Abstract
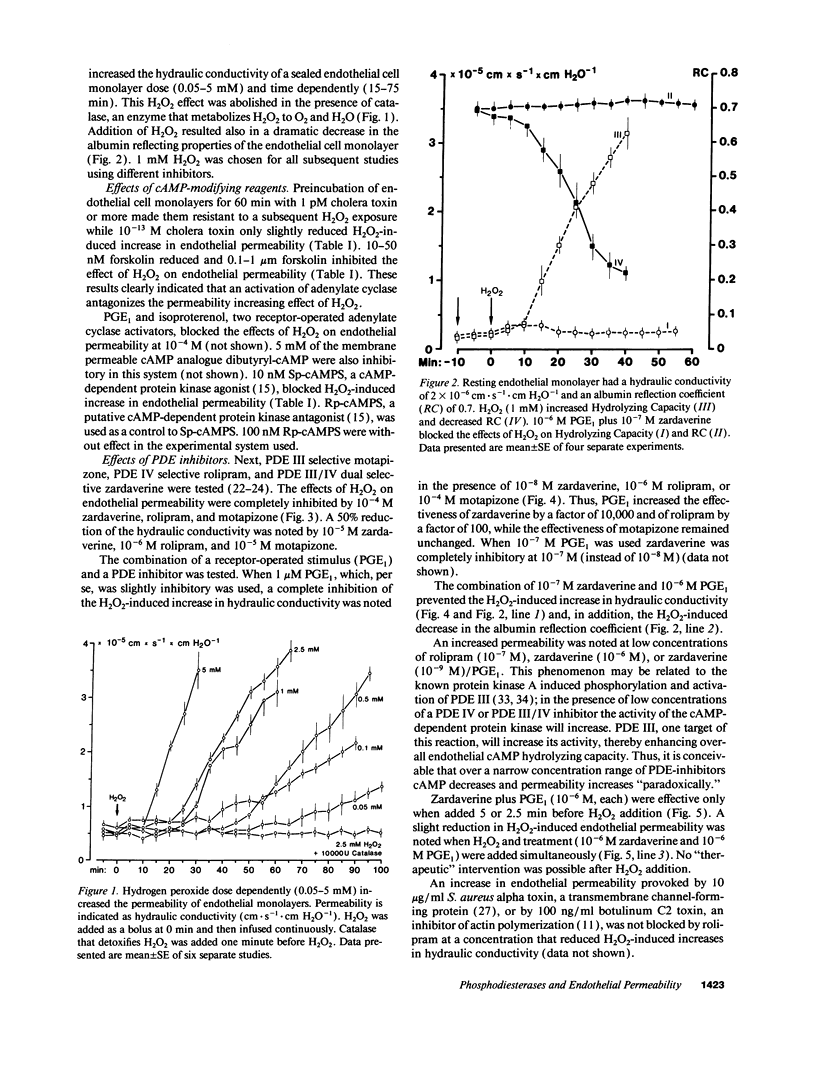
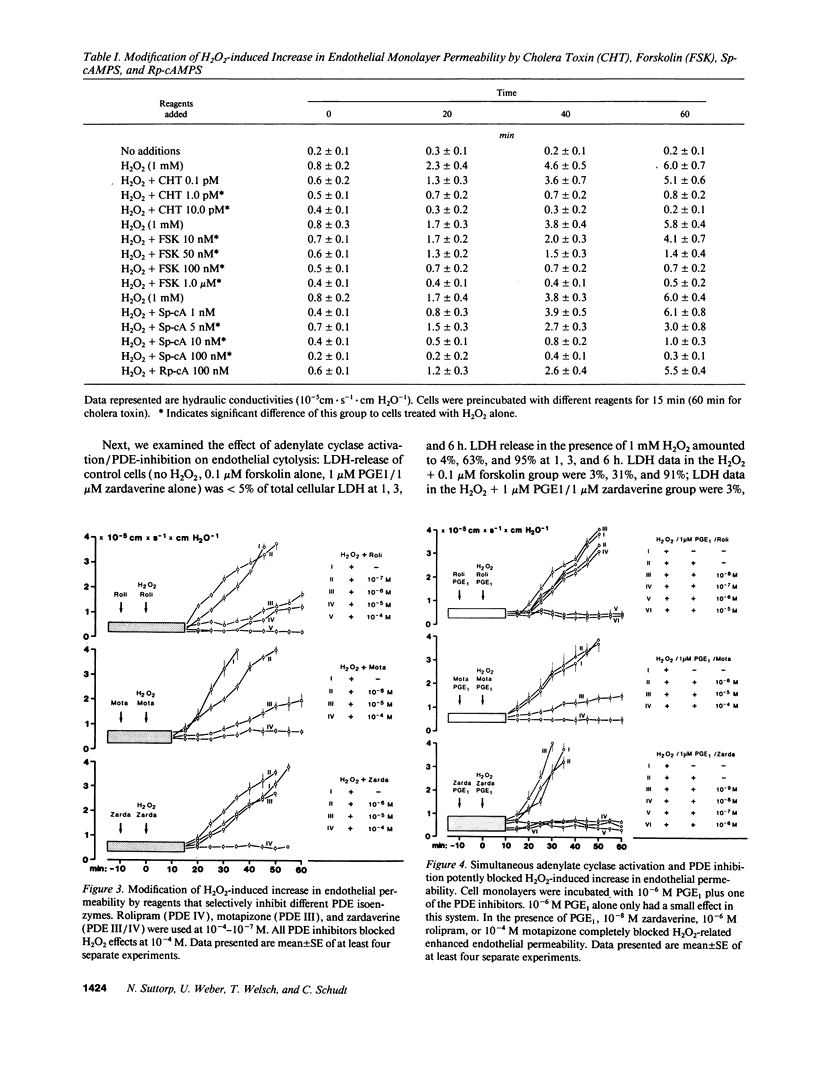
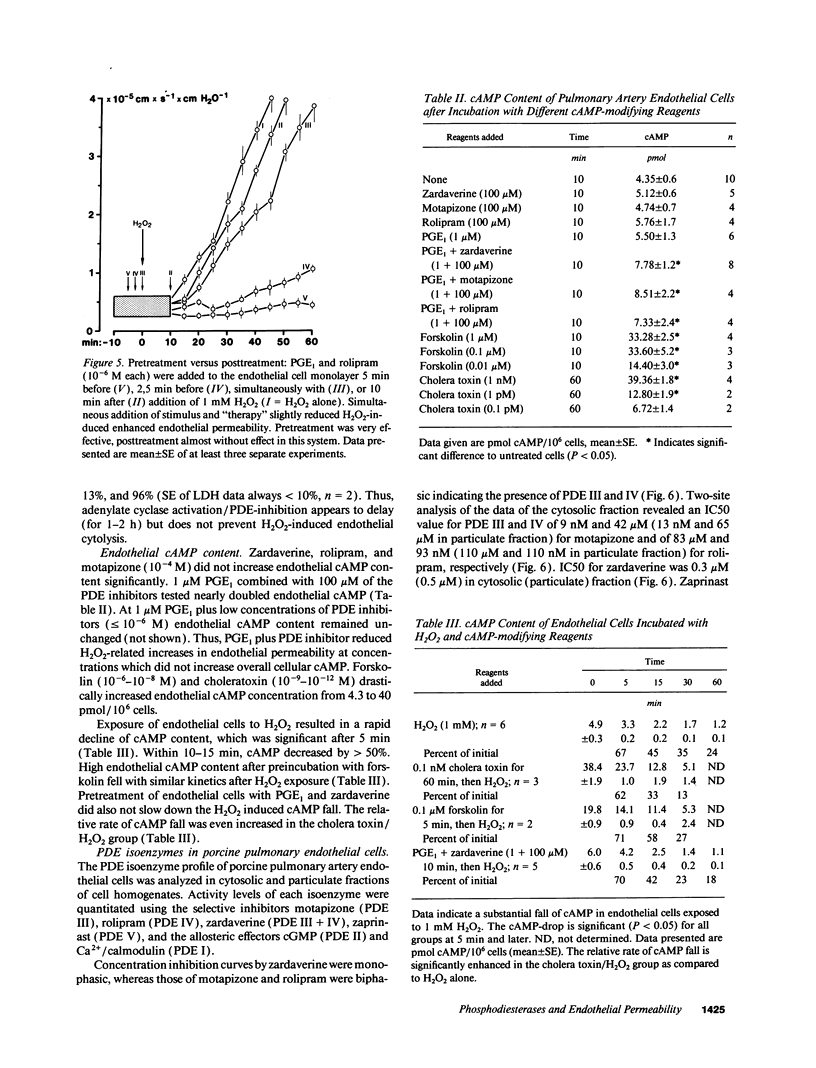
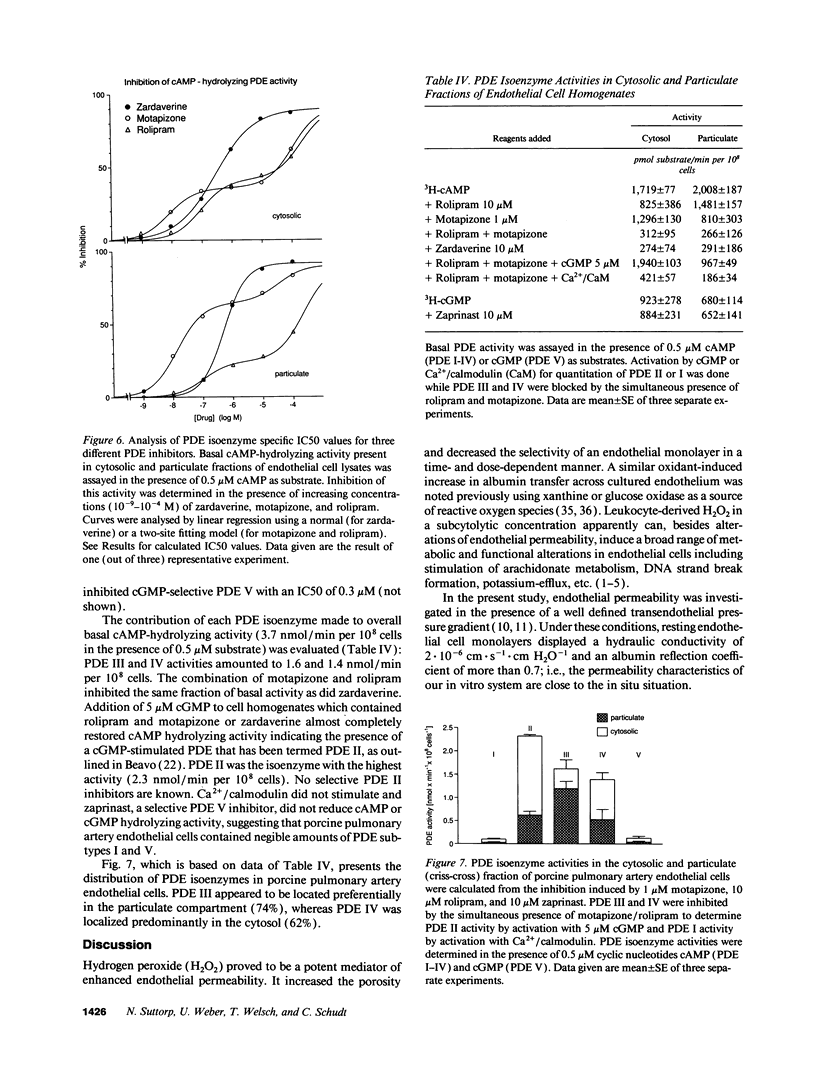
Neutrophil-derived hydrogen peroxide (H2O2) is believed to play an important role in the pathogenesis of vascular injury and pulmonary edema. H2O2 time- and dose-dependently increased the hydraulic conductivity and decreased the selectivity of an endothelial cell monolayer derived from porcine pulmonary arteries. Effects of H2O2 on endothelial permeability were completely inhibited by adenylate cyclase activation with 10(-12) M cholera toxin or 0.1 microM forskolin. 10(-8) M Sp-cAMPS, a cAMP-dependent protein kinase A agonist, was similarly effective. The phosphodiesterase (PDE) inhibitors motapizone (10(-4) M), rolipram (10(-6) M), and zardaverine (10(-8) M), which specifically inhibit PDE-isoenzymes III, IV, and III/IV potently blocked H2O2-induced endothelial permeability when combined with 10(-6) M prostaglandin E1. Overall cellular cAMP content and inhibition of H2O2 effects on endothelial permeability were poorly correlated. H2O2 exposure resulted in a rapid and substantial decrease in endothelial cAMP content. The analysis of the PDE isoenzyme spectrum showed high activities of isoenzymes II, III, and IV in porcine pulmonary endothelial cells. The data suggest that adenylate cyclase activation/PDE inhibition is a powerful approach to block H2O2-induced increase in endothelial permeability. This concept appears especially valuable when endothelial PDE isoenzyme pattern and PDE inhibitor profile are matched optimally.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Gordon J. L. Differential effects of hydrogen peroxide on indices of endothelial cell function. J Exp Med. 1984 Feb 1;159(2):592–603. doi: 10.1084/jem.159.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Barsony J., Marx S. J. Immunocytology on microwave-fixed cells reveals rapid and agonist-specific changes in subcellular accumulation patterns for cAMP or cGMP. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1188–1192. doi: 10.1073/pnas.87.3.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Füssle R., Tranum-Jensen J. Staphylococcal alpha-toxin: oligomerization of hydrophilic monomers to form amphiphilic hexamers induced through contact with deoxycholate detergent micelles. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5475–5479. doi: 10.1073/pnas.78.9.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton A. F., Macfarlane D. E., Hoak J. C. Prostacyclin biosynthesis in vascular endothelium is not inhibited by cyclic AMP. Studies with 3-isobutyl-1-methylxanthine and forskolin. Thromb Res. 1982 Dec 1;28(5):637–647. doi: 10.1016/0049-3848(82)90155-4. [DOI] [PubMed] [Google Scholar]
- Carson M. R., Shasby S. S., Shasby D. M. Histamine and inositol phosphate accumulation in endothelium: cAMP and a G protein. Am J Physiol. 1989 Oct;257(4 Pt 1):L259–L264. doi: 10.1152/ajplung.1989.257.4.L259. [DOI] [PubMed] [Google Scholar]
- Gottlieb A. I., Langille B. L., Wong M. K., Kim D. W. Structure and function of the endothelial cytoskeleton. Lab Invest. 1991 Aug;65(2):123–137. [PubMed] [Google Scholar]
- Grant P. G., Mannarino A. F., Colman R. W. cAMP-mediated phosphorylation of the low-Km cAMP phosphodiesterase markedly stimulates its catalytic activity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9071–9075. doi: 10.1073/pnas.85.23.9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Callahan K. S. Role of hydrogen peroxide in the neutrophil-mediated release of prostacyclin from cultured endothelial cells. J Clin Invest. 1984 Aug;74(2):442–448. doi: 10.1172/JCI111440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw D. B., Sklar L. A., Bohl B., Schraufstatter I. U., Hyslop P. A., Rossi M. W., Spragg R. G., Cochrane C. G. Cytoskeletal and morphologic impact of cellular oxidant injury. Am J Pathol. 1986 Jun;123(3):454–464. [PMC free article] [PubMed] [Google Scholar]
- Holman R. G., Maier R. V. Oxidant-induced endothelial leak correlates with decreased cellular energy levels. Am Rev Respir Dis. 1990 Jan;141(1):134–140. doi: 10.1164/ajrccm/141.1.134. [DOI] [PubMed] [Google Scholar]
- Hyslop P. A., Hinshaw D. B., Halsey W. A., Jr, Schraufstätter I. U., Sauerheber R. D., Spragg R. G., Jackson J. H., Cochrane C. G. Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J Biol Chem. 1988 Feb 5;263(4):1665–1675. [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. Regulation of smooth muscle contractile elements by second messengers. Annu Rev Physiol. 1989;51:299–313. doi: 10.1146/annurev.ph.51.030189.001503. [DOI] [PubMed] [Google Scholar]
- Kennedy T. P., Michael J. R., Hoidal J. R., Hasty D., Sciuto A. M., Hopkins C., Lazar R., Bysani G. K., Tolley E., Gurtner G. H. Dibutyryl cAMP, aminophylline, and beta-adrenergic agonists protect against pulmonary edema caused by phosgene. J Appl Physiol (1985) 1989 Dec;67(6):2542–2552. doi: 10.1152/jappl.1989.67.6.2542. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Kobayashi T., Fukushima M. Effects of dibutyryl cAMP on pulmonary air embolism-induced lung injury in awake sheep. J Appl Physiol (1985) 1987 Dec;63(6):2201–2207. doi: 10.1152/jappl.1987.63.6.2201. [DOI] [PubMed] [Google Scholar]
- Langeler E. G., van Hinsbergh V. W. Norepinephrine and iloprost improve barrier function of human endothelial cell monolayers: role of cAMP. Am J Physiol. 1991 May;260(5 Pt 1):C1052–C1059. doi: 10.1152/ajpcell.1991.260.5.C1052. [DOI] [PubMed] [Google Scholar]
- Lugnier C., Schini V. B. Characterization of cyclic nucleotide phosphodiesterases from cultured bovine aortic endothelial cells. Biochem Pharmacol. 1990 Jan 1;39(1):75–84. doi: 10.1016/0006-2952(90)90650-a. [DOI] [PubMed] [Google Scholar]
- Macphee C. H., Reifsnyder D. H., Moore T. A., Lerea K. M., Beavo J. A. Phosphorylation results in activation of a cAMP phosphodiesterase in human platelets. J Biol Chem. 1988 Jul 25;263(21):10353–10358. [PubMed] [Google Scholar]
- Minnear F. L., DeMichele M. A., Moon D. G., Rieder C. L., Fenton J. W., 2nd Isoproterenol reduces thrombin-induced pulmonary endothelial permeability in vitro. Am J Physiol. 1989 Nov;257(5 Pt 2):H1613–H1623. doi: 10.1152/ajpheart.1989.257.5.H1613. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Kelley G., Douglas J. S. Interactions between Ca2+ and cAMP messenger system in regulation of airway smooth muscle contraction. Am J Physiol. 1990 Jun;258(6 Pt 1):L279–L288. doi: 10.1152/ajplung.1990.258.6.L279. [DOI] [PubMed] [Google Scholar]
- Schnittler H. J., Wilke A., Gress T., Suttorp N., Drenckhahn D. Role of actin and myosin in the control of paracellular permeability in pig, rat and human vascular endothelium. J Physiol. 1990 Dec;431:379–401. doi: 10.1113/jphysiol.1990.sp018335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstätter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Glutathione cycle activity and pyridine nucleotide levels in oxidant-induced injury of cells. J Clin Invest. 1985 Sep;76(3):1131–1139. doi: 10.1172/JCI112068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schudt C., Winder S., Forderkunz S., Hatzelmann A., Ullrich V. Influence of selective phosphodiesterase inhibitors on human neutrophil functions and levels of cAMP and Cai. Naunyn Schmiedebergs Arch Pharmacol. 1991 Dec;344(6):682–690. doi: 10.1007/BF00174752. [DOI] [PubMed] [Google Scholar]
- Schudt C., Winder S., Müller B., Ukena D. Zardaverine as a selective inhibitor of phosphodiesterase isozymes. Biochem Pharmacol. 1991 Jun 21;42(1):153–162. doi: 10.1016/0006-2952(91)90694-z. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert A. F., Thompson W. J., Taylor A., Wilborn W. H., Barnard J., Haynes J. Reversal of increased microvascular permeability associated with ischemia-reperfusion: role of cAMP. J Appl Physiol (1985) 1992 Jan;72(1):389–395. doi: 10.1152/jappl.1992.72.1.389. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Lind S. E., Shasby S. S., Goldsmith J. C., Hunninghake G. W. Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood. 1985 Mar;65(3):605–614. [PubMed] [Google Scholar]
- Shasby D. M., Yorek M., Shasby S. S. Exogenous oxidants initiate hydrolysis of endothelial cell inositol phospholipids. Blood. 1988 Aug;72(2):491–499. [PubMed] [Google Scholar]
- Souness J. E., Diocee B. K., Martin W., Moodie S. A. Pig aortic endothelial-cell cyclic nucleotide phosphodiesterases. Use of phosphodiesterase inhibitors to evaluate their roles in regulating cyclic nucleotide levels in intact cells. Biochem J. 1990 Feb 15;266(1):127–132. doi: 10.1042/bj2660127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spragg R. G. DNA strand break formation following exposure of bovine pulmonary artery and aortic endothelial cells to reactive oxygen products. Am J Respir Cell Mol Biol. 1991 Jan;4(1):4–10. doi: 10.1165/ajrcmb/4.1.4. [DOI] [PubMed] [Google Scholar]
- Stelzner T. J., Weil J. V., O'Brien R. F. Role of cyclic adenosine monophosphate in the induction of endothelial barrier properties. J Cell Physiol. 1989 Apr;139(1):157–166. doi: 10.1002/jcp.1041390122. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Flöer B., Schnittler H., Seeger W., Bhakdi S. Effects of Escherichia coli hemolysin on endothelial cell function. Infect Immun. 1990 Nov;58(11):3796–3801. doi: 10.1128/iai.58.11.3796-3801.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttorp N., Galanos C., Neuhof H. Endotoxin alters arachidonate metabolism in pulmonary endothelial cells. Am J Physiol. 1987 Sep;253(3 Pt 1):C384–C390. doi: 10.1152/ajpcell.1987.253.3.C384. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Hessz T., Seeger W., Wilke A., Koob R., Lutz F., Drenckhahn D. Bacterial exotoxins and endothelial permeability for water and albumin in vitro. Am J Physiol. 1988 Sep;255(3 Pt 1):C368–C376. doi: 10.1152/ajpcell.1988.255.3.C368. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Polley M., Seybold J., Schnittler H., Seeger W., Grimminger F., Aktories K. Adenosine diphosphate-ribosylation of G-actin by botulinum C2 toxin increases endothelial permeability in vitro. J Clin Invest. 1991 May;87(5):1575–1584. doi: 10.1172/JCI115171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttorp N., Simon L. M. Lung cell oxidant injury. Enhancement of polymorphonuclear leukocyte-mediated cytotoxicity in lung cells exposed to sustained in vitro hyperoxia. J Clin Invest. 1982 Aug;70(2):342–350. doi: 10.1172/JCI110623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttorp N., Toepfer W., Roka L. Antioxidant defense mechanisms of endothelial cells: glutathione redox cycle versus catalase. Am J Physiol. 1986 Nov;251(5 Pt 1):C671–C680. doi: 10.1152/ajpcell.1986.251.5.C671. [DOI] [PubMed] [Google Scholar]
- Varani J., Phan S. H., Gibbs D. F., Ryan U. S., Ward P. A. H2O2-mediated cytotoxicity of rat pulmonary endothelial cells. Changes in adenosine triphosphate and purine products and effects of protective interventions. Lab Invest. 1990 Nov;63(5):683–689. [PubMed] [Google Scholar]
- Wilson J., Winter M., Shasby D. M. Oxidants, ATP depletion, and endothelial permeability to macromolecules. Blood. 1990 Dec 15;76(12):2578–2582. [PubMed] [Google Scholar]



