Abstract
There is significant individual variability in cognitive decline during aging, suggesting the existence of “vulnerability factors” for eventual deficits. Neuroinflammation may be one such factor; increased glial reactivity is a common outcome of aging, which in turn is associated with numerous neurodegenerative conditions. Early-life infection leads to cognitive impairment in conjunction with an inflammatory challenge in young adulthood, which led us to explore whether it might also accelerate the cognitive decline associated with aging. Rats were treated on postnatal day 4 with PBS or E. coli, and then tested for learning & memory at 2 or 16 month of age, using 2 fear conditioning tasks (context pre-exposure and ambiguous cue), and a spatial water maze task. Neonatally-infected rats exhibited memory impairments in both the ambiguous cue fear-conditioning task and in the water maze, but only at 16 month. There were no differences in anxiety between groups. Neonatally-infected rats also exhibited greater aging-induced increases in glial markers (CD11b and MHC II on microglia, and GFAP on astrocytes), as well as selective changes in NMDA receptor subunit expression within the hippocampus, but not in amygdala or parietal cortex compared to controls. Taken together, these data suggest that early-life infection leads to less successful cognitive aging, which may be linked to changes in glial reactivity.
Introduction
Cognitive decline is one of the primary consequences of aging. Deficits very often include specific impairments in hippocampal-dependent learning and memory (Robitsek, Fortin, Koh, Gallagher, and Eichenbaum, 2008). However, there is significant individual variability in cognitive decline during aging, as some individuals age more successfully than others, a phenomenon that has been well characterized in rodents (Lee, Min, Gallagher, and Kirkwood, 2005; Nicholson, Yoshida, Berry, Gallagher, and Geinisman, 2004). Similarly, only a subset of the population will eventually develop dementias such as Alzheimer’s disease, the risk factors for which are not well defined. Such variability suggests the existence of “vulnerability factors” for eventual deficits.
Neuroinflammation may be one such factor. For instance, exaggerated microglial cell activation, the primary immune cells of the CNS, and associated increased cytokine expression are associated with virtually every characterized neurodegenerative condition, including Alzheimer’s disease (Lynch, 2010). However, neuroinflammatory changes are a component of normal aging, including major histocompatibility (MHC II) expression upregulation on microglia, astrogliosis, and increased expression of pro-inflammatory cytokines (e.g., interleukin [IL]-1β; Godbout and Johnson, 2009) which in turn influence neural function (e.g., long-term-potentiation (LTP); Lynch, 2010). Thus, the factors that create a transition from normal to pathological within one individual, and not in another, remain unclear.
Many neuroinflammatory/neurodegenerative conditions are now widely believed to have a developmental component (Bilbo and Schwarz, 2009). The field of developmental or “fetal programming” has emerged based on the idea that experience during the perinatal period may modulate or “program” the normal course of development, with the result that adult outcomes are significantly and often permanently altered (Bennet and Gunn, 2006). For instance, bacterial infection in neonatal rats is associated with cognitive dysfunction in adulthood. These impairments, however, are only observed if a peripheral immune challenge (lipopolysaccharide [LPS]) is administered around the time of learning (Bilbo, Barrientos, Eads, Northcutt, Watkins, Rudy, and Maier, 2008; Bilbo, Biedenkapp, Der-Avakian, Watkins, Rudy, and Maier, 2005a; Bilbo, Levkoff, Mahoney, Watkins, Rudy, and Maier, 2005b; Bilbo, Newsum, Sprunger, Watkins, Rudy, and Maier, 2007; Bilbo, Rudy, Watkins, and Maier, 2006). LPS is a potent inducer of cytokines within the brain, and exaggerated or prolonged expression of cytokines in response to the LPS may impair synaptic processes and hippocampal-dependent memories (Barrientos, Higgins, Sprunger, Watkins, Rudy, and Maier, 2002; Gibertini, Newton, Friedman, and Klein, 1995; Vereker, Campbell, Roche, McEntee, and Lynch, 2000). Consistent with this possibility, neonatally-infected rats exhibit exaggerated microglial activation and IL-1β responses to LPS within the brain compared to control rats, and preventing the synthesis of IL-1β completely prevents the memory impairment (Bilbo et al., 2005a). Importantly, the same LPS challenge has no influence on memory in controls. Thus, neonatal infection appears to act as a “vulnerability” factor, by altering the adult rat’s response to the LPS challenge, which then influences memory.
These combined data led to the prediction that early-life infection may result in less successful aging. Thus, aging itself, and its associated neuroinflammatory changes, may be exaggerated or accelerated in early-infected rats, thus acting as the “second hit”, akin to LPS, which impairs cognition in early-infected animals.
Materials and Methods
Animals
Adult male and female Sprague-Dawley rats (70 days) were obtained from Harlan (Indianapolis, IN) and housed in same sex pairs in individually ventilated, microisolator polypropylene cages, with ad libitum access to food and filtered water. The colony was maintained at 22°C on a 12:12-h light:dark cycle (lights on at 0700h). Following acclimation to laboratory conditions, males and females were paired into breeders. Sentinel animals were housed in the colony room and screened periodically for the presence of common rodent diseases; all screens were negative. All experiments were conducted with protocols approved by the Duke University Animal Care and Use Committee.
General Procedures
Male rats were treated on postnatal day (P) 4 with PBS or E. coli, and were weaned on P21 into sibling pairs. Rats remained undisturbed until testing at either 2 month (n=6/group) or 16 month (n=9/group) of age. Three aging PBS rats died at 15 month, reducing that group size to 6. Thus, we selected 16 month in part because of the aforementioned loss of animals, and because it is on the earlier end of when aging-related cognitive decline typically emerges in rats (Johanson, Messier, Miller, Pascale, Vasudevan, Donahue, Stopa, Sabo, Caralopoulos, Klinge, Brinker, and Silverberg, 2009; Nieves-Martinez, Sonntag, Wilson, Donahue, Molina, Brunso-Bechtold, and Nicolle, 2010; Rech, de Lima, Dornelles, Garcia, Alcalde, Vedana, and Schroder, 2010), although this likely varies by strain. Nonetheless, the selection of “middle-aged” rats decreased the likelihood of severe cognitive deficits in the controls, allowing the detection of possible deficits in the early-infected rats (if aging is indeed accelerated in these animals). Sixteen-month-old rats were bred 14 months earlier than 2-month-old rats, to enable close to simultaneous testing with the two age groups (e.g., during the same week, although the two age groups were run in separate cohorts). Due to the limited availability of older rats, all rats were run in every behavioral paradigm: fear conditioning (context pre-exposure followed 7 days later by ambiguous cue paradigms), followed 3 d later by water maze, followed 3 d later by elevated plus maze. At the conclusion of testing, rats were sacrificed by cardiac perfusion, and brains were collected for later analysis of gene expression changes: 1) markers of glial activation, and 2) N-methyl-D-aspartate (NMDA) receptor subunit expression. The NMDA receptor is critical for learning and memory, and aging-related memory impairments are correlated with changes in subunit composition (Magnusson, 2000; Magnusson, Nelson, and Young, 2002), as well as decreased receptor-mediated perforant path responses and long-term-potentiation (LTP) induction and maintenance (Rosenzweig and Barnes, 2003).
Neonatal Infection Methods
Bacterial Culture
E. coli culture (ATCC 15746; American Type Culture Collection, Manassas, VA) vial contents were hydrated and grown overnight in 30 ml of brain-heart infusion (BHI; Difco Labs, Detroit, MI) at 37°C. Cultures were aliquoted into 1 ml stock vials supplemented with 10% glycerol and frozen at −20°C. One day before injections, a stock culture was thawed and incubated overnight in 40 ml of BHI at 37°C. The number of bacteria in cultures was read using a microplate reader (Bio-Tek Instruments, Inc., Winooski, VT) and quantified by extrapolating from previously determined growth curves. Cultures were centrifuged for 15 min at 4000 rpm, the supernatants were discarded, and the bacteria were re-suspended in the dose-appropriate volume of sterile Dulbecco’s PBS (Invitrogen Corp., Carlsbad, CA).
Injections
Female breeders were visually examined daily for confirmation of pregnancy, and male breeders were removed from cages prior to the birth of pups (=P0). Litters were culled on P4 to a maximum of 10 pups/litter, retaining 2 female and as many male pups as possible. Male pups were injected subcutaneously (30G needle) on P4 with either 0.1 × 106 colony forming units (CFU) of live bacterial E. coli/g suspended in 0.1 ml PBS, or 0.1 ml PBS. This dose results in sustained (~48–72 h) increased plasma cytokines (IL-1β, IL-6) and corticosterone, as well as specific increases in IL-1β and microglial activation within the brain (Bilbo et al., 2005a). All pups were removed from the mother at the same time and placed into a clean cage with bedding, weighed and injected individually, and returned to the mother as a group. Elapsed time away from the mother was less than 5 min. All pups from a single litter received the same treatment due to concerns over possible cross-contamination from E. coli. All injections were given between 1530 and 1600 hr. All male pups for adult analyses were weaned on P21 and housed in sibling pairs; remaining female pups were culled. To control for litter effects, a maximum of two pups/litter across a minimum of 4 different litters were assigned to a single experimental group.
Behavioral Paradigms
Context pre-exposure paradigm
Memory was first tested using a modified version of fear conditioning known as the context pre-exposure paradigm. This paradigm, described in detail previously (Bilbo et al., 2005a; Bilbo et al., 2005b; Bilbo et al., 2006), assesses the rat’s memory for a recently explored context, which depends critically on the hippocampus (Matus-Amat, Higgins, Barrientos, and Rudy, 2004; Rudy, Huff, and Matus-Amat, 2004).
Apparatus
The conditioning context consisted of one of two identical black Plexiglas boxes with open fronts (41.9 L × 40.6 W × 46.2 H, cm) to allow viewing of animals. The conditioning chambers (30.5 L × 26.7 W × 33 H, cm), placed inside each black box, were made of 2 clear Plexiglas walls (front and back) and 2 metal sides (Coulbourn Instruments). Each chamber had a ceiling-mounted infrared activity monitor connected to a PC. A 2 s, 1.5 mA shock was delivered through a removable floor of stainless steel rods. Each rod is 0.5 cm in diameter, spaced 1.75 cm center to center and is wired to a shock generator and scrambler. The chamber was cleaned with 70% alcohol and then water before each animal was placed inside.
Procedure
This paradigm consists of 3 phases: (I) exposure to the context, (II) immediate shock, and (III) fear test. (I) Adult rats were transported two at a time in a lidded, black ice bucket to the experimental testing room where they were placed into the conditioning context, allowed to freely explore for several minutes, and were then transported back to their home cage where they remained for 40 s before the next pre-exposure. This procedure was repeated 4 times. Animals remained in the novel context for 5 min on the first exposure and for 40 s on the three subsequent exposures. The rats were transported in the black bucket throughout the experiment. (II) 24 h following context pre-exposure, each rat was taken individually from their home cage and transported in the black bucket to the conditioning context. There, they received a single 2 s footshock immediately after being placed in the context. They were quickly removed from the chamber and transported back to their home cage. (III) Contextual fear was assessed 24 h following immediate shock by placing the rat in the conditioning context for 6 min. Each rat was observed by an observer blind to experimental conditions and judged as either freezing or active every 10 s, at the instant the sample is taken. Freezing in these experiments was defined as the absence of all visible movement, except for respiration. Figures represent the percent of time spent freezing for each animal, which is then averaged across groups. Scoring began 5 s after the animal was placed into the chamber. Total activity during both the pre-exposure period and test were also measured using the infrared monitors.
Ambiguous Cue Fear Conditioning
This task assesses emotional memory by requiring the animal to distinguish between 2 cues, one that is partially predictive of a shock, and a second that is always predictive of a shock.
Apparatus
Conditioning occurred in a similar apparatus as described above except that the walls were all clear Plexiglas, the ambient light was red (instead of natural white), and a speaker capable of emitting a 2976-Hz tone presented at 76 dB was mounted on the ceiling of each outer black box. The chamber was cleaned with non-toxic disinfectant (10% QTB, Caltech Industries, Midland, MI) and then water before each animal was placed inside.
Procedure
Rats were placed into the conditioning chamber and allowed to explore the chamber for 2 min before the onset of a series of tones and lights (the white house light) (15 sec duration for each). The light was presented 3 times, always immediately prior to the onset of a 2 sec 1 mA footshock. The tone was presented 5 times, 2 times in isolation (i.e., at least 2 min separating it from any other cue or event), and 3 times immediately prior to each of the light-shock pairings. Thus, the light served as a perfect cue of the shock (always predictive), whereas the tone was an imperfect predictor (ambiguous). Rats were tested 48 h and 7 d later for cued fear in a novel chamber for 12 min, consisting of 2 min prior to cue presentation (pre-CS), 5 min with the tone, and 5 min with the light. Presentation order of the tone vs light was alternated and counter-balanced across groups, and day of testing. The novel chamber consisted of triangular, blue corrugated walls, without rod floors, and white ambient light. Freezing was assessed as described above for the context pre-exposure paradigm.
Water Maze
This task assesses spatial learning and memory, which may be particularly susceptible to aging-related decline (Robitsek et al., 2008).
Apparatus
The maze was a circular pool approximately 1.8 m in diameter and filled with room temperature water. A circular platform 10 cm in diameter was placed 3 cm above the water (visible platform) or submerged 2 cm below the surface of the water (hidden platform), and the water was clouded by non-toxic black tempera paint to ensure that the rats could not see the platform. The visible platform had bright white tape around its perimeter to make it more visible above the black water. For the spatial task, the platform was placed in one quadrant of the pool and was maintained there throughout acquisition of the task. There were many prominent distal visual cues that remained constantly positioned around the testing room. A video camera was mounted on the ceiling and connected to a laptop computer with ANYmaze software (Stoelting Co., Wood Dale, IL) to record and analyze all behavior.
Procedures
Visible Platform
On day 1 of testing, each rat was placed onto the visible platform, which was positioned directly in the center of the pool, and allowed to look around the room for 1 min. Each rat was then placed into the pool proximate to the platform, and gently guided to the platform by the experimenter. Next, rats were trained to find the visible platform across 5 subsequent trials, following release at one of several randomly selected release points along the wall (snout facing the wall). Latencies to reach the platform were recorded.
Hidden Platform
On days 2–4 of testing, each rat received a total of 8–10 acquisition trials per day over 3 consecutive days. Two-month old rats received 24 total trials (8 per day), whereas 15-month-olds received 28 (10, 10, & 8 per day, respectively). Older rats received more trials because we expected them to take longer to acquire the task, and we wanted to avoid overtraining in the young (2 month) rats. Each trial started by placing the rat into the water at any of three randomly chosen quadrants (excluding the platform quadrant) with its snout facing the wall of the pool. The rat was allowed to search for the platform for up to 60 s and when located, allowed to remain on the platform for 10 s. The rat was placed on the platform if it failed to locate the platform within 60 s, and remained for 10 s. The rat was returned to a holding cage until the next trial, with an approximate inter-trial interval of 5 min. One, 24 or 96 h following the last acquisition trial on day 3, a retention probe trial (20s) was employed to measure rats’ selective search pattern, in the absence of the platform. After the 1 and 24 h probes, rats were given a single reminder trial with the platform present, prior to subsequent probe testing.
Elevated Plus Maze (EPM)
This task assesses anxiety. Apparatus: The maze consisted of two open arms and two closed arms (50.8 × 12.7 cm). The closed arms had 45 cm high black walls, and the maze was mounted 86 cm off the floor. Behavior was recorded and analyzed using Anymaze. Procedure: Rats were placed into the central start area and allowed to explore the maze for 5 min. The number of visits to each arm, and the time spent on each arm, as well as the central start area were recorded and analyzed.
Experimental Techniques
Tissue Collection
At the conclusion of behavioral testing, adult rats were deeply anesthetized with a ketamine/xylazine cocktail and were transcardially perfused with ice-cold saline for 2 min in order to clear brain vessels of blood and peripheral cells. Brains were extracted and rapidly frozen via immersion in isopentane, wrapped in foil and stored at −80°C until sectioning. All brains were collected between 9 and 11 am. Brains were cryostat sectioned (Leica 1850) at 50 μm, and discrete tissue punches of the hippocampal regions (CA1, CA3, and dentate gyrus (DG)), amygdala (AMY), pre-frontal cortex (PFC) and posterior parietal cortex (PAR) were excised using a brain punch tool (1 mm dia). Tissue punches were stored at −80°C until mRNA extraction.
Quantitative Real-Time PCR
We analyzed mRNA expression for the following genes, all of which have been demonstrated to change with aging (Lynch, 2010): CD11b and MHC II (markers of microglial activation), GFAP (a marker for astrocytes), and NR1, NR2A, and NR2B (NMDA receptor subunits).
Total RNA was isolated from tissue punches based on the TRIzol method of Chomczynski and Sacchi (Chomczynski and Sacchi, 1987). Following RNA isolation and enrichment, samples were DNASE-treated and cDNA was synthesized using the Reverse Transcriptase kit from Qiagen (Valencia, CA). cDNA (1 μl) was added to a reaction master mix (20 μl) containing 5 mM MgCl2, HotStar Taq DNA polymerase, SYBR Green I, dNTPs, fluorescein (10 nM) and gene specific primers (500 nM each of forward and reverse primer). Formation of PCR product was monitored in real time using an Eppendorf realplex. Threshold cycle (CT; number of cycles to reach threshold of detection) was determined for each reaction, and relative gene expression was determined using the 2−ΔΔCt method (Livak and Schmittgen, 2001; Pfaffl, 2001) as follows: 1) we normalized the cycle threshold (CT) of the target gene of interest (T) to that of the reference (housekeeping) gene (H), for both the test (A) and calibrator samples (B): ΔCT(A) = CT(T,A) − CT(H,A) and ΔCT(B) = CT(T,B) − CT(H,B). (We define the calibrator sample as that with the lowest (baseline) expression.) 2) We normalized the ΔCT of A to B: ΔΔCT = ΔCT(A) − ΔCT(B), and 3) we calculated the expression ratio: 2−ΔΔCT = Normalized expression ratio.
Primer Specifications
cDNA sequences were obtained from Genbank at the National Center for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov). Primer sequences were designed using an online Oligo Analysis & Plotting Tool (Qiagen) and tested for sequence specificity using the Basic Local Alignment Search Tool at NCBI. Primers were obtained from Sigma, and specificity was verified by melt curve analysis. We estimated the efficiency of each primer set based on serial dilution standard curves. All primer sets were estimated as greater than 90% efficient, and within 5% of each other. Primer sequences are listed in Table 1.
Table 1.
| Gene | Forward Primer 5′-3′ |
|---|---|
| CD11b | CTGGGAGATGTGAATGGAG |
| MHC II | AGAGACCATCTGGAGACTTG |
| GFAP | AGGGACAATCTCACACAGG |
| NR1 | GGAGTTCACAGTCAATGGTG |
| NR2A | TCCATTCTTCTGTCATCCTGC |
| NR2B | TGCACAATTACTCCTCGACG |
| GAPDH | GTTTGTGATGGGTGTGAACC |
Data Analysis
All data were analyzed using Statview statistical software, and significance was set at p<0.05 for all tests. For the ambiguous cue task, we calculated the amount of freezing to the tone and to the light for each animal, and subtracted from each the amount of freezing prior to any cue presentation (=“no cue freezing”). This treatment reduced inter-animal variability by accounting for individual differences in baseline freezing, which was always less than 15% and did not significantly differ by neonatal treatment, age, or time of test (p>0.05). These data were then analyzed as a function of age and neonatal group (PBS vs E. coli) using repeated measures (tone vs light) ANOVAs. For water maze, latencies to find the platform during training were analyzed as a function of neonatal treatment across all trials using repeated measures ANOVAs. Because 2 and 16 month old rats received different numbers of training trials, these data were analyzed separately for each age group. For the probe trials, we calculated and analyzed a “difference score”, defined as the amount of time spent in the platform quadrant, minus the amount of time spent in the “next best” quadrant for each animal (i.e., the quadrant with the next highest latency for a given animal), a conservative estimate of selective searching. These data were analyzed as a function of age and neonatal group across all probe trials using a repeated measures ANOVA. The number of platform location crossings, initial heading error to the platform location, latency to first entry of the target quadrant, swim speed, and swim path, were all recorded and analyzed as well. All gene expression data were analyzed using 2-way (age × neonatal group) ANOVAs for AMY, PFC, & PAR. Hippocampal tissue was analyzed between ages and neonatal treatment using repeated measures (CA1, CA3, & DG) ANOVAs for each gene.
Results
Aging reveals a cognitive deficit in the ambiguous cue fear task in rats infected as neonates
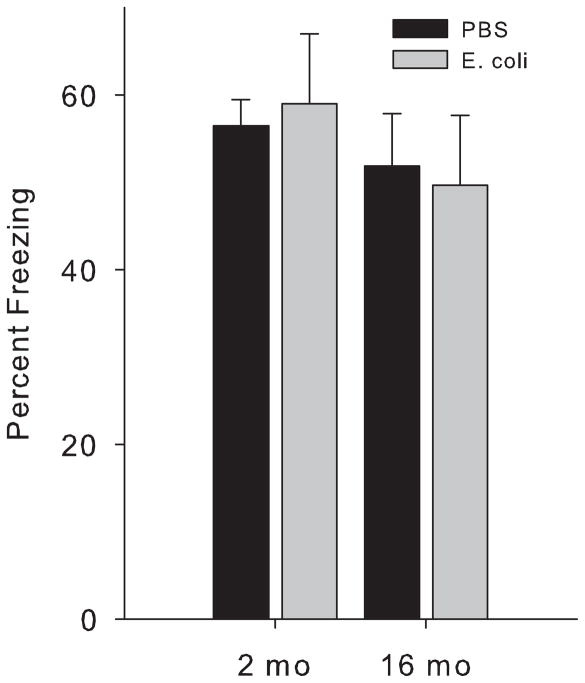
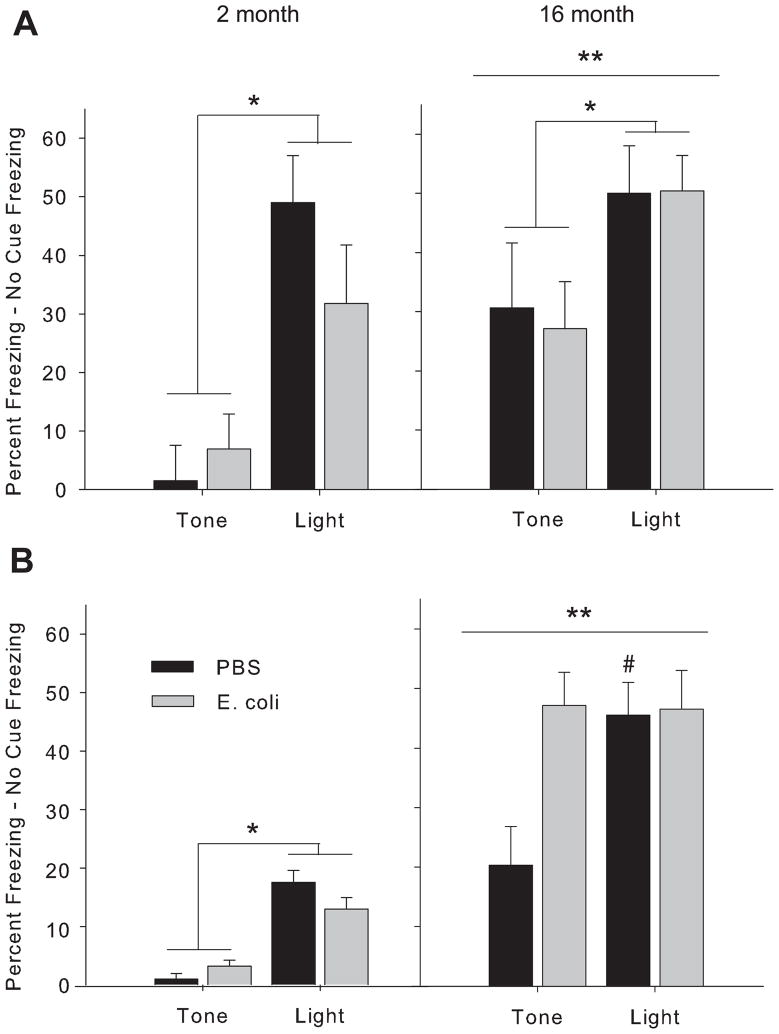
There were no significant differences by age or neonatal treatment in performance in the context pre-exposure task (p>0.05, Fig 1). In the ambiguous cue task, there were significant effects of age and cue at the 48 h test (F(1,23)= >5.5, p<0.05, for both, Fig 2A). All rats froze more to the purely predictive light cue than to the ambiguous tone cue, as expected, with no differences by neonatal treatment (p<0.05). Older rats also froze more overall than the 2 month rats (p<0.05). At the 1 week test (Fig 2B), there were significant effects of age and cue (F(1,23)=>7.5, p<0.01, for both), as well as age × neonatal group and cue × neonatal group interactions (F(1,23)= >4.8, p<0.05, for both). Again, older rats froze more overall than the 2 month rats (p<0.05). However, 16 month old rats infected as neonates froze equally high to both cues, whereas older control rats continued to freeze more to the light (p<0.05).
Figure 1. Percent freezing to the context in the pre-exposure task does not differ among groups.
Rats were treated on postnatal day (P) 4 with PBS or E. coli, and tested for memory at 2 month or 16 month of age using a task which requires the animal to make a simple association between a shock and exploration of a novel environment the previous day. There were no significant differences as a consequence of either age or neonatal treatment.
Figure 2. Older rats infected as neonates exhibit a cognitive impairment in the ambiguous cue task.
Rats were treated on P4 with PBS or E. coli, and tested for memory at 2 month or 16 month of age using a task that requires the animal to distinguish between multiple cues that predict a shock with varying fidelity. Figures represent percent freezing to each cue minus baseline (“no cue”) freezing at 48 hr (A) or 1 wk (B) after conditioning. *freezing to the light is significantly different overall from freezing to the tone; **freezing is overall higher in 16 month compared to 2 month old rats; #significantly different from PBS/Tone group; p<0.05 for all.
Aging and neonatal infection interact to influence learning and memory in the water maze task
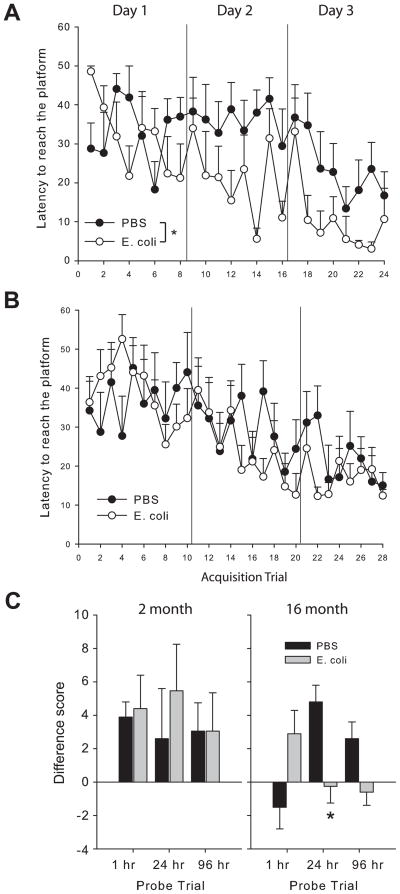
There were no significant differences in latency to find the visible platform at either age (data not shown). At 2 months, rats infected as neonates had significantly lower latencies to reach the hidden platform across the 24 trials of training in the water maze (F(1,287)=5.1, p<0.05, Fig 3A). In contrast, at 16 months, latency to find the platform during training did not differ by neonatal group (p>0.05; Fig 3B). For performance during the probe trials, there was a significant 3-way interaction (trial × age × neonatal group) for difference score (=amount of time spent in the platform quadrant, minus the amount of time spent in the “next best” quadrant for a given animal) (F(1,46)=3.1, p<0.05; Fig 3C). Post-hocs revealed that 2 month old rats performed better overall at the 1 hr probe compared to older rats (F(1,23)=4.7, p<0.05). At the 24 h probe, there was a significant age × group interaction (F(1,23)=4.4, p<0.05); 16 month old control rats performed better than neonatally-infected rats at this age, whereas there was no difference in the 2 month old rats. There was a significant effect of age on number of platform location crossings as well (F(1,46)=15.3, p<0.01), with 2 month old rats making more crossings across all probe trials than older rats (due to the fact that older rats very rarely made platform crossings, indicating the search strategy was more precise in the younger rats; data not shown), but this did not differ by neonatal group. Performance during the probe trials did not significantly differ on any other measure analyzed, including initial heading error to the platform location, latency to first entry of the target quadrant, swim speed, and swim path.
Figure 3. Water maze performance depends on neonatal treatment and age.
Rats were treated on P4 with PBS or E. coli, and tested for spatial learning & memory at 2 month or 16 month of age using a hidden platform task. Rats infected as neonates acquired the task faster than PBS controls at 2 months (A) but not at 16 months (B) of age. (C) Performance during the probe trials: Difference score = time in the platform quadrant minus time in the next best quadrant for each rat, indicative of selective searching in the target platform; *significantly different from PBS at the same time point, p<0.05.
Younger rats are less anxious than older rats, but there are no neonatal group differences
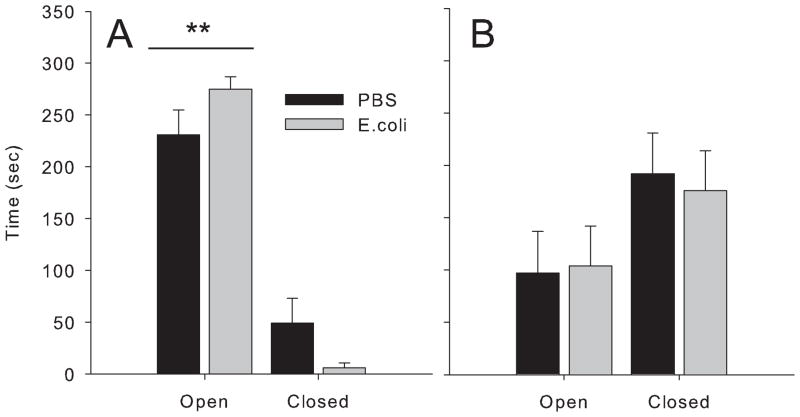
There was a significant arm × age interaction in the elevated plus maze (F(1,23)=15.3, p<0.01); 2 month old rats spent more time in the open arms compared to 16 month olds (Fig 4). There were no significant differences in the elevated plus maze between neonatal treatment groups. Interestingly, young rats spent the majority of time in the open arms of the maze, which is unusual for this task, but is likely due to the fact that these animals were heavily handled (fear conditioning and water maze testing) prior to this task.
Figure 4. Older rats exhibit more anxiety than young rats.
Rats were treated on P4 with PBS or E. coli, and tested for anxiety at 2 months (A) or 16 months (B) of age. Time spent in the open vs. closed arms of the elevated plus maze did not differ between neonatal treatment groups at either age, but 2 month old rats spent significantly more time in the open arms than older rats, **p<0.05.
Neonatal infection exaggerates aging-related changes in gene expression within the brain
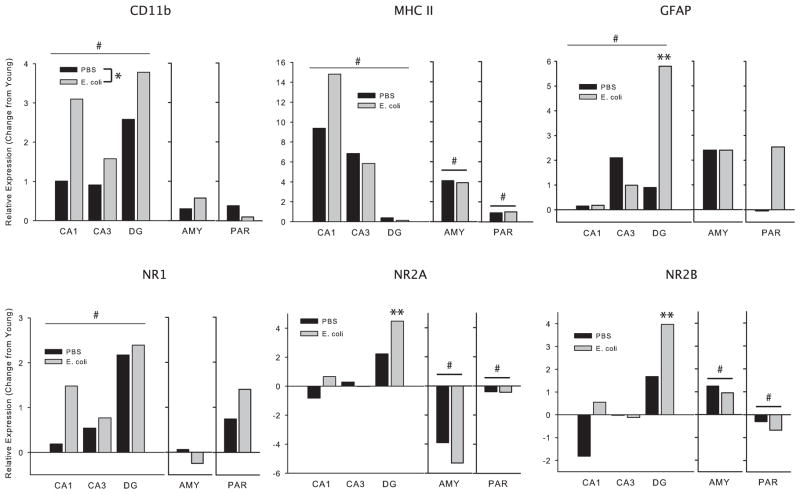
We assessed whether markers of glial activation/reactivity (CD11b, MHC II, & GFAP) and NMDA receptor subunit expression (NR1, NR2A, & NR2B) would change with age, and further whether such changes may be modulated by early life infection. There were no significant changes in any gene within the PFC, and therefore these data are not discussed. There was a significant main effect of age on CD11b expression within the hippocampus (F(1,46)=38.6, p<0.01; 16 month > 2 month), as well as a significant main effect of neonatal treatment, with expression overall higher in neonatally-infected rats (F(1,46)=4.7, p<0.05, Fig 5). There were no significant changes in AMY or PAR. For MHC II, there were significant main effects of age within the hippocampus (F(1,46)=8.1, p<0.01), AMY, and PAR (F(1,23)>11.5, p<0.01, for both), with expression higher in the 16 month olds. For GFAP, there was a significant main effect of age (F(1,46)=11.1, p<0.01), as well as a significant 3-way interaction (age × group × region) (F(2,46)=3.3, p<0.05) for expression within the hippocampus. Post-hocs revealed that expression was higher in the DG of neonatally-infected rats than in controls (p<0.05). There were no significant changes in AMY or PAR. For NR1, there was a significant main effect of age within the hippocampus only, with expression higher in the 16 month olds (F(1,46)=18.7, p<0.01). For NR2A and 2B, there were significant 3-way interactions (age × group × region) (F(2,46)>2.9, p<0.05, for each) for expression within the hippocampus; post-hocs revealed that expression was highest within the DG of neonatally-infected rats (p<0.05, for both). There were significant main effects of age for NR2A and 2B within the AMY and PAR as well; NR2A expression decreased in both regions with age (F(1,23)>4.8, p<0.05, for each), whereas NR2B expression increased in the AMY (F(1,23)=31.55, p<0.01) and decreased in the PAR (F(1,23)=8.8, p<0.01) with age.
Figure 5. Glial markers and NMDA receptor subunit composition are altered with age.
Rats treated on P4 with PBS or E. coli were sacrificed at 2 or 16 month of age, following the completion of behavioral testing. All statistics were performed on raw data, but data are presented here as relative changes in gene expression in the 16 month olds compared to 2 month olds in order to better illustrate age-associated changes. #significantly different overall from 2 month olds, *significantly different overall from PBS, **significantly different from PBS within the dentate gyrus (DG), p<0.05 for all. CD11b=complement 3 receptor, a marker for microglia; MHC II=major histocompatibility complex, a marker for microglia; GFAP=glial fibrillary acidic protein, a marker for astrocytes; AMY=Amygdala; PAR=parietal cortex.
Discussion
Aging is associated with marked cognitive decline in a subset of individuals, the precipitating factors for which are not well defined. Neuroinflammatory changes, including increased glial reactivity and pro-inflammatory cytokine production, appear to occur as a part of normal aging, which in turn may negatively impact neural function (Lynch, 2010). We hypothesized that early postnatal infection, which acts as a vulnerability factor for young adult LPS-induced memory impairments, may accelerate neuroinflammatory changes and thus result in less successful cognitive aging in these animals.
All 2 month and 16 month old rats performed equally well in the context pre-exposure task (Fig 1), which is consistent with our previous data using this paradigm, as impairments are only observed in the early-infected rats if combined with a mild inflammatory challenge around the time of learning (Bilbo et al., 2005a; Bilbo et al., 2005b; Bilbo et al., 2007; Bilbo et al., 2006; Bland, Beckley, Young, Tsang, Watkins, Maier, and Bilbo, 2009) In contrast, when we assessed memory using the ambiguous cue task, an arguably more complex task, we observed a neonatal group difference at 16 month, but not at 2 month (Fig 2). All 2 month old rats froze more to the purely predictive light cue than to the ambiguous tone cue at both 48 h and at 1 wk. Freezing at the 1 wk test was lower overall compared to 48 h at this age, suggesting that extinction had occurred. A different pattern emerged in the 16 month old rats; at 48 h, all rats froze more to the light than to the tone, although the gradient was not as distinct as in the younger rats. By 1 wk, however, early-infected rats no longer distinguished between the 2 cues, but froze equally high to both, whereas the control rats maintained a distinction between the two. Thus, it appears that the early-infected rats began to generalize over time, an outcome which only emerged with increased age.
Other groups have used the ambiguous cue task as a putative measure of anxiety in rodents, in which enhanced freezing to ambiguous cues occurs in more anxious individuals (Crestani, Lorez, Baer, Essrich, Benke, Laurent, Belzung, Fritschy, Luscher, and Mohler, 1999; Tsetsenis, Ma, Lo Iacono, Beck, and Gross, 2007). We therefore measured anxiety in our rats, but found no differences in the EPM between neonatal treatment groups at either age, consistent with our previous findings in young animals (Fig 4; (Bilbo et al., 2007; Bilbo et al., 2006). It should be noted that 2 month old rats spent an unusually high percentage of time in the open arms, likely due to prolonged handling in these rats (due to repeated behavioral testing prior to testing in the EPM). Thus, interestingly, anxiety clearly increased with age in both groups, as this pattern flipped at 16 month (i.e., they spent more time in the closed arms). Nonetheless, the lack of difference between neonatal treatment groups remains, and is consistent with our previous results in the EPM, which we have assessed more than once (Bilbo et al., 2007; Bilbo et al., 2006). Thus, we believe the behavioral difference at 16 month reflects forgetting (or some other aspect of encoding and/or retrieval) in the early-infected rats, and not an increase in anxiety (compared to controls). As retention intervals increase after fear conditioning, rats tend to show enhanced generalized fear to altered contexts and discrete cues (Biedenkapp and Rudy, 2007; Riccio, Ackil, and Burch-Vernon, 1992).
We also assessed performance in the water maze task. At 2 month, early-infected rats unexpectedly acquired the task significantly faster than PBS controls; however, this facilitation in learning did not extend to memory, as there were no significant differences in any measure during the probe trials, which is consistent with our previous data in the water maze (Fig 3; Bilbo et al., 2007). At 16 month, there were no differences in acquisition, whereas PBS controls remembered the platform location for longer intervals compared to the early-infected rats. Thus, these data are consistent with the data from the ambiguous cue task; that is, a neonatal group difference emerged with aging, but only at the longer retention intervals, suggestive of weaker encoding or retrieval in the early infection group.
There were no significant differences in glial markers in any brain region of the 2 month rats, which is largely consistent with previous findings, in which functional differences emerge only after a challenge in young adults (Bilbo et al., 2005a; Bland et al., 2009). However, there were a number of neuroinflammatory changes associated with aging (Fig 5). Glial surface marker mRNAs (CD11b, MHC II, & GFAP) were all increased with age in the hippocampus, which is likely indicative of increased reactivity/priming, consistent with previous reports (Frank, Barrientos, Biedenkapp, Rudy, Watkins, and Maier, 2006). MHC II expression increased with age in every brain region examined. These data are striking because the changes are several fold, and have occurred in much younger animals than previously reported (16 month vs 24 month; (Frank et al., 2006). Notably, the increase in CD11b, a marker for microglia, was overall larger in neonatally-infected rats, and the increase in GFAP, a marker for astrocytes, was selectively increased in the DG of neonatally-infected rats compared to controls.
These data are intriguing, as DG NMDA receptors are critical for pattern separation within the hippocampal network; that is, the ability to form distinct representations of similar but different episodes and their associated temporal and spatial relationships (McHugh, Jones, Quinn, Balthasar, Coppari, Elmquist, Lowell, Fanselow, Wilson, and Tonegawa, 2007). Such ability would be critical for successful performance in the ambiguous cue task. Similarly, NMDA receptors in the CA1 and DG are critical for long-term spatial memory (Lee and Kesner, 2002). Because glia are critical for NDMA receptor function (Lee, Mannaioni, Yuan, Woo, Gingrich, and Traynelis, 2007), their altered activity/reactivity in these regions could be influencing memory in the 16 month neonatally-infected rats. We assessed changes in NMDA receptor subunit mRNA expression in this study, as age related changes have been reported (Magnusson, 2000; Magnusson et al., 2002). Furthermore, neonatal LPS treatment alters subunit expression in adult rat hippocampus (Harre, Galic, Mouihate, Noorbakhsh, and Pittman, 2008). NR1 expression increased with age in the hippocampus in both groups, whereas there were group × region differences in the expression of NR2A and 2B. Both subunits increased more in the DG of neonatally-infected rats compared to controls. Changes in other regions, while influenced by age, did not differ by neonatal treatment. Others have largely reported decreases in receptor expression with age; however, those changes were reported in much older rats, and Magnusson et al (2002) reported an increase in NR2A in 10 month old mice, suggesting that middle age may be associated with compensatory increases.
In sum, there were a number of immune and plasticity-related gene changes, specifically within the hippocampus of the 16 month old rats infected as neonates. The functional consequences of selective changes in NMDA receptor subunits in older rats remain unclear, as they do for learning and memory in general. Furthermore, given the correlational nature of our data set, we are unable to draw conclusions at this point, but the early-infection plus age-induced changes are intriguing given the selectivity to the hippocampal regions and the nature of the behavioral deficits. Taken together, these data suggest that early-life infection may lead to less successful cognitive aging, and that the early-life environment of an individual should be considered when assessing the risk of age-related cognitive decline.
Acknowledgments
The author thanks Verne Tsang, Evan Bordt and Rishi Mistry for technical assistance. Supported in part by RO1 MH083698.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Bennet L, Gunn A. The fetal origins of adult mental illness. In: Wintour-Coghlan M, Owens J, editors. Early Life Origins of Health and Disease (Advances in Experimental Medicine and Biology) New York: Springer; 2006. pp. 204–211. [Google Scholar]
- Biedenkapp JC, Rudy JW. Context preexposure prevents forgetting of a contextual fear memory: implication for regional changes in brain activation patterns associated with recent and remote memory tests. Learn Mem. 2007;14:200–203. doi: 10.1101/lm.499407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Barrientos RM, Eads AS, Northcutt A, Watkins LR, Rudy JW, Maier SF. Early-life infection leads to altered BDNF and IL-1beta mRNA expression in rat hippocampus following learning in adulthood. Brain Behav Immun. 2008;22:451–455. doi: 10.1016/j.bbi.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005a;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci. 2005b;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Newsum NJ, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Differential effects of neonatal handling on early life infection-induced alterations in cognition in adulthood. Brain Behav Immun. 2007;21:332–342. doi: 10.1016/j.bbi.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Rudy JW, Watkins LR, Maier SF. A behavioural characterization of neonatal infection-facilitated memory impairment in adult rats. Behav Brain Res. 2006;169:39–47. doi: 10.1016/j.bbr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Beckley JT, Young S, Tsang V, Watkins LR, Maier SF, Bilbo SD. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy JM, Luscher B, Mohler H. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Gibertini M, Newton C, Friedman H, Klein TW. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-beta. Brain Behav Immun. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- Harre EM, Galic MA, Mouihate A, Noorbakhsh F, Pittman QJ. Neonatal inflammation produces selective behavioural deficits and alters N-methyl-D-aspartate receptor subunit mRNA in the adult rat brain. Eur J Neurosci. 2008;27:644–653. doi: 10.1111/j.1460-9568.2008.06031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson C, Messier A, Miller M, Pascale C, Vasudevan K, Donahue J, Stopa E, Sabo E, Caralopoulos I, Klinge P, Brinker T, Silverberg G. Periventricular destabilization and ventriculomegaly in aging rats: implications for reduced neurogenesis and cognition. Cerebrospinal Fluid Res. 2009;6(Suppl 2):S16. [Google Scholar]
- Lee CJ, Mannaioni G, Yuan H, Woo DH, Gingrich MB, Traynelis SF. Astrocytic control of synaptic NMDA receptors. J Physiol. 2007;581:1057–1081. doi: 10.1113/jphysiol.2007.130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Min SS, Gallagher M, Kirkwood A. NMDA receptor-independent long-term depression correlates with successful aging in rats. Nat Neurosci. 2005;8:1657–1659. doi: 10.1038/nn1586. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential contribution of NMDA receptors in hippocampal subregions to spatial working memory. Nat Neurosci. 2002;5:162–168. doi: 10.1038/nn790. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Age-related neuroinflammatory changes negatively impact on neuronal function. Front Aging Neurosci. 2010 doi: 10.3389/neuro.24.006.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KR. Declines in mRNA expression of different subunits may account for differential effects of aging on agonist and antagonist binding to the NMDA receptor. J Neurosci. 2000;20:1666–1674. doi: 10.1523/JNEUROSCI.20-05-01666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KR, Nelson SE, Young AB. Age-related changes in the protein expression of subunits of the NMDA receptor. Brain Res Mol Brain Res. 2002;99:40–45. doi: 10.1016/s0169-328x(01)00344-8. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y. Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J Neurosci. 2004;24:7648–7653. doi: 10.1523/JNEUROSCI.1725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves-Martinez E, Sonntag WE, Wilson A, Donahue A, Molina DP, Brunso-Bechtold J, Nicolle MM. Early-onset GH deficiency results in spatial memory impairment in mid-life and is prevented by GH supplementation. J Endocrinol. 2010;204:31–36. doi: 10.1677/JOE-09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech RL, de Lima MN, Dornelles A, Garcia VA, Alcalde LA, Vedana G, Schroder N. Reversal of age-associated memory impairment by rosuvastatin in rats. Exp Gerontol. 2010 doi: 10.1016/j.exger.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Riccio DC, Ackil J, Burch-Vernon A. Forgetting of stimulus attributes: methodological implications for assessing associative phenomena. Psychol Bull. 1992;112:433–445. doi: 10.1037/0033-2909.112.3.433. [DOI] [PubMed] [Google Scholar]
- Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, Eichenbaum H. Cognitive aging: a common decline of episodic recollection and spatial memory in rats. J Neurosci. 2008;28:8945–8954. doi: 10.1523/JNEUROSCI.1893-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Tsetsenis T, Ma XH, Lo Iacono L, Beck SG, Gross C. Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat Neurosci. 2007;10:896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereker E, Campbell V, Roche E, McEntee E, Lynch MA. Lipopolysaccharide inhibits long term potentiation in the rat dentate gyrus by activating caspase-1. J Biol Chem. 2000;275:26252–26258. doi: 10.1074/jbc.M002226200. [DOI] [PubMed] [Google Scholar]