Abstract
In thalassemia, fetal hemoglobin (HbF) augmentation with hydroxycarbamide (also known as hydroxyurea) is not always successful. The expected parallel effects on RBC membrane deformability, cell hydration and membrane phospholipid organization, all important for extending RBC life span and increasing Hb, have been infrequently examined. We analyzed these characteristics in 15 non-transfused E/β 0 thalassemia patients treated with HU (mean 10.2 months). Membrane deformability and cell hydration mildly improved in association with increased HbF levels approaching statistical significance (r = 0.51, p=0.06). All measures improved considerably splenectomized patients. These findings underscore the disappointing results of hydroxyurea treatment in clinical trials; and the importance of examining the effect on red cell characteristics for the development and understanding of HbF-enhancing agents.
Keywords: Thalassemia, E/beta Thalassemia, Fetal hemoglobin augmentation, Hydroxyurea, RBC deformability
INTRODUCTION
The excess of unpaired α globin chains in β thalassemia interact with the red cell (RBC) membrane, causing oxidative damage to membrane skeletal components. This interaction results in a rigid, mechanically unstable membrane which causes increased apoptosis and shortened RBC survival, marked by ineffective erythropoiesis and anemia. (1–3). Agents like hydroxycarbamide (also known as hydroxyurea), which enhance fetal hemoglobin (α2γ2) synthesis, can reduce unpaired α chains accumulation. This in turn is thought to lower oxidant stress on the membrane, reduce RBC damage, decrease apoptosis, and potentially improve RBC life span in the circulation, thereby ameliorating disease severity (4, 5). However, clinical results have been disappointing, showing variable increase in fetal hemoglobin (HbF) and modest increases in Hb (6–8). Although the effect of hydroxycarbamide on HbF synthesis has been well studied, the corresponding change in measurable RBC abnormalities, a key element in understanding the impact of treatment, has not. We therefore investigated RBC corpuscular and membrane characteristics before and during hydroxycarbamide therapy, and their association with changes in HbF and Hb in patients with thalassemia intermedia.
RESULTS AND DISCUSSION
A total of 15 patients, 8 females, ages 3 to 27 years (mean= 13.7±6) with E/β0 thalassemia and a baseline hemoglobin of 7.3±1.0 gr/dL were treated with hydroxycarbamide (18–20mg/Kg) for 10.2±2 months. hydroxycarbamide was well tolerated and there was no indication of hydroxycarbamide -induced marrow toxicity.
Ecktacytometry
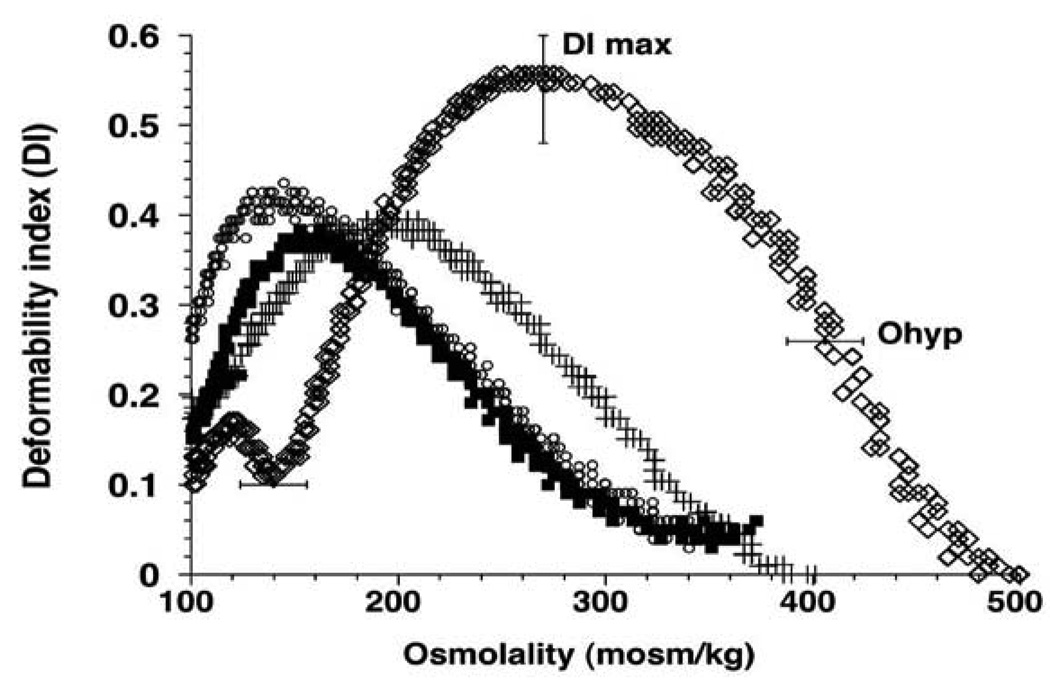
Ektacytometry yielded an osmotic deformability curve that was left-shifted to a lower osmolality (Figure 1), indicating the dehydrated state of the thalassemic RBC population (9). The variables that define the deformability-DImax, the maximal deformability index, the hypertonic osmolality at which the DI equals ½ DImax (Ohyp), and the hypotonic osmolality at which the DI equals ½ DImax (Olo) were significantly different between the patients and normal controls at baseline (0.36±0.06) vs. 0.54±0.06). Moreover, while maximum deformability in normal controls was observed at 290 mosmol, this value was lower in thalassemic RBC (194.0±31.4 mosmol). The reduction in DI max indicates a reduction in cell surface area and an increased intrinsic membrane stiffness (9). The Ohyp of the thalassemia patients (312.8 ±43 mosmol) was 80% of that for normal controls (420±20 mosmol) and the Olo in the thalassemic RBCs (114.1 ±12.2 mosmol) was 66% of that for the normal controls (170.7 mosmol).
Figure 1.
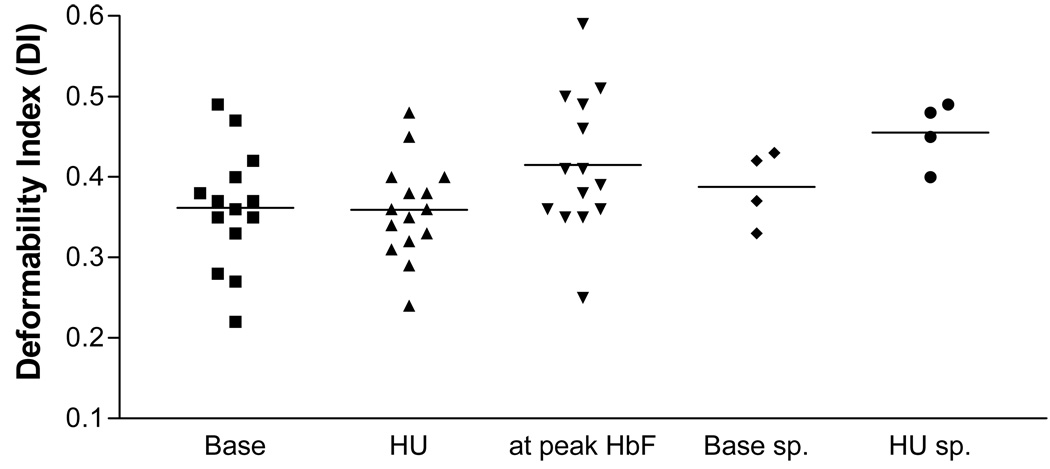
The osmotic deformability curves of normal controls shifted only minimally between the different time points of blood collection. However, thalassemia red cell deformability was highly variable when assessed during a steady dose of hydroxycarbamide. When the results obtained during hydroxycarbamide treatment were averaged, the values for DI max, Ohyp and Olo did not improve significantly over baseline. An association, approaching statistical significance, of the change of DImax with the change in HbF was noted at the time points when HbF levels peaked (r=0.51, p=0.06) (Figure 1). The transient increase in membrane deformability may be related to the increase in RBC volume and expansion of the membrane surface area, as shown by a parallel increase in MCV. When patients who had splenectomies (n=4) were assessed separately, baseline DImax was higher (0.39±0.02) and a more sustained improvement in DImax over baseline was noted (0.45±0.03; p=0.014). Both Ohyp and Olo showed a shift to normalization.
Red Cell Density Profile
The baseline distribution histograms showed a significantly lower MCH and MCV in the 15 patients as compared to normal controls (Table 1). A 9.4% increase in MCV was noted in most patients after 8–12 weeks of hydroxycarbamide treatment (58.6±8 to 64.2±9, p=0.04). MCH increased by 6.2% from baseline.
Table 1.
| Response of Hb to HU |
Hb (gr/dL) at baseline |
Average Hb change on HU (gr/dL) |
HbF (%) at baseline |
Average HbF change on HU (%) |
MCV (fl) | MCH (pg) | MCHC (gr/dL) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | HU | Baseline | HU | Baseline | HU | |||||
| ↑Hb >1gr/dL | 5.70 | 2.7 | 50 | 1.4 | 61.8 | 76.3 | 20.5 | 24.3 | 34.6 | 33.4 |
| 5.30 | 1 | 17 | 12.9 | 54.7 | 51 | 17 | 16.2 | 30 | 29.4 | |
| 6.50 | 1.4 | 37 | −2 | 47.7 | 56.4 | 16.3 | 20 | 33.6 | 33.8 | |
| 6.80 | 1.7 | 53 | 1.0 | 77.9 | 73 | 23 | 23.6 | 31.6 | 33.9 | |
| 7.20 | 1.1 | 28 | 14.7 | 53.8 | 71 | 18.4 | 22 | 28.2 | 31 | |
| 8.90 | 1.0 | 20 | 25.8 | 61.4 | 81 | 21 | 24.5 | 31 | 30 | |
| 6.5 | 1.4 | 48 | 3 | 67.2 | 69.6 | 22.3 | 23.2 | 35 | 35 | |
| Mean±SD | 6.7±1.2 | 1.5±0.6 | 36±15 | 8.1±10 | 60±10 | 68±10 | 20±2.6 | 22±3.0 | 32±2.5 | 32±2.2 |
| ↑Hb < 1gr/dL | 7.10 | 0.5 | 31 | 10.6 | 51.1 | 57 | 19.5 | 17.5 | 39 | 31.6 |
| 8.20 | 0 | 15 | 11.4 | 55.2 | 59.4 | 19.2 | 19 | 36 | 32.8 | |
| 8.10 | 0.4 | 14 | 3.2 | 59.9 | 49.8 | 20.5 | 16.7 | 35.7 | 33.3 | |
| 7.30 | 0.4 | 34 | 6.4 | 52 | 64 | 17 | 20.8 | 34.2 | 32.3 | |
| 8.00 | −0.4 | 31 | −2.6 | 67.4 | 67.9 | 20 | 20.7 | 31.8 | 31.4 | |
| 8.50 | 0.7 | 36 | 11.8 | 67 | 67.7 | 21 | 20.9 | 33 | 32.5 | |
| 8.30 | 0.4 | 29 | −1.6 | 55.9 | 52.8 | 17.5 | 17.1 | 30 | 31.3 | |
| 7.60 | 0.9 | 30 | 7.4 | 55 | 65.2 | 17 | 20.5 | 32.6 | 32 | |
| Mean±SD | 7.9±0.5 | 0.4±0.4 | 27.5±8 | 5.8±5.7 | 58±6 | 60.5±6.8 | 19±1.6 | 19.2±1.8 | 34±2.8 | 32±0.7 |
The change in MCV, although not significantly associated with the increase in HbF (r = 0.48, p = 0.07), was correlated with a sustained increase in total Hb. Patients in whom Hb increased ≥1gr/dl (n=8) had a 14.6% increase in MCV and a 12.6% increase in MCH, whereas patients in whom Hb increased less had only minimal changes (Table 1). The increases in MCV (21%) and MCH (12.5%) were more pronounced in patients who had splenectomies. MCHC did not decrease during hydroxycarbamide treatment, consistent with the lack of significant improvement in the cell hydration, as evidenced by the ektacytometry results.
Phosphatidylserine Membrane Exposure
Oxidant stress can affect inward and outward PS movement resulting in membrane PS externalization, an important marker for phagocytic removal of erythrocytes. It has also been implicated in increased erythrocyte apoptosis in thalassemia (3, 10). We found that the population of RBCs that exposed PS was increased at baseline (0.8±0.9 %) as compared to controls (<0.3%), and did not change significantly during hydroxycarbamide therapy (0.9±1.2%). The baseline population of RBCs that exposed PS was greater (2.3±1.9 %) in the four patients who had undergone splenectomies. It had significantly decreased to 0.4±0.2% during hydroxycarbamide therapy (p=0.007), and increased back to a baseline range (2.0±0.9%) 4–6 weeks after hydroxycarbamide treatment stopped.
Abnormal PS exposure was previously reported in patients with E/β thalassemia who underwent splenectomies (11, 12) and is likely related to the impaired ability to remove abnormal RBC. However, the effect of hydroxycarbamide on PS externalization in thalassemia has not been studied. Our results suggest that hydroxycarbamide diminishes PS externalization in the cell population which we detected only in splenectomized patients. Membrane PS exposure does not necessarily correlate with α globin aggregates and appears mostly in dehydrated mature cells (1, 11), suggesting that hydroxycarbamide -induced binding of excess α-globin may not necessarily have an apparent effect on PS exposure. Still, the notable effect of hydroxycarbamide on decreased PS externalization in the small cohort of splenectomized patients could be a result of increased α-globin binding. This is supported by the finding that three of the four splenectomized patients had the highest hydroxycarbamide -induced increase in HbF from baseline: 17.5±7%.
Phosphatidylserine exposure activates the prothrombin complex and can lead to hypercoagulablity (13). To determine if coagulation activation was present, we measured plasma levels of F 1.2. The levels were elevated at baseline (2.8±2.0 nmolar vs 0.4–1.1 nmolar in controls) and mildly decreased during hydroxycarbamide treatment (2.4±1.5 nmolar). They were higher at baseline in patients who had splenectomies (3.8±2.0 nmolar) and decreased to 2.9±0.2 nmolar during hydroxycarbamide treatment. The change in F1.2 corresponds to the population of PS-exposing RBCs in patients who have had splenectomies; a finding that underscores the presence of a chronic low-grade coagulation activation mostly in splenectomized E/β thalassemia patients(14).
The clinical variability of E/β thalassemia is enormous and not well understood (3). Similarly the in vitro response to hydroxycarbamide has been shown to be variable; HbF ranged from 3 to 30% in E/β0 thalassemia cell cultures treated with hydroxycarbamide and a variable response of in vitro cultures of erythroid precursors were demonstrated (15) (16). Though hydroxycarbamide treatment is expected to result in an overall increase in HbF and Hb levels, it may act differently on different patients and have inconsistent effect on their erythrocytes as demonstrated in this study.
Absence of spleen provides an opportunity to detect changes in the RBCs that are not easily recognized because RBCs are more efficiently removed from the circulation by an intact spleen. Our results show a variable and overall positive effect of hydroxycarbamide treatment on MCV and on PS externalization, both of which were most pronounced in patients who had splenectomies. These results are consistent with clinical observations of greater increases in HbF and Hb in patients who underwent splenectomies and were treated with hydroxycarbamide (7, 17).
Our study found no significant shift to normalization in RBC membrane deformability during hydroxycarbamide treatment. Decreased deformability accounts for increased RBC destruction and is a major factor that determines the life span of red cells (18). This lack of a considerable and steady effect could underlie the relatively modest change in Hb in thalassemia as a result of hydroxycarbamide treatment as previously noted (18).
Finally, the results of this study imply that attempts at alleviating α-globin precipitation have a favorable effect on erythrocytes as seen by a trend of improved rheologic properties mostly when HbF and total Hb increase. However this effect needs to be further intensified: A more meaningful HbF production can be expected to target the full range of abnormalities that cause the short RBC life span in thalassemia. Clinical trials with HbF-enhancing agents should evaluate the effect on RBC characteristics and cell survival.
STUDY DESIGN
The institutional review board approved the protocol and written informed consent was obtained from all participants. The study population consisted of 15 E/β0 thalassemia patients who had not received transfusions and who were enrolled in a larger cohort study of HbF-augmenting treatment. The clinical results for this cohort have been published elsewhere (7). After 6 months of dose escalation, patients had received hydroxycarbamide (Bristol Meyer Squib-BMS) at 18–20 mg/kg/day for twelve months, the time period used for this analysis. Laboratory values had been obtained at baseline and then every 4 weeks, or every 8 weeks for RBC analysis, and continued for 3 months after discontinuation of hydroxycarbamide. Results of RBC analysis were compared to an average of 50 normal controls.
Fetal Hb and RBC Analysis
Fetal Hb was determined by high performance liquid chromatography (HPLC) (19). RBC osmotic deformability was measured using a custom built Ektacytometer (EKTA) (9). Cells were subjected to increasing osmolality (100–500mOsm/Kg) at a constant shear stress (100 dyne/cm2), or to increasing shear stress (0–250 dyne/cm2) at constant osmolality (290 mosmol) (20). Histograms of RBC hydration were obtained with the automated blood cell analyzer Technicon H3 (Tarrytown, NY) according to the manufacturer’s protocol and were used to calculate mean cellular hemoglobin (MCH), mean cellular hemoglobin concentration (MCHC) and mean cellular volume (MCV). Phosphatidylserine (PS) exposure on the RBC membrane was determined by annexin V labeling (Roche, Indianapolis IN) (11). Prothrombin fragment 1.2 (F1.2) was analyzed with an Enzygnost kit from Dade Behring (Marburg, Germany). All results were compared to normal controls values.
Statistical Analysis
The results during hydroxycarbamide treatment were averaged and descriptive statistics were computed for each variable (SASv9.1). Student’s paired t-test was used to determine differences from baseline to hydroxycarbamide treatment. Pearson correlation coefficients were used to assess associations between HbF and the measured RBC variables. P values of < 0.05 were considered significant. Data are reported as means ± standard deviations.
Acknowledgements
We thank Margert Lo, Carmen Rodwell and Christina Oliver for their help in performing and analyzing these assays and Ginny Gildengorin for statistical analysis. We thank all the patients who participated in the study.
This work was supported by the National Institute of Health (NIH) grants 5 R01 HL611686 and HL070583 and by NIH CTSA grant UL1 RR024131.
REFERENCES
- 1.Schrier SL, Mohandas N. Globin-chain specificity of oxidation-induced changes in red blood cell membrane properties. Blood. 1992;79:1586–1592. [PubMed] [Google Scholar]
- 2.Advani R, Rubin E, Mohandas N, Schrier SL. Oxidative red blood cell membrane injury in the pathophysiology of severe mouse beta-thalassemia. Blood. 1992;79:1064–1067. [PubMed] [Google Scholar]
- 3.Pootrakul P, Sirankapracha P, Hemsorach S, Moungsub W, Kumbunlue R, Piangitjagum A, Wasi P, Ma L, Schrier SL. A correlation of erythrokinetics, ineffective erythropoiesis, and erythroid precursor apoptosis in thai patients with thalassemia. Blood. 2000;96:2606–2612. [PubMed] [Google Scholar]
- 4.Nienhuis AW, Ley TJ, Humphries RK, Young NS, Dover G. Pharmacological manipulation of fetal hemoglobin synthesis in patients with severe beta-thalassemia. Ann N Y Acad Sci. 1985;445:198–211. doi: 10.1111/j.1749-6632.1985.tb17189.x. [DOI] [PubMed] [Google Scholar]
- 5.Olivieri NF. Fetal erythropoiesis and the diagnosis and treatment of hemoglobin disorders in the fetus and child. Semin Perinatol. 1997;21:63–69. doi: 10.1016/s0146-0005(97)80021-3. [DOI] [PubMed] [Google Scholar]
- 6.Fucharoen S, Siritanaratkul N, Winichagoon P, Chowthaworn J, Siriboon W, Muangsup W, Chaicharoen S, Poolsup N, Chindavijak B, Pootrakul P, Piankijagum A, Schechter AN, Rodgers GP. Hydroxyurea increases hemoglobin F levels and improves the effectiveness of erythropoiesis in beta-thalassemia/hemoglobin E disease. Blood. 1996;87:887–892. [PubMed] [Google Scholar]
- 7.Singer ST, Kuypers FA, Olivieri NF, Weatherall DJ, Mignacca R, Coates TD, Davies S, Sweeters N, Vichinsky EP. Fetal haemoglobin augmentation in E/beta(0) thalassaemia: clinical and haematological outcome. Br J Haematol. 2005;131:378–388. doi: 10.1111/j.1365-2141.2005.05768.x. [DOI] [PubMed] [Google Scholar]
- 8.Fathallah H, Sutton M, Atweh GF. Pharmacological induction of fetal hemoglobin: Why haven't we been more successful in thalassemia? Ann N Y Acad Sci. 2005;1054:228–237. doi: 10.1196/annals.1345.029. [DOI] [PubMed] [Google Scholar]
- 9.Mohandas N, Clark MR, Jacobs MS, Shohet SB. Analysis of factors regulating erythrocyte deformability. J Clin Invest. 1980;66:563–573. doi: 10.1172/JCI109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiefer CR, Snyder LM. Oxidation and erythrocyte senescence. Curr Opin Hematol. 2000;7:113–116. doi: 10.1097/00062752-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kuypers FA, Yuan J, Lewis RA, Snyder LM, Kiefer CR, Bunyaratvej A, Fucharoen S, Ma L, Styles L, de Jong K, Schrier SL. Membrane phospholipid asymmetry in human thalassemia. Blood. 1998;91:3044–3051. [PubMed] [Google Scholar]
- 12.Atichartakarn V, Angchaisuksiri P, Aryurachai K, Onpun S, Chuncharunee S, Thakkinstian A, Atamasirikul K. Relationship between hypercoagulable state and erythrocyte phosphatidylserine exposure in splenectomized haemoglobin E/beta-thalassaemic patients. Br J Haematol. 2002;118:893–898. doi: 10.1046/j.1365-2141.2002.03711.x. [DOI] [PubMed] [Google Scholar]
- 13.Eldor A, Rachmilewitz EA. The hypercoagulable state in thalassemia. Blood. 2002;99:36–43. doi: 10.1182/blood.v99.1.36. [DOI] [PubMed] [Google Scholar]
- 14.Angchaisuksiri P, Atichartakarn V, Aryurachai K, Archararit N, Chuncharunee S, Tiraganjana A, Rattanasiri S. Hemostatic and thrombotic markers in patients with hemoglobin E/beta-thalassemia disease. Am J Hematol. 2007;82:1001–1004. doi: 10.1002/ajh.20945. [DOI] [PubMed] [Google Scholar]
- 15.Watanapokasin R, Sanmund D, Winichagoon P, Muta K, Fucharoen S. Hydroxyurea responses and fetal hemoglobin induction in beta-thalassemia/HbE patients' peripheral blood erythroid cell culture. Ann Hematol. 2006;85:164–169. doi: 10.1007/s00277-005-0049-1. [DOI] [PubMed] [Google Scholar]
- 16.Amoyal I, Goldfarb A, Fibach E. Flow cytometric analysis of hydroxyurea effects on fetal hemoglobin production in cultures of beta-thalassemia erythroid precursors. Hemoglobin. 2003;27:77–87. doi: 10.1081/hem-120021539. [DOI] [PubMed] [Google Scholar]
- 17.Bradai M, Abad MT, Pissard S, Lamraoui F, Skopinski L, de Montalembert M. Hydroxyurea can eliminate transfusion requirements in children with severe beta-thalassemia. Blood. 2003;102:1529–1530. doi: 10.1182/blood-2003-01-0117. [DOI] [PubMed] [Google Scholar]
- 18.Orringer EP, Blythe DS, Johnson AE, Phillips G, Jr, Dover GJ, Parker JC. Effects of hydroxyurea on hemoglobin F and water content in the red blood cells of dogs and of patients with sickle cell anemia. Blood. 1991;78:212–216. [PubMed] [Google Scholar]
- 19.Eastman JW, Wong R, Liao CL, Morales DR. Automated HPLC screening of newborns for sickle cell anemia and other hemoglobinopathies. Clin Chem. 1996;42:704–710. [PubMed] [Google Scholar]
- 20.Kuypers FA, Scott MD, Schott MA, Lubin B, Chiu DT. Use of ektacytometry to determine red cell susceptibility to oxidative stress. J Lab Clin Med. 1990;116:535–545. [PubMed] [Google Scholar]