Abstract
The conserved frizzled (fz) pathway regulates planar cell polarity in both vertebrate and invertebrate animals. This pathway has been most intensively studied in the wing of Drosophila, where the proteins encoded by pathway genes all accumulate asymmetrically. Upstream members of the pathway accumulate on the proximal, distal, or both cell edges in the vicinity of the adherens junction. More downstream components including Inturned and Multiple Wing Hairs accumulate on the proximal side of wing cells prior to hair initiation. The Mwh protein differs from other members of the pathway in also accumulating in growing hairs. Here we show that the two Mwh accumulation patterns are under different genetic control with the early proximal accumulation being regulated by the fz pathway and the latter hair accumulation being largely independent of the pathway. We also establish recruitment by proximally localized Inturned to be a putative mechanism for the localization of Mwh to the proximal side of wing cells. Genetically inturned (in) acts upstream of mwh (mwh) and is required for the proximal localization of Mwh. We show that Mwh can bind to and co-immunoprecipitate with Inturned. We also show that these two proteins can function in close juxtaposition in vivo. An In∷Mwh fusion protein provided complete rescue activity for both in and mwh mutations. The fusion protein localized to the proximal side of wing cells prior to hair formation and in growing hairs as expected if protein localization is a key for the function of these proteins.
THE frizzled (fz) signaling pathway regulates tissue planar cell polarity (PCP) in the epidermis of both vertebrate and invertebrate animals (Lawrence et al. 2007; Montcouquiol 2007; Wang and Nathans 2007; Zallen 2007). PCP is dramatic in the cuticle of insects such as Drosophila, which is decorated with arrays of hairs and sensory bristles.
The genetic basis for tissue polarity has been most extensively studied in the fly wing (Wong and Adler 1993). The Planar Polarity (PCP) genes of the fz pathway (also known as the core PCP genes), the planar polarity effector (PPE) genes and the multiple wing hairs (mwh) gene encode key components that regulate planar polarity in the wing. fz, disheveled (dsh), prickle/spiny leg (pk/sple), Van Gogh (Vang) (aka strabismus), starry night (stan) (aka flamingo) and diego (dgo) are members of the PCP group (Vinson and Adler 1987; Wong and Adler 1993; Taylor et al. 1998; Wolff and Rubin 1998; Chae et al. 1999; Gubb et al. 1999; Usui et al. 1999). A distinctive feature of these genes is that their protein products accumulate asymmetrically on the distal (Fz, Dsh, and Dgo) (Axelrod 2001; Feiguin et al. 2001; Shimada et al. 2001; Strutt 2001), proximal (Vang, Pk)(Tree et al. 2002; Bastock et al. 2003), or both distal and proximal (Stan) (Usui et al. 1999) sides of wing cells. These genes/proteins act as a functional group and are corequirements for the asymmetric accumulation of the others.
The PPE includes inturned (in), fuzzy (fy), and fritz (frtz) (Park et al. 1996; Collier and Gubb 1997; Collier et al. 2005). These genes are thought to function downstream of the PCP genes and the proteins encoded by these genes also accumulate asymmetrically in wing cells (Adler et al. 2004; Strutt and Warrington 2008). As is the case for the PCP genes, the PPE genes/proteins also appear to be a functional group and to be corequirements for the asymmetric accumulation of the others. Several observations support the hypothesis that the PPE genes are essential downstream effectors of the PCP genes. The earliest appreciation of this came from careful observations of the mutant phenotypes. A common feature of mutations in all of these genes is that they do not result in a randomization of hair polarity, but rather in a similar complicated and abnormal stereotypic pattern (Gubb and Garcia-Bellido 1982; Adler et al. 2000). That the abnormal patterns were so similar suggested that these genes all functioned in the same process (Wong and Adler 1993). The mutant phenotypes differed in that the vast majority of PCP mutant wing cells form a single hair, while many PPE mutant wing cells form two or three hairs. Mutations in PPE genes are epistatic to both loss- and gain-of-function mutations in PCP genes (Wong and Adler 1993; Lee and Adler 2002). Further evidence that the PPE genes function downstream of the PCP genes comes from the analysis of protein localization. PPE gene function is not needed for the proper asymmetric localization of PCP proteins (Usui et al. 1999; Strutt 2001; Tree et al. 2002; Collier et al. 2005) but in contrast PCP gene function is essential for the asymmetric accumulation of PPE proteins (Adler et al. 2004; Strutt and Warrington 2008). Further, the PCP genes/proteins instruct the localization of the PPE proteins (Adler et al. 2004).
The multiple-wing-hairs (mwh) gene is thought to function downstream of both the PCP and PPE genes (Wong and Adler 1993). This conclusion comes from analyses that are similar to those that established that the PPE genes function downstream of the PCP genes. The overall hair polarity pattern of mwh mutant wings shares the same complicated and abnormal stereotypic hair polarity pattern seen in PCP and PPE mutants. However, mwh cells differ by producing a larger number of hairs (typically three to four hairs) (Wong and Adler 1993). mwh mutations are epistatic to mutations in both the PCP and PPE genes and mwh is not required for the asymmetric accumulation of either PCP or PPE proteins (Usui et al. 1999; Strutt 2001; Adler et al. 2004; Strutt and Warrington 2008).
The mwh gene was recently determined to encode a novel G protein binding–formin homology 3 (GBD-FH3) protein with a complex accumulation pattern in wing cells (Strutt and Warrington 2008; Yan et al. 2008). Prior to hair initiation Mwh accumulates along the proximal side of wing cells and during hair growth Mwh accumulates in the growing hair. Temperature-shift experiments with a temperature-sensitive allele provided evidence for two temporally separate mwh functions and it was proposed that the two accumulation patterns were associated with the two temporal functions (Yan et al. 2008). Here we show that the early proximal accumulation of Mwh requires the function of the PCP and PPE genes (a result also seen previously in Strutt and Warrington 2008), while the hair accumulation of Mwh is largely independent of these two groups of genes providing further genetic evidence for Mwh having two independent functions.
How does the Mwh protein accumulate proximally? An obvious possibility is that Mwh interacts directly with one or more of the upstream proteins and in this way is recruited to the proximal side. The PPE proteins are strong candidates to interact directly with Mwh, as they function genetically in between the PCP gene and Mwh (Wong and Adler 1993). Consistent with this possibility we found that In and Mwh interacted in the yeast two-hybrid system and that these two proteins co-immunoprecipated from wing cells. This interaction was found not to be dependent on the function of the PCP genes consistent with the data from genetic studies that both in and mwh retain at least partial function in a fz mutant wing (Wong and Adler 1993). The hypothesis that Mwh is recruited to the proximal side by interacting with In predicts that these two proteins function in close proximity to one another. Consistent with these expectations we found that an In∷Mwh fusion protein provided both In and Mwh function.
MATERIALS AND METHODS
Fly genetics:
All flies were raised at 25° unless otherwise stated. Mutant stocks were obtained from the Drosophila stock center at University of Indiana, were generated in our lab, or were generous gifts from J. Axelrod, D. Strutt, T, Uemura, and T. Wolff. The FLP/FRT technology was used to generate genetics mosaics (Xu and Rubin 1993). To direct transgene expression, we used the Gal4/UAS system (Brand and Perrimon 1993). For temperature-shift experiments the relevant animals were collected as white prepupae, placed into fresh food vials or petri dishes, and moved to the proper incubator. In line with FlyBase usage we use Starry night instead of flamingo and Van Gogh instead of strabismus.
Immunostaining:
A standard staining procedure was used (Yan et al. 2008). Secondary antibodies and fluorescent phalloidin were obtained from Molecular Probes/Invitrogen.
Plasmid constructs: mwh and inturned construct subcloning for two-hybrid assays:
Full length of mwh cDNA was subcloned into pGADT7 vectors from NdeI–EcoRI. The following primers were used: AD-mwh5, GGAATTCCATATGGCTCCCAGTGTGTGCG and AD-mwh3new, CCGGAATTCT-TAGTAGAGGCCGGATGGCAG. To get the truncated form of mwh cDNA, we used AD-mwh5′, CCGGAATTCCATGTTTCTCAACACGTTCATTGA and the same AD-mwh3new primer. The truncated mwh cDNA was subcloned into pGADT7 vectors from NdeI–BamHI. Full length of inturned cDNA was subcloned into pGBKT7 vectors as NdeI–BamHI fragment. The following primers were used: Inturn-th5, TCTAGGGAATTTCCATATGCGCAAATCGCCGG-CCAG and Inturn-th3, GATCGCGGATCCATGTCATCCCATTGAGAAGAAGGA.
The in∷mwh fusion gene was generated using PCR to place restriction sites up- and downstream of the coding regions of each gene. We introduced a NotI site 5′ to the in coding sequence (AAGGAAAAAA GCGGCCGCCATGCGCAAATCGCCGGCCA) and NheI and KpnI sites 3′ (CGGGGTACCCTAGCTAGCTCCCATTGAGAAGAAGGA). For mwh we introduced a 5′ NheI site (CTAGCTAGCATGGCTCCCAGTGTGTGCGA) and a 3′ XbaI site (CTAGTCTAGATTAGTAGAGGCCGGATGGCAGAT). The in cDNA was inserted into pUAST using the NotI and KpnI sites. The mwh cDNA was then inserted into this plasmid using the NheI site introduced into in and the XbaI site found in the pUAST polylinker.
Antibodies:
Polyclonal anti-Mwh antibodies generated in our lab have been described previously (Yan et al. 2008) as have monoclonal anti-In antibodies (Adler et al. 2004). Alexa 488- and Alexa 568-conjugated secondary antibodies were purchased from Molecular Probes. Alexa 568 and 647 phalloidin were purchased from Molecular Probes.
Co-immunoprecipitation and Western blotting:
The following flies were used in co-immunoprecipitations:
w; hs-HA-inturned/ptc-Gal4 UAS-mwh
w; UAS-HA-inturned/ptc-Gal4 UAS-mwh
w; UAS-HA-inturned/ptc-Gal4
Wing discs (100–150) were dissected from third instar larvae and we followed procedures described previously. Polyclonal anti-Mwh antibody (from rabbit) was applied in co-immunoprecipitations. Polyclonal anti-Mwh antibody (from rat) and monoclonal anti-Inturned antibody (or monoclonal anti-GFP antibody, BD) were used in Western blotting.
Several different protein size markers were used in the various Western blotting experiments. These included the HiMark pre-stained high-molecular-weight protein standard (Invitrogen) and the SeeBlue Plus2 pre-stained standard (Invitrogen). For standard protein gels we used premade 4–20% Tris-glycine gels (Invitrogen) and for the high-molecular-weight protein separations we used 3–8% premade NuPAGE Tris-acetate gels (Invitrogen).
Quantitative analysis of Mwh accumulation patterns:
The intensity of Mwh antibody staining was quantified using Image J on unmodified confocal images. We used individual optical sections from around the level of the adherens junctions that contained the region of interest in focus. For hair staining we used optical sections that contained hairs in focus. Three types of measurements were made. To assess general cellular staining a group of cells was outlined and the mean staining intensity measured. We routinely made measurements on clones and on neighboring wild-type cells. To assess proximal accumulation of Mwh we drew a 5-pixel-wide line that went across a proximal cell edge and obtained a plot of intensity. The maximum value along the plot profile was used in our analysis. For hair staining of Mwh we drew a 5-pixel line along the proximal distal axis of the hair and obtained a plot profile of intensity. As for the edge measurements we used the maximum value obtained. To get an accurate assessment of the change in immunostaining level in mutants it is essential to correct for nonspecific staining (Waters 2009). To do this we did similar measurements on clones of mwh1, a null allele. This gave us an estimate that allowed us to correct for the nonspecific staining when assessing the decrease in Mwh staining in mutants. The correction was done as follows using general cell staining as an example (see supporting information, Figure S1, as an example). For general cell staining the mean ratio of average intensity for mwh null/neighboring wild-type cells was 0.55. We take this to be an estimate of the decline in intensity due to a 100% decline in Mwh levels. The fluorescence in the mutant cells is likely due to the effects of nonspecific staining, camera background, and autofluorescence. Thus, 0.45 (1 − 0.55) is the fraction of florescence in the neighboring wild-type cells due to bona fide immunostaining of Mwh. For a fy mutant clone the intensity ratio was 0.69 (mutant/wt). From this we estimate that 0.14 (0.69 − 0.55) of the fluorescence in the fy mutant cells was due to bona fide staining and that this represented approximately 31% (0.14/0.45) of the endogenous Mwh. Hence we estimate that in a fy mutant cell the level of Mwh declined by 69% (1 − 0.31).
RESULTS
The frizzled pathway instructs Mwh localization:
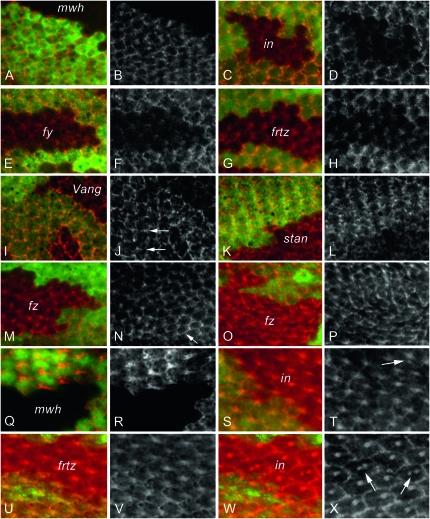
To determine if the frizzled pathway was required for either or both of the subcellular locations where Mwh accumulated, we immunolocalized Mwh in cells mutant for the PCP genes fz, Vang, and stan and the PPE genes fy, frtz, and in. We first considered the proximal accumulation seen just prior to and at hair initiation (32 hr). The accumulation of Mwh appeared decreased in all of the mutant clones (Figure 1), particularly in the PPE mutant clones (Figure 1, C–H). Our results are consistent with those reported by Strutt and Warrington (2008). We extended the analysis and quantified the decrease in two ways (see materials and methods for details). In the first we compared the mean fluorescence intensity for mutant clone cells and neighboring wild-type cells (Table 1). In all of these cases there was a significant decrease in Mwh level in mutant cells and this was greater in PPE mutant clones than in PCP clones. However, such measurements underestimated the actual decline in Mwh levels as nonspecific staining/autofluorescence produced a background that cannot be specifically measured and corrected for directly in these experiments. To assess the level of nonspecific staining we examined wings that contained clones mutant for a null allele of mwh. The cells in such clones lacked any Mwh protein and provided a measure of the extent of nonspecific staining. This could then be used to correct our estimates of the relative decline in Mwh accumulation in cells mutant for PCP and PPE genes (see materials and methods). Our data indicated that some Mwh protein remained in the PPE mutant cells. We estimated that the amount of Mwh protein declined by approximately 70% in the PPE mutant cells (Table 1). The decline in Mwh levels seen in PCP clones was less than 50%, but still substantial. The decrease was significantly greater in Vang clones than in fz clones. Although Mwh protein was present in PCP mutant cells the pattern of preferential proximal accumulation was largely if not completely disrupted (Figure 1, I–N).
Figure 1.—
Mwh accumulation requires the function of the PCP and PPE genes. (A, C, E, G, I, K, M, O, Q, S, U, and W) Clones of mutant cells marked by a loss of GFP and stained with anti-GFP (green) and anti-Mwh (red) antibodies. The remaining parts show Mwh staining in grayscale for greater contrast. (A and B) A mwh1 clone around the time of hair initiation, (C and D) an inIH56 clone prior to hair formation, (E and F) a fy2 clone prior to hair formation, (G and H) a frtz2 clone around the time of hair initiation, (I and J) a vangTBS42 clone prior to hair formation, (K and L) a stanVT13 clone prior to hair formation, (M and N) a fzP21 clone around the time of hair initiation, (O and P) a fzP21 clone during hair morphogenesis, (Q and R) a mwh1 clone during hair morphogenesis, (S and T) an inIH56 clone during hair morphogenesis, (U and V) a frtz2 clone during hair morphogenesis, (W and X) an inIH56 clone at late stages in hair morphogenesis (36 hr apf). Note the decreased levels of Mwh in all of the clones (particularly in C–H). As expected some of the in mutant clone cells formed multiple hairs (arrows in T and X).
TABLE 1.
mwh localization in the hair is regulated differently than localization at the proximal edge of wing cells
| Bulk Mwh |
Edge Mwh |
Hair Mwh |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutation | Ratio mut/wt Bulk 32 hr | na | Pb | Corrected % decease | ratio mut/wt edge 32 hr | n | P | Corrected % decrease | P bulk vs. edge | Ratio mut/wt hair 34–36 hr | n | P | Corrected % decrease | P bulk vs. hair | P edge vs. hair |
| mwh1 | 0.55 (0.036) | 12 | <0.001 | NR | 0.515 (0.009) | 40 | <0.001 | NR | 0.84 | 0.31 (0.011) | 138 | <0.001 | NR | <0.001 | <0.001 |
| inIH56 | 0.66 (0.032) | 11 | <0.001 | 76 | 0.640 (0.012) | 50 | <0.001 | 74 | 0.61 | 0.92 (0.019) | 64 | <0.001 | 12 | <0.001 | <0.001 |
| fy2 | 0.69 (0.058) | 10 | 0.002 | 69 | 0.673 (0.008) | 50 | <0.001 | 67 | 0.33 | ND | |||||
| frtz2 | 0.70 (0.027) | 11 | <0.001 | 67 | 0.608 (0.025) | 30 | <0.001 | 82 | 0.06 | 0.88 (0.017) | 48 | <0.001 | 17 | <0.001 | <0.001 |
| VangTBS42 | 0.79 (0.088) | 13 | <0.001 | 47 | 0.65 (012) | 40 | <0.001 | 72 | <0.001 | ND | |||||
| fzP21 | 0.88 (0.085) | 12 | <0.001 | 27 | 0.808 (0.11) | 50 | <0.001 | 40 | 0.01 | 0.90 (0.016) | 58 | <0.001 | 14 | 0.38 | <0.001 |
| stanvt13 | 0.80 (0.095) | 6 | 0.04 | 40 | 0.67 (0.017) | 20 | <0.001 | 68 | <0.001 | ND | |||||
ND, not done.
The number of measurements made for that sample. Note that n is much higher for edge and hair staining as multiple measurements were made for each clone, while for the bulk measurement only one measurement was made per clone.
P value from t-test or rank sum test (for samples that did not have equal variances).
At early stages the proximal concentration of Mwh may be more important than total cellular Mwh levels. We therefore also specifically measured proximal Mwh staining in PPE mutant clones (see materials and methods). Once again there was a substantial and significant decline in Mwh levels (Table 1). We estimated the amount of proximal edge Mwh was decreased by 67–82% in PPE mutant cells. For PCP mutant cells the decrease in edge staining ranged from 40 to 72%. For the three PCP mutants tested the decrease in edge Mwh was greater than total Mwh. As was the case for total Mwh staining the decline was less in fz mutant cells than in Vang or stan.
Immunostaining of older pupal wings (34–36 hr) showed that Mwh accumulated in growing hairs (Yan et al. 2008). To determine if this was affected by fz pathway mutations we immunostained clone-bearing wings that contained short- to medium-length hairs (Figure 1, O–X)). In some wings in this experiment we could still detect decreased level of Mwh at the edge and in the general cytoplasm of mutant cells. In some clones we detected a slightly lower level of Mwh staining in hairs and in some cases there was no clear difference. We examined null mwh clones to assess the amount of nonspecific staining (Figure 1, Q and R) and found the mutant/wild-type ratio was 0.31, significantly lower than we had seen for nonspecific staining at earlier stages. We suspected this was due to the stronger immunostaining signal in the hair (i.e., stronger signal vs. equivalent noise). The decline in Mwh staining in in, frtz, and fz mutant hairs while significant was dramatically less than in younger wings (Table 1 and Figure 1, O–X). We estimated the decline in Mwh hair levels ranged from 12 to 17%, and in contrast to the situation at earlier developmental stages we did not see a substantial difference between the decline for fz and in. When we compared the staining for in and frtz hairs vs. that in younger wings we found that the decline in hair staining was significantly lower (Table 1). The slight decline in hair staining in mutants compared to wild type could be a consequence of the decreased Mwh levels prior to hair initiation reducing the amount of preexisting Mwh protein that could relocate to the hair. We interpreted these results as evidence that the early proximal accumulation and the later hair accumulation of Mwh were differently regulated. The early proximal edge accumulation of Mwh was dependent on the fz pathway, while the accumulation in the hair was largely not.
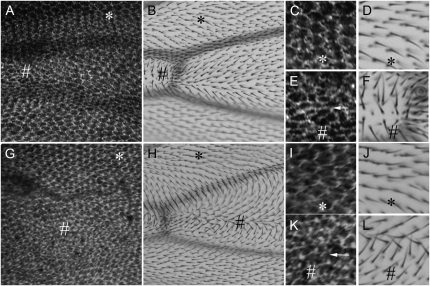
Clones of fz and Vang mutant cells display domineering nonautonomy in the wing (Vinson and Adler 1987; Taylor et al. 1998). This is manifested by changes in the locations where PCP and PPE proteins accumulate and where hairs form (Usui et al. 1999; Axelrod 2001; Feiguin et al. 2001; Strutt 2001; Adler et al. 2004). If the upstream fz pathway genes directed the preferential proximal accumulation of Mwh then we predicted that the pattern of Mwh accumulation would be affected in a nonautonomous fashion by fz and Vang clones. This appeared to be the case (see Figure 1, I, J, M, and N). As an alternative way to determine if the PCP genes/proteins instructed the asymmetric accumulation of Mwh, we utilized the ability of a gradient of PCP gene expression to repolarize wing hairs (Adler et al. 1997). This had the advantage that a larger and more consistent region of cells showed repolarized protein accumulation. For example, the expression of a PCP gene, such as fz under the control of the ptc driver, results in a medial to lateral gradient of expression within the ptc domain, which results in hairs pointing away from the midline (Figure 2B) (or toward the midline when PCP genes such as pk are expressed in this way (Figure 2H). This also results in a shift in the accumulation of PCP and PPE proteins from proximal/distal sides to anterior/posterior sides of wing cells (Usui et al. 1999; Axelrod 2001; Feiguin et al. 2001; Strutt 2001; Adler et al. 2004). We found that Mwh was preferentially relocalized to the anterior/posterior side of wing cells in such experiments (Figure 2, A, C, E, G, I, and K). It has previously been established that the function of the PPE genes is required for a gradient of expression of fz or any of the other PCP to repolarize wing hairs (Lee and Adler 2002). We also found that PPE gene function was required for a gradient of fz expression to affect Mwh accumulation, as this was indistinguishable in in vs. ptc-Gal4/UAS-fz; in/in wings (data not shown). Thus, the PCP genes and proteins instructed the asymmetric accumulation of Mwh and this required the function of the PPE genes and likely the repolarization of the PPE proteins.
Figure 2.—
PCP genes direct the accumulation of Mwh. (A–F) ptc-Gal4/UAS-fz wings. (A, C, and E) A 32-hr wing immunostained for Mwh. (B, D, and F) Adult wings. The same image is shown at two different magnifications. # indicates the middle of the ptc domain and * indicates the outside the domain. In the adult wings note that in the vicinity of the # the hairs tend to point away from the midline (point of highest Fz expression). Note also that the pattern of accumulation of Mwh in the pupal wings is altered in this region and no longer runs in the proximal distal direction (arrow). Outside of the ptc domain (*) hair polarity and Mwh accumulation are normal. (G–L) ptc-Gal4/UAS-pk wings. (I–L) Regions inside (#) and outside (*) the ptc domain. In this genotype hairs inside the domain tend to point toward the midline and once again hairs outside are not affected. The accumulation of Mwh is altered inside the domain (arrow) but not outside.
Mwh interacts with In:
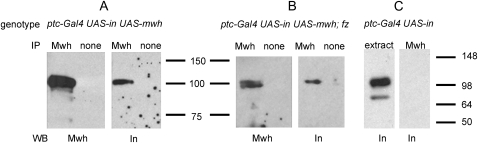
We hypothesized that one or more of the upstream, proximally localized proteins (e.g., Pk, Vang, In, Frtz) recruited Mwh, perhaps by a direct interaction. We considered In a prime candidate as it is a PDZ-containing cytopasmic protein (Yun et al. 1999) that functions genetically in between the PCP genes and mwh. Further the PPE proteins are reported to be essential for the phosphorylation of Mwh (Strutt and Warrington 2008). Using the yeast two-hybrid assay we found that Mwh could physically interact with In. Equivalent interactions were not seen with the other PPE proteins. Consistent with this interaction being important in the fly we found that the In protein could be co-immunoprecipitated with Mwh in transgenic flies (Figure 3), which indicated that these two proteins were part of a complex in wing disc cells. Our data suggested a model where Mwh was recruited to the proximal side of wing cells by interacting with the upstream In protein. Since endogenous Mwh and In did not perfectly overlap in wing cells (Strutt and Warrington 2008; Yan et al. 2008) the interaction might be transient. In other experiments we failed to see evidence for Mwh co-immunoprecipitating with the proximally localized PCP group proteins Vang or Pk (Figure 2S).
Figure 3.—
In and Mwh co-immunoprecipitate from wing disc cells. Co-immunoprecipitations of In and full-length Mwh in wild-type and fz mutant cells are shown. Wing discs from ptc-Gal4 UAS-mwh/UAS-HA-inturned (A), ptc-Gal4 UAS-mwh/UAS-HA-inturned; fzP21/fzP21 (B), or ptc-Gal4/UAS-HA-inturned (C). Wing disc extracts were incubated with anti-Mwh antibody (from rabbit). As a control, no anti-Mwh antibody was added to one sample. The protein complex was precipitated with protein A beads and then assayed by Western blotting using either anti-Mwh (from rat) or anti-In antibodies (mouse). Note that Mwh is able to pull down In and the interaction is independent of fz. In the absence of mwh expression In is not pulled down by anti-Mwh antibody (C). In some experiments we see a lower-molecular-weight band that presumably represents a breakdown product of In (C).
The subcellular localization of both In and Mwh was dependent on the upstream genes of the fz pathway. We found, however, that Mwh and In still co-immunoprecipitated from frizzled mutant fly wing disc cells (Figure 3). Thus, the interaction of In and Mwh did not depend on the function of the upstream genes nor the proximal accumulation of these proteins. That In and Mwh still interacted in fz mutant cells is not surprising. Mutations in PCP genes do not result in the large numbers of multiple hair cells seen in mwh and in mutants (Wong and Adler 1993). Thus In and Mwh must still be partially functional and able to inhibit ectopic hair initiation centers even when not localized to the proximal side of wing cells.
An In∷Mwh fusion protein has both in and mwh rescue activity:
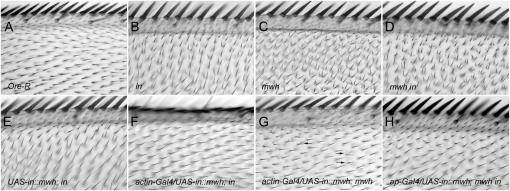
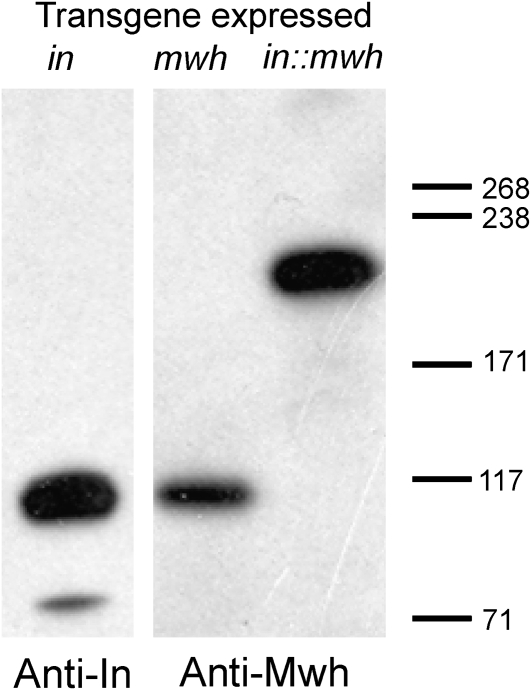
The requirement for in function for the proximal accumulation of Mwh and the co-immunoprecipitation of these two proteins suggested that the direct recruitment of Mwh by In was required for Mwh function. Indeed, it was also consistent with the suggestion that a (or the) major function of In was the proximal recruitment of Mwh and that these two proteins functioned while closely juxtaposed. As a test of this model we constructed a gene that encoded an In∷Mwh fusion protein and generated transgenic flies where this gene was under UAS control. On the basis of the model, our expectation was that this protein would localize to the proximal side of wing cells prior to hair formation and provide in rescue activity and rescue of the early mwh function. We found that the fusion protein provided both in and mwh rescue activity (Figure 4). It also provided rescue of a mwh in double mutant (Figure 4). The rescue of an in null allele by the fusion gene was close to complete when driven by actin-Gal4 (Figure 4F). Very few multiple hair cells were seen in rescued wings (<1%) and hair polarity was distal and indistinguishable from wild type. We quantified the rescue by scoring the fraction of cells in a defined region in the anterior proximal part of the wing. In this region 50% of cells in an in mutant wing form multiple hairs while in rescued wings 0% formed multiple cells. By immunostaining we found that prior to wing hair initiation the fusion protein was localized at the proximal edge of wing cells in the same pattern as wild-type In (Figure 5). We also found that the fusion protein provided substantial rescue of a mwh null allele when driven by actin-Gal4 (Figure 4G). The degree of rescue was similar to that seen when UAS-mwh expression was driven by actin-Gal4 (Yan et al. 2008). Hairs in mwh rescued wings showed normal distal polarity and there was a dramatic decline in the number of hairs per cell and the number of cells that formed multiple hairs. In our anterior test region 87% of cells in a mwh mutant formed multiple hairs while only 17% did so in the rescued wings. Most of the extra hairs in the rescued wings were quite small, suggesting that they were due to incomplete rescue of the late mwh function (Yan et al. 2008). It is worth noting here that the overexpression of in did not rescue a mwh mutation and that the overexpression of mwh did not rescue an in mutation. By Western blot analysis we confirmed that the fusion protein was of the expected size and that it was not cleaved into two parts in vivo (Figure 6). The rescue of the double mutant was not quite as good as the mwh mutant, although it was still dramatic (Figure 4H). The lesser rescue seems likely to be due to less than wild-type in activity in these wings enhancing the weak mwh phenotype that remains after rescue. Such interactions are seen among the planar polarity effector genes (Collier et al. 2005).
Figure 4.—
The In∷Mwh fusion protein provides both in and mwh rescue activity. The same region of the dorsal surface of the wing is shown in all images with one exception (D). This region is in the proximal part of the most anterior region of the wing (triple-row bristles are shown). Cells in this (and other) regions of an Ore-R (wt) wing (A) form a single distally pointing hair. In in (B) and mwh (C) mutant wings the hairs point toward the anterior margin and most cells form multiple hairs. The phenotype of mwh in double mutants is indistinguishable from mwh. D shows the ventral surface of a mwh in double mutant wing. H shows the dorsal surface of this wing. In the absence of a Gal4 driver we do not see substantial rescue by the fusion protein (E); however, in the presence of the actin-Gal4 driver we see essentially complete rescue of a null in allele (inIH56) (F) and almost complete rescue of a null mwh allele (mwh1) (G). Strong rescue of cells on the dorsal surface of a null mwh in double mutant wing is seen when expression is driven by ap-Gal4. As expected there, this rescue is not seen on the ventral surface of the same wing (D). Arrows point to cells in G that formed more than one hair.
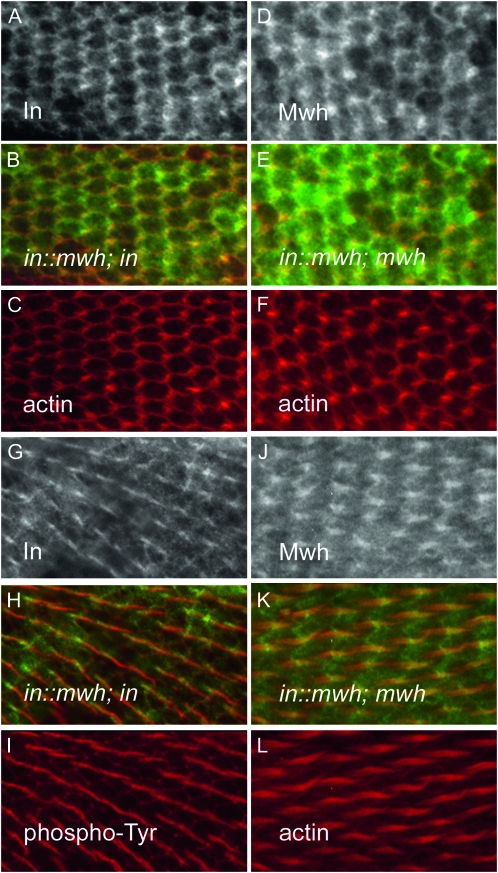
Figure 5.—
The In∷Mwh fusion protein accumulates at the proximal side of wing cells and in hairs. The In∷Mwh fusion protein localized in a zigzag pattern in pupal wing cells at and just before hair morphogenesis as detected by anti-In immunostaining (A–C) and anti-Mwh immunostaining (D–F). When anti-In immunostaining was used there was a null mutation in the endogenous in gene so that the only protein recognized by the anti-In antibody was the fusion protein. Similarly when anti-Mwh immunostaining was used there was a null mutation in the endogenous mwh gene. At later stages we found the fusion protein accumulated in hairs (G–L). In I the hair is stained using anti-Phosphotyrosine staining, which stains the surface of the hair. In L the hair is stained using phalloidin. Here the staining shows F-actin in the growing hair and also the actin rootlet that extends from the base of the hair toward the basal part of the cell. As is seen for the endogenous Mwh protein, accumulation is primarily in more proximal regions of the hair.
Figure 6.—
The In∷Mwh fusion protein is present as a full-length protein. Shown are Western blots of extracts made from wing discs that expressed a transgene. Note that the In and Mwh proteins have similar molecular weights. When overexpressed we sometimes see a small amount of an In breakdown product as is seen in this experiment. The fusion protein was the expected size and we did not see evidence for degradation.
The three PPE genes show strong genetic interactions (Lee and Adler 2002; Collier et al. 2005) and the proteins interact physically (Lee et al. 2000; Yan et al. 2007). One possible model to explain the interactions is that a complex between the In, Fy, and Frtz proteins is required for In to be able to bind Mwh. If so the In∷Mwh fusion protein might eliminate the need for fy and/or frtz. We tested this model but found that the In∷Mwh fusion protein was not able to rescue a fy or frtz loss-of-function mutation (this was also the case for the expression of In and Mwh separately; data not shown). Thus, the model is unlikely to be correct.
The wild-type Mwh protein accumulated in growing hairs while that was not seen for In (Adler et al. 2004; Strutt and Warrington 2008; Yan et al. 2008). The level of Mwh in hairs is higher than that seen elsewhere in the cell, suggesting that the protein is actively recruited to the hair, perhaps by binding to one or more proteins found in the hair. There does not appear to be a general barrier preventing cytoplasmic proteins from entering the growing hair as cytoplasmic GFP expressed from the ubiquitin promoter can sometimes be detected in hairs (Figure S3); however, it does not appear to preferentially accumulate there as does Mwh. In a similar manner, when in was overexpressed we could sometimes detect low levels in the hair. We found that the In∷Mwh fusion protein became enriched in growing hairs much like the wild-type Mwh protein (Figure 5, G and J). Thus, in the context of the fusion protein Mwh is epistatic to In with regard to hair accumulation.
DISCUSSION
How is Mwh recruited to its subcellular localizations?
Our data suggested that Mwh was recruited to the proximal side of wing cells by binding to proximally localized In. We established that in function was required for proximal Mwh localization, that the two proteins could be co-immunoprecipitated, and that they interacted in the yeast two-hybrid system. However, these two proteins did not precisely colocalize with Mwh showing a somewhat broader accumulation pattern (Strutt and Warrington 2008; Yan et al. 2008). We hypothesized that the lack of precise colocalization of In and Mwh was due to the dynamics of the system. For example, it is possible that the interaction is transient and cycles of binding and release leads to Mwh accumulating diffusely near the proximal edge of wing cells. It is also possible that the relative levels of the two proteins might not be compatible with all of each being in a common protein complex.
We found that a fusion protein containing the complete amino acid sequences of both In and Mwh provided both in and mwh function. Prior to hair formation the fusion protein localized to the proximal side of wing cells. This is seen for both In and Mwh so this was not a surprising result, although there is no way to be certain ahead of time that such a fusion protein will be functional. This early phase of proximal localization was presumably guided by the In part of the fusion protein as this is needed for in rescue activity and in acts upstream of mwh (Wong and Adler 1993; Lee and Adler 2002). Our data established that prior to hair initiation it was sufficient for all of the Mwh to be closely juxtaposed to In.
The accumulation of Mwh in the hair cannot be due to binding to In as this is genetically independent of in function and In is not found in the hair (Adler et al. 2004). It is likely recruited to the hair by one or more constituents of the cytoskeleton. We previously found that when expressed in striated muscle, Mwh accumulated in the region of the M band (Yan et al. 2008). Hence, we consider myosin-interacting proteins as candidates for interacting with Mwh. As noted previously (Yan et al. 2009) the C-terminal half of Mwh is sufficient and necessary for it to accumulate in hairs. This part of the protein is not conserved outside of insects and it does not contain any recognizable domains so the molecular biology of Mwh has not provided hints as to what causes it to accumulate in growing hairs. The In∷Mwh fusion protein accumulated in growing hairs in a manner similar to that of wild-type mwh. Thus, in the context of the fusion protein this Mwh accumulation pattern was epistatic to the In pattern. This was presumably mediated by the C-terminal part of Mwh in the fusion protein. The wild-type In protein is normally not found in hairs, but its presence as part of the fusion protein did not interfere with hair morphogenesis. One hypothesis for the function of In was that it functioned as an inhibitor of hair initiation (Wong and Adler 1993; Adler et al. 2004) (e.g., it could act as an inhibitor of actin polymerization). That its accumulation in growing hairs was of no consequence to hair morphogenesis argues against In being a general inhibitor of the cytoskeleton. An alternative hypothesis is that In functions to pull Mwh away from the region where hair initiation occurs. This hypothesis is consistent with the function of the fusion protein and the lack of consequence of In being present in growing hairs. Being bound to In might also be a requirement for the PPE-dependent phosphorylation of Mwh (Strutt and Warrington 2008).
The general approach of making fusion proteins should allow one to test various models for the action of proteins involved in tissue planar polarity as long as the fusion proteins are functional. Published data have shown that fusions of proteins involved in tissue planar polarity to fluorescent proteins are compatible with function (e.g., Axelrod 2001; Strutt 2001; Bastock et al. 2003; Shimada et al. 2006). Thus, there is reason to think that this approach will often be informative. The In∷Mwh protein is the first example of a functional fusion between two components of the fz pathway. In the case of the In∷Mwh fusion we had no way a priori to know how it would localize in wing cells. This will often be the case when a fusion protein contains tags that result in different localizations. For example, prior to doing the experiment it seemed possible that In would prevent the accumulation of Mwh in the hair after initiation. Such a result would have established that the In localization pattern was epistatic to that of Mwh. That result would have provided a test for the function of Mwh accumulation in the hair. Given the situation, alternative fusion strategies that were not subject to such uncertainty might be preferable although they often introduce alternative potential problems.
PPE and PCP genes and the accumulation of Mwh:
Immunostaining of wings bearing clones mutant for PPE or PCP genes showed that the upstream genes were essential for both the recruitment of Mwh to the proximal part of the cell and for the accumulation of normal levels of Mwh. In PPE mutant cells a similar decrease was seen when we measured overall and proximal edge accumulation. In contrast, in PCP mutant cells a much greater decrease was seen for Mwh specifically localized at the proximal edge. In addition, the decrease in Mwh levels was substantially greater in PPE than in PCP mutant cells. These data suggest that PPE gene function is important for both the accumulation and localization of Mwh, while PCP gene function is primarily important for localization and not accumulation. Consistent with this hypothesis we also found that Mwh and the PPE protein In could directly interact. We suggest that this interaction normally leads to both the proximal recruitment and stabilization of Mwh. In a PCP mutant cell In and Mwh would still interact although this would not be restricted to the proximal edge of the cell. These proteins being spread throughout the cell would lower their local concentration and might make the interaction less favored. This could explain the decreased level of Mwh seen in PCP mutant cells. Consistent with this hypothesis we found that In and Mwh still co-immunoprecipitated in a fz mutant cell. Further, on the basis of the multiple hair cell phenotype it has long been known that in and mwh retained some function in PCP mutants (Wong and Adler 1993) as essentially all mwh and many in mutant cells produce more than one hair. In contrast, about 98% of wing cells form a single hair in PCP mutants.
Acknowledgments
We thank Jeannette Charlton for help with many of the experiments. We thank colleagues in the fly community that provided fly stocks. This work was supported by a grant from the National Institute of General Medical Science to P.N.A.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.114066/DC1.
References
- Adler, P., J. Taylor and J. Charlton, 2000. The domineering non-autonomy of frizzled and van Gogh clones in the Drosophila wing is a consequence of a disruption in local signaling. Mech. Dev. 96 197–207. [DOI] [PubMed] [Google Scholar]
- Adler, P. N., R. E. Krasnow and J. Liu, 1997. Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr. Biol. 7 940–949. [DOI] [PubMed] [Google Scholar]
- Adler, P. N., C. Zhu and D. Stone, 2004. Inturned localizes to the proximal side of wing cells under the instruction of upstream planar polarity proteins. Curr. Biol. 14 2046–2051. [DOI] [PubMed] [Google Scholar]
- Axelrod, J., 2001. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 15 1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock, R., H. Strutt and D. Strutt, 2003. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development 130 3007–3014. [DOI] [PubMed] [Google Scholar]
- Brand, A. H., and N. Perrimon, 1993. Targetted gene expression as a means of altering cell fate and generating dominant phenotypes. Development 118 401–415. [DOI] [PubMed] [Google Scholar]
- Chae, J., M. J. Kim, J. H. Goo, S. Collier, D. Gubb et al., 1999. The Drosophila tissue polarity gene starry night encodes a member of the protocadherin family. Development 126 5421–5429. [DOI] [PubMed] [Google Scholar]
- Collier, S., and D. Gubb, 1997. Drosophila tissue polarity requires the cell-autonomous activity of the fuzzy gene, which encodes a novel transmembrane protein. Development 124 4029–4037. [DOI] [PubMed] [Google Scholar]
- Collier, S., H. Lee, R. Burgess and P. Adler, 2005. The WD40 repeat protein Fritz links cytoskeletal planar polarity to Frizzled subcellular localization in the Drosophila epidermis. Genetics 169 2035–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiguin, F., M. Hannus, M. Mlodzik and S. Eaton, 2001. The ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Dev. Cell 1 93–101. [DOI] [PubMed] [Google Scholar]
- Gubb, D., and A. Garcia-Bellido, 1982. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J. Embryol. Exp. Morphol. 68 37–57. [PubMed] [Google Scholar]
- Gubb, D., C. Green, D. Huen, D. Coulson, G. Johnson et al., 1999. The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes Dev. 13 2315–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, P. A., G. Struhl and J. Casal, 2007. Planar cell polarity: One or two pathways? Nat. Rev. Genet. 8 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H., and P. N. Adler, 2002. inturned and fuzzy are essential for the function of the frizzled pathway in the wing. Genetics 160 1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H., S. Collier and P. N. Adler, 2000. Interaction between inturned and fuzzy, two downstream genes of the fz pathway. A. Dros. Res. Conf. 41 558B. [Google Scholar]
- Montcouquiol, M., 2007. Planar polarity in mammals: similarity and divergence with Drosophila Melanosgaster. J. Soc. Biol. 201 61–67. [DOI] [PubMed] [Google Scholar]
- Park, W. J., J. Liu, E. J. Sharp and P. N. Adler, 1996. The Drosophila tissue polarity gene inturned acts cell autonomously and encodes a novel protein. Development 122 961–969. [DOI] [PubMed] [Google Scholar]
- Shimada, Y., T. Usui, S. Yanagawa, M. Takeichi and T. Uemura, 2001. Asymmetric colocalization of Flamingo, a seven-pass transmembrane cadherin, and Dishevelled in planar cell polarization. Curr. Biol. 11 859–863. [DOI] [PubMed] [Google Scholar]
- Shimada, Y., S. Yonemura, H. Ohkura, D. Strutt and T. Uemura, 2006. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev. Cell 10 209–222. [DOI] [PubMed] [Google Scholar]
- Strutt, D., 2001. Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol. Cell 7 367–375. [DOI] [PubMed] [Google Scholar]
- Strutt, D., and S. J. Warrington, 2008. Planar polarity genes in the Drosophila wing regulate the localisation of the FH3-domain protein Multiple Wing Hairs to control the site of hair production. Development 135 3103–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, J., N. Abramova, J. Charlton and P. N. Adler, 1998. Van Gogh: a new Drosophila tissue polarity gene. Genetics 150 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tree, D. R. P., J. M. Shulman, R. Rousset, M. P. Scott, D. Gubb et al., 2002. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell 109 1–11. [DOI] [PubMed] [Google Scholar]
- Usui, T., Y. Shima, Y. Shimada, S. Hirano, R. W. Burgess et al., 1999. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell 98 585–595. [DOI] [PubMed] [Google Scholar]
- Vinson, C. R., and P. N. Adler, 1987. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature 329 549–551. [DOI] [PubMed] [Google Scholar]
- Wang, Y., and J. Nathans, 2007. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development 134 647–658. [DOI] [PubMed] [Google Scholar]
- Waters, J. C., 2009. Accuracy and precision in quantitative fluorescence microscopy. J. Cell. Biol. 185 1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff, T., and G. Rubin, 1998. Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development 125 1149–1159. [DOI] [PubMed] [Google Scholar]
- Wong, L. L., and P. N. Adler, 1993. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J. Cell. Biol. 123 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, T., and G. M. Rubin, 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117 1223–1237. [DOI] [PubMed] [Google Scholar]
- Yan, J., D. Huen, T. Morely, G. Johnson, D. Gubb et al., 2008. The multiple-wing-hairs Gene Encodes a Novel GBD-FH3 Domain-Containing Protein That Functions Both Prior to and After Wing Hair Initiation. Genetics 180 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, J., C. Zhu, H. Lee and P. N. Adler, 2007. The function of inturned, fuzzy and frtiz in controlling planar polarity. A. Dros. Res. Conf. 48 465C. [Google Scholar]
- Yan, J., Q. Lu, X. Fang and P. N. Adler, 2009. Rho1 has multiple functions in Drosophila wing planar polarity. Dev. Biol. 333 186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, U. J., S. Y. Kim, J. Liu, P. N. Adler, E. Bae et al., 1999. The inturned protein of Drosophila melanogaster is a cytoplasmic protein located at the cell periphery in wing cells. Dev. Genet. 25 297–305. [DOI] [PubMed] [Google Scholar]
- Zallen, J. A., 2007. Planar polarity and tissue morphogenesis. Cell 129 1051–1063. [DOI] [PubMed] [Google Scholar]