Abstract
Objective
We sought to understand the link between low SEP and cardiovascular disease (CVD) by examining the association between SEP, health-related coping behaviors, and C-reactive protein (CRP), an inflammatory marker and independent risk factor for CVD in a US sample of adults.
Design
We used a multiple mediation model to evaluate how these behaviors work in concert to influence CRP levels and whether these relationships were moderated by gender and race/ethnicity.
Main outcome measures
CRP levels were divided into two categories: elevated CRP (3.1–10.0 mg/L) and normal CRP (≤ 3.0 mg/L).
Results
Both poverty and low educational attainment were associated with elevated CRP, and these associations were primarily explained through higher levels of smoking and lower levels of exercise. In the education model, poor diet also emerged as a significant mediator. These behaviors accounted for 87.9% of the total effect of education on CRP and 55.8% the total effect of poverty on CRP. We also found significant moderation of these mediated effects by gender and race/ethnicity.
Conclusion
These findings demonstrate the influence of socioeconomically-patterned environmental constraints on individual-level health behaviors. Specifically, reducing socioeconomic inequalities may have positive effects on CVD disparities through reducing cigarette smoking and increasing vigorous exercise.
Keywords: C-reactive protein, mediation, moderation, socioeconomic position, health behaviors
Introduction
The incidence of cardiovascular disease (CVD) is higher among individuals of lower as compared to higher socioeconomic position (SEP) (Kaplan & Keil, 1993; Winkleby, Jatulis, Frank, & Fortmann, 1992); however, the determinants of this association are not well understood (Lantz, et al., 1998; Winkleby, Cubbin, Ahn, & Kraemer, 1999). Exposure to social stressors (e.g., violence or daily hassles) is patterned by socioeconomic position (Wheaton, 1999) and may contribute to the distribution of CVD in the population. Stressors may influence CVD risk directly, through repeated activation of the stress-responsive neuroendocrine systems that also influence cardiac function, plaque formation, and sympathetic-adrenal function (McEwen, 1998). Stress may also influence CVD risk indirectly via health behaviors that are provoked as a means of responding to or coping with these challenges (Jackson & Knight, 2006; Jackson, Knight, & Rafferty, 2009).
Studies in both animals and humans have demonstrated that repeated or persistent exposure to stressors, whether physical or psychosocial, is associated with the dysregulation of neuroendocrine feedback loops governing the hypothalamic-pituitary-adrenal (HPA) axis (Crimmins, Kim, & Seeman, 2009; Sapolsky, Alberts, & Altmann, 1997; Taylor, Lehman, Kiefe, & Seeman, 2006). The HPA axis stimulates production of pro-inflammatory cytokines such as interleukin-6, which in turn stimulate C-reactive protein (CRP) (Papanicolaou, Wilder, Manolagas, & Chrousos, 1998), an acute-phase marker of systemic inflammation. Elevated CRP may be a consequence of this biological cascade, and chronically elevated CRP has been associated with increased risk of myocardial infarction and stroke (Pradhan, et al., 2002; Ridker, Hennekens, Buring, & Rifai, 2000; Schlager, et al., 2007). There is a growing body of research suggesting that there are differences in CRP levels among men and women, with women on average having higher levels of CRP, even after accounting for estrogen use (Beasley, et al., 2009; Khera, et al., 2005; Lakoski, et al., 2006). In addition, Whites typically have lower CRP levels than Blacks and Hispanics (Beasley, et al., 2009; Nazmi & Victora, 2007). The reasons, however, for these gender and race/ethnic differences are not clear.
Consistent with the patterning of incidence of CVD in the population, elevated CRP is also more prevalent in low SEP individuals (Loucks, et al., 2006; Nazmi & Victora, 2007; Ranjit, et al., 2007). Exposure to stressors like those associated with low SEP can evoke a variety of coping behaviors as a means of adapting or resisting the psychosocial threat presented by the exposure (Taylor & Armor, 1996), some of which have negative physical health consequences. Many CVD risk behaviors, including smoking, alcohol use, and consuming high-fat foods, (Albert, Glynn, & Ridker, 2003; Frohlich, et al., 2003; Nettleton, et al., 2006) have been shown to reduce feelings of anxiety associated with stress (Jackson & Knight, 2006; Krueger & Chang, 2008; Ng & Jeffery, 2003). Conversely, there are coping behaviors that are linked to positive health outcomes. For example, in addition to reducing chronic disease risk, exercise has been shown to improve perceived coping abilities and reduce anxiety (DiLorenzo, et al., 1999; Moses, Steptoe, Mathews, & Edwards, 1989; Steptoe, Edwards, Moses, & Mathews, 1989).
These same behaviors are also associated with CRP levels. Several studies have shown a significant dose-dependent association between cigarette smoking and elevated CRP (Bazzano, He, Muntner, Vupputuri, & Whelton, 2003; Dietrich, Garcia, de Pablo, Schulze, & Hoffmann, 2007), and high glycemic load and consumption of starchy foods have both been linked with increased CRP levels (Ford, 2002; Mora, Lee, Buring, & Ridker, 2006). Studies indicate there may be a U-shaped association between amount of alcohol consumption and elevated CRP levels (Imhof, et al., 2001; Pai, et al., 2006). CRP levels are generally found to be highest in heavy drinkers, but lower in moderate drinkers compared to those who do not drink. The higher levels seen in abstainers compared with moderate drinkers, however, may be due to high CRP levels among former drinkers (Averina, Nilssen, Arkhipovsky, Kalinin, & Brox, 2006). There is also evidence of a significant inverse association between exercise and CRP levels (Hickling, Hung, Knuiman, Divitini, & Beilby, 2008; Huffman, et al., 2007; Liu, et al., 2002). Studies have found that mean CRP levels and odds of elevated CRP decrease with increasing exercise intensity.
Health behaviors may therefore be a primary mediating pathway linking stress and consequent elevated CRP levels and increased CVD risk among groups overrepresented in lower SEP strata. Furthermore, the particular behaviors used to cope with stress may vary by race/ethnicity and gender. Societal views on gender roles may place constraints on women, limiting the types of coping behaviors (both positive and negative) that are socially acceptable. For example, some research suggests that women who drink heavily are viewed more negatively than men and may be more concerned with how others perceive their drinking (Armeli, Carney, Tennen, Affleck, & O’Neil, 2000; George, Gourmic, & McAfee, 1988). This is one hypothesized explanation for the finding that the association between chronic strain and drinking is stronger among men than women (Armeli, et al., 2000; Cooper, Russell, Skinner, Frone, & Mudar, 1992). In addition, historically disadvantaged minority groups are more likely to be of lower socioeconomic position and live in segregated, resource-poor neighborhoods with limited access to coping resources associated with positive health outcomes, and increased exposure to stressors and behaviors linked to negative health outcomes (LaVeist & Wallace, 2000; Moore & Diez Roux, 2006; Williams, 1999).
Existing research on the relationship between socioeconomic position, health behaviors, and CVD risk is limited in several ways. Previous research has generally relied on traditional multivariate regression modeling approaches, which do not allow for the simultaneous evaluation of predictors as mediators and moderators. Health behaviors often cluster together within individuals and thus it is essential to use analytic methods that explicitly examine the joint influences of these behaviors on CVD risk. This approach is also useful for identifying how behaviors interact with group-level characteristics (e.g., gender) that may have varying influence at different points in the causal chain. Such investigations can shed light on the factors that drive the differential distribution of CVD risk in the population and inform the tailoring of intervention efforts to specific subgroups which may have similar overall health burdens, but different underlying pathways.
This study utilized a multiple mediator model to assess the relative contribution of positive (vigorous exercise) and negative (cigarette smoking, alcohol use, and poor diet) health behaviors on the relationship between the chronic stress of low SEP and elevated CRP. Our analysis addressed three questions: (1) to what extent do health behaviors mediate the association between SEP and elevated CRP, (2) are these relationships invariant across different measures of SEP, specifically education and relative poverty, and (3) do the relative contributions of these multiple behavioral pathways linking SEP and CRP vary between men and women or across race/ethnic groups (Hispanics, non-Hispanic Blacks, and non-Hispanic Whites)?
Methods
Data source and study population
Data are from the fourth wave of the National Health and Nutrition Examination Surveys (NHANES IV) (Centers for Disease Control, 2004). Cross-sectional data were collected continually between 2001 and 2006. NHANES IV is a multi-stage stratified probability sample of US households, with an oversample of Blacks, Hispanics, and older adults which provides estimates for the national non-institutionalized population when sample weights are applied. National Center for Health Statistics Research Ethics Review Board approval was obtained for NHANES IV and informed consent was obtained from all participants.
The sample used in these analyses was restricted to black, white, and Hispanic participants aged 40 and older (N = 9508). This age group was chosen because of its relative stability in terms of socioeconomic attainment and high immediate risk of incident cardiovascular disease.
C-reactive protein
Serum CRP samples were analyzed by high-sensitivity latex-enhanced nephelometry using a BNII Nephelometer (Centers for Disease Control, 2004). For these analyses, CRP levels were divided into two categories: elevated CRP (3.1–10.0 mg/L) and normal CRP (≤ 3.0 mg/L). These groupings are based on recommendations by the American Heart Association (AHA) and the Centers for Disease Control and Prevention (CDC) that individuals with CRP levels greater than 3.0 mg/L are at increased risk for CVD (Pearson, et al., 2003). As noted earlier, there are gender and race/ethnic differences in CRP levels. However, it is not clear at this point whether or not these differences contribute to differences in CVD risk (Khera, et al., 2005), and research suggests that this cutoff is significantly associated with incident coronary heart disease in both men and women after adjusting for race/ethnicity (Cushman, et al., 2005). The recommendations by the AHA and CDC also suggested that CRP levels greater than 10.0 mg/L are indicative of acute infection and, thus, should not be used as indicators of persistent levels of this protein. Individuals in this category were excluded from the analyses.
Socioeconomic position
In order to investigate whether different facets of SEP have equivalent influence on levels of CRP, two measures of SEP were used in these analyses: poverty and education. Self-reported family income was used in NHANES IV to calculate a poverty-to-income ratio (PIR). The PIR is defined as the midpoint of the reported family income category divided by the official U.S. Census poverty threshold corresponding to the size of the household. Poverty was dichotomized as living in or near poverty (PIR < 1.25) versus not in poverty. Education was categorized based on self-report as completion of less than high school, high school, some college, and college or more.
Potential mediators
Vigorous exercise, cigarette smoking, poor diet, and heavy alcohol use were tested as potential behavioral pathways through which the stress of low socioeconomic position is associated with elevated CRP levels. Participants were asked whether or not they engaged in any vigorous exercise lasting 10 minutes or longer over the last 30 days, and this response was dichotomized as yes versus no. Cigarette smoking was dichotomized as current versus not current. Heavy alcohol consumption was defined as a report of both being a current drinker and having an average of more than two drinks on days when alcohol was consumed (nondrinkers and those consuming ≤ two drinks were the reference group).
Diet was assessed using a single 24-hour dietary recall. Based on previous studies of diet and stress coping (Dallman, et al., 2003; De Caterina, Zampolli, Del Turco, Madonna, & Massaro, 2006), poor diet was defined as having low adherence to recommended levels of total fat, saturated fat, carbohydrates, and cholesterol consumption, based on the United States Department of Agriculture guidelines (U.S. Department of Health and Human Services & U.S. Department of Agriculture, 2005). The measure of dietary adherence was created by assigning respondents one point for each USDA guideline that was met. Poor diet was dichotomized at the mean (meeting one of the recommended levels) with scores above the mean serving as the reference group.
Other covariates
The following factors have been shown to be associated with both SEP and CRP and therefore were included in the multivariate models as potential confounders: age, obesity, and chronic conditions. Age was measured continuously. Obesity was defined as having a body mass index (BMI) greater than or equal to 30 kg/m2. We explored several chronic conditions as potential confounders of the association between low SEP and elevated CRP, including self-reported hypertension, high cholesterol, diabetes, arthritis, myocardial infarction, angina pectoris, congestive heart failure, stroke, cancer, asthma, bronchitis, and emphysema. We only included disease conditions that were significantly associated with both poverty and high CRP at the 0.10 level using chi-square tests. According to these criteria, chronic disease was modeled dichotomously based on whether an individual had any of the following conditions: diabetes, arthritis, bronchitis, emphysema, congestive heart failure, heart attack, hypertension, or stroke. As discussed above, we also examined gender and race/ethnicity as moderators of the relationship among SEP, health behaviors, and CRP. Gender was dichotomized as male versus female, and race/ethnicity was categorized as Black, Hispanic, and White, with the latter serving as the reference group.
Data analysis
There were 9508 white, black, and Hispanic NHANES IV participants aged 40 years and older. Five pregnant participants were excluded because CRP levels may shift during gestation, making their measurements potentially misleading. An additional 1009 were excluded for having CRP levels above 10.0 mg/L. Of the 8494 participants remaining, 6313 (74.3%) had complete data available on all covariates used in the poverty analyses and 6688 (78.7%) had complete data for the education analyses.
Means and frequencies were calculated for all continuous and categorical covariates by poverty status, educational attainment, and CRP level, and t-tests and chi-square tests were used to estimate bivariate associations, taking into account the complex sampling design of NHANES.
Mediation analyses
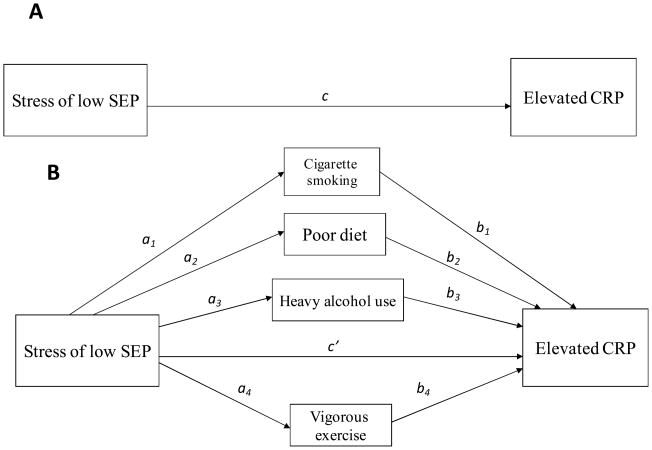
Two separate multiple mediation analyses, one for each indicator of socioeconomic position, were conducted to test the hypothesis that the association between SEP and elevated CRP is mediated by the four health behaviors. This mediation was tested using a path analytic approach. Path analysis is an appropriate technique for evaluating mediation because it allows for the simultaneous modeling of multiple regression relationships (see Figure 1). Figure 1A shows the total effect, c, of SEP on CRP. The estimate of the total effect, c, was disaggregated into individual indirect effects, a1–4 and b1–4, which represent the effects of the four mediating health behaviors, and the direct effect, c′, the effect of SEP that is independent of these mediators and age, gender, race/ethnicity, chronic conditions, and obesity.
Figure 1.
Illustration of proposed multiple mediation of the association between SEP and elevated CRP. (A) Total effect of poverty on CRP. (B) Hypothesized indirect effect of SEP on CRP through mediators and residual direct effect.
Structural equation modeling using probit regression was employed in these analyses. Probit regression is a log-linear approach analogous to logistic regression. In probit regression, the normal distribution is used instead of the logistic distribution, and predicted probits are z-scores instead of log-odds. Probit regression is believed to be more accurate for mediation with a categorical outcome (MacKinnon, 2008). Since predicted probits are z-scores, they are easily transformed into predicted probabilities. Thus, results are presented as coefficients (z-scores) and are then transformed into predicted probabilities for ease of interpretation.
Two probit regression models were used to assess mediation paths for each proposed mediator: first, the effect of poverty/educational attainment on each of the potential mediators (a1 through a4 in Figure 1B), and second, the effect of the potential mediators on high CRP mediators after accounting for poverty/educational attainment and all confounders (b1 through b4 in Figure 1B). In the first set of models, all mediator-CRP association models were adjusted for age, race/ethnicity, and sex. The vigorous exercise-CRP association model was further adjusted for obesity and chronic conditions. The second model was adjusted for age, race/ethnicity, sex, obesity, and chronic conditions. The product of the two coefficients, a*b, for each of the mediators is the estimate of the indirect effect of poverty/educational attainment on CRP through each specific mediator (Sobel, 1982). Significance tests of these mediation pathways were estimated by dividing each indirect effect by the corresponding standard error.
In order to account for multicollinearity, potential correlations between proposed mediators and confounders were assessed using modification indices. A modification index is the amount the chi-square will drop if a given parameter is included in the model. Strong correlations were found between smoking and vigorous activity and between smoking and heavy alcohol consumption; thus, these correlated variables were allowed to covary in the education and poverty mediation models. Overall model fit was assessed using the Comparative Fit Index (CFI) and the Root Mean Square Error of Approximation (RMSEA); it is recommended that the CFI be above 0.90 (Bentler, 1990) and the RMSEA be below 0.05 (Brown & Cudeck, 1993).
Moderated mediation analyses
Moderated mediation is defined as a mediated effect that varies across levels of a moderator, either on the path from the independent variable to the mediator or from the mediator to the outcome variable (Edwards & Lambert, 2007; Muller, Judd, & Yzerbyt, 2005). We investigated whether the mediation pathways linking SEP and CRP indicated by each health behavior differed for men and women or across race/ethnic groups. We evaluated the moderating influence of gender and race/ethnicity on those mediating pathways that were significant based on the mediation analysis.
We used moderated path analysis based on simple regression equations to assess moderated mediation (Edwards & Lambert, 2007). This involved the same two equations as those used to assess mediation, with the addition of the following interaction terms: the product of the SEP indicator and the moderator and the product of the mediator and the moderator (illustrated below). In each equation, X = main independent variable (poverty or education), Mo = moderator (gender or race/ethnicity), Me = mediator, and Y = main dependent variable (elevated CRP).
The first equation was used to assess moderation of the effect of socioeconomic position on each mediator (Path A). A separate model was estimated for each potential mediator:
| (1) |
The second equation examined moderation of each mediator’s effect on CRP as well as the residual effect of socioeconomic position on CRP (Path B). One model was run incorporating all interactions between the different potential mediators and a single moderator as well as between the socioeconomic indicator and the potential moderators:
| (2) |
The coefficient estimates from these equations were used to assess moderated mediation. Confounders were adjusted for in the same way as described above. Significant Path A moderation was defined as a significant β13 in Equation (1); significant Path B moderation was defined as a significant β25, β27, β29, or β31 in Equation (2). Significant indirect effect moderation was assessed by dividing the difference of the two indirect effects by the pooled standard error of the indirect effect from each group (MacKinnon, 2008).
Descriptive statistics and moderated mediation equations were estimated using Stata 10.1 (StataCorp, College Station, TX) and the multiple mediation analyses were conducted using Mplus (Muthén & Muthén, Los Angeles, CA).
Results
Table 1 presents the distribution of study variables by poverty status and educational attainment. Overall, most characteristics varied significantly by socioeconomic position, whether defined by poverty status or education. Low SEP respondents were more likely to have an elevated CRP level, to be older, to be female, to be Black or Hispanic, to have at least one chronic condition, and to be a heavy alcohol user and a current smoker relative to high SEP respondents.
Table 1.
Descriptive statistics by poverty status and educational attainment
| In or near poverty (1356) | Not in poverty (4953) | p-value | Less than high school (1848) | High school (1498) | Some college (1644) | College or more (1319) | p-value | |
|---|---|---|---|---|---|---|---|---|
| High CRP (%) | 41.6 | 30.4 | <0.0001 | 37.5 | 33.7 | 34.2 | 24.8 | <0.0001 |
| Mean CRP (mg/L)* | 3.1 (0.08) | 2.5 (0.04) | <0.0001 | 3.0 (0.09) | 2.7 (0.05) | 2.7 (0.07) | 2.2 (0.06) | <0.0001 |
| Age (years)* | 58.4 (0.6) | 56.7 (0.3) | 0.008 | 61.7 (0.6) | 58.1 (0.6) | 55.1 (0.4) | 54.8 (0.5) | <0.0001 |
| Gender (% male) | 44.4 | 51.3 | 0.0004 | 50.2 | 48.9 | 47.5 | 54.6 | 0.001 |
| Race (%) | <0.0001 | <0.0001 | ||||||
| Black | 15.8 | 8 | 16.8 | 7.9 | 9.1 | 5.5 | ||
| Hispanic | 19.5 | 6.3 | 22 | 6.6 | 6.4 | 3.1 | ||
| White | 64.7 | 85.7 | 61.2 | 85.5 | 84.5 | 91.3 | ||
| Chronic conditions (%) | 70.6 | 58.4 | <0.0001 | 73.8 | 65.3 | 56.7 | 54.4 | <0.0001 |
| Obese (%) | 34.9 | 31.6 | 0.13 | 34 | 33.8 | 33.7 | 27.7 | 0.005 |
| Vigorous exercise (%) | 15.5 | 31.3 | <0.0001 | 11.7 | 20.1 | 30.7 | 46 | <0.0001 |
| Low dietary adherence (%) | 59.1 | 58.3 | 0.7 | 60.1 | 61.4 | 58.3 | 54.7 | 0.02 |
| Heavy alcohol use (%) | 20.4 | 16.8 | 0.02 | 19 | 19.9 | 19.1 | 12.1 | <0.0001 |
| Current smoker (%) | 32.8 | 17.7 | <0.0001 | 29 | 23.4 | 21.6 | 9.1 | <0.0001 |
Values in parentheses are standard errors
Mediation
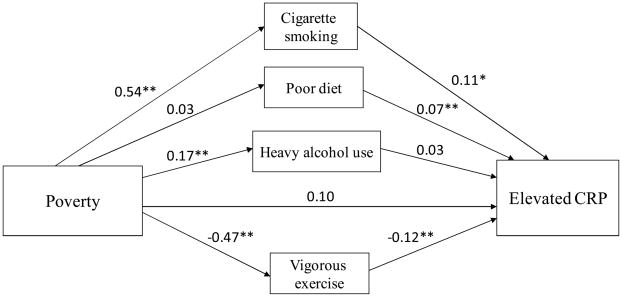
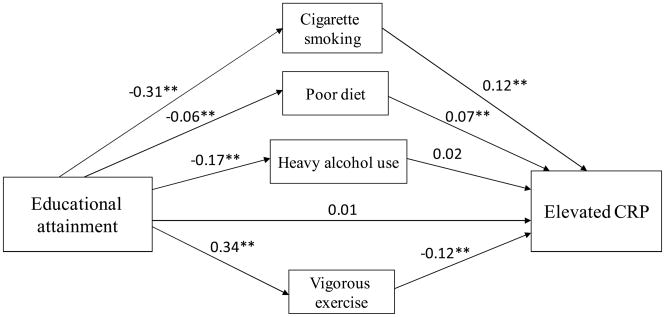
Because there was evidence of a linear association between educational attainment and the health behaviors (data not shown), we present our findings with education modeled ordinally. Figures 2 and 3 present the indirect and direct effects of our two measures of socioeconomic position, poverty status and educational attainment, respectively, on elevated CRP. Both models represent the data well (CFI: 0.95 for each model; RMSEA: 0.01 and 0.02). There was a significant total effect of poverty on elevated CRP (c = 0.22, p < 0.001) and of education on CRP (c = −0.07, p < 0.001). Thus, the predicted probability of elevated CRP was 7% higher for those living in poverty relative to those with higher income. The predicted probability of elevated CRP was 2% lower for each increasing level of education attained. The majority (55.8%) of the total effect of poverty on CRP was explained by the indirect effect (i.e., mediated by health behaviors), while the remaining 44.2% was accounted for by the direct effect. The indirect effect of education on CRP accounted for 87.9% of the total effect, while the direct effect accounted for just 12.1% of the total effect. The direct effect of poverty on elevated CRP was marginally significant (c′ = 0.10, p = 0.11), while the direct effect of education on elevated CRP was not statistically significant (c′ = 0.01, p = 0.57).
Figure 2.
Path analysis of mediation of poverty-CRP association after adjusting for age, gender, race, chronic conditions, and obesity.*p<0.05; **p<0.01
Figure 3.
Path analysis of mediation of education-CRP association after adjusting for age, gender, race, chronic conditions, and obesity. *p<0.05; **p<0.01
The relative mediating influence of particular health behaviors varied depending on the indicator of socioeconomic position. The results from the poverty analysis (Figure 2) suggest two indirect routes to elevated CRP levels: cigarette smoking (indirect effect path coefficient a*b = 0.06, p = 0.008) and lack of vigorous exercise (a*b = 0.06, p = 0.01). The predicted probability of being a current smoker increased by 16% for those living in or near poverty versus not. And, in turn, the predicted probability of elevated CRP increased by 3% for current smokers versus current non-smokers. The predicted probability of engaging in vigorous exercise decreased by 16% among those living in or near poverty versus not, and the probability of having elevated CRP decreased by 4% among those who exercised compared with those who did not.
Using educational attainment as a measure of socioeconomic position yielded similar results regarding the significant mediating pathways of cigarette smoking (a*b= −0.04, p=.005) and vigorous exercise (a*b = −0.04, p = 0.002). The predicted probability of smoking decreased by 10% with each increasing level of educational attainment, and the predicted probability of elevated CRP increased by 5% among smokers versus non-smokers. The predicted probability of exercise increased by 8% with each increasing level of education, and the predicted probability of elevated CRP decreased by 4% among those who exercised vigorously compared with those who did not. In contrast to the results using poverty as an indicator of SEP, poor diet was also a significant mediating pathways between education and CRP (a*b = −0.005, p = 0.01). The predicted probability of poor diet was 2% lower for each increasing level of educational attainment, and the predicted probability of elevated CRP was 3% higher among those with poor diet versus those with better diets.
Moderated mediation
We examined whether the behavioral pathways underlying the inverse associations between SEP and CRP differed by gender or race/ethnicity using moderated mediation analysis. We only assessed the moderating effects of race/ethnicity and gender for those behaviors that were significant in the mediation models. Table 2 presents the moderation results, including both composite and total indirect effects, for each race/ethnic and gender subgroup. There was no evidence that the influence of smoking varied by gender or differed for Blacks relative to Whites for either poverty or education. The effect of smoking, however, did differ for Hispanics relative to Whites; the inverse association between poverty and cigarette smoking was weaker among Hispanics as compared to Whites (a = 0.27 for Hispanics vs. a = 0.60 for Whites). The results for the path linking education and smoking were similar (a = −0.16 for Hispanics vs. a = −0.34 for Whites). The association between smoking and elevated CRP was negative and not significant for Hispanics (b = −0.18 in both models), the opposite of that seen for Whites (b = 0.26 in poverty model and b = 0.27 in education model).
Table 2.
Moderation effects of selected health behaviors on the association between socioeconomic position and elevated CRP
| Cigarette smoking | Vigorous exercise | Poor diet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Moderator | Education → Behavior | Behavior → CRP | Total indirect effect | Education → behavior | Behavior → CRP | Total indirect effect | Education → Behavior | Behavior → CRP | Total indirect effect |
| Race/ethnicity | |||||||||
| White | −0.34 (0.03)** | 0.27 (0.06)** | −0.09** | 0.35 (0.03)** | −0.30 (0.07)** | −0.11** | −0.10 (0.02)** | 0.12 (0.05)* | −0.01* |
| Black | −0.26 (0.04)** | 0.29 (0.11)* | −0.08* | 0.25 (0.05)** | −0.16 (0.14) | −0.04 | 0.02 a (0.04) | 0.14 (0.09) | 0.003 |
| Hispanic | −0.16 a (0.05)** | −0.18 a (0.13) | 0.03 a | 0.33 (0.05)** | 0.03 (0.16) | 0.01 | 0.09 a (0.05) | −0.05 (0.09) | −0.005 |
| Sex | |||||||||
| Women | −0.30 (0.03)** | 0.17 (0.09) | −0.05 | 0.31 (0.03)** | −0.25 (0.08)** | −0.08** | −0.11 (0.03)** | 0.20 (0.05)** | −0.02** |
| Men | −0.32 (0.03)** | 0.30 (0.08)** | −0.10** | 0.36 (0.03)** | −0.28 (0.07)** | −0.10** | −0.04 b (0.02) | 0.02 b (0.07) | −0.001 b |
| Poverty → Behavior | Behavior → CRP | Total indirect effect | Poverty → Behavior | Behavior → CRP | Total indirect effect | ||||
| Race/ethnicity | |||||||||
| White | 0.60 (0.08)** | 0.26 (0.06)** | 0.16** | −0.58 (0.11)** | −0.29 (0.07)** | 0.17** | |||
| Black | 0.59 (0.10)** | 0.26 (0.11)* | 0.15* | −0.39 (0.15)* | −0.14 (0.14) | 0.05 | |||
| Hispanic | 0.27 a (0.13)* | −0.18 a (0.14) | −0.05 a | −0.19 a (0.14) | 0.02 (0.15) | −0.004 a | |||
| Sex | |||||||||
| Women | 0.49 (0.09)** | 0.14 (0.09) | 0.07 | −0.42 (0.10)** | −0.24 (0.08)** | 0.10* | |||
| Men | 0.61 (0.08)** | 0.30 (0.08)** | 0.18** | −0.52 (0.10)** | −0.25 (0.08)** | 0.13** | |||
Values are probit coefficients (standard errors) and represent the expected change in the z-score of the outcome per unit change in the predictor.
P < 0.05
P < 0.01
Significantly different from Whites (P < 0.10)
Significantly different from women (P < 0.10)
There was also significant moderation of the mediated effect of vigorous exercise by race/ethnicity. Neither the association between poverty and exercise (a = −0.19, p=0.19) nor between exercise and elevated CRP (b = 0.02, p=0.91) were significant among Hispanics, in contrast to the results for Whites. The association between education and exercise was significant for Hispanics (a = 0.33, p<0.0001), but again in contrast with Whites the association between exercise and elevated CRP was not significant (b = 0.03, p=0.87). The associations between education and exercise (a = 0.25 for Blacks, a = 0.35 for Whites) and exercise and elevated CRP were weaker among Blacks compared to Whites (b = −0.16 for Blacks, b = −0.30 for Whites), though there was no significant difference in the Path B association, or in the total indirect effect.
There were both gender and race/ethnic differences in the mediating influence of poor diet on the association between education and elevated CRP. The associations between education and diet (a = −0.04 for men, a = −0.11 for women) and diet and CRP (b = 0.02 for men, b = 0.20 for women) were substantially weaker for men compared to women. The association between education and diet was significantly different for Blacks and Hispanics compared to Whites, with the association being positive in the two minority groups (a = 0.02 for Blacks, a = 0.09 for Hispanics) and negative among Whites (a = −0.10). In addition, the indirect effect of education on elevated CRP through diet was significantly different among Blacks compared to Whites (a*b = 0.003 for Blacks, a*b = −0.01 for Whites).
Discussion
These findings confirm that low socioeconomic position, whether indicated by educational attainment or relative poverty, is associated with elevated CRP, a clinically-relevant physiologic indicator of cardiovascular disease risk. This association was partially mediated by health behaviors that may be used to cope with stressors that are more common in the living and working conditions that characterize low SEP. Our results provide evidence that a subset of the posited health-related coping behaviors mediate the association between socioeconomic position and elevated CRP. Finally, the moderation analyses indicated that the relationships between SEP, health behaviors, and CRP vary by gender and race/ethnicity.
Living in poverty was significantly associated with elevated CRP after accounting for confounding factors, and this association was primarily mediated by higher prevalence of cigarette smoking and lower prevalence of vigorous exercise. Lower educational attainment was also significantly associated with elevated CRP, and this relationship was primarily mediated by cigarette smoking, decreased vigorous exercise, and poor dietary adherence. The mediated effect of diet was stronger for women than for men, and all indirect effects of the health behaviors were significantly weaker for Hispanics relative to Whites.
There was variation in the extent to which health behaviors mediated between SEP and elevated CRP. Health behaviors accounted for 87.9% of the total effect of education on CRP, but only 55.8% of the total effect of poverty on CRP. This suggests there may be differences in the pathways through which poverty and education affect CRP levels. For example, particularly for poverty behaviors other than the ones examined here, or factors such as psychosocial resources and neighborhood characteristics, may contribute to the relationship between socioeconomic position and CRP.
Our assessment of moderation by gender suggests that the indirect effect of poor diet is much stronger for women compared to men. There may be differences in the level of emotion-based consumption of high fat, high sugar foods, perhaps with the goal of reducing feeling of stress and anxiety (Jackson & Knight, 2006). Unfortunately measures of these possible motivating factors are not available in NHANES.
It is unclear why race/ethnicity moderates the association between low socioeconomic position and elevated CRP. The health behaviors examined here had weaker indirect effects on the association between SEP and CRP among Hispanics than among Whites, suggesting there may be only a weak overall association between SEP and CRP among Hispanics, or at least that the effect of SEP on CRP does not work via the behaviors assessed in our study. This finding is consistent with recent research that found no significant associations between education or income and CRP among Hispanics, particularly for those living in households where English was not the primary language (Ranjit, et al., 2007). This may suggest that the effects of SEP on CRP and the pathways linking the two may vary by level of acculturation, highlighting the importance of measuring aspects of the cultural and psychosocial environments.
Understanding the pathways linking SEP, behaviors, and cardiovascular risk – and how those pathways vary across social groups – is useful for developing effective intervention strategies (Winkleby, et al., 1999), and this study highlights that within socially-disadvantaged groups there is heterogeneity in the relative influence of health behaviors on CRP. The finding that both gender and race/ethnicity have moderating effects on the relationships between SEP, certain health behaviors, and CRP can inform the development of preventive interventions and health promotion strategies better tailored to these groups. For example, dietary intake was an important mediator between education and CRP risk for the study population overall, but its relative contribution to CRP was heterogeneous by gender, as shown by the moderation analysis. This suggests that efforts aimed at reducing cardiovascular risk among disadvantaged groups may need to approach health promotion efforts differently for men and women in order to respond to those pathways that are most relevant for CVD risk in each particular subgroup.
There have been several recent demonstrations of the effectiveness of targeting and/or tailoring intervention strategies to the needs of particular groups. For example, researchers who conducted a fruit and vegetable intervention tailored to Afrocentric ethnic identity found that those randomized to the tailored arm of the intervention increased their fruit and vegetable intake to a greater degree than those in the control arm (Resnicow, et al., 2009). Another study reported that nutritional education tailored to individual’s coping style was associated with marginal improvements in fruit and vegetable intake (Williams-Piehota, et al., 2009). Thus, tailoring interventions based on contextual (e.g., availability of fresh fruits and vegetables, neighborhood opportunities for physical activity) and individual factors (e.g., financial strains, familial and cultural roles and attitudes, stress coping styles) that may vary by race/ethnicity and gender may be more effective than generic strategies that attempt to reach a wide audience.
This study is not without limitations. If the conceptual model is correctly specified and the stress of low SEP leads to a particular patterning of health behaviors that in turn cause physiologic dysregulation and chronically elevated CRP levels, then we have identified true causal mediators. Unfortunately, cross-sectional data do not permit a definitive assessment of this hypothesized model. The lack of a direct measure of stress or stress appraisal is another limitation. Low SEP is associated with many forms of stress, including increased perceived stress, financial hardship, marital strain, negative life events, and daily hassles (Hilmert, et al., 2008; McLoyd, 1998; Taylor, Repetti, & Seeman, 1997), but without direct information on respondents’ reports of stressful events or their appraisal of them we cannot know with certainty that low SEP promotes engagement in poor health behaviors as a means of coping with these stressors. However, consistent with our findings, recent evidence indicates that psychosocial stressors mediated the associations between both low educational attainment and low income and current smoking and physical inactivity (Krueger & Chang, 2008; Schulz, et al., 2008). Future research should directly examine the role of experiences of stress and stress appraisal with health-related coping behaviors.
The implications of this study must be set in the context of the growing recognition in public health research that opportunities to engage in healthy behaviors are not equally afforded to all individuals in society. Many barriers make it more difficult for those living in disadvantaged areas to engage in coping behaviors associated with positive physical health outcomes, such as physical activity and healthy dietary intake. Similarly, these same environments may facilitate the adoption of behaviors linked to harmful consequences for physical health (Williams, 1990). Adults are less likely to exercise if they live in neighborhoods they perceive as unsafe (Wilson, Kirtland, Ainsworth, & Addy, 2004), and low income neighborhoods are more likely to have liquor stores, fast food restaurants, and billboards promoting cigarette smoking (Hackbarth, Silvestri, & Cosper, 1995; Larson, Story, & Nelson, 2009; LaVeist & Wallace, 2000; Moore & Diez Roux, 2006) than more advantaged areas. Physical exercise promotion and smoking cessation programs, along with healthy eating programs, are effective short-term targets for reducing socioeconomic disparities in CVD risk. Interventions, however, aimed at alleviating inequalities in SEP are likely to be more effective long-term approaches to eliminating these disparities.
Acknowledgments
This work was supported by the Michigan Center for Integrative Approaches to Health Disparities P60 MD00249. Briana Mezuk and Cleopatra Abdou also acknowledge funding from the Robert Wood Johnson Health and Society Scholars Program, the University of Michigan.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/hea
References
- Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003;107(3):443–447. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- Armeli S, Carney MA, Tennen H, Affleck G, O’Neil TP. Stress and alcohol use: a daily process examination of the stressor-vulnerability model. J Pers Soc Psychol. 2000;78(5):979–994. doi: 10.1037//0022-3514.78.5.979. [DOI] [PubMed] [Google Scholar]
- Averina M, Nilssen O, Arkhipovsky VL, Kalinin AG, Brox J. C-reactive protein and alcohol consumption: Is there a U-shaped association? Results from a population-based study in Russia. The Arkhangelsk study. Atherosclerosis. 2006;188(2):309–315. doi: 10.1016/j.atherosclerosis.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Bazzano LA, He J, Muntner P, Vupputuri S, Whelton PK. Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Ann Intern Med. 2003;138(11):891–897. doi: 10.7326/0003-4819-138-11-200306030-00010. [DOI] [PubMed] [Google Scholar]
- Beasley LE, Koster A, Newman AB, Javaid MK, Ferrucci L, Kritchevsky SB, et al. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity (Silver Spring) 2009;17(5):1062–1069. doi: 10.1038/oby.2008.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Brown MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Centers for Disease Control. NHANES 2001–2002, 2003–2004, and 2005–2006 documentation. 2004 Available from: < http://www.cdc.gov/nchs.htm>.
- Cooper ML, Russell M, Skinner JB, Frone MR, Mudar P. Stress and alcohol use: moderating effects of gender, coping, and alcohol expectancies. J Abnorm Psychol. 1992;101(1):139–152. doi: 10.1037//0021-843x.101.1.139. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Kim JK, Seeman TE. Poverty and Biological Risk: The Earlier “Aging” of the Poor. J Gerontol A Biol Sci Med Sci. 2009 doi: 10.1093/gerona/gln010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112(1):25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A. 2003;100(20):11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caterina R, Zampolli A, Del Turco S, Madonna R, Massaro M. Nutritional mechanisms that influence cardiovascular disease. Am J Clin Nutr. 2006;83(2):421S–426S. doi: 10.1093/ajcn/83.2.421S. [DOI] [PubMed] [Google Scholar]
- Dietrich T, Garcia RI, de Pablo P, Schulze PC, Hoffmann K. The effects of cigarette smoking on C-reactive protein concentrations in men and women and its modification by exogenous oral hormones in women. Eur J Cardiovasc Prev Rehabil. 2007;14(5):694–700. doi: 10.1097/HJR.0b013e328270b913. [DOI] [PubMed] [Google Scholar]
- DiLorenzo TM, Bargman EP, Stucky-Ropp R, Brassington GS, Frensch PA, LaFontaine T. Long-term effects of aerobic exercise on psychological outcomes. Prev Med. 1999;28(1):75–85. doi: 10.1006/pmed.1998.0385. [DOI] [PubMed] [Google Scholar]
- Edwards JR, Lambert LS. Methods for integrating moderation and mediation: A general analytical framework using moderated path analysis. Psychological Methods. 2007;12(1):1–22. doi: 10.1037/1082-989X.12.1.1. [DOI] [PubMed] [Google Scholar]
- Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology. 2002;13(5):561–568. doi: 10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- Frohlich M, Sund M, Lowel H, Imhof A, Hoffmeister A, Koenig W. Independent association of various smoking characteristics with markers of systemic inflammation in men. Results from a representative sample of the general population (MONICA Augsburg Survey 1994/95) Eur Heart J. 2003;24(14):1365–1372. doi: 10.1016/s0195-668x(03)00260-4. [DOI] [PubMed] [Google Scholar]
- George WH, Gourmic SJ, McAfee MP. Perceptions of postdrinking female sexuality: Effects of gender, beverage choice, and drink payment. J Appl Psychol. 1988;18:1295–1317. [Google Scholar]
- Hackbarth DP, Silvestri B, Cosper W. Tobacco and alcohol billboards in 50 Chicago neighborhoods: market segmentation to sell dangerous products to the poor. J Public Health Policy. 1995;16(2):213–230. [PubMed] [Google Scholar]
- Hickling S, Hung J, Knuiman M, Divitini M, Beilby J. Are the associations between diet and C-reactive protein independent of obesity? Prev Med. 2008;47(1):71–76. doi: 10.1016/j.ypmed.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Hilmert CJ, Schetter CD, Dominguez TP, Abdou C, Hobel CJ, Glynn L, et al. Stress and blood pressure during pregnancy: racial differences and associations with birthweight. Psychosom Med. 2008;70(1):57–64. doi: 10.1097/PSY.0b013e31815c6d96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman KM, Orenduff MC, Samsa GP, Houmard JA, Kraus WE, Bales CW. Dietary carbohydrate intake and high-sensitivity C-reactive protein in at-risk women and men. Am Heart J. 2007;154(5):962–968. doi: 10.1016/j.ahj.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357(9258):763–767. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- Jackson JS, Knight KM. Race and self-regulatory health behaviors: the role of the stress response and the HPA axis in physical and mental health disparities. In: Schaie KW, Cartensen L, editors. Social structures, aging, and self-regulation in the elderly. New York: Springer; 2006. pp. 189–207. [Google Scholar]
- Jackson JS, Knight KM, Rafferty JA. Race and Unhealthy Behaviors: Chronic Stress, the HPA Axis, and Physical and Mental Health Disparities Over the Life Course. Am J Public Health. 2009 doi: 10.2105/AJPH.2008.143446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88(4 Pt 1):1973–1998. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46(3):464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- Krueger PM, Chang VW. Being poor and coping with stress: health behaviors and the risk of death. Am J Public Health. 2008;98(5):889–896. doi: 10.2105/AJPH.2007.114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakoski SG, Cushman M, Criqui M, Rundek T, Blumenthal RS, D’Agostino RB, Jr, et al. Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am Heart J. 2006;152(3):593–598. doi: 10.1016/j.ahj.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA. 1998;279(21):1703–1708. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009;36(1):74–81. doi: 10.1016/j.amepre.2008.09.025. [DOI] [PubMed] [Google Scholar]
- LaVeist TA, Wallace JM., Jr Health risk and inequitable distribution of liquor stores in African American neighborhood. Soc Sci Med. 2000;51(4):613–617. doi: 10.1016/s0277-9536(00)00004-6. [DOI] [PubMed] [Google Scholar]
- Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002;75(3):492–498. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- Loucks EB, Sullivan LM, Hayes LJ, D’Agostino RB, Sr, Larson MG, Vasan RS, et al. Association of educational level with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2006;163(7):622–628. doi: 10.1093/aje/kwj076. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP. Introduction to statistical mediation analysis. New York: Lawrence Erlbaum Associates; 2008. [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McLoyd VC. Socioeconomic disadvantage and child development. American Psychologist. 1998;53(2):185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Moore LV, Diez Roux AV. Associations of neighborhood characteristics with the location and type of food stores. Am J Public Health. 2006;96(2):325–331. doi: 10.2105/AJPH.2004.058040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295(12):1412–1419. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- Moses J, Steptoe A, Mathews A, Edwards S. The effects of exercise training on mental well-being in the normal population: a controlled trial. J Psychosom Res. 1989;33(1):47–61. doi: 10.1016/0022-3999(89)90105-0. [DOI] [PubMed] [Google Scholar]
- Muller D, Judd CM, Yzerbyt VY. When moderation is mediated and mediation is moderated. Journal of Personality and Social Psychology. 2005;89(6):852–863. doi: 10.1037/0022-3514.89.6.852. [DOI] [PubMed] [Google Scholar]
- Nazmi A, Victora CG. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: a systematic review of population-based studies. BMC Public Health. 2007;7:212. doi: 10.1186/1471-2458-7-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2006;83(6):1369–1379. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DM, Jeffery RW. Relationships between perceived stress and health behaviors in a sample of working adults. Health Psychol. 2003;22(6):638–642. doi: 10.1037/0278-6133.22.6.638. [DOI] [PubMed] [Google Scholar]
- Pai JK, Hankinson SE, Thadhani R, Rifai N, Pischon T, Rimm EB. Moderate alcohol consumption and lower levels of inflammatory markers in US men and women. Atherosclerosis. 2006;186(1):113–120. doi: 10.1016/j.atherosclerosis.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128(2):127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, et al. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women’s Health Initiative observational study. JAMA. 2002;288(8):980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Diez-Roux AV, Shea S, Cushman M, Ni H, Seeman T. Socioeconomic position, race/ethnicity, and inflammation in the multi-ethnic study of atherosclerosis. Circulation. 2007;116(21):2383–2390. doi: 10.1161/CIRCULATIONAHA.107.706226. [DOI] [PubMed] [Google Scholar]
- Resnicow K, Davis R, Zhang N, Tolsma D, Alexander G, Wiese C, et al. Tailoring a fruit and vegetable intervention on ethnic identity: results of a randomized study. Health Psychol. 2009;28(4):394–403. doi: 10.1037/a0015217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Alberts SC, Altmann J. Hypercortisolism associated with social subordinance or social isolation among wild baboons. Arch Gen Psychiatry. 1997;54(12):1137–1143. doi: 10.1001/archpsyc.1997.01830240097014. [DOI] [PubMed] [Google Scholar]
- Schlager O, Exner M, Mlekusch W, Sabeti S, Amighi J, Dick P, et al. C-reactive protein predicts future cardiovascular events in patients with carotid stenosis. Stroke. 2007;38(4):1263–1268. doi: 10.1161/01.STR.0000259890.18354.d2. [DOI] [PubMed] [Google Scholar]
- Schulz AJ, House JS, Israel BA, Mentz G, Dvonch JT, Miranda PY, et al. Relational pathways between socioeconomic position and cardiovascular risk in a multiethnic urban sample: complexities and their implications for improving health in economically disadvantaged populations. J Epidemiol Community Health. 2008;62(7):638–646. doi: 10.1136/jech.2007.063222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic intervals for indirect effects in structural equations models. In: Lieinhart S, editor. Sociological methodology. San Francisco: Jossey-Bass; 1982. pp. 290–312. [Google Scholar]
- Steptoe A, Edwards S, Moses J, Mathews A. The effects of exercise training on mood and perceived coping ability in anxious adults from the general population. J Psychosom Res. 1989;33(5):537–547. doi: 10.1016/0022-3999(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Armor DA. Positive illusions and coping with adversity. J Pers. 1996;64(4):873–898. doi: 10.1111/j.1467-6494.1996.tb00947.x. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol Psychiatry. 2006;60(8):819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Repetti RL, Seeman T. Health psychology: What is an unhealthy environment and how does it get under the skin? Annual Review of Psychology. 1997;48:411–447. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, & U.S. Department of Agriculture. Dietary guidelines for Americans. 2005;Chapter 2 http://www.health.gov/dietaryguidelines/dga2005/document/pdf/DGA2005.pdf.
- Wheaton B. The nature of stressors. In: Scheid AVHaTL., editor. A handbook for the study of mental health: Social contexts, theories, and systems. New York: Cambridge University Press; 1999. pp. 176–197. [Google Scholar]
- Williams-Piehota P, Latimer AE, Katulak NA, Cox A, Silvera SA, Mowad L, et al. Tailoring messages to individual differences in monitoring-blunting styles to increase fruit and vegetable intake. J Nutr Educ Behav. 2009;41(6):398–405. doi: 10.1016/j.jneb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR. Socioeconomic differentials in health: A review and redirection. Soc Psychol Q. 1990;53:81–99. [Google Scholar]
- Williams DR. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann N Y Acad Sci. 1999;896:173–188. doi: 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
- Wilson DK, Kirtland KA, Ainsworth BE, Addy CL. Socioeconomic status and perceptions of access and safety for physical activity. Ann Behav Med. 2004;28(1):20–28. doi: 10.1207/s15324796abm2801_4. [DOI] [PubMed] [Google Scholar]
- Winkleby MA, Cubbin C, Ahn DK, Kraemer HC. Pathways by which SES and ethnicity influence cardiovascular disease risk factors. Ann N Y Acad Sci. 1999;896:191–209. doi: 10.1111/j.1749-6632.1999.tb08116.x. [DOI] [PubMed] [Google Scholar]
- Winkleby MA, Jatulis DE, Frank E, Fortmann SP. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health. 1992;82(6):816–820. doi: 10.2105/ajph.82.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]