sites is necessary to abolish the yeast two-hybrid interaction between EGL-15 and SEM-5. These data indicate that SEM-5 can interact with EGL-15 via either Y1009 or Y1087.
The two SEM-5 binding sites in the EGL-15 CTD redundantly mediate SM chemoattraction:
Two types of transgenic rescue assays were used to assess the mechanism by which the CTD mediates SM chemoattraction. In the first of these, a genomic rescuing construct was used to identify CTD sites that are necessary for SM chemoattraction. The wild-type EGL-15 genomic construct (NH#112) rescues both the larval arrest and the SM migration defects of egl-15(null) animals when introduced in transgenic assays (Figure 2A). By contrast, a construct that contains the mutation found in egl-15(n1457) that truncates the CTD rescues only the larval arrest defect and fails to rescue the SM migration defect, resulting in severely posteriorly displaced SMs (Figure 2B). The results of a series of CTD deletion and truncation constructs tested using this assay indicated the presence of a site in the alternatively spliced region that can efficiently mediate SM chemoattraction (Figure S4). These results are consistent with a possible role of Y1087 in SM chemoattraction.
The second transgenic rescue assay tested whether portions of the CTD are sufficient for mediating SM chemoattraction. This assay has the benefit of eliminating the complexities due to redundancy and alternative splicing. We created a CTD-sufficiency “cassette” in which we deleted all egl-15 sequences downstream of the kinase domain within the genomic rescuing fragment and appended the commonly used 3′ UTR sequences from the unc-54 gene (Figure 3). This deleted the EGL-15 CTD and all C-terminal alternative splicing from the egl-15 genomic rescuing fragment. Fragments could be tested for sufficiency by introducing them into a unique HpaI site located just after the kinase domain. The “empty cassette” (without any fragments inserted), when expressed in an egl-15(null) strain, can rescue the essential function, but not the SM chemoattraction function of EGL-15 (Figure 3A). Thus, short sequences can be inserted into this “cassette” to serve as an artificial CTD to test whether they are sufficient to restore proper SM chemoattraction. To distinguish this assay from the normal genomic rescuing assay, we refer to this as the “cassette assay.” All constructs tested in this assay rescue the essential function of EGL-15.
Individual DNA fragments spanning the Y1009 SEM-5 binding site (Figure 3B) and the Y1087 SEM-5 binding site (Figure 3D) are each capable of efficient SM chemoattraction rescue. SMs in these rescued lines are centered, unlike the empty vector control where the SMs are posteriorly displaced. To test the relevance of the SEM-5 binding sites within these fragments, we introduced tyrosine-to-phenylalanine mutations into the respective fragments. The mutated constructs fail to rescue SM migration (Figure 3, C and E), resulting in SMs that are posteriorly displaced to a similar extent to those observed in the empty cassette control lines. Thus, Y1009 and Y1087 are specifically required for these fragments to rescue, suggesting that SEM-5 binding at these sites can provide the links to EGL-15 that mediate SM chemoattraction.
Removal of both C-terminal domain SEM-5 binding sites abolishes SM chemoattraction:
To test the roles of the Y1009CND and Y1087YNT binding sites in SM chemoattraction, we introduced tyrosine-to-phenylalanine mutations into the genomic rescuing construct, singly and in combination, and determined the effects on SM migration guidance. Mutation of Y1009 and Y1087 together abolishes SM chemoattraction rescue (Figure 2H). By contrast, individual mutations do not show the same dramatic effect on SM positions (Figure 2, D and E). Consistent with the inability of Y884 to bind SEM-5 in the yeast two-hybrid assay, the presence of Y884 does not permit SM chemoattaction (Figure 2H), and mutation of Y884 to phenylalanine does not significantly affect SM positions, either alone or in combination with other tyrosine-to-phenylalanine mutations (Figure 2, C, F, G, and I). Thus, our yeast two-hybrid data show that SEM-5 can bind directly to EGL-15 at both Y1009 and Y1087. Both Y1009 and Y1087 are consensus Y-X-N-X SEM-5 binding sites. Analyses of these sites in the cassette assay demonstrate that both are sufficient for SM chemoattraction, while the genomic assays demonstrate that the sites act redundantly to mediate SM chemoattraction.
An additional factor is implicated in the EGL-15-SEM-5 fluid homeostasis pathway:
SEM-5 is a required EGL-15 signaling component not only for SM chemoattraction, but also for fluid homeostasis. While mutations in sem-5 result in a Soc phenotype, the egl-15(n1457) truncation of the EGL-15 CTD, which is predicted to eliminate both SEM-5 binding sites, does not confer a Soc phenotype (Goodman et al. 2003). The phenotype of this mutant thus indicates that an additional site must be able to recruit SEM-5 to EGL-15, either directly or indirectly.
Y884 is another predicted direct binding site for the SEM-5 SH2 domain, even though it cannot bind SEM-5 in the yeast two-hybrid system. To test formally whether it might function to recruit SEM-5 to mediate fluid homeostasis, the EGL-15(ΔSEM-5) transgene, which mutates all three potential SEM-5 SH2 binding sites, was expressed in a clr-1(e1745ts); egl-15(null) background. clr-1(e1745ts); egl-15(null); ayEx[EGL-15(ΔSEM-5)] animals are Clr at the nonpermissive temperature for clr-1(e1745ts), formally demonstrating that EGL-15(ΔSEM-5) efficiently mediates fluid homeostasis. Thus, EGL-15 transduces its signal to SEM-5 independently of all of its canonical SEM-5 SH2 binding sites, implicating another mechanism that links EGL-15 to SEM-5 to mediate the essential/fluid homeostasis function.
One possible alternate mechanism could utilize an additional adaptor protein. The yeast two-hybrid EGL-15 interactors might provide this additional link to SEM-5. These were tested using either RNAi or existing viable mutations in a background that truncates the SEM-5 binding sites in the EGL-15 CTD. None of the nine other putative interactors confers a Soc phenotype when tested in a clr-1(e1745ts); egl-15(n1457) background, suggesting that none provides the link to SEM-5 (data not shown).
Another candidate that could serve as the adaptor that links EGL-15 to SEM-5 is rog-1, the C. elegans FRS2 homolog. FRS2 is a key component of the mammalian FGFR signaling pathway that acts as the main adaptor protein linking FGF receptors to downstream signaling molecules (Hadari et al. 2001). FRS2 is constitutively complexed with FGF receptors. Upon FGF receptor activation, tyrosines present within FRS2 are phosphorylated, enabling the recruitment of additional signaling components. Phosphorylation of FRS2 on Y196, Y306, Y349, and Y392 allows Grb2 to bind (Kouhara et al. 1997), thus linking activated FGF receptors to Grb2. In C. elegans, rog-1 encodes a 599-amino-acid protein containing an N-terminal PTB domain that is 34% identical and 60% similar to the PTB domain of murine FRS2α; the carboxy-terminal 350 amino acids are less similar (Figure S1) (Matsubara et al. 2007).
We tested whether rog-1 in C. elegans plays a similar role to FRS2 in mammals and serves as an adaptor protein linking SEM-5 to EGL-15. Mutation of rog-1 alone does not confer any egl-15-like phenotypes (Table S2) (Matsubara et al. 2007; Bennett 2009). Both rog-1(RNAi) and the putative null allele, rog-1(tm1031), are sterile (Table S2) and have been shown to have defects in a Ras/MAP kinase pathway that mediates germline progression (Matsubara et al. 2007; Bennett 2009). These tests did not result in either a Soc or SM migration phenotype (Table S2). However, for the fluid homeostasis function, this might be because of redundancy with the direct binding of SEM-5 via the EGL-15 CTD. To circumvent this potential redundancy, we tested whether compromising rog-1 in a clr-1(e1745ts); egl-15(n1457) background could suppress the Clr phenotype (Soc). clr-1(e1745ts); rog-1(RNAi); egl-15(n1457) animals are not Soc, indicating that ROG-1 does not play a role in a redundant mechanism. These animals were sterile, similar to the rog-1(tm1031) single mutant, confirming the effectiveness of the rog-1(RNAi). These data support the model that ROG-1 is not the adaptor that links SEM-5 to EGL-15 for the fluid homeostasis pathway.
DISCUSSION
The C. elegans Grb2 ortholog, SEM-5, was originally identified in screens for EGF receptor signaling processes and the fluid homeostasis function mediated by the FGF receptor, EGL-15 (Clark et al. 1992). SEM-5 binding sites on the C. elegans LET-23 EGF receptor itself mediate some of the functions of this receptor tyrosine kinase, similar to the mechanism by which the EGF receptor and GRB2 function in mammalian systems (Lesa and Sternberg 1997). By contrast, Grb2 is linked to the activated FGF receptor signaling complex via the FRS2 adaptor protein (Hadari et al. 2001). Here we show that SEM-5 can bind directly to EGL-15 to mediate SM chemoattraction and that SEM-5 associates with EGL-15 via a different mechanism for the signal transduction mechanism that regulates fluid homeostasis.
The direct association between SEM-5 and EGL-15 was initially indicated by the results of a yeast two-hybrid screen designed to identify C. elegans proteins with the potential to interact directly with the intracellular domain of EGL-15. This screen identified 10 potential direct interactors, including SEM-5 and 5 other interactors with SH2 domains. Although a number of these potential interactors have known functions that offer tantalizing possible connections to migration guidance or other signaling functions of EGL-15, none, in addition to SEM-5, could be corroborated with genetic evidence linking them either to SM chemoattraction or fluid homeostasis. For example, Abl has a well-studied role in actin remodeling (Koleske et al. 1998; Plattner et al. 1999; Grevengoed et al. 2001; Sini et al. 2004), while Csk plays a role in integrin-mediated cell adhesion and migration in human colon cancer cells (Rengifo-Cam et al. 2004). Vav, like other Dbl homology-containing Rho-GEFs, has a cytoskeletal role and also has known roles in F-actin polymerization (Fischer et al. 1998) and Rac-mediated lamellipodia formation (Crespo et al. 1996, 1997). The AGE-1 adaptor protein, AAP-1, serves to link C. elegans PI3-kinase (Paradis et al. 1999) to the DAF-2 insulin receptor and potentially could recruit AGE-1 to EGL-15. PI3 kinase has been shown to act downstream of vertebrate FGFRs; however, no role for PI3 kinase has been established in EGL-15 functions in C. elegans. As tantalizing as these possibilities seem, a variety of genetic tests failed to corroborate the association indicated by the yeast two-hybrid results. It is possible that some of these potential interactors are involved in some of the other functions of EGL-15.
By contrast, not only does SEM-5 have well-established roles in the functions mediated by EGL-15, but a large amount of evidence points to the specificity of the yeast two-hybrid interaction. First, not all SH2-containing proteins interact with the yeast two-hybrid “bait” EGL-15(Intra I), which was used in this screen. C. elegans CED-2 and PTP-2 each have SH2 domains that fail to interact with EGL-15(Intra I), although they interact strongly with EGL-15(Intra-IV) (Figure S3). This demonstrates some degree of specificity of the screen itself. Second, the interaction between SEM-5 and EGL-15 requires the EGL-15 CTD, the domain required for SM chemoattraction. Third, the binding to EGL-15(Intra I) is specific to Y1009, and the binding to EGL-15(Intra-IV) specifically requires a pair of tyrosine residues, Y1009 and Y1087; the conservative mutation of these tyrosines to phenylalanines completely abolishes the interaction. Fourth, the interaction is dependent on EGL-15 kinase activity and thus likely requires the tyrosine phosphorylation known to be essential for SH2 binding. These characteristics of the yeast two-hybrid interaction indicate its relevance to the physiological functions of EGL-15 and SEM-5.
The requirement of the EGL-15 CTD for its interaction with SEM-5 suggested that the interaction would be necessary for SM chemoattraction, which specifically requires the EGL-15 CTD. Using two different transgenic rescue assays, we were able to show that both of these SEM-5 binding tyrosines are important for SM chemoattraction. Alternative splicing generates a number of EGL-15 isoforms that differ in the C-terminal portion of their CTDs (Goodman et al. 2003). Y1009 is located in a region that is common to all CTD isoforms. Although Y1009 is sufficient to allow SM chemoattraction, it is not necessary. By contrast, Y1087 is specific to the type IV CTD, but is also sufficient for SM chemoattraction. These two sites function redundantly for SM chemoattraction; only when both the Y1009 and the Y1087 binding sites are mutated is SM chemoattraction abolished. The presence of both sites within the type IV isoform suggests that this isoform may play a particularly important role in SM chemoattraction. The conservation of this site within Caenorhabditis briggsae EGL-15 provides further support for its importance. Thus, despite the prevalence of type I transcripts within mixed cDNA isolates, it is possible that the type IV isoform might be more highly expressed in the migrating SMs. The average relative abundance of the isoforms might be accounted for by a skew toward type I expression within the hypodermis, which is a large syncytium that contains many nuclei.
The intracellular domain of EGL-15 contains an additional YXNX motif at Y884 that could act as another binding site for the SH2 domain of SEM-5. However, Y884 does not appear to play a role in recruiting SEM-5 or in SM chemoattraction. First, SEM-5 does not associate with EGL-15 via this site by yeast two-hybrid analysis. Second, mutation of the two CTD SEM-5 binding sites abolishes SM chemoattraction, indicating that Y884 is not sufficient to carry out this recruiting function. The inability of Y884 to recruit SEM-5 is likely due to the structural role that it plays within the highly conserved G α-helix of the EGL-15 kinase domain. The structural importance of this residue is underscored by the fact that the corresponding residue in all human FGFRs is phenylalanine instead of tyrosine. By contrast, Y1009 and Y1087 are C-terminal to the kinase domain and are likely to be in flexible segments that can be readily phosphorylated to trigger SEM-5 recruitment.
Other EGL-15 functions require a different mechanism to signal to SEM-5:
Similar to the egl-15(n1457) mutation that truncates the EGL-15 CTD, disruption of the two CTD SEM-5 binding sites, Y1009 and Y1087, specifically affects SM chemoattraction, leaving intact other common functions shared by SEM-5 and EGL-15. One of these shared roles is the SM chemorepulsion mechanism. Although SEM-5 is required for the gonad-dependent repulsion, removing the CTD binding sites does not compromise this function. Therefore, the mechanism by which SEM-5 and EGL-15 mediate SM chemorepulsion has yet to be determined. Similarly, SEM-5 and EGL-15 also have a shared role in the fluid homeostasis/essential function. Mutations in SEM-5 can suppress the Clr phenotype; however, the egl-15(ΔSEM-5) mutation cannot, suggesting that SEM-5 must link to EGL-15 by a different mechanism for this function. While it is possible that SEM-5 could interact directly with EGL-15 via its SH3 domains to mediate these functions, the lack of two-hybrid interactions in the absence of the SH2 binding sites in the CTD suggests that this is not the case. Rather, these functions may require an additional adaptor protein to transduce signals between EGL-15 and SEM-5. We have identified a number of potential candidates that could play this role, including the rog-1-encoded FRS2-like adaptor and the nine remaining yeast two-hybrid interactors. Genetic tests failed to reveal the involvement of these genes in EGL-15-mediated processes. The multi-substrate adaptor protein SOC-1 is known to function in EGL-15-mediated fluid homeostasis (Schutzman et al. 2001). It is possible that it serves to link SEM-5 to EGL-15 independently of the CTD direct binding sites, although the mechanism by which SOC-1 itself links to EGL-15 remains to be elucidated.
Conclusions:
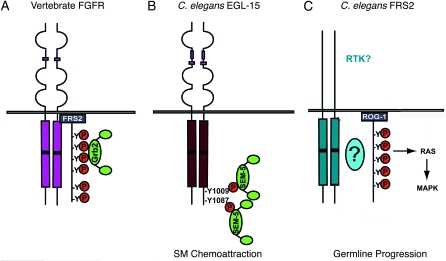
Many signal transduction components have been conserved throughout the evolution of metazoa. Nevertheless, there is considerable flexibility in how they are used (Figure 4). Data in C. elegans indicate that the FRS2-like protein, ROG-1, is not involved in FGF signal transduction, but rather is involved in a different RAS/MAP kinase pathway that is critical for germline progression. D. melanogaster also has an FRS2-like protein, but, like C. elegans, no evidence links it to FGF signaling. Instead, the Dof protein is critical to FGF signal transduction in Drosophila (Michelson et al. 1998; Vincent et al. 1998; Imam et al. 1999). C. elegans does not have a Dof-like gene in its genome and thus must utilize yet other mechanisms. Here we show that C. elegans uses direct binding to link SEM-5 to EGL-15 for SM chemoattraction, obviating the need for the FRS2-like multi-substrate adaptor protein for this function. For other EGL-15 functions, an additional, yet to be discovered, mechanism links SEM-5 to the EGL-15 signaling complex. Identifying that mechanism will further show the breadth of evolutionary flexibility in these signal transduction systems.
Figure 4.—
Comparison of FGFR and FRS2 signaling complexes in vertebrates and C. elegans. (A) The interaction between the vertebrate FGF receptor and Grb2 requires the signaling adaptor protein, FRS2. (B) The C. elegans FGF receptor EGL-15 can bind SEM-5 directly at Y1009 and Y1087, which are required for SM chemoattraction. (C) The C. elegans FRS2 homolog, ROG-1, is involved in a RAS/MAP kinase signaling pathway that is required for germline progression, but does not involve EGL-15.
Acknowledgments
We thank J. Schutzman for initiating the EGL-15 yeast two-hybrid experiments, I. Sasson for initiating the ROG-1 experiments, Y. Kohara for cDNAs, S. Hubbard for discussions about the structure of the EGL-15 kinase domain, and members of our lab for critical reading of this manuscript. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) National Center for Research Resources. tm1031 was provided by the National Bioresource Project for C. elegans (Tokyo). This work was supported by NIH grant GM50504 (M.J.S.) and by the following NIH training grants: T32 GM07223 (T-W.L. and D.C.B) and T32 DK07259 (S.J.G.).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.113373/DC1.
References
- Bennett, D. C., 2009. Identifying interactors of C. elegans FGFR signaling. Ph.D. Thesis, Yale University, New Haven, CT.
- Borland, C. Z., J. L. Schutzman and M. J. Stern, 2001. Fibroblast growth factor signaling in Caenorhabditis elegans. Bioessays 23 1120–1130. [DOI] [PubMed] [Google Scholar]
- Branda, C. S., 2001. Mechanisms of sex myoblast migration in C. elegans hermaphrodites. Ph.D. Thesis, Yale University, New Haven, CT.
- Branda, C. S., and M. J. Stern, 2000. Mechanisms controlling sex myoblast migration in Caenorhabditis elegans hermaphrodites. Dev. Biol. 226 137–151. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulow, H. E., T. Boulin and O. Hobert, 2004. Differential functions of the C. elegans FGF receptor in axon outgrowth and maintenance of axon position. Neuron 42 367–374. [DOI] [PubMed] [Google Scholar]
- Burdine, R. D., E. B. Chen, S. F. Kwok and M. J. Stern, 1997. egl-17 encodes an invertebrate fibroblast growth factor family member required specifically for sex myoblast migration in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 94 2433–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdine, R. D., C. S. Branda and M. J. Stern, 1998. EGL-17(FGF) expression coordinates the attraction of the migrating sex myoblasts with vulval induction in C. elegans. Development 125 1083–1093. [DOI] [PubMed] [Google Scholar]
- Chen, E. B., and M. J. Stern, 1998. Understanding cell migration guidance: lessons from sex myoblast migration in C. elegans. Trends Genet. 14 322–327. [DOI] [PubMed] [Google Scholar]
- Chen, E. B., C. S. Branda and M. J. Stern, 1997. Genetic enhancers of sem-5 define components of the gonad-independent guidance mechanism controlling sex myoblast migration in Caenorhabditis elegans hermaphrodites. Dev. Biol. 182 88–100. [DOI] [PubMed] [Google Scholar]
- Clark, S. G., M. J. Stern and H. R. Horvitz, 1992. C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature 356 340–344. [DOI] [PubMed] [Google Scholar]
- Crespo, P., X. R. Bustelo, D. S. Aaronson, O. A. Coso, M. Lopez-Barahona et al., 1996. Rac-1 dependent stimulation of the JNK/SAPK signaling pathway by Vav. Oncogene 13 455–460. [PubMed] [Google Scholar]
- Crespo, P., K. E. Schuebel, A. A. Ostrom, J. S. Gutkind and X. R. Bustelo, 1997. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature 385 169–172. [DOI] [PubMed] [Google Scholar]
- Dalton, S., and R. Treisman, 1992. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell 68 597–612. [DOI] [PubMed] [Google Scholar]
- DeVore, D. L., H. R. Horvitz and M. J. Stern, 1995. An FGF receptor signaling pathway is required for the normal cell migrations of the sex myoblasts in C. elegans hermaphrodites. Cell 83 611–620. [DOI] [PubMed] [Google Scholar]
- Dixon, S. J., M. Alexander, R. Fernandes, N. Ricker and P. J. Roy, 2006. FGF negatively regulates muscle membrane extension in Caenorhabditis elegans. Development 133 1263–1275. [DOI] [PubMed] [Google Scholar]
- Eswarakumar, V. P., I. Lax and J. Schlessinger, 2005. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 16 139–149. [DOI] [PubMed] [Google Scholar]
- Fire, A., S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver et al., 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391 806–811. [DOI] [PubMed] [Google Scholar]
- Fischer, K. D., Y. Y. Kong, H. Nishina, K. Tedford, L. E. Marengere et al., 1998. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr. Biol. 8 554–562. [DOI] [PubMed] [Google Scholar]
- Fleming, T. C., F. W. Wolf and G. Garriga, 2005. Sensitized genetic backgrounds reveal a role for C. elegans FGF EGL-17 as a repellent for migrating CAN neurons. Development 132 4857–4867. [DOI] [PubMed] [Google Scholar]
- Goodman, S. J., C. S. Branda, M. K. Robinson, R. D. Burdine and M. J. Stern, 2003. Alternative splicing affecting a novel domain in the C. elegans EGL-15 FGF receptor confers functional specificity. Development 130 3757–3766. [DOI] [PubMed] [Google Scholar]
- Gotoh, N., K. Manova, S. Tanaka, M. Murohashi, Y. Hadari et al., 2005. The docking protein FRS2alpha is an essential component of multiple fibroblast growth factor responses during early mouse development. Mol. Cell. Biol. 25 4105–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevengoed, E. E., J. J. Loureiro, T. L. Jesse and M. Peifer, 2001. Abelson kinase regulates epithelial morphogenesis in Drosophila. J. Cell Biol. 155 1185–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadari, Y. R., H. Kouhara, I. Lax and J. Schlessinger, 1998. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol. Cell. Biol. 18 3966–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadari, Y. R., N. Gotoh, H. Kouhara, I. Lax and J. Schlessinger, 2001. Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways. Proc. Natl. Acad. Sci. USA 98 8578–8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman, R. K., 1988. Genetics, pp. 17–45 in The Nematode Caenorhabditis elegans, edited by W. B. Wood. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Huang, P., and M. J. Stern, 2004. FGF signaling functions in the hypodermis to regulate fluid balance in C. elegans. Development 131 2595–2604. [DOI] [PubMed] [Google Scholar]
- Huang, P., and M. J. Stern, 2005. FGF signaling in flies and worms: more and more relevant to vertebrate biology. Cytokine Growth Factor Rev. 16 151–158. [DOI] [PubMed] [Google Scholar]
- Imam, F., D. Sutherland, W. Huang and M. A. Kransnow, 1999. stumps, a Drosophila gene required for fibroblast growth factor (FGF)-directed migrations of tracheal and mesodermal cells. Genetics 152 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokel, M., C. Z. Borland, L. DeLong, H. R. Horvitz and M. J. Stern, 1998. clr-1 encodes a receptor tyrosine phosphatase that negatively regulates an FGF receptor signaling pathway in Caenorhabditis elegans. Genes Dev. 12 1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleske, A. J., A. M. Gifford, M. L. Scott, M. Nee, R. T. Bronson et al., 1998. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron 21 1259–1272. [DOI] [PubMed] [Google Scholar]
- Kouhara, H., Y. R. Hadari, T. Spivak-Kroizman, J. Schilling, D. Bar-Sagi et al., 1997. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89 693–702. [DOI] [PubMed] [Google Scholar]
- Lax, I., A. Wong, B. Lamothe, A. Lee, A. Frost et al., 2002. The docking protein FRS2alpha controls a MAP kinase-mediated negative feedback mechanism for signaling by FGF receptors. Mol. Cell 10 709–719. [DOI] [PubMed] [Google Scholar]
- Lesa, G. M., and P. W. Sternberg, 1997. Positive and negative tissue-specific signaling by a nematode epidermal growth factor receptor. Mol. Biol. Cell 8 779–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, J. Z., and H. R. Horvitz, 1993. Three new classes of mutations in the Caenorhabditis elegans muscle gene sup-9. Genetics 135 53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo, T. W., C. S. Branda, P. Huang, I. E. Sasson, S. J. Goodman et al., 2008. Different isoforms of the C. elegans FGF receptor are required for attraction and repulsion of the migrating sex myoblasts. Dev. Biol. 318 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara, Y., I. Kawasaki, S. Urushiyama, T. Yasuda, M. Shirakata et al., 2007. The adaptor-like protein ROG-1 is required for activation of the Ras-MAP kinase pathway and meiotic cell cycle progression in Caenorhabditis elegans. Genes Cells 12 407–420. [DOI] [PubMed] [Google Scholar]
- Michelson, A. M., S. Gisselbrecht, E. Buff and J. B. Skeath, 1998. Heartbroken is a specific downstream mediator of FGF receptor signalling in Drosophila. Development 125 4379–4389. [DOI] [PubMed] [Google Scholar]
- Moore, D. S., and G. P. Mccabe, 1993. Introduction to the Practice of Statistics. Freeman and Company, New York.
- Ong, S. H., G. R. Guy, Y. R. Hadari, S. Laks, N. Gotoh et al., 2000. FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol. Cell. Biol. 20 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz, D. M., and N. Itoh, 2001. Fibroblast growth factors. Genome Biol. 2 REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis, S., M. Ailion, A. Toker, J. H. Thomas and G. Ruvkun, 1999. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev. 13 1438–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson, T., 2004. Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell 116 191–203. [DOI] [PubMed] [Google Scholar]
- Plattner, R., L. Kadlec, K. A. DeMali, A. Kazlauskas and A. M. Pendergast, 1999. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 13 2400–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanska, U. M., D. G. Fernig and T. Kinnunen, 2009. Extracellular interactome of the FGF receptor-ligand system: complexities and the relative simplicity of the worm. Dev. Dyn. 238 277–293. [DOI] [PubMed] [Google Scholar]
- Rengifo-Cam, W., A. Konishi, N. Morishita, H. Matsuoka, T. Yamori et al., 2004. Csk defines the ability of integrin-mediated cell adhesion and migration in human colon cancer cells: implication for a potential role in cancer metastasis. Oncogene 23 289–297. [DOI] [PubMed] [Google Scholar]
- Rose, D. M., F. Winston and P. Hieter, 1990. Laboratory Course Manual for Methods in Yeast Genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- Roubin, R., K. Naert, C. Popovici, G. Vatcher, F. Coulier et al., 1999. let-756, a C. elegans fgf essential for worm development. Oncogene 18 6741–6747. [DOI] [PubMed] [Google Scholar]
- Schutzman, J. L., C. Z. Borland, J. C. Newman, M. K. Robinson, M. Kokel et al., 2001. The Caenorhabditis elegans EGL-15 signaling pathway implicates a DOS-like multisubstrate adaptor protein in fibroblast growth factor signal transduction. Mol. Cell. Biol. 21 8104–8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sini, P., A. Cannas, A. J. Koleske, P. P. Di Fiore and G. Scita, 2004. Abl-dependent tyrosine phosphorylation of Sos-1 mediates growth-factor-induced Rac activation. Nat. Cell Biol. 6 268–274. [DOI] [PubMed] [Google Scholar]
- Songyang, Z., S. E. Shoelson, M. Chaudhuri, G. Gish, T. Pawson et al., 1993. SH2 domains recognize specific phosphopeptide sequences. Cell 72 767–778. [DOI] [PubMed] [Google Scholar]
- Stern, M. J., and H. R. Horvitz, 1991. A normally attractive cell interaction is repulsive in two C. elegans mesodermal cell migration mutants. Development 113 797–803. [DOI] [PubMed] [Google Scholar]
- Stern, M. J., L. E. Marengere, R. J. Daly, E. J. Lowenstein, M. Kokel et al., 1993. The human GRB2 and Drosophila Drk genes can functionally replace the Caenorhabditis elegans cell signaling gene sem-5. Mol. Biol. Cell 4 1175–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston, J. E., and H. R. Horvitz, 1977. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 56 110–156. [DOI] [PubMed] [Google Scholar]
- Sundaram, M., J. Yochem and M. Han, 1996. A Ras-mediated signal transduction pathway is involved in the control of sex myoblast migration in Caenorhabditis elegans. Development 122 2823–2833. [DOI] [PubMed] [Google Scholar]
- Szewczyk, N. J., and L. A. Jacobson, 2003. Activated EGL-15 FGF receptor promotes protein degradation in muscles of Caenorhabditis elegans. EMBO J. 22 5058–5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J. H., M. J. Stern and H. R. Horvitz, 1990. Cell interactions coordinate the development of the C. elegans egg-laying system. Cell 62 1041–1052. [DOI] [PubMed] [Google Scholar]
- Vincent, S., R. Wilson, C. Coelho, M. Affolter and M. Leptin, 1998. The Drosophila protein dof is specifically required for FGF signaling. Mol. Cell 2 515–525. [DOI] [PubMed] [Google Scholar]
- Xu, H., K. W. Lee and M. Goldfarb, 1998. Novel recognition motif on fibroblast growth factor receptor mediates direct association and activation of SNT adapter proteins. J. Biol. Chem. 273 17987–17990. [DOI] [PubMed] [Google Scholar]