Abstract
UNC-6/Netrin is an evolutionarily conserved, secretory axon guidance molecule. In Caenorhabditis elegans, UNC-6 provides positional information to the axons of developing neurons, probably by establishing a concentration gradient from the ventral to the dorsal side of the animal. Although the proper localization of UNC-6 is important for accurate neuronal network formation, little is known about how its localization is regulated. Here, to examine the localization mechanism for UNC-6, we generated C. elegans expressing UNC-6 tagged with the fluorescent protein Venus and identified 13 genes, which are involved in the cellular localization of Venus∷UNC-6. For example, in unc-51, unc-14, and unc-104 mutants, the neurons showed an abnormal accumulation of Venus∷UNC-6 in the cell body and less than normal level of Venus∷UNC-6 in the axon. An aberrant accumulation of Venus∷UNC-6 in muscle cells was seen in unc-18 and unc-68 mutants. unc-51, unc-14, and unc-104 mutants also showed defects in the guidance of dorso-ventral axons, suggesting that the abnormal localization of UNC-6 disturbed the positional information it provides. We propose that these genes regulate the process of UNC-6 secretion: expression, maturation, sorting, transport, or exocytosis. Our findings provide novel insight into the localization mechanism of the axon guidance molecule UNC-6/Netrin.
A variety of axon guidance molecules and their receptors are critical for pathfinding axons to reach their precise targets (Tessier-Lavigne and Goodman 1996; Yu and Bargmann 2001; Dickson 2002; Chilton 2006; Killeen and Sybingco 2008). Axon guidance molecules, providing the positional information to axons, are expressed either on the surface of cells or secreted into the extracellular space. The axons, receiving positional information from the axon guidance molecules, express axon guidance receptors at the growth cone.
Netrin is an evolutionarily conserved axon guidance molecule that has both axonal attraction and repulsion activities (Serafini et al. 1994; Colamarino and Tessier-Lavigne 1995). UNC-6 of Caenorhabditis elegans is a member of the Netrin family (Ishii et al. 1992). During C. elegans development, UNC-6 is expressed in the ventral cells, including epidermoblasts, glia, neurons, muscle cells, and vulval precursor cells (VPCs) (Wadsworth et al. 1996; Asakura et al. 2007). UNC-6 is thought to establish a concentration gradient from the ventral to the dorsal side of the animal (Wadsworth 2002), to provide ventral-dorsal positional information to attract some axons ventrally while repelling others to extend dorsally (Hedgecock et al. 1990; McIntire et al. 1992; Wadsworth 2002). In addition, UNC-6 provides positional information for cell migration (Hedgecock et al. 1990), synapse formation (Colón-Ramos et al. 2007; Poon et al. 2008), and cell polarity (Adler et al. 2006; Ziel et al. 2009). However, little is known about the molecular mechanisms of UNC-6 localization.
To examine the localization mechanisms of UNC-6, we generated C. elegans expressing UNC-6 tagged with the fluorescent protein Venus (Asakura et al. 2007) and identified 13 genes required for the cellular localization of Venus∷UNC-6, including unc-51, unc-14, unc-104, unc-18, and unc-68. In addition to being involved in the localization of UNC-6, unc-51, unc-14, and unc-104 mutants also showed defects in UNC-6-mediated axon guidance, suggesting that the inappropriate UNC-6 localization disturbed the positional information available to the axons. Our findings provide novel insight into the localization mechanisms of the axon guidance molecule UNC-6/Netrin.
MATERIALS AND METHODS
The general methods for growing and handling the C. elegans worms were described by Brenner (1974). The Bristol strain N2 was used as the standard wild-type strain.
Mutations used:
Linkage group (LG) I: unc-14(e57), unc-73(e936), unc-40(n324), unc-11(e47), unc-13(e51), and unc-101(m1).
LG II: unc-104(e1265), unc-53(e404), syd-1(ju82), rrf-3(pk1426), and unc-10(y250).
LG III: unc-25(e156), unc-36(e251), unc-64(e246), unc-116(rh24, e2310), hpl-2(ok917), and snt-1(md290).
LG IV: unc-5(e53), unc-44(e362), unc-33(mn407), egl-19(n582), osm-3(p802), and ghIs9(Venus∷unc-6; str-3p∷dsRed2).
LG V: unc-51(e369), unc-68(e540, r1162), snb-1(md247), unc-31(e169), nrx-1(ds1), and rpm-1(js410).
LG X: unc-6(ev400), unc-18(e234), nuIs9(unc-5∷GFP), and unc-10(e102).
Imaging:
To analyze the UNC-6 localization in vivo, we used ghIs9(Venus∷unc-6; str-3p∷dsRed2) (Asakura et al. 2007) as an integrant strain. Each animal was mounted on a 2.5% agarose pad in M9 buffer containing 5% sodium azide and was observed using a fluorescence microscope (Axioplan2, Zeiss). Images were taken using a confocal microscope LSM510 (Zeiss).
Mutagenesis and genetic mapping:
The gh alleles were isolated in a screen performed according to standard protocols (Anderson 1995). Briefly, ghIs9(Venus∷unc-6) was mutagenized with ethylmethane sulphonate (EMS), and the F2 generation was screened for animals that exhibited localization defects of Venus∷UNC-6. We screened ∼3000 haploid genomes. The gh36 mutation was dominant, and the other mutations were recessive. Single nucleotide polymorphism (SNP) mapping was used for genetic mapping in the CB4856 strain (Wicks et al. 2001; Davis et al. 2005). The map position was further refined by a complementation test.
RNAi analysis:
Analysis using unc-6 RNAi was performed as described by Asakura et al. (2007). Experiments using RNAi against autophagy-related genes were performed as described by Ogura and Goshima (2006). In this article, the RNAi-hypersensitive double mutant rrf-3(pk1426); hpl-2(ok917) was used (Wang et al. 2005).
Molecular analysis:
We used KOD-Plus (Toyobo, KOD-201) for the PCR experiments. The unc-18 ORF was amplified from pPCR2.1F27D9#F1R1 (Gengyo-Ando et al. 1993), and inserted into the mCherry (McNally et al. 2006) expression vector pNW5 (myo-3 promoter∷mCherry) or pNW19 (H20 promoter∷mCherry), resulting in the myo-3p∷unc-18∷mCherry (pNW7) and H20p∷unc-18∷mCherry (pNW20) constructs.
Transformation of C. elegans:
Transformation was performed as described by Mello et al. (1991). myo-2p∷mRFP (Campbell et al. 2002) (pmy2P-mR) was used as the marker (10 ng/μl). pBluescript SK+ was used to equalize the amount of DNA in the transformations. Mixtures of [pBluescript SK+ (40 ng/μl), pmy2P-mR (10 ng/μl), and pNW7 (50 ng/μl)] or [pBluescript SK+ (40 ng/μl), pmy2P-mR (10 ng/μl), and pNW20 (50 ng/μl)] were injected into the YC81[unc-18(e234); ghIs9] adult gonad, resulting in YC84[unc-18(e234); ghIs9; ghEx20(myo-3p∷unc-18∷mCherry; myo-2p∷mRFP)] or YC85[unc-18(e234); ghIs9; ghEx21(H20p∷unc-18∷mCherry; myo-2p∷mRFP)].
RESULTS
Genetic screening to identify genes that regulate the Venus∷UNC-6 localization:
To identify genes that regulate the UNC-6 localization, we used strain ghIs9, which expresses functional and visible Venus∷UNC-6 (Asakura et al. 2007). On the wild-type background, Venus∷UNC-6 was mainly detected in ventral cells, including epidermoblasts, glia, neurons, muscle cells, and vulval precursor cells (Figure 1). Venus∷UNC-6 was detected in dorsal muscle cells in the tail (Figure 1). In male worms, Venus∷UNC-6 was expressed in the ray (data not shown). The general distribution pattern of Venus∷UNC-6 in the wild-type genetic background was similar to that of 3xHA-tagged UNC-6, reported previously (Wadsworth et al. 1996), except for our additional observation of Venus∷UNC-6 expression in P6.p descendants, ventral muscle, dorsal muscle in the tail, and in the ray of the male tail (Figure 1, data not shown). These differences were probably due to the different fixation methods used, because with Bouin's fixative (Nonet et al. 1997), HA staining of urIs1 (3xHA∷UNC-6, Wadsworth et al. 1996) showed an identical pattern to ghIs9 (data not shown). In addition, an unc-6 promoter∷mRFP fusion gene also showed the same pattern (data not shown).
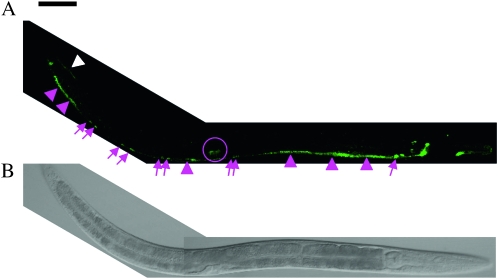
Figure 1.—
Expression of Venus∷UNC-6 in living C. elegans. An L4 worm. Right lateral view, anterior is to the right. (A) Expression of Venus∷UNC-6. (B) DIC image of the same worm. Bar, 50 μm. As described previously (Wadsworth et al. 1996), ventral neurons expressed Venus∷UNC-6 (magenta arrows). In addition, ventral muscle (magenta arrowheads), dorsal muscle (white arrowheads), and vulval cells (magenta circle) expressed Venus∷UNC-6. Venus∷UNC-6 expressed by the ventral muscle in the central part of the worm is not visible, because the intensity of the Venus∷UNC-6 in these cells was very low.
Since UNC-6 is a secreted protein, we expected that some Venus∷UNC-6 would be detected outside of the cells. However, we could not detect any extracellular Venus∷UNC-6, probably owing to its weak fluorescence intensity. Therefore, we focused our analysis on the cellular Venus∷UNC-6 localization, and so in this article, the “localization” of Venus∷UNC-6 refers to not the extracellular but the cellular localization of Venus∷UNC-6. We believe that the observed cellular Venus∷UNC-6 localization largely reflects the process of its secretion.
To identify the genes responsible for the proper localization of Venus∷UNC-6, we took two approaches: (1) we performed EMS mutagenesis screening with ghIs9 to isolate mutant alleles in which the mislocalization of Venus∷UNC-6 was observed, and (2) we examined the localization of Venus∷UNC-6 in existing mutants of genes related to vesicular transport and secretion. From these experiments, we isolated or identified 13 genes required for the proper localization of Venus∷UNC-6 (Table 1). These mutants had no morphological defects on cell shapes except for axons and the penetrance of the localization phenotype in each mutant was 100% (data not shown).
TABLE 1.
Summary of mutants displaying Venus∷UNC-6 localization defects
| Gene | Allele | LG | Mammalian homolog | Reference or source |
|---|---|---|---|---|
| Accumulated unevenly in the cell body of neurons | ||||
| unc-14 | e57, gh34 | I | — | Ogura et al. (1997) |
| unc-51 | e369 | V | ULKI | Ogura et al. (1994) |
| Accumulated evenly in the cell body of neurons | ||||
| unc-104 | e1265 | II | KIFIA | Otsuka et al. (1991) |
| ND | gh23 | II | ND | |
| Accumulated in muscle cells | ||||
| ND | gh33 | I | ND | |
| unc-68 | e540, gh21, gh22, gh28, gh29, gh32, gh37, r1162 | V | Ryanodine receptor | Maryon et al. (1996);Sakube et al. (1997) |
| ND | gh27, gh38 | V | ND | |
| ND | gh26 | X | ND | |
| unc-18 | e234 | X | Sec1/Munc18 | Gengyo-Ando et al. (1993) |
| syd-1; rpm-1 | ju82; ju44 | II/V | SYDEI; Pam/Highwire | Nakata et al. (2005) |
| Accumulated in vulval precursor cells and vulval cells | ||||
| ND | gh25 | IV | ND | |
| Strongly expressed in unc-6-expressing cells | ||||
| ND | gh36 | IV | ND | |
ND, not determined.
Mutants showing an abnormal localization of Venus∷UNC-6 in neurons:
In the neurons, Venus∷UNC-6 on a wild-type background showed a punctate distribution pattern throughout the cytoplasm and axons and was excluded from the nucleus (Figure 2A). We identified four genes required for this localization of Venus∷UNC-6 within the neurons (Figure 2, B–E; Table 1). In these mutant worms, Venus∷UNC-6 was expressed abnormally in the neurons, but the expression in other cell types was similar to that in the wild type (data not shown), suggesting that UNC-6 localization in the neurons is regulated by a unique mechanism. In unc-51(e369) and unc-14(e57) mutants, the Venus∷UNC-6 was accumulated unevenly in the neuronal cell body, and little Venus∷UNC-6 was present in the axon (Figure 2, B and C). UNC-51 is a serine/threonine kinase homologous to yeast Atg1, which is required for autophagy (Ogura et al. 1994; Matsuura et al. 1997; Straub et al. 1997; Mizushima 2007). UNC-14, a RUN domain protein, is the binding partner of UNC-51 (Ogura et al. 1997). Although the precise molecular functions are unknown, UNC-51 and UNC-14 have been implicated in membrane trafficking and localization of UNC-5, which is the receptor for UNC-6 (Ogura and Goshima 2006).
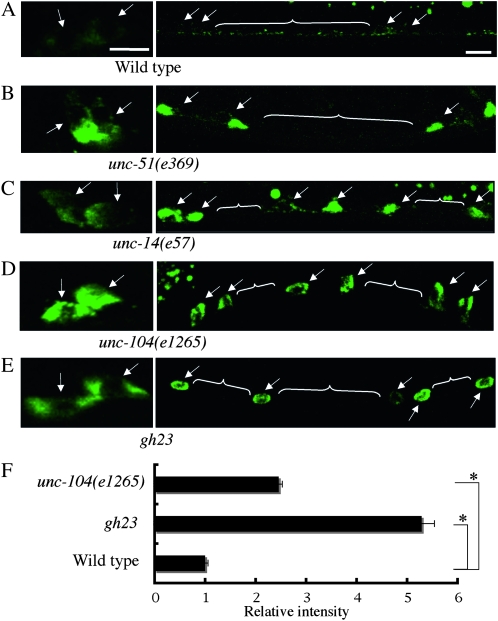
Figure 2.—
Mutants that exhibit abnormal Venus∷UNC-6 localization in neurons. (A) Wild type. (B) unc-51(e369) mutant. (C) unc-14(e57) mutant. (D) unc-104(e1265) mutant. (E) gh23 mutant. Arrows indicate cell bodies. White lines indicate axons. Anterior is to the right, lateral view. Bars, 5 μm. In the wild-type background, Venus∷UNC-6 showed a punctate distribution throughout the cell body and axon, except for the nucleus. In the unc-51(e369), unc-14(e57), unc-104(e1265), and gh23 mutants, Venus∷UNC-6 was accumulated in the neural cell bodies, and little Venus∷UNC-6 was in the axons. (F) Relative fluorescence intensities of the Venus∷UNC-6 in neuronal cell of unc-104(e1265) and gh23 mutants to wild-type worms. In each case, 20 neuronal cell bodies were examined and the results were averaged. Error bars show the standard error. *P < 0.01 (Student's t-test). In unc-51(e369) mutants and unc-14(e57) mutants, Venus∷UNC-6 is accumulated in some part of the neuronal cell body. Therefore, we could not compare the fluorescence intensity between these mutants.
UNC-51 has been reported to be involved in the autophagy in C. elegans (Meléndez et al. 2003). However, it is unlikely that defects in the traditional autophagy pathway caused the abnormal Venus∷UNC-6 localization, because the RNAis of other genes required for autophagy (bec-1/atg-6, atg-7, lgg-1/atg-8, and atg-18) in an RNAi-hypersensitive mutant strain showed the normal Venus∷UNC-6 localization (Table 2). In unc-104(e1265) mutants, the Venus∷UNC-6 was accumulated evenly throughout the neuronal cell body, and little Venus∷UNC-6 was present in the axon (Figure 2, D and F). unc-104 encodes a kinesin motor protein, which is homologous to KIF1A in vertebrate (Otsuka et al. 1991; Hirokawa and Noda 2008). These findings therefore suggest that UNC-6 might be transported by the motor protein UNC-104 from the neuronal cell body to the axon. In gh23 mutants, Venus∷UNC-6 in neurons also accumulated evenly in the cell body, with very little appearing in the axon (Figure 2, E and F), which was similar to that in unc-104(e1265) mutants (Figure 2D). Complementation analysis revealed that gh23 was not an allele of unc-104. The similar expression patterns indicated that the responsible gene product of gh23 might be involved in the molecular function of UNC-104 in UNC-6 transport.
TABLE 2.
Summary of mutants displaying normal Venus∷UNC-6 localization
| Gene | Allele | Mammalian homolog | Reference or source | |
|---|---|---|---|---|
| Molecular motors | ||||
| unc-116 | e2310, rh24 | KIF5 | Patel et al. (1993) | |
| osm-3 | p802 | KIF17 | Shakir et al. (1993) | |
| Exocytosis-related proteins | ||||
| snt-1 | md290 | Synaptotagnmin | Nonet et al. (1993) | |
| snb-1 | md247 | Synaptobrevin | Nonet et al. (1998) | |
| unc-64 | e246 | Syntaxin | Ogawa et al. (1998); Saifee et al. (1998) | |
| unc-31 | e169, e928 | CAPS | Speese et al. (2007) | |
| unc-13 | e51 | Munc13 | Maruyama and Brenner (1991) | |
| egl-19 | n582 | L-type Ca2+ channel | Lee et al. (1997) | |
| nrx-1 | ds1 | Neurexin | Shen et al. (2007) | |
| unc-10 | e102 | RIM | Koushika et al. (2001) | |
| rab-3 | y250 | Rab3A | Nonet et al. (1997) | |
| UNC-6/netrin receptors | ||||
| unc-5 | e53 | UNC5H | Leung-Hagesteijn et al. (1992) | |
| unc-40 | n324 | DCC | Chan et al. (1996) | |
| Autophagy-related proteins | ||||
| bec-l/atg-6 | RNAi | Atg6/beclin1 | Meléndez et al. (2003) | |
| atg-7 | RNAi | Atg7 | Meléndez et al. (2003) | |
| lgg-1/atg-8 | RNAi | Atg8/LC3 | Meléndez et al. (2003) | |
| atg-18 | RNAi | Atg18/WIPI1 | Meléndezet al. (2003) | |
| Others | ||||
| unc-25 | e156 | Glutamate decarboxylase 1 | Jin et al. (1999) | |
| unc-33 | e204, mn407 | CRMP2 | Li et al. (1992) | |
| unc-44 | e362 | Ankyrin | Otsuka et al. (1995) | |
| unc-16 | jul46 | JIP3 | Byrd et al. (2001) | |
| unc-101 | ml | AP-1 | Lee et al. (1994) | |
| unc-53 | e234 | NAV | Stringham et al. (2002) |
Mutants showing an abnormal localization of Venus∷UNC-6 in muscle cells:
In the muscle cells, Venus∷UNC-6 on a wild-type background was distributed throughout the cytoplasm and was excluded from the nucleus (Figure 3A). We identified seven genes required for this Venus∷UNC-6 localization within muscle cells (Table 1). In these mutant worms, Venus∷UNC-6 was accumulated abnormally in the muscle cells (Figure 3, B–D), but a normal distribution pattern in other cell types was observed (data not shown), suggesting that, like neurons, a unique mechanism for controlling UNC-6 localization exists in muscle cells. Complementation analysis with existing mutants revealed that gh21, gh22, gh28, gh29, gh32, and gh37 were alleles of unc-68. The reference alleles unc-68(e540) and unc-68(r1162) exhibited the same phenotype (Figure 3B). UNC-68 is homologous to ryanodine receptors (RyRs), which regulate body-wall muscle contraction by controlling Ca2+ release from the endoplasmic reticulum (ER) (Maryon et al. 1996; Sakube et al. 1997; Zalk et al. 2007). This release of Ca2+ mediated by UNC-68 is also required for regulating both spontaneous and evoked neurotransmitter release (Liu et al. 2005). These results therefore suggest that the Ca2+ release from the ER mediated by UNC-68 is required for proper UNC-6 localization in muscle.
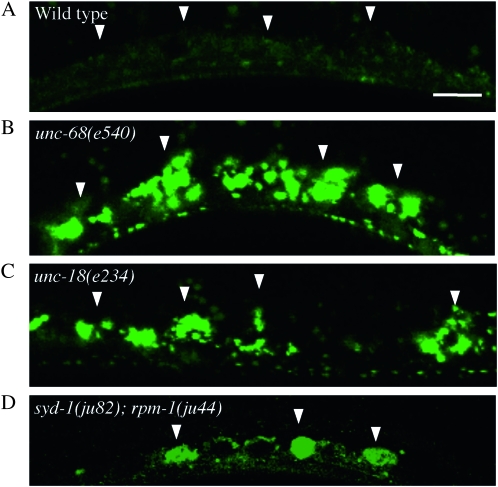
Figure 3.—
Mutants that exhibit abnormal Venus∷UNC-6 localization in muscle cells. Ventral muscle. (A) Wild type. (B) unc-68(e540) mutant. (C) unc-18(n234) mutant. (D) syd-1(ju82); rpm-1(js410) double mutant. Arrowheads indicate ventral muscle cells. Anterior is to the right, lateral view. Bar, 10 μm. Within the muscle cells, Venus∷UNC-6 showed a punctate distribution throughout the cell body except for the nucleus. In unc-68(e540), unc-18(e234), and syd-1(ju82); rpm-1(js410), Venus∷UNC-6 accumulated as fluorescent clusters in the muscle cells.
Complementation analysis revealed that gh27 and gh38 were allelic mutants. Since gh27 and gh38 are mapped to LGV, in which unc-68 is located (supporting information, Figure S1), we performed complementation analysis with unc-68(e540). Venus∷UNC-6 accumulated in the muscle cells of the trans-heterozygous strain e540/gh27, but the level was clearly lower than that of the homozygous strains e540/e540, gh27/gh27, and gh38/gh38 (data not shown). Since unc-68(e540) is thought to be a null allele (Sakube et al. 1997), interallelic complementation of the sort observed in hypomorphic alleles, such as for unc-5 (Merz et al. 2001), was unlikely. In addition, unc-68(e540) shows Unc phenotype, but gh27 and gh38 did not. These findings indicated that gh27 and gh38 interacted genetically with unc-68, but were not allelic to it. Complementation analysis revealed that gh33 and gh26 were alleles of unidentified genes (Table 1).
Venus∷UNC-6 was also accumulated in the muscle cells of the unc-18(e234) mutants (Figure 3C). UNC-18, which is homologous to the SM (Sec1/Munc18-like) proteins, regulates a multistep vesicle exocytosis process in neurons, in cooperation with SNARE proteins (Gengyo-Ando et al. 1993; Malsam et al. 2008; Südhof and Rothman 2009). Therefore, we next examined whether neuronal UNC-18 regulates the localization of Venus∷UNC-6 in muscle.
UNC-18 in neurons acts non-cell autonomously to regulate the localization of Venus∷UNC-6 in muscle:
Although Venus∷UNC-6 was accumulated abnormally in the muscle cells of the unc-18(e234) mutant, UNC-18 is expressed by the neurons, not by the muscles (Gengyo-Ando et al. 1993). To analyze the cell autonomy of this gene, we examined whether muscle- or neuron-specific expression of unc-18 could rescue the Venus∷UNC-6 localization defect in unc-18(e234) mutants. In these rescue experiments, we used the myo-3 promoter for muscle-specific expression (Okkema et al. 1993) and the H20 promoter for neuron-specific expression (Shioi et al. 2001).
The muscle-specific expression of unc-18 did not rescue the abnormal Venus∷UNC-6 accumulation in the muscle cells of unc-18(e234) mutants (Figure 4A), but its neuron-specific expression did (Figure 4B). These results suggested that neuronal UNC-18 cell nonautonomously regulates the localization of UNC-6 in muscle. The possible involvement of presynaptic input in the regulation of UNC-6's localization in muscle is also supported by the phenotypic defects we observed in the syd-1(ju82); rpm-1(ju44) double mutant, in which Venus∷UNC-6 accumulated in the muscle cells (Figure 3D). The syd-1(ju82); rpm-1(ju44) mutant shows a severe defect in locomotion, since the number of synapses is reduced and presynaptic components are disrupted (Nakata et al. 2005). Our findings therefore suggest that synaptic activity is required for the proper localization of UNC-6 in the muscle.
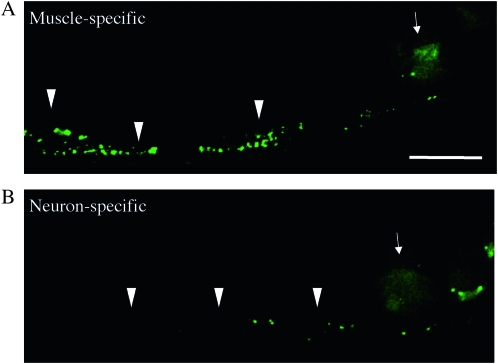
Figure 4.—
UNC-18 functions cell nonautonomously in neurons to regulate the Venus∷UNC-6 localization in muscle. Venus∷UNC-6 in a worm expressing muscle-specific UNC-18 (A) or neuron-specific UNC-18 (B). YC84[unc-18(e234); ghIs9(Venus∷unc-6); ghEx20(myo-3p∷unc-18∷mCherry; myo-2p∷mRFP)] worms were used for the muscle-specific expression, and YC85[unc-18(e234); ghIs9(Venus∷unc-6); ghEx21(H20p∷unc-18∷mCherry; myo-2p∷mRFP)] worms were used for the neuron-specific expression. Arrowheads indicate the accumulated Venus∷UNC-6 in muscle cells. Arrows indicate the myo-2p∷mRFP fluorescence used as an expression marker. Anterior is to the right. Bar, 20 μm. The accumulation of Venus∷UNC-6 was observed in worms expressing muscle-specific UNC-18 (A), but little accumulation of Venus∷UNC-6 was observed with the neuron-specific expression (B).
Since unc-18 and unc-68 are known to regulate the exocytosis of synaptic vesicles in neurons, we examined the localization of Venus∷UNC-6 in the mutants of other exocytosis-related genes, such as snt-1/synaptotagmin, snb-1/synaptobrevin, unc-64/syntaxin, and unc-13/Munc13 (Maruyama and Brenner 1991; Nonet et al. 1993; Nonet et al. 1998; Ogawa et al. 1998; Saifee et al. 1998; Malsam et al. 2008; Südhof and Rothman 2009). However, the localization of Venus∷UNC-6 was not altered in these mutants (Table 2). Thus, the mechanism by which UNC-18 regulates the UNC-6 localization remains an unsolved question.
Mutants showing the abnormal localization of Venus∷UNC-6 in VPCs:
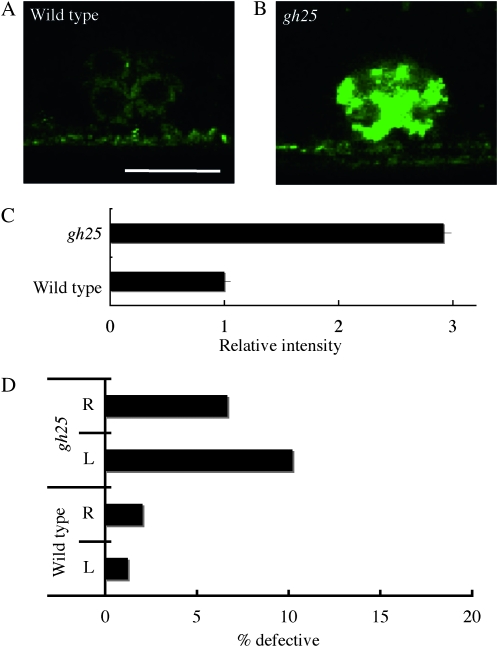
We identified one gene required for the localization of Venus∷UNC-6 in VPCs. In gh25 mutants, the intensity of Venus∷UNC-6 was increased in the VPCs and vulval cells (Figure 5, B and C; Table 1), but its distribution was normal in the other cell types (data not shown), suggesting that VPCs have a specific mechanism for secreting UNC-6.
Figure 5.—
A mutant that exhibits abnormal Venus∷UNC-6 localization in the vulval precursor cells (VPCs). P6.p descendants (VPCs) at the eight-cell stage. (A) Wild type. (B) gh25 mutant. Anterior is to the right, lateral view. Bar, 10 μm. In the gh25 mutant, Venus∷UNC-6 accumulated abnormally in the VPCs. (C) Relative fluorescence intensities of Venus∷UNC-6 in the gh25 mutants to wild-type worms. In each case, 10 VPCs were examined and the results were averaged. Error bars show the standard error. *P < 0.01 (Student's t-test). (D) The percentage of HSN axons showing guidance defects. R represents HSN R. L represents HSN L. tph-1p∷gfp (Sze et al. 2000) was used to visualize the HSN neuron. In gh25 mutants, HSN axon guidance defects were observed.
Since the VPCs are the UNC-6 source for HSN axon guidance (Asakura et al. 2007), we analyzed the HSN axon morphology in gh25 mutants. We found that gh25 mutants had HSN guidance defects (Figure 5D), suggesting that the abnormal Venus∷UNC-6 expression in gh25 mutants result in the impaired UNC-6 secretion and the HSN axon guidance defects.
Mutants showing a high level of Venus∷UNC-6 expression in all the UNC-6-expressing cells:
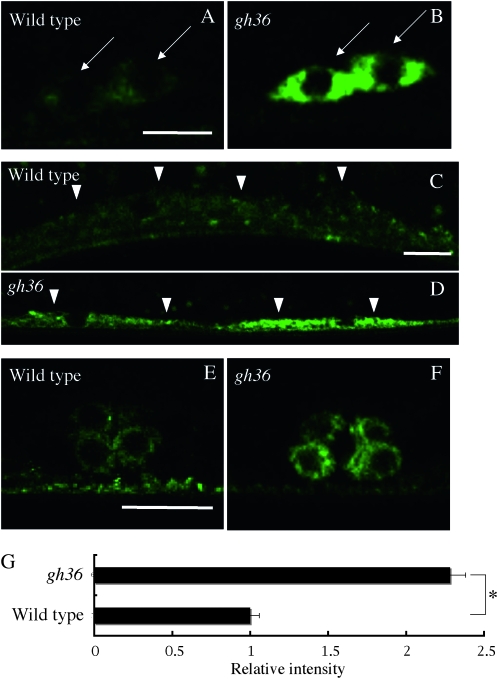
We identified one gene required globally for the normal expression level of Venus∷UNC-6. In gh36 mutants, Venus∷UNC-6 fluorescence intensity was increased in all of the Venus∷UNC-6-expressing cells (Figure 6, B, D, F, G; Table 1). However, we found that UNC-5∷GFP (Killeen et al. 2002) fluorescence intensity was not increased in gh36 mutants (Figure S2), indicating that gh36 did not affect transgene expression or fluorescence levels in general. Unlike the other mutants, the distribution of the intracellular Venus∷UNC-6 expression was normal for all cell types. Therefore, the responsible gene product of gh36 may negatively regulate unc-6 expression.
Figure 6.—
A mutant that exhibits a high level of Venus∷UNC-6 expression in all the cells that express it. (A and B) Cell bodies of ventral neurons. (C and D) Ventral muscle cells. (E and F) P6.p descendants (VPCs) at the eight-cell stage. (A, C, and E) Wild type. (B, D, and F) gh36 mutants. Arrows indicate neuronal cell bodies. Arrowheads indicate muscle cells. Anterior is to the right, lateral view. Bars, 5 μm (A and B); 10 μm (C–F). In the gh36 mutant, the Venus∷UNC-6 level was increased in all of the cells that normally express it. (G) Relative fluorescence intensities of the Venus∷UNC-6 in the neuronal cell bodies of gh36 mutants to wild-type worms. In each case, 20 neuronal cell bodies were examined and the results were averaged. Error bars show the standard error. *P < 0.01 (Student's t-test).
unc-104, unc-18, and unc-68 are required for anterior ventral microtubule cell ventral guidance:
Given that UNC-6 is required for dorso-ventral axon guidance, we predicted that the altered localization of Venus∷UNC-6 in these identified mutants probably caused or reflected UNC-6 secretion defects, which should result in dorso-ventral axon guidance defects. In support of this hypothesis, unc-51 and unc-14 mutants show defective dorsally directed axon pathfinding by DD/VD neurons (McIntire et al. 1992). In 2006, we reported that unc-51 and unc-14 interact genetically with unc-6 to influence DD/VD axon guidance (Ogura and Goshima 2006). To verify this hypothesis further, we examined ventrally directed anterior ventral microtubule cell (AVM) axon guidance in unc-104, unc-18, and unc-68 mutants.
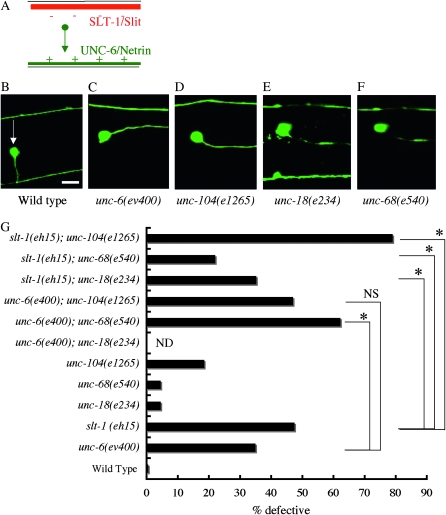
The AVM cell body is located laterally, and its axon grows ventrally to the ventral nerve cords (Figure 7, A and B). The pathfinding of the AVM axon is affected by two parallel guidance cues, UNC-6/Netrin and Slt-1/Slit. Ventral UNC-6 attracts and dorsal SLT-1 repels the AVM axon (Figure 7A; Hao et al. 2001). The AVM axon grows ventrally at L1 stage. We confirmed that unc-104, unc-18, and unc-68 mutants had localization defects of Venus∷UNC-6 at the L1 stage as well (Figure S3).
Figure 7.—
AVM axon guidance defects in UNC-6/Netrin-localization mutants. (A) Schematic drawing of the ventral guidance signals for the AVM (Hao et al. 2001). The AVM axon grows ventrally, attracted by ventral UNC-6/Netrin (green) and repelled by dorsal SLT-1/Slit (red). (B–F) The AVM morphology. (B) Wild type. (C) unc-6(ev400). (D) unc-104(1265). (E) unc-18(e234). (F) unc-68 (e540). zdIs5(mec-4∷gfp) was used to visualize the AVM neuron (Clark and Chiu 2003). An arrow indicates the AVM cell body. Right lateral view, anterior is to the right. Bar, 10 μm. In the wild-type worm, the AVM neuron extended its axon ventrally and then anteriorly. In the unc-6(ev400), unc-104(1265), unc-18(e234), and unc-68 (e540) mutants shown, the AVM neuron extended anteriorly without navigating ventrally. (G) The AVM axon guidance defects in unc-6(ev400), slt-1(eh15), unc-18(e234), unc-68(e540), and unc-104(e1265) and their double mutants. The genetic distance between unc-6 and unc-18 is too small to make double mutants. n = 200–967. *P < 0.01 (Student's t-test). NS, not significant. unc-104(e1265) did not enhance unc-6(ev400), but it strongly enhanced slt-1(eh15). unc-68(e540) enhanced unc-6(ev400). unc-68(e540) and unc-18(e234) suppressed slt-1(eh15).
In unc-104(e1265), unc-18(e234), and unc-68(e540) mutants, minor defects of the AVM axon guidance were observed (Figure 7G). Although unc-104; unc-6 double mutants exhibited no enhancement in AVM defects compared to unc-6 single mutants, unc-104; slt-1 double mutants exhibited enhanced defects compared to slt-1 single mutants. These results suggest that unc-104 has a role in the unc-6-pathway and is consistent with our hypothesis that the UNC-6 localization defect reflects the axon guidance defect of the AVM neuron.
Interestingly, unc-68; unc-6 double mutants exhibited enhanced defects compared to unc-6 single mutants (Figure 7G). unc-18; slt-1 and unc-68; slt-1 double mutants exhibited suppressed defects compared to slt-1 single mutants (Figure 7G). The enhancement in unc-68; unc-6 double mutants may result from the accumulation of other axon guidance molecules, such as SLT-1, in unc-68 mutants. The absence of enhancement in unc-18; slt-1 and unc-68; slt-1 double mutants suggests that UNC-6 expressed by the muscle cells do not participate in the AVM ventral guidance.
UNC-6 is also required for synaptic development of the DA9 neuron (Poon et al. 2008). We analyzed the synaptic development of the DA9 neuron in unc-18 and unc-68 mutants. However, we did not find the defects. It is also reported that synaptic development of the DA9 neuron is normal in unc-18 mutants (data not shown; Zhao and Nonet 2000). These suggest that UNC-6 expressed by muscle cells does not participate in the synaptic development of the DA9 neuron. UNC-6 is also required for dorsal migration of distal tip cells (Hedgecock et al. 1990). However, we did not find the dorsal migration defects of the distal tip cells in unc-18 and unc-68 mutants as well (data not shown). We could not find clear defects on the UNC-6 function in unc-18 and unc-68 mutants.
DISCUSSION
Identification of genes required for proper Venus∷UNC-6 localization:
The secretory axon guidance molecule UNC-6/Netrin provides positional information for axon guidance (Tessier-Lavigne and Goodman 1996; Yu and Bargmann 2001; Dickson 2002; Chilton 2006; Killeen and Sybingco 2008). The temporal and spatial expression of UNC-6/Netrin has been well documented and plays an important role in neural network formation (Wadsworth et al. 1996; Watanabe et al. 2006; Asakura et al. 2007). A model for the patterning mechanism, in which the Netrin receptor frazzled rearranges secreted Netrin in Drosophila melanogaster, has been proposed (Hiramoto et al. 2000). However, little is known about the localization/secretion mechanisms of UNC-6/Netrin.
In this study, we identified 13 genes required for the cellular localization of Venus∷UNC-6 in C. elegans. Four genes were required specifically for the proper localization of Venus∷UNC-6 in neurons, 7 for its localization in muscle, 1 for its localization in VPCs, and 1 for controlling the global expression level of Venus∷UNC-6. We propose that, in these cells, these 13 genes regulate the processes associated with the secretion of UNC-6: expression, maturation, sorting, transport, and exocytosis, and that each of these cell types has a specific mechanism for regulating UNC-6 localization.
Genes required for the UNC-6 localization in neurons:
Venus∷UNC-6 is expressed in neurons, including PVT, AVG, RIF, AVA, AVB, PVQ, VA, and VB(Wadsworth et al. 1996; Asakura et al. 2007). In unc-51, unc-14, unc-104, and gh23 mutants, Venus∷UNC-6 accumulated in the neuronal cell bodies, but there was little fluorescence in the axons, suggesting that these genes regulate the transport of UNC-6 from the neuronal cell body to the axon. In unc-51, unc-14, and unc-104 mutants, UNC-6/Netrin-mediated dorso-ventral axonal guidance is defective (McIntire et al. 1992; this study). In addition, unc-51, unc-14, and unc-104 interact genetically with unc-6 (Ogura and Goshima 2006; this study). These findings suggest that the defects in UNC-6 transport in these mutants disrupted the normal secretion of UNC-6 from the neurons.
UNC-104 is a homolog of the kinesin motor protein, KIF1A, which transports the precursors of synaptic vesicles (SVs) and dense core vesicles (DCVs) from neuronal cell bodies to synapses (Hall and Hedgecock 1991; Otsuka et al. 1991; Yonekawa et al. 1998; Zahn et al. 2004; Hirokawa and Noda 2008). The pattern of accumulation of Venus∷UNC-6 was very similar to that of SVs and DCVs in unc-104 mutants (Hall and Hedgecock 1991; Nonet 1999; Zahn et al. 2004), supporting our hypothesis that UNC-104 transports vesicles containing UNC-6 from the neuronal cell body to the axon. In addition, UNC-6 may be secreted by neurons at the synapse, since UNC-104/KIF1A transports the precursors of synaptic vesicles (Hirokawa and Noda 2008). The phenotype of the gh23 mutant was very similar to that of unc-104, therefore the responsible gene product of gh23 may be involved in UNC-104 function. In mutants of unc-116 and osm-3, which encode the kinesin motor proteins UNC-116/KIF5 and OSM-3/KIF17, respectively (Patel et al. 1993; Shakir et al. 1993), the localization of Venus∷UNC-6 was identical to that in wild-type animals, indicating that these kinesin motor proteins are not involved in the transport of UNC-6. This is consistent with the results of a series of recent studies showing that the kinesin and dynein motor proteins use specific adaptor or scaffold proteins to recognize and bind different cargoes (Hirokawa and Takemura 2005).
The Venus∷UNC-6 accumulation phenotype in unc-51 and unc-14 mutants was different from that in unc-104 mutant. In unc-51 and unc-14 mutants, Venus∷UNC-6 accumulated unevenly in the cell body, whereas in the unc-104 mutant, it accumulated evenly throughout the cell body except in the nucleus. The difference may reflect the roles of these gene products in the regulation of UNC-6 localization. UNC-51 is a serine/threonine kinase, which binds UNC-14, a RUN domain protein (Ogura et al. 1994, 1997). UNC-51 and UNC-14 are predicted to play important roles in vesicle trafficking. UNC-51 can bind VAB-8, a kinesin-like protein, and phosphorylate VAB-8 in vitro (Wolf et al. 1998; Lai and Garriga 2004). UNC-51 and VAB-8 cooperatively regulate the posterior axonal outgrowth of CAN neurons. UNC-14 can bind UNC-16/JIP, and together they cooperate with a kinesin motor protein UNC-116/KIF5 to transport synaptic vesicles (Sakamoto et al. 2005). The UNC-51 of D. melanogaster phosphorylates UNC-76/FEZ1, a kinesin heavy chain adaptor protein, to regulate axonal transport (Toda et al. 2008). In addition, UNC-51 and UNC-14 regulate the localization (trafficking) of UNC-5, a receptor for UNC-6, in neurons (Ogura and Goshima 2006). Therefore, UNC-51 and UNC-14 may regulate the processing involved in UNC-6's localization in neuronal cell bodies, including UNC-6's maturation, selection, or transport (Figure 8A). Furthermore, UNC-104 may function as a motor protein in concert with the activities of UNC-51 and UNC-14 to transport vesicles containing UNC-6 from cell bodies to axons.
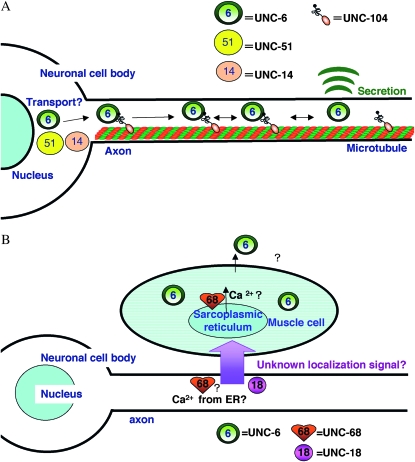
Figure 8.—
Models of the UNC-6/Netrin localization. (A) Model for the localization of UNC-6/Netrin in neurons: UNC-104/KIF1A transports UNC-6/Netrin-containing vesicles along the axon. UNC-51 and its binding partner UNC-14 are required for the maturation, selection, or transport of UNC-6/Netrin. (B) Model for the localization of UNC-6/Netrin localization in muscle: UNC-6/Netrin secretion requires an unknown UNC-18/Sec1-mediated signal from neurons. The UNC-6/Netrin secretion also requires an UNC-68/RyR-mediated process, which may involve calcium release through UNC-68/RyR from the ER in neurons and/or muscle cells.
UNC-51 is also required for autophagy in C. elegans (Meléndez et al. 2003). However, the traditional autophagy pathway probably does not participate in the Venus∷UNC-6 localization, since the RNAi of other genes required for autophagy resulted in normal Venus∷UNC-6 localization.
Genes required for appropriate UNC-6 localization in muscle cells:
We identified seven genes required for the proper localization of Venus∷UNC-6 in muscle cells. These were unc-18, unc-68, syd-1, rpm-1, and the responsible genes of gh33, (gh27, gh38), and gh26. In the unc-18 mutant, Venus∷UNC-6 accumulated in muscle cells. This finding was unexpected, because UNC-18 belongs to the SM (Sec1/Munc18-like) protein family (Gengyo-Ando et al. 1993; Malsam et al. 2008; Südhof and Rothman 2009), which regulates vesicle exocytosis by interacting with syntaxin, a member of the SNARE proteins in neurons (Sassa et al. 1999; Weimer et al. 2003; Malsam et al. 2008; Südhof and Rothman 2009). Indeed, UNC-18 is expressed in neurons but not in muscle (Gengyo-Ando et al. 1993). We showed that the muscle-specific expression of unc-18 did not rescue the Venus∷UNC-6 accumulation in unc-18 mutants, whereas the neuron-specific expression of UNC-18 did. These results suggested that UNC-18 in neurons regulates the UNC-6 localization in muscle.
How does UNC-18 regulate the Venus∷UNC-6 localization in muscle? We showed that, except for UNC-18, mutations in the genes encoding SNARE proteins and other proteins that are essential for the exocytosis of neurotransmitters (Malsam et al. 2008; Südhof and Rothman 2009) did not cause the abnormal localization of Venus∷UNC-6 (Table 2), indicating that the traditional machinery for neurotransmitter release is not involved in the UNC-18 function. In addition, there were no defects in the localization of Venus∷UNC-6 in the muscle of the unc-13, unc-25, and unc-31 mutants (Table 2). UNC-13/Munc13, UNC-25/glutamate decarboxylase 1, and UNC-31/CAPS are, respectively, required for acetylcholine (ACh) secretion (Maruyama and Brenner 1991), GABA synthesis (Jin et al. 1999), and neuropeptide secretion (Berwin et al. 1998; Speese et al. 2007). These findings therefore indicate that traditional neurotransmitters such as ACh, GABA, or neuropeptides are also not involved in the UNC-18 function. The normal Venus∷UNC-6 localization observed in the unc-25 mutant indicated that the muscle homeostasis regulated by GABA (Garcia et al. 2007) is not involved in the UNC-18 function. It is also unlikely that the abnormal Venus∷UNC-6 localization in unc-18 resulted from the severe defect in muscle contraction observed in this mutant, since the unc-13 mutant, which also displayed a paralyzed phenotype, showed normal localization of Venus∷UNC-6.
We propose that an unknown signal from neurons, mediated by UNC-18, regulates the UNC-6 localization in muscle (Figure 8B). The unknown signal is probably secreted at synapses, since Venus∷UNC-6 also accumulated in the muscles of syd-1; rpm-1 mutants, in which presynaptic components are reduced and disrupted (Nakata et al. 2005). The secretion machinery associated with this unknown signal is probably different from that of traditional neurotransmitters. Although the signal and its secretory machinery remain unidentified, the characterization of gh27, gh33, or gh26 may reveal the mechanisms responsible for the proper localization of UNC-6 in muscle.
Venus∷UNC-6 also accumulated in muscle in the unc-68 mutant. UNC-68 is homologous to ryanodine receptors (RyRs), a class of Ca2+ channels (Maryon et al. 1996; Sakube et al. 1997; Zalk et al. 2007). UNC-68 plays important roles in muscle contraction, mediating Ca2+-induced Ca2+ release (CICR) from the endoplasmic reticulum (ER). In addition, CICR in neurons is required for regulating both spontaneous and evoked neurotransmitter release (Liu et al. 2005). Therefore, the simplest explanation for the UNC-68 function is that, in the muscle cells and/or neurons, CICR from the ER mediated by UNC-68 is required for the proper localization of UNC-6 in muscle. Unfortunately, we could not make an UNC-68 construct that expressed specifically in the muscle or neurons, because of the large size of the ORF (15.6 kb). It therefore remains unclear where and how UNC-68 regulates the localization of UNC-6 in muscle cells.
We found that unc-18 and unc-68 mutants had localization defects of Venus∷UNC-6 in muscle cells. However, our results suggest that unc-18 and unc-68 do not appear to participate in known UNC-6 functions including AVM axon guidance, DA9 synaptic development, and cell migration of distal tip cells. We think that UNC-6 expressed by muscle cells may have unknown functions, or that functions of UNC-6 expressed by muscle cells may be masked by UNC-6 expressed by neurons. Another possibility is that, although unc-18 and unc-68 mutants showed Venus∷UNC-6 localization defects in muscle cells, UNC-6 secretion from the muscle cells could be normal in these mutants.
Genes required for the UNC-6 localization in vulval precursor cells:
UNC-6 expressed by the VPCs is essential for proper HSN axon guidance (Asakura et al. 2007). Venus∷UNC-6 was accumulated in the VPCs in the gh25 mutant. In addition, HSN axon guidance defects were observed in the gh25 mutants. Taken together, these findings suggest that the responsible gene of gh25 plays an important role in the UNC-6 secretion by the VPCs.
The Venus∷UNC-6 accumulation in the gh25 mutant resembled the EGL-17/FGF accumulation in EGL-17-secretion defective mutants (Kamikura and Cooper 2003, 2006). EGL-17 is a secreted protein that is expressed in the VPCs and attracts sex myoblasts (Burdine et al. 1998). The UNC-6 secretion by the VPCs may be mediated by a mechanism similar to that for EGL-17 secretion.
Genes required for UNC-6 expression:
In mice, the transient expression of Netrin 1 at the dorsal spinal cord is required for the accurate axon guidance of primary sensory axons (Watanabe et al. 2006), and transcription factors such as Runx3 are also involved in the axon guidance of primary sensory axons (Inoue et al. 2002). In C. elegans, the upregulation of UNC-6 in the vulval precursor cells is essential for the complex axon guidance of the HSN neurons (Asakura et al. 2007). These observations suggest that accurate construction of the nervous system requires axon guidance molecules to be expressed at the proper time, place, and concentration.
In mutant gh36, the Venus∷UNC-6 fluorescence intensity was increased in all the cells that expressed Venus∷UNC-6, without any alteration in its intracellular distribution. Therefore, the responsible gene of this mutant may negatively regulate unc-6 expression. The analysis of the gh36 mutant may provide information about how UNC-6 expression is regulated.
Other mechanisms of Netrin localization:
In D. melanogaster, a model for Netrin's patterning mechanism has been proposed, in which Netrin's localization is regulated by interaction with its receptor, Frazzled/UNC-40 (Hiramoto et al. 2000). In C. elegans, we could not find any alteration in the localization of Venus∷UNC-6 in unc-40 mutants (data not shown). Since the nervous system of C. elegans is extremely simple compared to that of D. melanogaster, such a redistribution mechanism of UNC-6 might not be required for it to form. Determining whether such a redistribution mechanism for Netrin is conserved in mammalian species is an important issue to be addressed in the future.
Finally, we used strong loss-of-function alleles or RNAi to examine the localization of Venus∷UNC-6 on the known mutants or genes. Since all of them are not null alleles, it remains possible that the genes listed in Table 2 could be involved in regulating the localization of UNC-6. In addition, all the new mutants except for gh36 were recessive alleles, therefore, we think that these mutants except for gh36 are loss-of-function alleles. However, they could be gain-of-function alleles, since we did not identify the responsible genes.
Acknowledgments
We thank Takeshi Ishihara for the H20 promoter clone, Roger Y. Tsien for the mRFP clone, Jon Audhya for the mCherry clone, Andrew Fire for the C. elegans expression vectors, Keiko Gengyo-Ando for the unc-18 clone, Yuji Kohara for the C. elegans EST clones, Joseph G. Culotti for nuIs9, Takako Okada for technical support, Sandy Chen and Ayako Asakura for reading the manuscript, and members of the Goshima laboratory for suggestions and helpful discussion. Some nematode strains used in this study were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources. This work was supported by Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency (JST) (Y.G.), grants-in-aid for scientific research in a priority area (Y.G. and K.O.) from the Ministry of Education, Science, Sports and Culture, and the Yokohama Foundation for Advancement of Medical Science (T.A. and K.O.).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.116293/DC1.
References
- Adler, C. E., R. D. Fetter and C. I. Bargmann, 2006. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat. Neurosci. 9 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, P., 1995. Mutagenesis. Methods Cell. Biol. 48 31–58. [PubMed] [Google Scholar]
- Asakura, T., K. Ogura and Y. Goshima, 2007. UNC-6 expression by the vulval precursor cells of Caenorhabditis elegans is required for the complex axon guidance of the HSN neurons. Dev. Biol. 304 800–810. [DOI] [PubMed] [Google Scholar]
- Berwin, B., E. Floor and T. F. Martin, 1998. CAPS (mammalian UNC-31) protein localizes to membranes involved in dense-core vesicle exocytosis. Neuron 21 137–145. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdine, R. D., C. S. Branda and M. J. Stern, 1998. EGL-17(FGF) expression coordinates the attraction of the migrating sex myoblasts with vulval induction in C. elegans. Development 125 1083–1093. [DOI] [PubMed] [Google Scholar]
- Byrd, D. T., M. Kawasaki, M. Walcoff, N. Hisamoto, K. Matsumoto et al., 2001. UNC-16, a JNK-signaling scaffold protein, regulates vesicle transport in C. elegans. Neuron 32 787–800. [DOI] [PubMed] [Google Scholar]
- Campbell, R. E., O. Tour, A. E. Palmer, P. A. Steinbach, G. S. Baird et al., 2002. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99 7877–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, S. S., H. Zheng, M. W. Su, R. Wilk, M. T. Killeen et al., 1996. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell 87 187–195. [DOI] [PubMed] [Google Scholar]
- Chilton, J. K., 2006. Molecular mechanisms of axon guidance. Dev. Biol. 292 13–24. [DOI] [PubMed] [Google Scholar]
- Clark, S. G., and C. Chiu, 2003. C. elegans ZAG-1, a Zn-finger-homeodomain protein, regulates axonal development and neuronal differentiation. Development 130 3781–3794. [DOI] [PubMed] [Google Scholar]
- Colamarino, S. A., and M. Tessier-Lavigne, 1995. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell 81 621–629. [DOI] [PubMed] [Google Scholar]
- Colón-Ramos, D. A., M. A. Margeta and K. Shen, 2007. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science 318 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M. W., M. Hammarlund, T. Harrach, P. Hullet, S. Olsen et al., 2005. Rapid single polymorphism in C. elegans. BMC Genomics 6 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson, B. J., 2002. Molecular mechanisms of axon guidance. Science 298 1959–1964. [DOI] [PubMed] [Google Scholar]
- Garcia, S. M., M. O. Casanueva, M. C. Silva, M. D. Amaral and R. I. Morimoto, 2007. Neuronal signaling modulates protein homeostasis in Caenorhabditis elegans post-synaptic muscle cells. Genes Dev. 21 3006–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengyo-Ando, K., Y. Kamiya, A. Yamakawa, K. Kodaira, K. Nishiwaki et al., 1993. The C. elegans unc-18 gene encodes a protein expressed in motor neurons. Neuron 11 703–711. [DOI] [PubMed] [Google Scholar]
- Hall, D. H., and E. M. Hedgecock, 1991. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell 65 837–847. [DOI] [PubMed] [Google Scholar]
- Hao, J. C., T. W. Yu, K. Fujisawa, J. G. Culotti, K. Gengyo-Ando et al., 2001. C. elegans slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron 32 25–38. [DOI] [PubMed] [Google Scholar]
- Hedgecock, E. M., J. G. Culotti and D. H. Hall, 1990. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4 61–85. [DOI] [PubMed] [Google Scholar]
- Hiramoto, M., Y. Hiromi, E. Giniger and Y. Hotta, 2000. The Drosophila Netrin receptor Frazzled guides axons by controlling Netrin distribution. Nature 406 886–889. [DOI] [PubMed] [Google Scholar]
- Hirokawa, N., and Y. Noda, 2008. Intracellular transport and kinesin superfamily proteins, KIFs: structure, function, and dynamics. Physiol. Rev. 88 1089–1118. [DOI] [PubMed] [Google Scholar]
- Hirokawa, N., and R. Takemura, 2005. Molecular motors and mechanisms of directional transport in neurons. Nat. Rev. Neurosci. 6 201–214. [DOI] [PubMed] [Google Scholar]
- Inoue, K., S. Ozaki, T. Shiga, K. Ito, T. Masuda et al., 2002. Runx3 controls the axonal projection of proprioceptive dorsal root ganglion neurons. Nat. Neurosci. 10 946–954. [DOI] [PubMed] [Google Scholar]
- Ishii, N., W. G. Wadsworth, B. D. Stern, J. G. Culotti and E. M. Hedgecock, 1992. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron 9 873–881. [DOI] [PubMed] [Google Scholar]
- Jin, Y., E. Jorgensen, E. Hartwieg and H. R. Horvitz, 1999. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J. Neurosci. 19 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikura, D. M., and J. A. Cooper, 2003. Lipoprotein receptors and a disabled family cytoplasmic adaptor protein regulate EGL-17/FGF export in C. elegans. Genes Dev. 17 2798–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikura, D. M., and J. A. Cooper, 2006. Clathrin interaction and subcellular localization of Ce-DAB-1, an adaptor for protein secretion in Caenorhabditis elegans. Traffic 7 324–336. [DOI] [PubMed] [Google Scholar]
- Killeen, M. T., and S. S. Sybingco, 2008. Netrin, Slit and Wnt receptors allow axons to choose the axis of migration. Dev. Biol. 323 143–151. [DOI] [PubMed] [Google Scholar]
- Killeen, M., J. Tong, A. Krizus, R. Steven, I. Scott et al., 2002. UNC-5 function requires phosphorylation of cytoplasmic tyrosine 482, but its UNC-40-independent functions also require a region between the ZU-5 and death domains. Dev. Biol. 251 348–366. [DOI] [PubMed] [Google Scholar]
- Koushika, S. P., J. E. Richmond, G. Hadwiger, R. M. Weimer, E. M. Jorgensen et al., 2001. A post-docking role for active zone protein Rim. Nat. Neurosci. 4 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, T., and G. Garriga, 2004. The conserved kinase UNC-51 acts with VAB-8 and UNC-14 to regulate axon outgrowth in C. elegans. Development 131 5991–6000. [DOI] [PubMed] [Google Scholar]
- Lee, J., G. D. Jongeward and P. W. Sternberg, 1994. unc-101, a gene required for many aspects of Caenorhabditis elegans development and behavior, encodes a clathrin-associated protein. Genes Dev. 8 60–73. [DOI] [PubMed] [Google Scholar]
- Lee, R. Y., L. Lobel, M. Hengartner, H. R. Horvitz and L. Avery, 1997. Mutations in the alpha1 subunit of an L-type voltage-activated Ca2+ channel cause myotonia in Caenorhabditis elegans. EMBO J. 16 6066–6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung-Hagesteijn, C., A. M. Spence, B. D. Stern, Y. Zhou, M. W. Su et al., 1992. UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell 71 289–299. [DOI] [PubMed] [Google Scholar]
- Li, W., R. K. Herman and J. E. Shaw, 1992. Analysis of the Caenorhabditis elegans axonal guidance and outgrowth gene unc-33. Genetics 132 675–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q., B. Chen, M. Yankova, D. K. Morest, E. Maryon et al., 2005. Presynaptic ryanodine receptors are required for normal quantal size at the Caenorhabditis elegans neuromuscular junction. J. Neurosci. 25 6745–6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malsam, J., S. Kreye and T. H. Söllner, 2008. Membrane fusion: SNAREs and regulation. Cell. Mol. Life Sci. 65 2814–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama, I. N., and S. Brenner, 1991. A phorbol ester/diacylglycerol-binding protein encoded by the unc-13 gene of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 88 5729–5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryon, E. B., R. Coronado and P. Anderson, 1996. unc-68 encodes a ryanodine receptor involved in regulating C. elegans body-wall muscle contraction. J. Cell Biol. 134 885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura, A., M. Tsukada, Y. Wada and Y. Ohsumi, 1997. Apg1p, a novel protein kinase required for the autophagic process in Saccharomyces cerevisiae. Gene 192 245–250. [DOI] [PubMed] [Google Scholar]
- McIntire, S. L., G. Garriga, J. White, D. Jacobson and H. R. Horvitz, 1992. Genes necessary for directed axonal elongation or fasciculation in C. elegans. Neuron 2 307–322. [DOI] [PubMed] [Google Scholar]
- McNally, K., A. Audhya, K. Oegema and F. J. McNally, 2006. Katanin controls mitotic and meiotic spindle length. J. Cell Biol. 175 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meléndez, A., Z. Tallóczy, M. Seaman, E. L. Eskelinen, D. H. Hall et al., 2003. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 301 1387–1391. [DOI] [PubMed] [Google Scholar]
- Mello, C. C., J. M. Kramer, D. Stinchcomb and V. Ambros, 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz, D. C., H. Zheng, M. T. Killeen, A. Krizus and J. G. Culotti, 2001. Multiple signaling mechanisms of the UNC-6/netrin receptors UNC-5 and UNC-40/DCC in vivo. Genetics 158 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima, N., 2007. Autophagy: process and function. Genes Dev. 21 2861–2873. [DOI] [PubMed] [Google Scholar]
- Nakata, K., B. Abrams, B. Grill, A. Goncharov, X. Huang et al., 2005. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell 120 407–420. [DOI] [PubMed] [Google Scholar]
- Nonet, M. L., 1999. Visualization of synaptic specializations in live C. elegans with synaptic vesicle protein-GFP fusions. J. Neurosci. Methods 89 33–40. [DOI] [PubMed] [Google Scholar]
- Nonet, M. L., K. Grundahl, B. J. Meyer and J. B. Rand, 1993. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell 73 1291–1305. [DOI] [PubMed] [Google Scholar]
- Nonet, M. L., J. E. Staunton, M. P. Kilgard, T. Fergestad, E. Hartwieg et al., 1997. Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J. Neurosci. 21 8061–8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet, M. L., O. Saifee, H. Zhao, J. B. Rand and L. Wei, 1998. Synaptic transmission deficits in Caenorhabditis elegans synaptobrevin mutants. J. Neurosci. 18 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, H., S. Harada, T. Sassa, H. Yamamoto and R. Hosono, 1998. Functional properties of the unc-64 gene encoding a Caenorhabditis elegans syntaxin. J. Biol. Chem. 273 2192–2198. [DOI] [PubMed] [Google Scholar]
- Ogura, K., and Y. Goshima, 2006. The autophagy-related kinase UNC-51 and its binding partner UNC-14 regulate the subcellular localization of the Netrin receptor UNC-5 in Caenorhabditis elegans. Development 133 3441–3450. [DOI] [PubMed] [Google Scholar]
- Ogura, K., C. Wicky, L. Magnenat, H. Tobler, I. Mori et al., 1994. Caenorhabditis elegans unc-51 gene required for axonal elongation encodes a novel serine/threonine kinase. Genes Dev. 8 2389–2400. [DOI] [PubMed] [Google Scholar]
- Ogura, K., M. Shirakawa, T. M Barnes, S. Hekimi and Y. Ohshima, 1997. The UNC-14 protein required for axonal elongation and guidance in Caenorhabditis elegans interacts with the serine/threonine kinase UNC-51. Genes Dev. 11 1801–1811. [DOI] [PubMed] [Google Scholar]
- Okkema, P. G., S. W. Harrison, V. Plunger, A. Aryana and A. Fire, 1993. Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics 135 385–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka, A. J., A. Jeyaprakash, J. Garcia-Anoveros, L. Z. Tang, G. Fisk et al., 1991. The C. elegans unc-104 gene encodes a putative kinesin heavy chain-like protein. Neuron 6 113–122. [DOI] [PubMed] [Google Scholar]
- Otsuka, A. J., R. Franco, B. Yang, K. H. Shim, L. Z. Tang et al., 1995. An ankyrin-related gene (unc-44) is necessary for proper axonal guidance in Caenorhabditis elegans. J. Cell Biol. 129 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, N., D. Thierry-Mieg and J. R. Mancillas, 1993. Cloning by insertional mutagenesis of a cDNA encoding Caenorhabditis elegans kinesin heavy chain. Proc. Natl. Acad. Sci. USA 90 9181–9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon, V. Y., M. P. Klassen and K. Shen, 2008. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature 455 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifee, O., L. Wei and M. L. Nonet, 1998. The Caenorhabditis elegans unc-64 locus encodes a syntaxin that interacts genetically with synaptobrevin. Mol. Biol. Cell 9 1235–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto, R., D. T. Byrd, H. M. Brown, N. Hisamoto, K. Matsumoto et al., 2005. The Caenorhabditis elegans UNC-14 RUN domain protein binds to the kinesin-1 and UNC-16 complex and regulates synaptic vesicle localization. Mol. Biol. Cell 16 483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakube, Y., H. Ando and H. Kagawa, 1997. An abnormal ketamine response in mutants defective in the ryanodine receptor gene ryr-1 (unc-68) of Caenorhabditis elegans. J. Mol. Biol. 267 849–864. [DOI] [PubMed] [Google Scholar]
- Sassa, T., S. Harada, H. Ogawa, J. B. Rand, I. N. Maruyama et al., 1999. Regulation of the UNC-18-Caenorhabditis elegans syntaxin complex by UNC-13. J. Neurosci. 19 4772–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini, T., T. E. Kennedy, M. J. Galko, C. Mirzayan, T. M. Jessell et al., 1994. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78 409–424. [DOI] [PubMed] [Google Scholar]
- Shakir, M. A., T. Fukushige, H. Yasuda, J. Miwa and S. S. Siddiqui, 1993. C. elegans osm-3 gene mediating osmotic avoidance behaviour encodes a kinesin-like protein. Neuroreport 4 891–894. [DOI] [PubMed] [Google Scholar]
- Shen, L. L., Y. Wang and D. Y. Wang, 2007. Involvement of genes required for synaptic function in aging control in C. elegans. Neurosci. Bull. 23 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi, G., M. Shoji, M. Nakamura, T. Ishihara, I. Katsura et al., 2001. Mutations affecting nerve attachment of Caenohabditis elegans. Genetics 157 1611–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speese, S., M. Petrie, K. Schuske, M. Ailion, K. Ann et al., 2007. UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans. J. Neurosci. 27 6150–6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub, M., M. Bredschneider and M. Thumm, 1997. AUT3, a serine/threonine kinase gene, is essential for autophagocytosis in Saccharomyces cerevisiae. J. Bacteriol. 179 3875–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringham, E., N. Pujol, J. Vandekerckhove and T. Bogaert, 2002. unc-53 controls longitudinal migration in C. elegans. Development 129 3367–3379. [DOI] [PubMed] [Google Scholar]
- Südhof, T. C, and J. E. Rothman, 2009. Membrane fusion: grappling with SNARE and SM proteins. Science 323 474–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze, J. Y., M. Victor, C. Loer, Y. Shi and G. Ruvkun, 2000. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 403 560–564. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne, M., and C. S. Goodman, 1996. The molecular biology of axon guidance. Science 274 1123–1133. [DOI] [PubMed] [Google Scholar]
- Toda, H., H. Mochizuki, R. Flores, III, R. Josowitz, T. B. Krasieva et al., 2008. UNC-51/ATG1 kinase regulates axonal transport by mediating motor-cargo assembly. Genes Dev. 23 3292–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth, W. G., 2002. Moving around in a worm: netrin UNC-6 and circumferential axon guidance in C. elegans. Trends Neurosci. 8 423–429. [DOI] [PubMed] [Google Scholar]
- Wadsworth, W. G., H. Bhatt and E. M. Hedgecock, 1996. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron 16 35–46. [DOI] [PubMed] [Google Scholar]
- Wang, D., S. Kennedy, D. Conte, Jr., J. K. Kim, H. W. Gabel et al., 2005. Somatic misexpression of germline P granules and enhanced RNA interference in retinoblastoma pathway mutants. Nature 436 593–597. [DOI] [PubMed] [Google Scholar]
- Watanabe, K., N. Tamamaki, T. Furuta, S. L. Ackerman, K. Ikenaka et al., 2006. Dorsally derived netrin 1 provides an inhibitory cue and elaborates the 'waiting period' for primary sensory axons in the developing spinal cord. Development 133 1379–1387. [DOI] [PubMed] [Google Scholar]
- Weimer, R. M., J. E. Richmond, W. S. Davis, G. Hadwiger, M. L. Nonet et al., 2003. Defects in synaptic vesicle docking in unc-18 mutants. Nat. Neurosci. 6 1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks, S. R., R. T. Yeh, W. R. Gish, R. H. Waterson and R. H. Plasterk, 2001. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 28 160–164. [DOI] [PubMed] [Google Scholar]
- Wolf, F. W., M. S. Hung, B. Wightman, J. Way and G. Garriga, 1998. vab-8 is a key regulator of posteriorly directed migrations in C. elegans and encodes a novel protein with kinesin motor similarity. Neuron 20 655–666. [DOI] [PubMed] [Google Scholar]
- Yonekawa, Y., A. Harada, Y. Okada, T. Funakoshi, Y. Kanai et al., 1998. Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J. Cell Biol. 141 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, T. W., and C. I. Bargmann, 2001. Dynamic regulation of axon guidance. Nat. Neurosci. 4 1169–1176. [DOI] [PubMed] [Google Scholar]
- Zahn, T., J. Angleson, M. MacMorris, E. Domke, J. Hutton et al., 2004. Dense core vesicle dynamics in Caenorhabditis elegans neurons and the role of kinesin UNC-104. Traffic 5 544–559. [DOI] [PubMed] [Google Scholar]
- Zalk, R., S. E. Lehnart and A. R. Marks, 2007. Modulation of the ryanodine receptor and intracellular calcium. Annu. Rev. Biochem. 76 367–385. [DOI] [PubMed] [Google Scholar]
- Zhao, H., and M. L. Nonet, 2000. A retrograde signal is involved in activity-dependent remodeling at a C. elegans neuromuscular junction. Development. 127 1253–1266. [DOI] [PubMed] [Google Scholar]
- Ziel, J. W., E. J. Hagedorn, A. Audhya and D. R. Sherwood, 2009. UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat. Cell Biol. 11 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]