Abstract
On the basis of the free radical and rate of living theories of aging, it has been proposed that decreased metabolism leads to increased longevity through a decreased production of reactive oxygen species (ROS). In this article, we examine the relationship between mitochondrial energy metabolism and life span by using the Clk mutants in Caenorhabditis elegans. Clk mutants are characterized by slow physiologic rates, delayed development, and increased life span. This phenotype suggests that increased life span may be achieved by decreasing energy expenditure. To test this hypothesis, we identified six novel Clk mutants in a screen for worms that have slow defecation and slow development and that can be maternally rescued. Interestingly, all 11 Clk mutants have increased life span despite the fact that slow physiologic rates were used as the only screening criterion. Although mitochondrial function is decreased in the Clk mutants, ATP levels are normal or increased, suggesting decreased energy utilization. To determine whether the longevity of the Clk mutants results from decreased production of ROS, we examined sensitivity to oxidative stress and oxidative damage. We found no evidence for systematically increased resistance to oxidative stress or decreased oxidative damage in the Clk mutants despite normal or elevated levels of superoxide dismutases. Overall, our findings suggest that decreased energy metabolism can lead to increased life span without decreased production of ROS.
MUTATIONS in clk-1 have been shown to increase longevity in both worms and mice, suggesting that these mutations affect an evolutionarily conserved mechanism of life span extension (Lakowski and Hekimi 1996; Liu et al. 2005; Lapointe et al. 2009). The CLK-1 protein encodes a hydroxylase involved in the synthesis of ubiquinone (Ewbank et al. 1997), a multifunctional, lipid-like molecule that transfers electrons in the electron transport chain and may also act as an intracellular antioxidant (Maroz et al. 2009). clk-1 was originally identified in worms in a screen for maternally rescued mutations that result in abnormal development and behavior. In addition to slow development and slow defecation, clk-1 mutants show decreased brood size, a decreased rate of thrashing, and a decreased rate of pharyngeal pumping (Wong et al. 1995). It was a surprise, however, that clk-1 worms also displayed extended longevity, because, at the time that it was discovered, only two other mutants, age-1 and daf-2, with very different phenotypes, had been found to extend longevity (Friedman and Johnson 1988; Kenyon et al. 1993).
It is currently uncertain how mutations in clk-1 result in the overall slowing of development and physiologic rates as well as an extended life span. One classic theory of aging, called the rate of living theory, postulates the existence of a link between energy metabolism and aging (Pearl 1922; Speakman 2005). This theory proposes that what determines the life span of an organism is the rate at which it produces and uses energy at the cellular level. Thus, the fact that clk-1 worms exhibit slow physiologic rates and development suggests a decrease in the rate that these worms utilize energy, and, by the rate of living theory, this could account for their long life span.
In support of the rate of living theory, the loss of clk-1 has been shown to result in decreased whole-worm oxygen consumption (Felkai et al. 1999; Yang et al. 2007) and decreased electron transfer from complex I to complex III in the electron transport chain (Kayser et al. 2004b), although this has not been observed by all investigators (Miyadera et al. 2001). While some reports have suggested that energy consumption is not reduced in clk-1 worms, at least under liquid culture conditions (Braeckman et al. 2002), the observation that clk-1 worms have higher levels of ATP than wild-type worms (Braeckman et al. 1999) suggests a decreased use of energy in clk-1 worms regardless of whether energy production is normal or decreased. It has also been found that clk-1 double-mutant combinations that exhibit slower development than clk-1 worms live even longer than clk-1 worms (Lakowski and Hekimi 1996). In addition, overexpression of clk-1 prevents the slowing of the defecation rate with age, increases mitochondrial function, and decreases life span (Felkai et al. 1999).
Drawing on ideas from the free radical theory of aging (Harman 1956), it has been suggested that a possible mechanism underlying the rate of living theory is that decreased metabolism results in a lower rate of production of reactive oxygen species (ROS). As the free radical theory of aging proposes that aging results from the accumulation of molecular damage caused by ROS, then lower ROS production should result in slower aging. In clk-1 worms, it has not been possible to directly measure levels of ROS in vivo; however, measurement of hydrogen peroxide production from submitochondrial particles has demonstrated increased ROS generation in clk-1 mitochondria compared to wild type (Yang et al. 2009). In addition, the superoxide production potential is increased in clk-1 worms compared to wild-type N2 worms (Braeckman et al. 2002). Despite showing increased levels of ROS production, clk-1 worms have been found to have normal or decreased levels of oxidative damage (Kayser et al. 2004a; Yang et al. 2007, 2009) and decreased accumulation of lipofuscin (Braeckman et al. 2002). The decrease in oxidative damage that occurs in spite of increased ROS production likely results from increased antioxidant defenses. In support of this conclusion, sod-2 and sod-3 mRNA are increased in clk-1 worms compared to wild type (Yang et al. 2007).
Clearly, the levels of ROS production and antioxidant defense are altered in clk-1 worms and likely contribute to the physiology and life span of these worms. Evidence supporting a role for altered ROS levels in determining the clk-1 phenotype comes from the demonstration that increasing the levels of ROS through decreasing superoxide dismutase expression has been shown to modulate a variety of phenotypes in clk-1 worms (Shibata et al. 2003; Yang et al. 2007). It is important to note, however, that the decrease in oxidative damage in clk-1 worms appears not to contribute to their long life as it is possible to experimentally increase oxidative damage in clk-1 worms beyond wild-type levels without reducing life span (Yang et al. 2007).
In addition to clk-1, four other genes have been identified that yield a clk-1-like phenotype (Clk phenotype), which includes slow development, slow defecation, slow pharyngeal pumping, decreased brood size and long life span coupled with maternal rescue (homozygous mutants from heterozygous mothers are phenotypically normal) (Hekimi et al. 1995; Lemieux et al. 2001). The Clk phenotype has been studied in most detail in clk-1 worms (Wong et al. 1995) and, subsequently, with gro-1 (Lemieux et al. 2001), clk-2 (Benard et al. 2001), and tpk-1 worms (de Jong et al. 2004), while clk-3 worms have not been extensively studied [although clk-3 worm energy metabolism and oxygen consumption have been examined (Braeckman et al. 2002; Shoyama et al. 2009)]. Despite the phenotypic similarity of these mutants, the mutations that have been identified thus far have been shown to occur in genes encoding proteins with a wide range of functions with no obvious relationship to one another. gro-1 encodes a tRNA-modifying enzyme (Lemieux et al. 2001), clk-2 encodes a homolog of yeast Tel2p and a regulator of several PI3K-related protein kinases (Ahmed et al. 2001; Benard et al. 2001; Jiang et al. 2003; Takai et al. 2007), and tpk-1 encodes thiamine pyrophosphokinase, which is necessary for the assimilation of thiamine (vitamin B1) (de Jong et al. 2004).
All of the Clk mutants that have been identified exhibit slow physiologic rates and increased life span, suggesting that one may be sufficient for the other. To test this hypothesis, we identified six novel Clk mutants and demonstrate that these strains bear all of the characteristic features of the Clk phenotype, including extended longevity. We further show that mitochondrial function is decreased in the Clk mutants but that this decrease does not result in increased resistance to oxidative stress or decreased oxidative damage. Our results provide a plausible explanation for the extended life span observed in the Clk mutants and support aspects of the rate of living theory of aging while casting further doubt on the free radical theory of aging.
MATERIALS AND METHODS
General methods and strains:
Caenorhabditis elegans strains were cultured as described (Brenner 1974). Animals were maintained on nematode growth medium (NGM) agar plates, which were seeded with OP50, a slow-growing mutant of Escherichia coli. All strains were maintained at 20°. Wild-type animals were N2 Bristol strain. Mutations used were in the Bristol background unless specified. The following mutations, mutant combinations, and strains were used for mapping: LGI—unc-11(e47), unc-54(e190), unc-35(e259); LGII—dpy-10(e128), clk-3(qm38), unc-52(e444), dpy-10(e128) unc-4(e120), unc-4(e120) bli-1(e769), unc-85(e1414) dpy-10(e128), unc-4(e120) daf-19(m86), unc-85(e1414); LGIII—dpy-17(e164), unc-32(e189), unc-25(e156), unc-45(e286), dpy-1(e1), clk-1(qm30), dpy-17(e164) unc-32(e189), unc-93(e1500sd) dpy-17(e164), dpy-17(e164) unc-79(e1068), clk-2(qm37), gro-1(e2400); DA438—bli-4(e937)I; rol-6(e187)II; daf-2(e1368) vab-7(e1562)III; unc-31(e928)IV; dpy-11(e224)V; lon-2(e678)X; RW7000—Bergerac strain that contains ∼500 Tc1 loci, used for STS mapping (Williams 1995). During these experiments we also generated the following double-mutant strains: daf-2;clk-2, daf-2;clk-3, daf-2;clk-4, daf-2;clk-5, daf-2;clk-7, daf-2;clk-8, daf-2;clk-9, daf-2;clk-10, and daf-2;gro-1.
Isolation of mutants:
Screening for Clk mutants was carried out as previously described (Wong et al. 1995). Briefly, wild-type animals (P0) at the L4 or young adult stage were mutagenized with ethyl methanesulfonate. Groups of 15 mutagenized relatively young adult hermaphrodites (P0) were plated on 9-cm petri dishes and allowed to self-fertilize. They were removed after having laid ∼300 eggs (20 eggs each). The resulting F1 animals were left on the plate until they had laid a total of ∼6000–10,000 F2 eggs and were then removed from the plates. When most resulting F2 animals had grown to adulthood, 30 adults with no detectable morphological or behavioral mutant phenotype were transferred onto individual small plates. Strains issued from those F2 animals that produced an entire brood of slow growing, but anatomically wild-type, worms that also had a slow defecation cycle were analyzed further.
To determine further the heritability of these mutations, hermaphrodites from candidate mutant strains (m/m) were mated to N2 males and three or four of the resulting cross-progeny (F1) were selected. The self-progeny (F2) of these individuals were then examined for the presence of phenotypically wild-type animals. When ∼25% of the F2's showed the mutant phenotype, the strain was discarded because this suggested that the animal originally picked was an escaper, that is, an animal carrying a fully zygotic mutation that was not strongly expressed. When substantially <25% of the F2 animals displayed the mutant phenotype, 24 phenotypically wild-type F2's were picked onto individual plates and their F3 progeny were examined. In most cases, ∼25% of the F3 broods were composed almost entirely of animal displaying a mutant phenotype. When none of the F3 broods displayed a fully mutant phenotype, the procedure was repeated. When the repeated procedure was still unsuccessful at obtaining fully mutant F3 broods, we considered that the phenotype of the original strain was synthetic in origin, that is, due to more than one independently segregating mutation, and the strain was discarded.
A total of 10,600 F2 animals and their progeny were screened in the way described above, and six new clk mutants were isolated. All of the mutants were outcrossed with the wild type at least three times. One of the mutants described here [(clk-10(qm169)] was isolated serendipitously in a screen for slow-growing mutants without maternal effect.
Mapping of mutations:
Mutations were linked to chromosomes in one of three ways. Initially, linkage analysis was performed by using the DA438 strain, which carries at least one marker for each chromosome (Avery 1993). When this did not yield a significant result, linkage to single-marker mutations was performed, or linkage to STSs by PCR analysis (for qm151) was performed as described (Williams 1995). Once linkage to a chromosome was established, some mutations were mapped more precisely using standard 2- and 3-point mapping strategies.
Assays for developmental and behavioral phenotypes:
All analyses were carried out at 20°.
Embryonic lethality:
Ten young, egg-laying adult hermaphrodites that had reached adulthood before they were picked were placed on fresh plates and allowed to lay eggs for 4–6 hr. A number of eggs were then picked from this plate onto a fresh plate, and the number of unhatched eggs was counted after 48 hr.
Embryonic development:
Adult hermaphrodites were placed in a drop of M9 buffer and cut open with a razor blade to release the eggs. Two-celled eggs were chosen and placed onto fresh plates. The embryos were monitored every hour until hatching.
Self-brood size:
L4 worms were placed onto fresh plates and incubated until they had matured and had laid the first few eggs. The hermaphrodites were then transferred onto fresh plates daily to prevent overcrowding until egg laying ceased. The total number of progeny produced were counted once the offspring had matured to adulthood.
Life span:
Life-span studies were completed as previously described (Yang et al. 2007; Van Raamsdonk and Hekimi 2009) on either normal NGM plates or plates supplemented with 100 μm fluorodeoxyuridine (FUdR). Animals were scored as dead when they no longer responded with movement to light prodding on the head.
Defecation:
Young adult hermaphrodites were placed singly onto fresh plates, and the length of the defecation cycle of the animals was measured as the time between consecutive pBoc contractions (the pBoc contraction is the contraction of the posterior body muscle that initiates defecation).
Mobility span:
Mobility-span assays were completed on NGM plates supplemented with 100 μm FUdR. To assess mobility, a circle was drawn around each worm and the plate was left for ∼5 min. Worms that were visibly mobile or had moved outside of the circle were scored as mobile. Worms still present in the circle were scored as immobile. Worms that were able to move only their head were scored as immobile. Twenty worms per plate were examined with three trials per strain.
Scoring of maternal rescue:
Defecation:
Wild-type males were mated to homozygous mutant hermaphrodites, and 10 of the resulting wild-type F1 hermaphrodites (clk/+) were pooled and allowed to lay self-fertilized eggs for a 4-hr period. F2 animals were scored and singled. Subsequent analysis of the phenotype of the F3 broods, which issued from each F2 animal, allowed us to determine which F2's were maternally rescued homozygotes, as indicated by the slow growth of the entire F3 brood.
Post-embryonic development:
Wild-type males were mated to homozygous mutant hermaphrodites, and 10 of the resulting wild-type F1 hermaphrodites (clk/+) were pooled and allowed to lay F2 eggs for a 4-hr period. About 200 eggs were picked and left for 3 hr to hatch. All larvae that had hatched during that period were placed onto individual fresh plates and monitored once every 3 hr until the final development of the vulva. Their genotype was determined subsequently as for defecation.
Oxygen consumption and ATP levels:
Oxygen consumption was measured as previously described with minor modifications (Suda et al. 2005). Briefly, five first-day adult worms were picked into 0.25 μl M9 buffer of a 0.5-μl sealed tiny chamber at 22°. A fiber optical oxygen sensor (AL300 FOXY probe from Ocean Optics) was inserted into this chamber, and oxygen partial pressure was monitored for 15–30 min. The change in oxygen levels during this period was calculated as oxygen consumption. Oxygen consumption is presented per milligram protein. To quantify protein levels, worms were frozen and stored at −80°. Subsequently, frozen worm pellets were broken to powder in liquid nitrogen with mortar and pestle. The resulting powder was dissolved in 200 μl NET buffer (50 mm Tris–HCl, pH 7.5, 150nM NaCl, 0,1% NP-40, 1 mm EDTA). After brief centrifugation, the supernatant was transferred to a new tube, and protein concentration was determined by the Bradford method using a Bio-Rad protein assay kit.
For ATP measurement, ∼200 synchronized young adult worms were collected in M9 buffer and washed three times. Worm pellets were treated with three freeze/thaw cycles and boiled for 15 min to release ATP and destroy ATPase activity and then spun at 4° and 11,000 × g for 10 min. ATP contents were measured with an ATP detection kit according to the manufacturer's instructions (Invitrogen, Carlsbad, CA).
Western blotting for SOD-1 and SOD-2 protein:
One hundred young adult worms of each genotype were picked, lysed in 2× loading buffer, electrophoresed on 12% SDS–polyacrylamide gels, and then blotted onto nitrocellulose membrane (Bio-Rad). After applying primary antibody (1:1000, anti-SOD1 or anti-SOD2) and secondary antibody (1:10,000 mouse anti-rabbit IgG; Invitrogen), the membranes were incubated with the ECL plus detection reagent (Amersham Biosciences) and scanned using a Typhoon trio plus scanner. The anti-SOD1 and anti-SOD2 antibodies were generated in our laboratory (Yang et al. 2007).
Generation of daf-2 double mutants:
To isolate Clk mutants on a daf-2 background, daf-2 males were crossed with Clk mutant hermaphrodites. The resulting F1 progeny were left to self-fertilize, and the F2 worms were singled out and grown at 25°. The F2 worms producing slow-growing dauer [daf-2(e1370) are dauer constitutive at 25°] progeny were considered to be clk; daf-2 double mutants. For Clk genes linked to the same chromosome as daf-2 mutants, dauer non-Unc recombinants of daf-2unc-32/clk hermaphrodites were picked at 25° and then transferred to 15° to recover from the dauer stage. The recombinants were then singled out on plates at 20°. The plates with slow F2 worms were considered to contain a clk;daf-2 double mutant. The genotype of all double mutants was checked through a complementation test.
Sensitivity to oxidative stress and oxidative damage:
Juglone sensitivity was assessed in 1-day-old adult worms on plates containing 240 μm juglone (Sigma). For this assay, plates were made fresh on the day of the assay as the toxicity of juglone decreases rapidly over time. Survival was monitored for 4 hr. To assess the ability of worms to develop under oxidative stress, a minimum of 40 eggs were placed on plates containing either 0.2 or 0.3 mm paraquat and seeded with OP50 bacteria.
Oxidative damage was assessed using an Oxyblot assay kit (Millipore) to detect carbonylated proteins. In this assay, carbonyl groups are derivatized to 2,4-dinitrophenylhydrazone (DNP-hydrazone), which can then be detected by Western blotting with a DNP-specific antibody. The Oxyblot assay was completed according to the manufacturer's protocol. Protein (7 μg) was used in each lane. After detection, membranes were stripped and reprobed with an anti-tubulin antibody to control for loading.
Statistical analysis:
Survival plots were compared using the log-rank test. Significance between strains for juglone sensitivity assays were assessed by ANOVA. The effect of SOD RNA interference (RNAi) on development was assessed using a Student's t-test.
RESULTS
Clk mutants exhibit slow development and slow physiologic rates:
The Clk phenotype in C. elegans is characterized by slow development, slow defecation, decreased brood size, slow pharyngeal pumping. and increased life span (slow aging), all of which are maternally rescued. Thus far, five Clk mutants have been identified (clk-1, clk-2, clk-3, gro-1, and clk-8/tpk-1). All five Clk mutants have extended longevity despite the fact that they were identified based on their slow development and physiology. This suggests that slowing development and physiology may be sufficient to extend life span in C. elegans. To further investigate this hypothesis, we sought to determine whether we could identify additional long-lived mutants by screening for worms with slow development and slow defecation that are maternally rescued. Accordingly, we repeated the screen used to identify clk-1, clk-2, and clk-3 worms (Hekimi et al. 1995; Wong et al. 1995). In total, we screened the progeny of 10,600 F2's and identified six novel morphologically normal mutants with slow development and defecation [we also identified clk-8 in this screen, which we have partially described elsewhere (de Jong et al. 2004); this strain will be referred to as clk-8/tpk-1].
Having identified the six new Clk mutants in the screen, we next sought to characterize their phenotype in detail in comparison to the wild type (N2) and clk-1(qm30) worms. As with clk-1 worms, all of the newly identified mutants exhibited slow embryonic development, slow post-embryonic development (PED), slow defecation, and a reduced brood size (Figure 1, A–D; Figure S1; Table S1). Post-embryonic phenotypes (PED and defecation) were wild type in heterozygous animals issued from homozygous mothers, thereby indicating that the mutations are all recessive (data not shown). In addition, we confirmed that these phenotypes are maternally rescued by assessing embryonic development and defecation in homozygous mutant offspring of heterozygous mothers (Figure 1, B and D; Table S1). Overall, all 11 Clk mutants exhibit slow development and physiologic rates, which is consistent with decreased energy utilization.
Figure 1.—
Clk mutants exhibit an overall slowing of development and physiologic rates. Embryonic development time (A), post-embryonic development time (B), and defecation cycle length (D) are increased in all Clk mutants compared to wild-type N2 worms. Post-embryonic development time (B) and defecation cycle length (D) show almost complete maternal rescue. Self-brood size (C) is also decreased in all Clk mutants compared to wild-type worms. All of these phenotypes suggest decreased energy expenditure in the Clk mutants. ***P < 0.001.
Clk mutants show extended life span:
To determine whether the slow development and physiology of the Clk mutants is sufficient for increased longevity, we examined the life span of the Clk mutants. The life span of clk-1, clk-2, clk-3, and gro-1 have previously been shown to be increased (Lakowski and Hekimi 1996). Despite the fact that long life was not one of the screening criteria at any time, all 11 Clk mutants exhibited significantly increased life span (Table S1, Figure S2A). The fact that the mutants identified in the screen bear all of the characteristics of the Clk phenotype suggests that we have identified six novel Clk mutants, thereby bringing the total number of Clk mutants to 11. Complementation tests were carried out between all 11 mutants and no allelism was found. In addition, all 11 genes were found to map to distinct locations (Hekimi et al. 1995) (mapping data are summarized in Table S2).
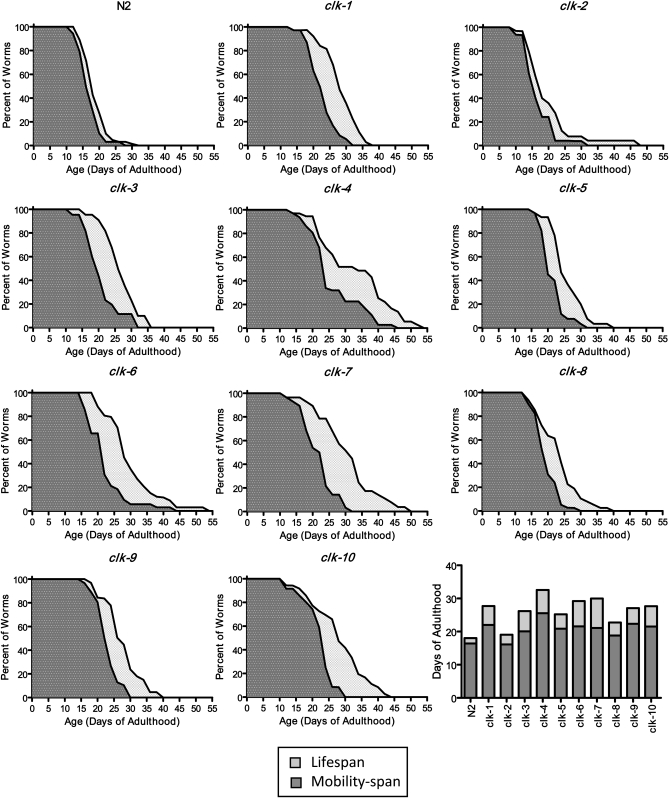
In addition to living longer, we sought to determine whether the physiologic fitness (also referred to as health span) of the Clk mutants was increased compared to wild type. For this purpose, we measured the mobility span of the Clk mutants. Mobility span is a measure of how long the worms remain active, as death in worms is normally preceded by a period of immobility of variable length (Huang et al. 2004). Wild-type worms remained mobile for most of their life span and died within one and a half days of becoming immobile (Figure 2). With the exception of clk-2 mutant worms, which remained mobile as long as wild-type worms, all of the Clk mutants had a longer period of mobility than wild-type worms (Figure 2). In addition, after the point in time when worms became immobile, all of the Clk mutants survived longer than wild-type worms. Thus, Clk mutants exhibit a longer life span and health span compared to wild-type worms.
Figure 2.—
Clk mutants have increased life span and mobility span. To determine whether the slowing of physiologic rates and development resulted in early immobility and thus a reduced health span, we examined the mobility span of Clk mutants. The mobility span was defined as the period of time where a worm exhibited voluntary, directional movement. Wild-type N2 worms remain mobile for most of their life span and become immobile only 1–2 days before they die. With the exception of clk-2 worms, the Clk mutants exhibited increased mobility spans compared to wild-type worms (dark shading). The Clk mutants also exhibited increased survival in a state of immobility compared to N2 worms (light shading). In the bar graph, the height of the darkly shaded bar indicates the mobility span, the height of the lightly shaded bar indicates the period of immobility, and the total bar height indicates the life span.
Clk mutants have decreased energy utilization:
To gain further support for decreased energy utilization in the Clk mutants, we measured mitochondrial function as an estimate of energy production, and steady-state levels of ATP. It has previously been shown that clk-1 mutants have decreased mitochondrial function (Felkai et al. 1999; Yang et al. 2007) and increased levels of ATP (Braeckman et al. 1999). Measurement of whole-worm oxygen consumption in day 1 adult worms revealed that all of the Clk mutants except for clk-3 showed decreased oxygen consumption compared to wild-type worms, indicating decreased mitochondrial function (Figure 3A, Figure S2B). As others have observed decreased oxygen consumption in clk-3 mutants (Shoyama et al. 2009), this indicates that under certain conditions all 11 Clk mutants have decreased oxygen consumption despite the fact that they were identified in a screen for slow development and defecation. This decrease in mitochondrial function provides a plausible mechanism for the long life of the Clk mutants as decreased expression of mitochondrial genes has been shown to result in increased life span (Dillin et al. 2002; Lee et al. 2003).
Figure 3.—
Clk mutants have decreased energy production but normal levels of ATP. To determine whether energy utilization is decreased in Clk mutants, we examined mitochondrial function and ATP levels. (A) Whole-worm oxygen consumption was measured to assess mitochondrial function in day 1 adult worms. With the exception of clk-3, all of the Clk mutants showed decreased oxygen consumption compared to wild-type N2 worms. This suggests that Clk mutants produce less energy than wild-type worms. (B) Measurement of ATP levels in day 1 adult worms revealed that Clk mutants do not have decreased ATP. In combination with their decreased mitochondrial function, this finding suggests decreased energy utilization. (C) We also examined the decline in mitochondrial function among the Clk mutants. With the exception of clk-8/tpk-1 worms, we found that oxygen consumption declined more gradually in the Clk mutants than in wild type, suggesting a more balanced production of energy throughout their life span than in wild-type worms. *P < 0.05, **P < 0.01, ***P < 0.001.
Next, we examined ATP levels to determine if the decreased mitochondrial function resulted in decreased levels of ATP. Examination of ATP levels revealed that none of the Clk mutants have decreased levels of ATP (Figure 3B). In fact, clk-6 mutants have significantly increased ATP levels, and there is a trend toward increase in clk-1 and clk-10 mutant worms. The fact that ATP levels are normal or increased despite decreased mitochondrial function suggests that the Clk mutants have decreased energy expenditure compared to wild-type worms.
To determine whether the mitochondrial function of the Clk mutants remains low throughout their life span, we examined oxygen consumption in day 7 adults (a comparison of oxygen consumption at day 1 and day 7 is shown in Figure S3). In N2 worms, oxygen consumption declines with age such that at day 7 the oxygen consumption is ∼60% of its day 1 oxygen consumption (Figure 3C). In contrast, all of the Clk mutants except the clk-8 worms do not show any decrease in oxygen consumption from day 1 to day 7 (Figure 3C). Thus, while mitochondrial function declines with age in wild-type worms, it remains relatively constant during the first week of adulthood in the Clk mutants. A more gradual decline in oxygen consumption over time in long-lived mutants has also been observed in isp-1 and daf-2 worms (Shoyama et al. 2009) and in mouse mutants with only one copy of Mclk1, the mouse homolog of clk-1 (Lapointe et al. 2009).
Clk mutants do not show increased resistance to oxidative stress:
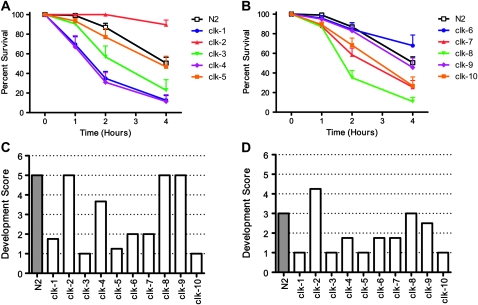
The decreased mitochondrial function and slow development and physiology of the Clk mutants suggest a slow rate of living, which may result in decreased production of ROS. According to the free radical theory of aging, decreased production of ROS could account for the extended life span of the Clk mutants. As the direct measurement of ROS levels in vivo can be unreliable (Lambert and Brand 2009; Murphy 2009), we chose instead to examine sensitivity to oxidative stress, which assesses the combination of ROS production and antioxidant defenses. To test whether the Clk mutants had increased resistance to oxidative stress, we exposed the Clk mutants to juglone, a compound known to raise intracellular concentrations of superoxide. If ROS levels were decreased in the Clk mutants, we would expect that these worms would be more resistant to increasing levels of ROS caused by exposure to juglone than wild-type N2 worms. However, we find no evidence of increased resistance to oxidative stress in the Clk mutants.
On exposure to 240 μm juglone, wild-type N2 worms begin to die after 2 hr and approximately half are dead after 4 hr on juglone plates (Figure 4A). Examination of the sensitivity to oxidative stress among the Clk mutants reveals that only clk-2 worms are more resistant to oxidative stress than wild-type N2 worms (Figure 4, A and B). The resistance of clk-2 worms to juglone-induced oxidative stress has been previously reported (Johnson et al. 2001). In contrast, clk-1, clk-3, clk-4, clk-7, clk-8/tpk-1, and clk-10 worms are more sensitive to oxidative stress than wild-type worms (Figure 4, A and B). Thus, the long life observed in the Clk mutants does not result from increased resistance to oxidative stress. It is also important to note that the increased sensitivity to oxidative stress observed in the Clk mutants does not result in a reduced life span.
Figure 4.—
Clk mutants are not resistant to oxidative stress. (A and B) Sensitivity to oxidative stress of the Clk mutants was assessed by exposing adult worms to 240 μM juglone. Of the 10 Clk mutants assessed, only clk-2 worms showed increased resistance to oxidative stress compared to wild-type N2 worms. In contrast, clk-1, clk-3, clk-4, clk-7, clk-8, and clk-10 worms were more sensitive to oxidative stress than N2 worms. Sensitivity to oxidative stress during development was assessed by transferring eggs to plates containing either 0.2 mM (C) or 0.3 mM (D) paraquat. Under these conditions, only clk-2 worms were able to develop to a developmental stage further than wild-type worms. In contrast, clk-1, clk-3, clk-4, clk-5, clk-6, clk-7, and clk-10 worms all arrested at an earlier stage in development than N2 worms. Overall, Clk mutants are not more resistant to oxidative stress than wild-type worms either during development or during adulthood. In most cases, Clk mutants were more sensitive to oxidative stress than wild-type worms.
Next, we examined the development of Clk mutants on plates containing paraquat, another compound that increases intracellular levels of superoxide, to determine whether Clk mutants might be more resistant to oxidative stress during development. For this experiment, we transferred eggs onto NGM plates supplemented with 0.2 or 0.3 mm paraquat and determined the latest developmental stage achieved. We utilized a semiquantitative approach whereby each strain was given a score according to the furthest developmental stage attained (1 = L1/L2, 2 = L3/L4, 4 = adult, 5 = fertile adult). As with adult worms, we found no evidence that Clk mutants were more resistant to oxidative stress during development than wild-type worms and found in many cases that the Clk mutants were more sensitive than wild-type worms.
At 0.2 mm paraquat, clk-2, clk-8/tpk-1, and clk-9 worms developed as well as wild-type worms while clk-1, clk-3, clk-4, clk-5, clk-6, clk-7, and clk-10 worms showed early developmental arrest in response to oxidative stress (Figure 4C). At a higher concentration of 0.3 mm paraquat, we found that only clk-2 worms grew to a further developmental stage than wild-type worms (Figure 4D). In contrast, clk-1, clk-3, clk-4, clk-5, clk-6, clk-7, clk-9, and clk-10 worms all showed developmental deficits compared to N2 worms (Figure 4D). Thus, aside from clk-2 and clk-8/tpk-1, all of the Clk mutants appear to be more sensitive to oxidative stress during development than wild-type worms, indicating that, as in adulthood, most of the Clk mutants do not have increased resistance to oxidative stress.
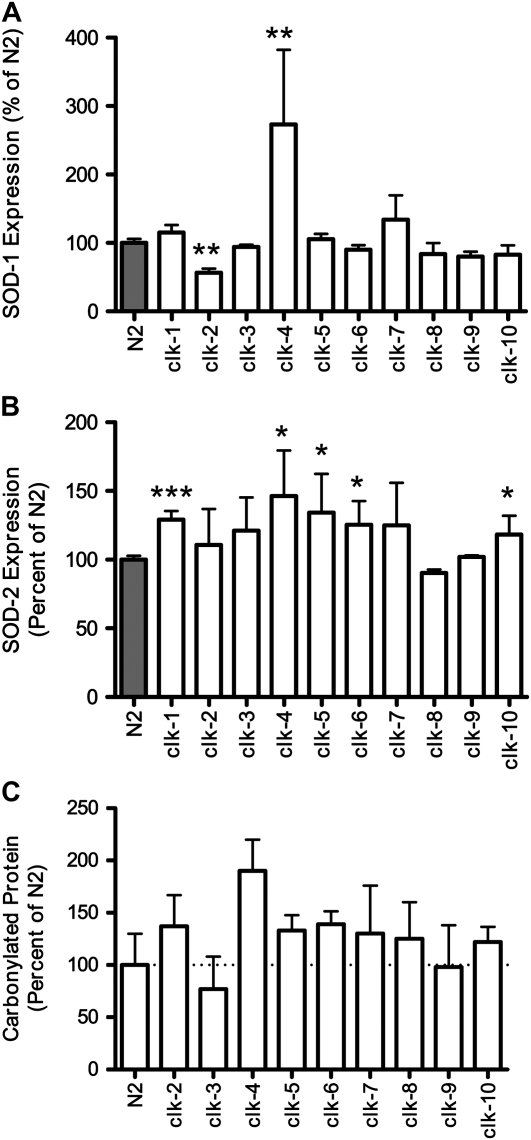
While increased sensitivity to oxidative stress suggests increased levels of ROS, this may also result from a decrease in antioxidant defense mechanisms. To investigate this possibility, we examined the levels of SOD-1 and SOD-2 protein in each of the Clk mutants. Examination of SOD-1 levels by Western blotting revealed a significant increase in SOD-1 expression in clk-4 and gro-1 worms and a significant decrease in SOD-1 protein in clk-2 worms (Figure 5A, Figure S2C). In the remainder of the Clk mutants, SOD-1 levels were unchanged from wild-type levels (Figure 5A). In contrast, SOD-2 levels appeared to be increased in most of the Clk mutants, although the increase was significant only in the case of clk-1, clk-4, clk-5, clk-6, clk-10, and gro-1 worms (Figure 5B, Figure S2C). Only two Clk mutants, clk-8/tpk-1 and clk-9, failed to show any increase in SOD-2. Overall, SOD protein levels appear to be unchanged or increased among the Clk mutants. The increase in SOD-2 protein levels suggests that ROS generation may be increased in the mitochondria, which is consistent with defects in mitochondrial function.
Figure 5.—
Clk mutants have increased expression of SOD2 and no decrease in oxidative damage. On the basis of their increased sensitivity to oxidative stress, we examined the levels of SOD1 and SOD2 protein to determine whether the increased sensitivity resulted from a down-regulation of anti-oxidant defense proteins. (A) Examination of SOD-1 protein levels revealed increased SOD-1 in clk-4 worms but decreased levels in clk-2 worms. (B) SOD-2 levels were increased in most of the Clk mutants with the difference reaching significance in clk-1, clk-4, clk-5, clk-6, and clk-10 worms. The fact that SOD protein levels were not decreased in Clk mutants suggests that the increase in sensitivity to oxidative stress results from increased levels of ROS production and not from decreased anti-oxidant defense. (C) Next we sought to determine whether the increase in SOD2 expression was sufficient to limit or reduce oxidative damage in the Clk mutants. By Western blotting for carbonylated proteins, we found no evidence for decreased oxidative damage in the Clk mutants. Thus, Clk mutants appear to have increased ROS production, which results in upregulation of SOD2 protein, but this increase in anti-oxidant capacity is not enough to reduce levels of oxidative damage.
Since SOD levels were significantly increased in some of the Clk mutants, we sought to determine whether this increase was sufficient to result in decreased oxidative damage. If this were the case, then the extended longevity could potentially be explained by an increase in antioxidant defenses. To examine oxidative damage, we performed a Western blot for carbonylated proteins. Quantification of the resulting blots revealed no evidence for decreased oxidative damage in the Clk mutants (Figure 5C). In most cases, we observed a trend toward increased oxidative damage in the Clk mutants. Thus, it appears that the Clk mutants are more susceptible to oxidative damage than wild-type worms and that any compensatory upregulation of antioxidant defense is not sufficient to reduce oxidative damage.
Synergistic effects of Clk mutants and daf-2 life span:
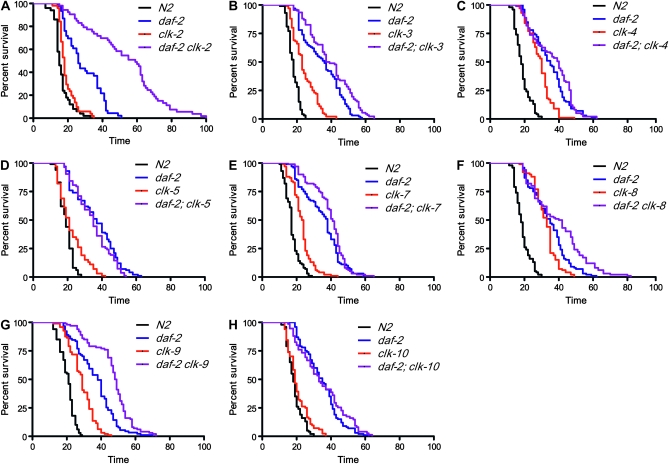
Deletion of clk-1 has been shown to markedly extend the life span of daf-2 worms such that clk-1;daf-2 worms live much longer than if the already extended life spans of clk-1 and daf-2 were added together (Lakowski and Hekimi 1996; Hekimi et al. 2001; Burgess et al. 2003). If the synergism between clk-1 and daf-2 life spans occurs at the phenotypic level, we would expect the other Clk mutants to similarly extend the life span of daf-2 worms, while if the mechanism occurs at a molecular level, this marked extension of daf-2 life span would be expected to be specific to clk-1. To test these predictions, we constructed all double-mutant combinations of Clk mutations and daf-2(e1370) and scored their life spans (Figure 6, Figure S2D). We found three main types of interactions with daf-2:
Strongly synergistic interaction is shown by clk-1 and clk-2. This interaction is characterized by life-span extension well beyond the addition of the two single-mutant life spans. This is especially notable in the case of clk-2, which by itself lived only marginally longer than wild-type N2 worms in these experiments.
Fully or partially additive interactions with daf-2 are exhibited by clk-3, clk-7, clk-8, clk-9, clk-10, and gro-1.
No additivity was observed for clk-4 and clk-5.
Figure 6.—
Interaction of clk mutations with the insulin/IGF-signaling pathway of life-span extension. On the basis of the dramatic extension of daf-2 life span by clk-1, we crossed the other Clk mutants with daf-2 worms to determine whether these mutations also exhibited synergistic effects on life-span extension. clk-2 (A) mutation was found to dramatically increase the life span of daf-2 worms despite only marginally increasing life span in a wild-type background. clk-3 (C), clk-7 (E), clk-8 (F), clk-9 (G, and clk-10 (H) mutations exhibited additive effects on daf-2 life span in that they extend daf-2 life span and wild-type life span by similar amounts. In contrast, clk-4 (D) and clk-5 (E) mutations did not increase the life span of daf-2 worms and thus are non-additive.
Correlations between phenotypes:
Having completed the phenotypic characterization of the Clk mutants, we examined the data set for correlations between the different phenotypes with a particular focus on life span (Table S3). The strongest phenotypes correlating with life span were embryonic development time (R2 = 0.42) and mobility span (R2 = 0.41). Mobility has previously been suggested as a good predictor of life span (Huang et al. 2004). Oxygen consumption on day 1 appeared to be correlated with post-embryonic development, brood size, and especially defecation cycle length (R2 = 0.43, 0.41, 0.79). Interestingly, SOD1 and SOD2 protein levels correlated with mobility span and with the ratio of oxygen consumption on day 7 to that on day 1.
DISCUSSION
In this article, we describe the identification of 6 novel Clk mutants and further characterize the 5 previously described Clk mutants. We find that all 11 Clk mutants exhibit slow development, slow defecation, decreased brood size, and long life span. The Clk mutants also have decreased mitochondrial function (this study and Shoyama et al. 2009), which does not lead to increased resistance to oxidative stress or decreased oxidative damage. Our findings support the rate of living theory of aging and suggest that the mechanism by which decreased energy utilization leads to long life is not the result of increased resistance to oxidative stress.
Mutations in diverse and molecularly unrelated genes lead to a Clk phenotype:
In the screen described here, we identified six previously undescribed mutants that possess all of the characteristic phenotypes of Clk mutants, including maternal rescue. On the basis of the number of Clk mutants that have been identified thus far, it is clear that many genes can mutate to yield the Clk phenotype. Furthermore, the group of genes that can mutate to yield the Clk phenotype appears to be quite diverse as the genes with currently defined functions appear to be unrelated (see the Introduction for a description of gene functions). The observation of maternal rescue in all of these strains suggests a critical role of normal gene function during development in preventing the Clk phenotype because the maternally donated gene product in a single egg is sufficient to normalize the phenotype of a 1000-cell adult. The fact that all of the Clk phenotypes occur together as linked phenotypes suggests the possibility of a common process, or response, as the basis of the Clk phenotype.
Clk mutants have decreased mitochondrial function:
Examination of mitochondrial function revealed that oxygen consumption is decreased in all of the Clk mutants, except for clk-3. It has been previously noted that clk-3 worms show certain abnormalities such as a large body size (Braeckman et al. 2002), which may contribute to our inability to detect a decrease in oxygen consumption in these worms. It is also possible that these worms have altered mitochondrial function but not an overall decrease in oxygen consumption and that these changes in mitochondrial function have a similar effect on the alterations in the other Clk mutants. The fact that other researchers have observed decreased mitochondrial function in clk-3 worms under different conditions and at different time points than we used here suggests that at least under certain conditions and at certain time points clk-3 worms have decreased oxygen consumption (Shoyama et al. 2009). Overall, decreased mitochondrial function appears to be a common feature among the Clk mutants.
Interestingly, it has previously been shown in C. elegans that decreases in mitochondrial function generally result in increased life span. isp-1 encodes an iron sulfur protein in mitochondrial complex III, and mutations in this protein result in decreased respiration, slow development, slow defecation, decreased brood size, and increased life span (Feng et al. 2001). Similarly, mutations in sod-2, which encodes the primary mitochondrial superoxide dismutase, result in decreased respiration, slow development, slow defecation, decreased brood size, and increased life span (Van Raamsdonk and Hekimi 2009). In fact, the association of disturbed mitochondrial function and increased life span has been clearly demonstrated in RNAi screens (Lee et al. 2003) and for proteins in all parts of the electron transport chain except complex II (Dillin et al. 2002). The effect of inhibition of mitochondrial function by RNAi appears to be most critical during development as RNAi against mitochondrial proteins fails to extend life span in adult worms (Dillin et al. 2002; Rea et al. 2007). Thus, the maternal rescue observed in the Clk mutants can perhaps be explained by the presence of maternally deposited gene product at a critical period of development. In addition, it has recently been proposed that altered mitochondrial membrane potential may be a common mechanism underlying multiple longevity pathways (Lemire et al. 2009).
For those clk genes that have been identified, there exists at least some evidence linking their function to mitochondrial energy metabolism. While the connection of CLK-1 to the mitochondria is straightforward, CLK-2 has a somewhat more indirect link to mitochondrial function. Vertebrate CLK-2 regulates a number of different phosphatidylinositol 3-kinase-related protein kinase targets (Hayashi et al. 2006), one of which, the energy-sensing mTOR, is known to regulate and be regulated by mitochondrial function (Schieke and Finkel 2007). gro-1 acts in both the cytoplasm and mitochondria; however, it is interesting to note that the mutant phenotype has been shown to be due only to the absence of GRO-1 in mitochondria (Lemieux et al. 2001). Finally, TPK-1 is involved in the synthesis of the cofactor thiamine pyrophosphate, which is necessary for several crucial biochemical reactions involved in mitochondrial energy metabolism (de Jong et al. 2004).
Clk mutants are not more resistant to oxidative stress:
When C. elegans mutants with decreased mitochondrial function were initially found to have increased life span, it was suggested that this may result from decreased production of ROS [e.g., isp-1 (Feng et al. 2001)]. However, since other mutations that decrease mitochondrial function result in increased ROS [e.g., mev-1 and gas-1 (Ishii et al. 1990; Kayser et al. 2001)], the effect of ROS production appears to depend on how mitochondrial function is decreased. As direct measurement of ROS levels in vivo can be unreliable (Lambert and Brand 2009; Murphy 2009), we instead chose to examine sensitivity to oxidative stress in the Clk mutants. While increased sensitivity to oxidative stress could result from either increased ROS production or decreased antioxidant defenses, in both cases the net result should be an increase in oxidative damage. We found that, with the exception of clk-2 worms, which have previously been shown to be resistant to oxidative stress (Johnson et al. 2001), all of the Clk mutants exhibited increased sensitivity to oxidative stress during development, during adulthood, or both. As increased sensitivity to oxidative stress could result from increased ROS production or decreased antioxidant defense, we measured the levels of SOD protein and observed normal or increased levels in all of the Clk mutants except clk-2 worms, which showed a decrease in SOD-1 protein levels. In fact, many of the Clk mutants exhibited a significant increase in SOD-2 levels, suggesting that increased mitochondrial ROS may be triggering the upregulation of SOD-2. The fact that SOD protein levels were not decreased suggests that the increased sensitivity to oxidative stress in the Clk mutants results from increased ROS production, although this was not directly tested. In addition, the levels of carbonylated proteins were not decreased in any of the Clk mutants, suggesting that their longevity is not dependent on lowering oxidative damage.
It is interesting to note that many of the Clk mutants exhibited increased expression of SOD-2 in combination with increased sensitivity to oxidative stress. While this appears to be counter-intuitive, there are two possible explanations. In cases such as clk-5 and clk-6, where sensitivity to oxidative stress is observed in development but not in adulthood, it is possible that the upregulation of SOD-2 (which was measured in adult worms) occurs in response to increased oxidative stress during development, thereby leading to normal stress resistance in adulthood. In cases where sensitivity to oxidative stress persists to adulthood, it is possible that the increased expression of SOD-2 is effective at eliminating superoxide but the levels of hydrogen peroxide generated exceed the worms capacity to convert hydrogen peroxide to water. In support of this idea, it has been demonstrated that worms overexpressing sod-1 exhibit increased sensitivity to paraquat, which is eliminated by increasing catalase activity (Doonan et al. 2008).
Synergistic effect on life span reveals molecular interaction of clk-1 and clk-2 with the insulin/IGF-signaling pathway:
To examine the interaction between life-span-extending mechanisms, we generated double mutants with daf-2 worms, which have decreased insulin/IGF signaling (Kenyon et al. 1993). Our laboratory has previously shown that clk-1 acts in a distinct pathway from daf-2 since the two mutations have synergistic effects on life span in clk-1;daf-2 double mutants (Lakowski and Hekimi 1996). In contrast to clk-1, two other genes, isp-1 and sod-2, which are thought to increase life span through decreasing mitochondrial function, did not show synergistic effects with daf-2 life span (Feng et al. 2001; Van Raamsdonk and Hekimi 2009), thereby suggesting that the marked interaction between clk-1 and daf-2 life-span extension does not result simply from decreased mitochondrial function but more likely results from a specific molecular interaction of clk-1 and the insulin/IGF-signaling pathway. This conclusion is further supported by our present findings. We found that only clk-1 and clk-2 exhibit synergistic effects on the life span of daf-2 worms. Similar to our previous study examining the clk-1;daf-2 life span (Lakowski and Hekimi 1996), the effect of clk-2 deletions on the daf-2 life span is particularly dramatic as clk-2 worms show the smallest increase in longevity of all the Clk mutants.
Rate of living theory and free radical theory of aging:
The rate of living theory of aging proposes that organismal life span is inversely correlated with the rate of energy expenditure (Pearl 1922). Originally, this theory was supported by the observation that individuals belonging to species that have lower mass-specific metabolic rates tend to live longer than individuals belonging to species with higher mass-specific metabolic rates. While counter examples have cast doubt on the universality of this theory, there is also considerable support for this theory (Speakman 2005). In worms, there is a general trend that long-lived worms also have slow physiologic rates. For example, clk-1, isp-1, eat-2, and sod-2 worms all develop slowly and show extended longevity. However, examples also exist of long-lived worms that have normal physiologic rates (e.g., age-1) and of worms with a slow rate of living that have a shortened life span (e.g., mev-1, gas-1). Note that most, if not all, worms in the latter category tend to be sick in appearance, as mutations with a toxic effect can shorten life span and tend to slow physiologic rates.
Our results provide further support for the rate of living theory in showing a link between energy metabolism and aging. In screening only for mutants with slow development and slow defecation, we found that 10 of 10 Clk mutants exhibited extended life span. gro-1 mutant worms, which display the Clk phenotype but were not identified in our screens, also have extended life span. For comparison, Kim and Sun (2007) completed an RNAi screen for worms that are resistant to oxidative stress induced by paraquat and assessed which of the resulting worms had a 10% or greater increase in life span. They found that 13.8% (84/608) of paraquat-resistant worms had increased life span. While this success rate is much higher than random, our study suggests that slow development and slow defecation are a much better predictor of life span than resistance to oxidative stress.
The free radical theory of aging proposes that aging results from the accumulation of molecular damage caused by ROS, which eventually leads to cellular dysfunction and organismal death (Harman 1956). This theory has been extensively tested, and while numerous experiments support the theory, numerous others refute it (Muller et al. 2007). In fact, a number of recent articles on C. elegans clearly demonstrate that increasing oxidative damage does not shorten life span (Yang et al. 2007; Doonan et al. 2008; Honda et al. 2008; Van Raamsdonk and Hekimi 2009; Yen et al. 2009). Here, we show that the long-lived Clk mutants are not more resistant to oxidative stress but in most cases are in fact more sensitive to oxidative stress. This emphasizes the fact that increasing sensitivity to oxidative stress does not result in decreased life span and is, in fact, compatible with long life. Similarly, we have recently shown that deletion of sod-2 results in increased sensitivity to oxidative stress and increased life span (Van Raamsdonk and Hekimi 2009). Thus, our work here provides additional examples that contradict the predictions of the free radical theory of aging and suggests that decreased energy utilization can extend life span without decreasing oxidative damage.
Acknowledgments
We thank the Caenorhabditis Genetics Center for providing strains used in this research. J.M.V.R. is supported by the Canadian Institutes of Health Research, the Hereditary Disease Foundation, and the McGill Tomlinson Fellowships. S.H. is supported by grants from the Canadian Institutes of Health Research (216377, 216376, and 218649) and by McGill University. S.H. is Campbell Chair of Developmental Biology and Strathcona Chair of Zoology.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.115378/DC1.
References
- Ahmed, S., A. Alpi, M. O. Hengartner and A. Gartner, 2001. C. elegans RAD-5/CLK-2 defines a new DNA damage checkpoint protein. Curr. Biol. 11 1934–1944. [DOI] [PubMed] [Google Scholar]
- Avery, L., 1993. The genetics of feeding in Caenorhabditis elegans. Genetics 133 897–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard, C., B. McCright, Y. Zhang, S. Felkai, B. Lakowski et al., 2001. The C. elegans maternal-effect gene clk-2 is essential for embryonic development, encodes a protein homologous to yeast Tel2p and affects telomere length. Development 128 4045–4055. [DOI] [PubMed] [Google Scholar]
- Braeckman, B. P., K. Houthoofd, A. De Vreese and J. R. Vanfleteren, 1999. Apparent uncoupling of energy production and consumption in long-lived Clk mutants of Caenorhabditis elegans. Curr. Biol. 9 493–496. [DOI] [PubMed] [Google Scholar]
- Braeckman, B. P., K. Houthoofd, K. Brys, I. Lenaerts, V. A. De et al., 2002. No reduction of energy metabolism in Clk mutants. Mech. Ageing Dev. 123 1447–1456. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, J., A. K. Hihi, C. Y. Benard, R. Branicky and S. Hekimi, 2003. Molecular mechanism of maternal rescue in the clk-1 mutants of Caenorhabditis elegans. J. Biol. Chem. 278 49555–49562. [DOI] [PubMed] [Google Scholar]
- de Jong, L., Y. Meng, J. Dent and S. Hekimi, 2004. Thiamine pyrophosphate biosynthesis and transport in the nematode Caenorhabditis elegans. Genetics 168 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillin, A., A. L. Hsu, N. Rantes-Oliveira, J. Lehrer-Graiwer, H. Hsin et al, 2002. Rates of behavior and aging specified by mitochondrial function during development. Science 298 2398–2401. [DOI] [PubMed] [Google Scholar]
- Doonan, R., J. J. McElwee, F. Matthijssens, G. A. Walker, K. Houthoofd et al., 2008. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev. 22 3236–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank, J. J., T. M. Barnes, B. Lakowski, M. Lussier, H. Bussey et al., 1997. Structural and functional conservation of the Caenorhabditis elegans timing gene clk-1. Science 275 980–983. [DOI] [PubMed] [Google Scholar]
- Felkai, S., J. J. Ewbank, J. Lemieux, J. C. Labbe, G. G. Brown et al., 1999. CLK-1 controls respiration, behavior and aging in the nematode Caenorhabditis elegans. EMBO J. 18 1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, J., F. Bussiere and S. Hekimi, 2001. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev. Cell 1 633–644. [DOI] [PubMed] [Google Scholar]
- Friedman, D. B., and T. E. Johnson, 1988. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 118 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman, D., 1956. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11 298–300. [DOI] [PubMed] [Google Scholar]
- Hayashi, H., O. Matsuzaki, S. Muramatsu, Y. Tsuchiya, T. Harada et al., 2006. Centaurin-alpha1 is a phosphatidylinositol 3-kinase-dependent activator of ERK1/2 mitogen-activated protein kinases. J. Biol. Chem. 281 1332–1337. [DOI] [PubMed] [Google Scholar]
- Hekimi, S., P. Boutis and B. Lakowski, 1995. Viable maternal-effect mutations that affect the development of the nematode Caenorhabditis elegans. Genetics 141 1351–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekimi, S., J. Burgess, F. Bussiere, Y. Meng and C. Benard, 2001. Genetics of life span in C. elegans: molecular diversity, physiological complexity, mechanistic simplicity. Trends Genet. 17 712–718. [DOI] [PubMed] [Google Scholar]
- Honda, Y., M. Tanaka and S. Honda, 2008. Modulation of longevity and diapause by redox regulation mechanisms under the insulin-like signaling control in Caenorhabditis elegans. Exp. Gerontol. 43 520–529. [DOI] [PubMed] [Google Scholar]
- Huang, C., C. Xiong and K. Kornfeld, 2004. Measurements of age-related changes of physiological processes that predict life span of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 101 8084–8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii, N., K. Takahashi, S. Tomita, T. Keino, S. Honda et al., 1990. A methyl viologen-sensitive mutant of the nematode Caenorhabditis elegans. Mutat. Res. 237 165–171. [DOI] [PubMed] [Google Scholar]
- Jiang, N., C. Y. Benard, H. Kebir, E. A. Shoubridge and S. Hekimi, 2003. Human CLK2 links cell cycle progression, apoptosis, and telomere length regulation. J. Biol. Chem. 278 21678–21684. [DOI] [PubMed] [Google Scholar]
- Johnson, T. E., E. de Castro, S. Hegi de Castro, J. Cypser, S. Henderson et al., 2001. Relationship between increased longevity and stress resistance as assessed through gerontogene mutations in Caenorhabditis elegans. Exp. Gerontol. 36 1609–1617. [DOI] [PubMed] [Google Scholar]
- Kayser, E. B., P. G. Morgan, C. L. Hoppel and M. M. Sedensky, 2001. Mitochondrial expression and function of GAS-1 in Caenorhabditis elegans. J. Biol. Chem. 276 20551–20558. [DOI] [PubMed] [Google Scholar]
- Kayser, E. B., M. M. Sedensky and P. G. Morgan, 2004. a The effects of complex I function and oxidative damage on life span and anesthetic sensitivity in Caenorhabditis elegans. Mech. Ageing Dev. 125 455–464. [DOI] [PubMed] [Google Scholar]
- Kayser, E. B., M. M. Sedensky, P. G. Morgan and C. L. Hoppel, 2004. b Mitochondrial oxidative phosphorylation is defective in the long-lived mutant clk-1. J. Biol. Chem. 279 54479–54486. [DOI] [PubMed] [Google Scholar]
- Kenyon, C., J. Chang, E. Gensch, A. Rudner and R. Tabtiang, 1993. A C. elegans mutant that lives twice as long as wild type. Nature 366 461–464. [DOI] [PubMed] [Google Scholar]
- Kim, Y., and H. Sun, 2007. Functional genomic approach to identify novel genes involved in the regulation of oxidative stress resistance and animal life span. Aging Cell 6 489–503. [DOI] [PubMed] [Google Scholar]
- Lakowski, B., and S. Hekimi, 1996. Determination of life-span in Caenorhabditis elegans by four clock genes. Science 272 1010–1013. [DOI] [PubMed] [Google Scholar]
- Lambert, A. J., and M. D. Brand, 2009. Reactive oxygen species production by mitochondria. Methods Mol. Biol. 554 165–181. [DOI] [PubMed] [Google Scholar]
- Lapointe, J., Z. Stepanyan, E. Bigras and S. Hekimi, 2009. Reversal of the mitochondrial phenotype and slow development of oxidative biomarkers of aging in long-lived Mclk1+/− mice. J. Biol. Chem. 284 20364–20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. S., R. Y. Lee, A. G. Fraser, R. S. Kamath, J. Ahringer et al., 2003. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat. Genet. 33 40–48. [DOI] [PubMed] [Google Scholar]
- Lemieux, J., B. Lakowski, A. Webb, Y. Meng, A. Ubach et al., 2001. Regulation of physiological rates in Caenorhabditis elegans by a tRNA-modifying enzyme in the mitochondria. Genetics 159 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire, B. D., M. Behrendt, A. DeCorby and D. Gaskova, 2009. C. elegans longevity pathways converge to decrease mitochondrial membrane potential. Mech. Ageing Dev. 130 461–465. [DOI] [PubMed] [Google Scholar]
- Liu, X., N. Jiang, B. Hughes, E. Bigras, E. Shoubridge et al., 2005. Evolutionary conservation of the clk-1-dependent mechanism of longevity: loss of mclk1 increases cellular fitness and life span in mice. Genes Dev. 19 2424–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroz, A., R. F. Anderson, R. A. Smith and M. P. Murphy, 2009. Reactivity of ubiquinone and ubiquinol with superoxide and the hydroperoxyl radical: implications for in vivo antioxidant activity. Free Radic. Biol. Med. 46 105–109. [DOI] [PubMed] [Google Scholar]
- Miyadera, H., H. Amino, A. Hiraishi, H. Taka, K. Murayama et al., 2001. Altered quinone biosynthesis in the long-lived clk-1 mutants of Caenorhabditis elegans. J. Biol. Chem. 276 7713–7716. [DOI] [PubMed] [Google Scholar]
- Muller, F. L., M. S. Lustgarten, Y. Jang, A. Richardson and H. Van Remmen, 2007. Trends in oxidative aging theories. Free Radic. Biol. Med. 43 477–503. [DOI] [PubMed] [Google Scholar]
- Murphy, M. P., 2009. How mitochondria produce reactive oxygen species. Biochem. J. 417 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl, R., 1922. The Biology of Death. J. B. Lippincott, Philadelphia.
- Rea, S. L., N. Ventura and T. E. Johnson, 2007. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 5 e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieke, S. M., and T. Finkel, 2007. TOR and aging: less is more. Cell Metab. 5 233–235. [DOI] [PubMed] [Google Scholar]
- Shibata, Y., R. Branicky, I. O. Landaverde and S. Hekimi, 2003. Redox regulation of germline and vulval development in Caenorhabditis elegans. Science 302 1779–1782. [DOI] [PubMed] [Google Scholar]
- Shoyama, T., Y. Shimizu and H. Suda, 2009. Decline in oxygen consumption correlates with life span in long-lived and short-lived mutants of Caenorhabditis elegans. Exp. Gerontol. 44 784–791. [DOI] [PubMed] [Google Scholar]
- Speakman, J. R., 2005. Body size, energy metabolism and life span. J. Exp. Biol. 208 1717–1730. [DOI] [PubMed] [Google Scholar]
- Suda, H., T. Shouyama, K. Yasuda and N. Ishii, 2005. Direct measurement of oxygen consumption rate on the nematode Caenorhabditis elegans by using an optical technique. Biochem. Biophys. Res. Commun. 330 839–843. [DOI] [PubMed] [Google Scholar]
- Takai, H., R. C. Wang, K. K. Takai, H. Yang and T. de Lange, 2007. Tel2 regulates the stability of PI3K-related protein kinases. Cell 131 1248–1259. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk, J. M., and S. Hekimi, 2009. Deletion of the mitochondrial superoxide dismutase sod-2 extends life span in Caenorhabditis elegans. PLoS Genet. 5 e1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, B. D., 1995. Genetic mapping with polymorphic sequence-tagged sites. Methods Cell Biol. 48 81–96. [DOI] [PubMed] [Google Scholar]
- Wong, A., P. Boutis and S. Hekimi, 1995. Mutations in the clk-1 gene of Caenorhabditis elegans affect developmental and behavioral timing. Genetics 139 1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W., J. Li and S. Hekimi, 2007. A measurable increase in oxidative damage due to reduction in superoxide detoxification fails to shorten the life span of long-lived mitochondrial mutants of Caenorhabditis elegans. Genetics 177 2063–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. Y., J. A. Gangoiti, M. M. Sedensky and P. G. Morgan, 2009. The effect of different ubiquinones on life span in Caenorhabditis elegans. Mech. Ageing Dev. 130 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen, K., H. B. Patel, A. L. Lublin and C. V. Mobbs, 2009. SOD isoforms play no role in life span in ad lib or dietary restricted conditions, but mutational inactivation of SOD-1 reduces life extension by cold. Mech. Ageing Dev. 130 173–178. [DOI] [PubMed] [Google Scholar]