Abstract
Aim
Sex differences are evident in human skeletal muscle as the cross-sectional area of individual muscle fibres is greater in men as compared to women. We have recently shown that resistance exercise stimulates mTOR signalling and muscle protein synthesis in humans during early post-exercise recovery. Therefore, the aim of this study was to determine if sex influences the muscle protein synthesis response during recovery from resistance exercise.
Methods
Seventeen subjects, 9 male and 8 female, were studied in the fasted state before, during and for two hours following a bout of high-intensity leg resistance exercise. Mixed muscle protein fractional synthetic rate (FSR) was measured using stable isotope techniques and mTOR signalling was assessed by immunoblotting from repeated vastus lateralis muscle biopsy samples.
Results
Post-exercise muscle protein synthesis increased by 52% in the men and by 47% in the women (P<0.05) and was not different between groups (P>0.05). Akt phosphorylation increased in both groups at 1 hr post-exercise (P<0.05) and returned to baseline during 2 hr post-exercise with no differences between groups (P>0.05). Phosphorylation of mTOR and its downstream effector S6K1 increased significantly and similarly between groups during post-exercise recovery (P<0.05). eEF2 phosphorylation decreased at 1- and 2 hrs post-exercise (P<0.05) to a similar extent in both groups.
Conclusion
The contraction-induced increase in early post-exercise mTOR signalling and muscle protein synthesis is independent of sex and appears to not be playing a role in the sexual dimorphism of leg skeletal muscle in young men and women.
Keywords: Protein Metabolism, Muscle Contraction, Gender, mTORC1 signalling
Introduction
On average, men have a larger skeletal muscle mass than women. This difference has been attributed to higher (~10 fold) levels of circulating testosterone in males. The sexual dimorphism of skeletal muscle begins during puberty when testosterone production increases significantly and is sustained by the greater blood concentrations that ensue. Testosterone has profound effects on muscle cell size and protein synthesis (Urban et al. 1995, Bhasin et al. 2001). Although circulating concentrations of testosterone may maintain the larger skeletal muscle mass in males, it is possible that sex-differences in muscle fibre size may be entirely attributed to changes that occur during puberty. However, it is not known whether the protein anabolic response to resistance exercise also contributes to the sexual dimorphism of human skeletal muscle.
Skeletal muscle hypertrophy occurs when the rate of muscle protein synthesis exceeds that of muscle protein breakdown over time. High-intensity resistance exercise stimulates both muscle protein synthesis and breakdown during the post-exercise recovery period, however, the magnitude of the change in each is not proportional with increases in synthesis exceeding that of breakdown (Biolo et al. 1995). The increase in muscle protein synthesis can be measured within hours following a single bout of heavy resistance exercise (Dreyer et al. 2006, Dreyer et al. 2008) and remains elevated for up to 24 hours in trained individuals (MacDougall et al. 1995) and for up to 48 (Phillips et al. 1997) and 72 hours in untrained individuals (Miller et al. 2005).
The acute increase in muscle protein synthesis appears to be mediated primarily through changes in translation rather than changes in transcription. In particular, the mammalian target of rapamycin (mTOR) signaling pathway appears to be a major regulator of overall muscle fibre size by controlling translation initiation and elongation (Bodine et al. 2001). We have shown that the activation of the mTOR signalling pathway in human skeletal muscle is associated with an increased rate of muscle protein synthesis during the early recovery phase following a single bout of resistance exercise (Dreyer et al. 2006, Dreyer et al. 2008). The contraction-induced increase in muscle protein synthesis appears to be dependent on mTOR activation as we have recently shown that a specific mTOR inhibitor, given prior to exercise, completely blocks the post-exercise increase in muscle protein synthesis (Drummond et al. 2009).
We have included both men and women in several of our recent studies (Rasmussen et al. 2000, Dreyer et al. 2006, Dreyer et al. 2008, Fujita et al. 2009) examining the acute response of resistance exercise on muscle protein metabolism. However, those studies were not designed to test whether sex differences exist following an acute bout of resistance exercise. Little is known about sex-based differences in muscle protein synthesis, however, previous work has shown that basal rates of whole body protein synthesis (Volpi et al. 1998) and muscle protein synthesis are similar in healthy young men and women (Fujita et al. 2007, Smith et al. 2009) and that men and women have similar relative gains in muscle hypertrophy following resistance exercise training (Abe et al. 2000, Hubal et al. 2005, Kosek et al. 2006). Therefore, we hypothesized that the contraction-induced increase in post-exercise leg muscle protein synthesis and mTOR signalling would be similar in young men and women.
Materials and Methods
Subjects
We studied 17 young healthy subjects (9 males and 8 females) who were physically active but were not currently engaged in a resistance exercise training program. Physically active was defined as being normally active (i.e., not sedentary) and also not involved in aerobic/endurance training. All subjects gave informed written consent before participating in the study, which was approved by the Institutional Review Board of the University of Texas Medical Branch (which is in compliance with the Declaration of Helsinki). Screening of subjects was performed with clinical history, physical exam, and laboratory tests including complete blood count with differential, liver and kidney function tests, coagulation profile, fasting blood glucose and oral glucose tolerance test (OGTT), hepatitis B and C screening, HIV test, TSH, lipid profile, a pregnancy test for all females, urinalysis, drug screening, and ECG. All female subjects were not taking oral contraceptives and were studied during their follicular phase. Data from 7 male and 4 female subjects were included in a previous publication (Dreyer et al. 2006). The subjects’ physical characteristics are summarized in Table 1.
Table 1.
Subject Characteristics & Absolute and Relative Exercise Load
| Male | Female | P value | |
|---|---|---|---|
| N | 9 | 8 | |
| Age | 27.1 ± 2.1 | 26.0 ± 3.4 | 0.901 |
| Height (cm) | 175.9 ± 2.8 | 162.7 ± 2.8 | 0.005 |
| Body Weight (kg) | 77.9 ± 4.5 | 62.8 ± 2.7 | 0.014 |
| Body Mass Index (kg/m2) | 25.2 ± 1.4 | 23.8 ± 1.1 | 0.453 |
| Whole Body Lean Mass (kg) | 60.6 ± 3.1 | 43.0 ± 1.3 | 0.001 |
| Whole Body Fat Mass (kg) | 15.7 ± 2.6 | 17.7 ± 2.1 | 0.580 |
| % Body Fat | 19.4 ± 2.3 | 27.6 ± 2.2 | 0.020 |
| Leg Volume (L) | 11.4 ± 0.7 | 10.6 ± 0.6 | 0.400 |
| Single Leg Lean Mass (kg) | 10.4 ± 0.8 | 7.3 ± 0.3 | 0.002 |
| % of Total Leg Mass | 77 ± 3 | 63 ± 2 | |
| Single Leg Fat Mass (kg) | 2.7 ± 0.4 | 4.0 ± 0.4 | 0.050 |
| % of Total Leg Mass | 20 ± 2 | 34 ± 2 | |
| Total weight lifted (kg) | 6421 ± 535 | 5072 ± 350 | 0.053 |
| Total wt. lifted/Bilateral Leg Lean Mass | 303.4 ± 14.5 | 353.6 ± 18.9 | 0.053 |
Values are mean ± SE
Study design
Details of this study design have previously been published (Dreyer et al. 2006). Briefly, each subject’s bilateral lower extremity 1 repetition maximum (1RM) was determined on two separate occasions on a leg extension machine (Cybex-VR2, Medway, MA) which was located within the General Clinical Research Center’s (GCRC) Exercise Laboratory and used on the day of the study. In addition to the 1RM determination a Dual-energy X-ray absorptiometry (DEXA) scan (Hologic QDR 4500W, Bedford, MA) was performed to measure body composition and lean mass.
Each subject was admitted to the GCRC of the University of Texas Medical Branch (UTMB) the evening prior to the exercise study. All subjects were instructed to refrain from physical exercise for 24 hours prior to arriving and to maintain their regular diet prior to study participation. The subjects were all fed a standardized meal (12 kcal/kg of body weight; 60% carbohydrate, 20% fat, and 20% protein) prepared by the Bionutrition Division of the GCRC. Each subject was also offered a snack at 2200 hr and did not eat again until the end of the study the following day. The snack was provided at 2200 hr to avoid prolonged fasted during the study.
The morning of the study, polyethylene catheters were inserted into a forearm vein for tracer infusion, in the contralateral hand vein which was heated for arterialized blood sampling, and in the femoral artery and vein (retrograde placement) of the leg for blood sampling. The femoral lines were placed in the same leg from which muscle biopsies were obtained. The arterial catheter was also used for the infusion of indocyanine green (ICG, Akorn, Inc., Buffalo Grove, IL) to determine blood flow.
After drawing a background blood sample, a primed continuous infusion of L-[ring-2H5] phenylalanine(Cambridge Isotope Laboratories, Andover, MA) was begun (time = 0) and maintained at a constant rate until the end of the experiment. The priming dose for the labeled phenylalanine was 2 μmol· kg−1 and the infusion rates was 0.05 μmol · kg−1· min−1. All studies were begun between 0700 and 0800 hr.
There were four main periods of the study design: a baseline period (Baseline) which was the hour prior to exercise, an exercise period (Exercise), and then the first (1 hr Post) and second (2 hr Post) hours of post-exercise recovery. For each period, except during exercise, all subjects rested comfortably in the semi-recumbent position. The entirety of the study was conducted in the exercise room at the GCRC.
Marking the beginning of the baseline period (Baseline), and two hours after starting the tracer infusion, the 1st muscle biopsy was obtained from the lateral portion of the vastus lateralis of the leg with the biopsy site between 15 and 25 cm from the mid-patella. The biopsy was performed using a 5 mm Bergstrom biopsy needle, under sterile procedure and local anesthesia (1% lidocaine). Once harvested the muscle tissue was immediately blotted and frozen in liquid nitrogen (within seconds) and stored at −80°C until analysis. Immediately after the 1st biopsy, a continuous infusion of ICG was started in the femoral artery (0.5 mg/min) and maintained for 50 minutes. Ten minutes after ICG infusion was started, blood samples were drawn 4 times, at 10 minute intervals, from the femoral vein and the arterialized hand vein to measure ICG concentration. In addition to the blood obtained for the ICG measurement of blood flow, blood samples were also taken from the femoral artery and vein and from the arterialized hand vein to measure blood glucose, lactate, pH and phenylalanine concentrations. At the end of baseline, a 2nd biopsy was obtained; however, the biopsy needle was inclined at a different angle so that the second biopsy was taken approximately 5 cm apart from the first.
Following the 2nd biopsy, the subjects were seated in a Cybex leg extension machine to perform the exercise portion of the study (Exercise). After a brief warm up (22 kg × 10 reps), each subject performed 10 sets of 10 repetitions of bilateral leg extension exercise starting at 70% of 1RM. Each set was separated by 3 minutes, except during blood collection (performed following sets 3, 6, 8 and 10), which required additional time. During the course of the resistance exercise session the load was reduced as needed in order for each set of 10 to be completed. Ample verbal encouragement was also used to help ensure set completion. In general, subjects had difficulty with the last sets of exercise but all subjects did complete 10 sets of 10 repetitions. As during the baseline period, ICG was continually infused into the femoral artery during exercise in order to measure leg blood flow. Blood samples were again drawn for blood glucose, lactate, pH and phenylalanine concentrations. The 3rd muscle biopsy was immediately preceded by 10 repetitions at 70% of the 1RM and obtained with the subject seated in the Cybex leg extension machine (i.e., within seconds of completing the final muscle contraction) which marked the end of the exercise period. As with the 2nd biopsy, the needle was inserted into the same incision as the first two biopsies; however, the biopsy needle was inclined at a different angle so that the third biopsy was taken approximately 5 cm apart from the previous two biopsies.
During the 3rd period (1hr Post), ICG was again infused continuously (as during the 1st and 2nd periods) to measure leg blood flow and blood was drawn for the measurement of blood pH, glucose, lactate, and phenylalanine concentrations. Samples were obtained every 10 minutes (as during the 1st and 2nd periods). At the end of the first hour post-exercise, a 4th muscle biopsy was obtained through a new incision site approximately 5 cm proximal to the first incision. The 4th and final period was identical to the 3rd period but captured the second hour of post exercise recovery (2 hr Post). Blood samples were collected in the same manner as during the previous periods. At the end of that hour (2 hours following exercise), a final muscle biopsy was performed as described above from the second incision, however, the biopsy needle was again inclined at a different angle so that the muscle sample was obtained from tissue approximately 5 cm apart from the prior biopsy.
Femoral Blood flow, pH, Glucose and Lactate
Serum ICG concentration for the determination of leg blood flow was measured spectrophotometrically (Beckman Coulter, Fullerton, CA) at λ=805 nm (Jorfeldt et al. 1971). Blood pH was measured at the UTMB core laboratory using standard procedure. Plasma glucose and lactate concentration was measured using an automated glucose and lactate analyzer (YSI, Yellow Springs, OH) within minutes after drawing the sample.
Muscle Fractional Synthetic Rate
Vastus lateralis muscle tissue samples were ground, and intracellular free phenylalanine and muscle proteins were extracted as previously described (Wolfe 1992). Muscle intracellular free concentration and enrichment of phenylalanine was determined by gas chromatography-mass spectrometry (GCMS, 6890 Plus GC, 5973N MSD, 7683 autosampler, Agilent Technologies, Palo Alto, CA) using appropriate internal standards (Wolfe 1992). Mixed leg muscle protein-bound phenylalanine enrichment was analyzed by GCMS after protein hydrolysis and amino acid extraction (Wolfe 1992), using the external standard curve approach (Calder et al. 1992). We calculated the fractional synthetic rate of mixed muscle proteins (FSR) by measuring the incorporation rate of the phenylalanine tracer into the proteins (ΔEp/t) and using the precursor-product model to calculate the synthesis rate:
where ΔEp is the increment in protein-bound phenylalanine enrichment between two sequential biopsies, t is the time between the two sequential biopsies, and EM(1) + EM(2) are the phenylalanine enrichments in the free intracellular pool in the two sequential biopsies. Data are expressed as percent per hour.
SDS PAGE and Immunoblotting
Details to the immunoblotting procedures have been previously published (Dreyer et al. 2006). Aliquots from homogenates were loaded (equal amount of protein) per lane in duplicate and separated by SDS-PAGE. All proteins were run on 7.5% gels (BioRad, Hercules, CA) for 60 min at 150 V except for 4E-BP1 which was run on 15% gels for the same duration. A molecular weight ladder (BioRad, Precision Plus protein standard) and a rodent control, to compare across different blots, were also included on each gel. Following SDS-PAGE, proteins were transferred to polyvinylidene diflouride membranes (PVDF) (Hybond-P, Amersham Biosciences, Piscataway, NJ) at 50 V for 1 hr. We confirmed equal loading on each gel and that an equivalent amount of protein was transferred to the membrane by Coomassie and/or Ponceau S staining. Blots were also incubated with β-tubulin, which served as an internal loading control. Once transferred, PVDF membranes were placed in blocking buffer [5% nonfat dry milk (NFDM) in TBST (Tris-buffered saline and 0.1% Tween-20] for 1 hour. Following serial washes the membranes were incubated with primary antibody in 5% NFDM in TBST overnight at 4°C with constant agitation. The next morning, the blots were washed in TBST twice and incubated with secondary antibody for 1 hr in 5% NFDM in TBST at room temperature with constant agitation. After serial washes the blots were then incubated for 5 minutes with enhanced chemiluminescence reagent (ECL plus Western Blotting Detection System, Amersham Biosciences, Piscataway, NJ) to detect horseradish peroxidase activity. Images were obtained with a ChemiDoc XRS imaging system (BioRad, Hercules, CA). Once the appropriate image was captured, Densitometric analysis was performed using Quantity One 1-D analysis Software (Ver 4.5.2) (BioRad, Hercules, CA). Total protein was determined for each blot and did not change over the course of the experiment from baseline (data not shown). Therefore, data are presented as phosphorylation status relative to a standardized rodent control in arbitrary units.
Antibodies
The primary antibodies used were all purchased from Cell Signaling (Beverly, MA): phospho-mTOR (Ser2448; 1:1000); phospho-4E-BP1 (Thr37/46; 1:1000); phospho-Akt (Ser473; 1:1000); phospho-p70 S6K1 (S6K1) (Thr389; 1:500); phospho-eEF2 (Thr56; 1:1000); β-tubulin (1:50,000). Anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody was purchased from Amersham Bioscience (1:2000).
Statistical Analysis
All values are expressed as mean ± SE. All subjects were treated identically throughout the study. For subject characteristics and exercise load data an unpaired t-test was performed to determine differences between men and women. For muscle protein synthesis a paired t-test was performed on baseline FSR and compared to the average of the 2 hours of post-exercise recovery FSR. Differences in baseline and post-exercise FSR between groups were determined by an unpaired t-test. Comparisons were made using 2-way repeated measures ANOVA, the factors being group (Male, Female) and time (baseline, exercise, 1 hr post-exercise and 2 hr post-exercise) for the following variables: blood flow, blood glucose concentration, blood lactate and pH, blood and muscle phenylalanine concentrations and (baseline, 1 hr post-exercise and 2 hr post-exercise) for the signalling proteins. Post-hoc analyses were conducted using Bonferroni’s t-test for multiple comparisons versus baseline (Sigma Plot®; Systat Software Inc., San Jose, CA). Significance was set at P≤0.05.
Results
Subject Characteristics
Subject characteristics are provided in Table 1. Men were significantly taller, heavier and had more whole body lean mass and leg lean mass (P<0.05). Percent body fat and leg fat mass were greater in women (P<0.05).
Exercise Load
Exercise load data are provided in Table 1. Men demonstrated a trend (P=0.083) to have a lower 1RM per bilateral leg lean mass ratio compared to women. The actual load lifted as a % of their 1RM during the 10 sets of 10 repetitions was 67±0.02% for men and 66±0.01% for women. During bilateral leg extension resistance exercise there was a trend (P=0.053) for men to lift more total weight than women. However, the relative total weight lifted (total weight per bilateral leg lean mass) demonstrated a trend (P=0.053) to be higher in the women.
Femoral Blood flow, pH, Glucose and Lactate Concentrations
Leg blood flow was not different between groups at baseline (P>0.05; Table 2) and increased to a similar extent during exercise in both groups (P<0.05). Leg blood flow values returned to baseline values during post-exercise recovery with no differences between groups (P>0.05).
Table 2.
Blood metabolites and intracellular phenylalanine
| Baseline | Exercise | 1 hr Post | 2 hr Post | ||
|---|---|---|---|---|---|
| Male | Blood flow (ml • min−1 • 100mg of leg LM−1) | 4.40 ± 0.1 | 17.17 ± 2.1* | 5.90 ± 0.8 | 4.82 ± 0.5 |
| Glucose concentration (mg • dl−1) | |||||
| Femoral artery | 94 ± 2 | 107 ± 5*b | 96 ± 3 | 92 ± 3 | |
| Femoral vein | 93 ± 2 | 104 ± 5*b | 94 ± 3 | 88 ± 3 | |
| Lactate (mmol • L−1) | |||||
| Femoral artery | 0.75 ± 0.1 | 9.95 ± 0.9*b | 2.12 ± 0.3 | 0.86 ± 0.1 | |
| Femoral vein | 0.80 ± 0.1 | 12.10 ± 0.9*† | 2.31 ± 0.2* | 1.00 ± 0.1 | |
| Blood pH | |||||
| Femoral artery | 7.41 ± 0.01 | 7.31 ± 0.01* | 7.40 ± 0.01 | 7.41 ± 0.01 | |
| Femoral vein | 7.37 ± 0.01 | 7.17 ± 0.01*b | 7.36 ± 0.01 | 7.37 ± 0.01 | |
| Phenylalanine concentration (μmol • L−1) | |||||
| Femoral artery | 54 ± 2 | 56 ± 2 | 54 ± 2 | 54 ± 1 | |
| Femoral vein | 58 ± 2 | 57 ± 2 | 58 ± 2 | 57 ± 2 | |
| Muscle intracellular | 78 ± 5 | 86 ± 6* | 76 ± 5 | 64 ± 3 | |
| Female | Blood flow (ml • min−1 • 100mg of leg LM−1) | 5.10 ± 0.8 | 18.34 ± 3.5* | 7.50 ± 1.0 | 6.81 ± 1.0 |
| Glucose concentration (mg • dl−1) | |||||
| Femoral artery | 88 ± 2 | 98 ± 3* | 89 ± 2 | 87 ± 2 | |
| Femoral vein | 86 ± 2 | 95 ± 3* | 86 ± 3 | 83 ± 3 | |
| Lactate (mmol • L−1) | |||||
| Femoral artery | 0.64 ± 0.1 | 8.21 ± 0.5* | 1.94 ± 0.2 | 0.7 ± 0.1 | |
| Femoral vein | 0.68 ± 0.1 | 9.68 ± 0.6* | 2.21 ± 0.2a | 0.89 ± 0.1 | |
| Blood pH | |||||
| Femoral artery | 7.41 ± 0.01 | 7.32 ± 0.01* | 7.39 ± 0.01 | 7.40 ± 0.01 | |
| Femoral vein | 7.37 ± 0.01 | 7.20 ± 0.01* | 7.36 ± 0.01 | 7.36 ± 0.01 | |
| Phenylalanine concentration (μmol • L−1) | |||||
| Femoral artery | 54 ± 4 | 56 ± 4 | 54 ± 4 | 53 ± 4 | |
| Femoral vein | 59 ± 4 | 58 ± 5 | 58 ± 6 | 57 ± 4 | |
| Muscle intracellular | 73 ± 3 | 85 ± 3* | 68 ± 2 | 63 ± 4 | |
P<0.05 vs. Baseline;
P<0.05 vs. Female;
P<0.1 vs. Baseline;
P<0.1 vs. Female.
Femoral arterial glucose concentration was not different between groups at baseline (P>0.05; Table 2) and increased in both groups during exercise (P<0.05), however, men tended to have higher arterial glucose concentration during exercise than women (P=0.06). Femoral arterial glucose concentration returned to baseline during post-exercise recovery in both groups (P>0.05). Femoral vein glucose concentrations followed a similar pattern (Table 2).
Femoral arterial lactate concentration was not different between groups at baseline (P>0.05; Table 2) and increased during exercise in both groups (P<0.05) with men tending to have higher lactate concentrations than women (P=0.07). Femoral arterial lactate concentrations returned to baseline during post-exercise recovery in both groups (P>0.05). Femoral vein lactate concentrations were not different between groups at baseline (P>0.05; Table 2) and increased during exercise in both groups (P<0.05). However, men had significantly higher venous lactate concentrations during exercise than women (P<0.05). During the 1st hour of post-exercise recovery, femoral vein lactate concentration remained significantly elevated for men (P<0.05) and there was a trend for venous lactate to remain elevated for women (P=0.07). Femoral vein lactate concentration returned to baseline during the 2nd hour of post-exercise recovery in both groups (P>0.05).
Femoral arterial blood pH was not different between groups at baseline (P>0.05; Table 2) and decreased during exercise in both groups (P<0.05). Femoral arterial blood pH returned to baseline immediately following exercise in both groups. Femoral vein blood pH was also not different between groups at baseline (P>0.05; Table 2). During exercise, femoral vein pH decreased significantly from baseline in both groups (P<0.05). However, the decrease in pH tended (P=0.06) to be greater in men than women. Leg vein pH returned to baseline immediately following exercise in both groups (P>0.05).
Blood and Intracellular Phenylalanine Concentration
Femoral arterial and venous phenylalanine concentrations at baseline, during exercise, and for the two hours of post-exercise recovery were not different from baseline and were not different between groups (P>0.05; Table 2). Muscle intracellular phenylalanine concentrations were not different between groups at baseline (P>0.05) and increased (P<0.05) during resistance exercise, and then returned to baseline levels during the 2 hours of post-exercise recovery (P<0.05). Changes in muscle intracellular concentrations of phenylalanine were not different between men and women at any time point (P>0.05; Table 2)
Muscle Protein Synthesis
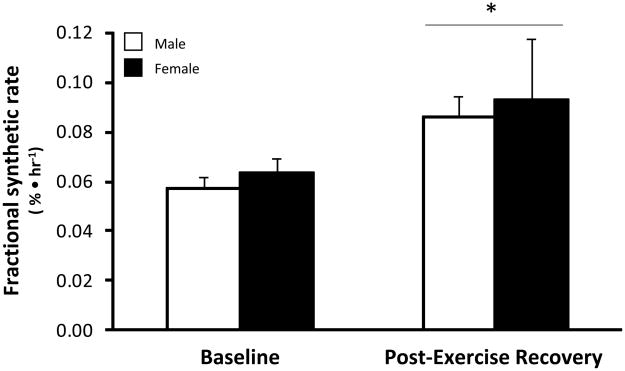
Mixed muscle (vastus lateralis) protein fractional synthetic rate (FSR) was not different between men and women at baseline (P>0.05). FSR increased during the two hours of post-exercise recovery in both groups (P<0.05; Figure 1). The increase in FSR during post-exercise recovery was not different between men and women (P>0.05).
Figure 1. Muscle protein synthesis (FSR).
Male (open bar) and female (closed bar) fractional synthesis rates (FSR) following an overnight fast (baseline) and the average FSR for the 2 hours following heavy resistance exercise (post-exercise recovery). Each subject performed 10 sets of 10 repetitions of bilateral knee extension in the seated position. Data are expressed as mean ± SE (N=9 men; N=8 women); *P<0.05 vs. Baseline.
Signalling
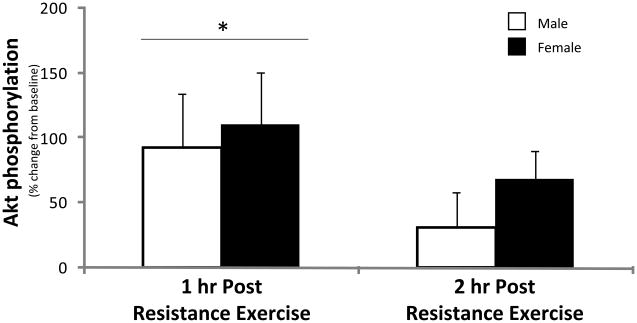
Akt phosphorylation at Ser473 was not different between men and women at baseline (P>0.05) and increased similarly in both men and women at 1 hour post-exercise (P<0.05, Figure 3). Akt phosphorylation returned to baseline at 2 hours post-exercise (P>0.05) in both groups.
Figure 3. Akt.
Phosphorylation status of Akt at Ser473 during the 1st and 2nd hour of post-exercise recovery. No difference was detected at baseline between men and women (data not shown). Data are expressed as percent change form baseline ± SE (N=9 men; N=8 women); *P<0.05 vs. Baseline, †P<0.05 vs. Male.
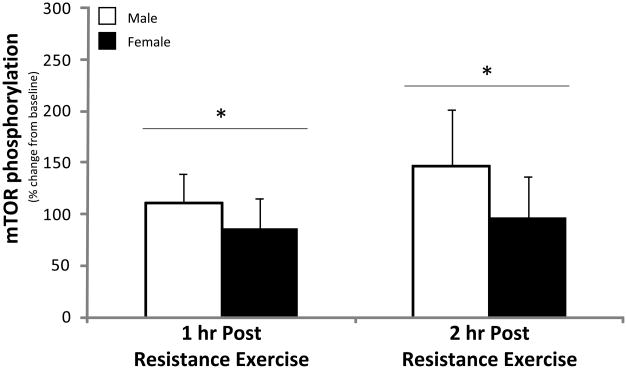
mTOR phosphorylation at Ser2448 was not different between men and women at baseline (P>0.05) and increased in both men and women during the 2 hours of post-exercise recovery (P<0.05; Figure 4). The change in mTOR phosphorylation was not different between men and women during post-exercise recovery (P>0.05).
Figure 4. mTOR.
Phosphorylation status of mTOR at Ser2448 during the 1st and 2nd hour of post-exercise recovery. No difference was detected at baseline between men and women (data not shown). Data are expressed as percent change form baseline ± SE (N=9 men; N=8 women); *P<0.05 vs. Baseline.
4E-BP1 phosphorylation at Thr37/46 was not different between men and women at baseline or during post-exercise recovery with exercise having no effect on post-exercise recovery 4E-BP1 phosphorylation (P>0.05; data not shown).
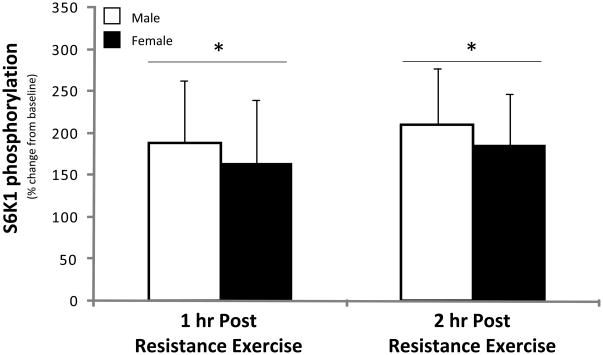
S6K1phosphorylation at Thr389 was not different between men and women at baseline (P>0.05) and increased significantly in both men and women during the 2 hours of post-exercise recovery (P<0.05; Figure 5). S6K1 phosphorylation was not different between groups during post-exercise recovery (P>0.05).
Figure 5. S6K1.
Phosphorylation status of S6K1 at Thr389 during the 1st and 2nd hour of post-exercise recovery. No difference was detected at baseline between men and women (data not shown). Data are expressed as percent change form baseline ± SE (N=6 men; N=6 women); *P<0.05 vs. Baseline.
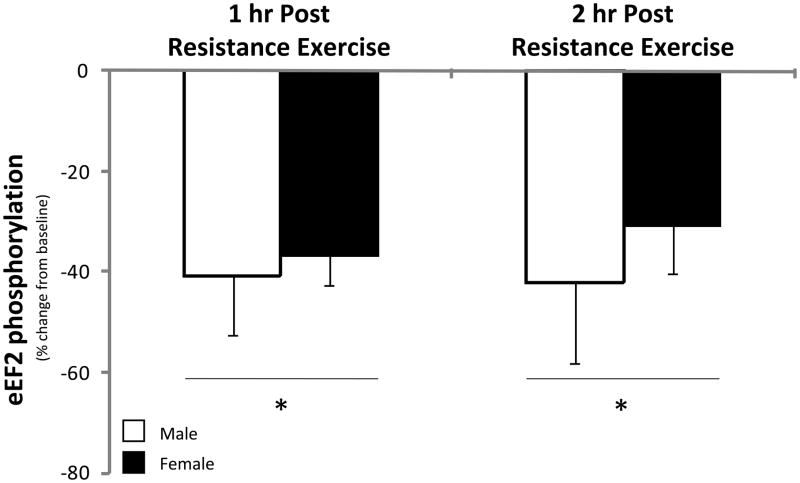
eEF2 phosphorylation at Thr56 was not different between men and women at baseline (P>0.05) and was significantly reduced in both men and women during the 2 hours of post-exercise recovery (P<0.05; Figure 6). eEF2 phosphorylation was not different between men and women during post-exercise recovery (P>0.05).
Figure 6. eEF2.
Phosphorylation status of eEF2 at Thr56 during the 1st and 2nd hour of post-exercise recovery. No difference was detected at baseline between men and women (data not shown). Data are expressed as percent change form baseline ± SE (N=9 men; N=7 women); *P<0.05 vs. Baseline.
Discussion
The primary and novel finding from our study is that the resistance exercise induced increase in muscle protein synthesis and mTOR signaling is independent of sex. Specifically, leg muscle protein synthesis was significantly increased by 52% in young men and by 47% in young women during the first 2 hours of post-exercise recovery (Figure 1). Our muscle protein synthesis data was also supported by similar changes detected in the phosphorylation status of several proteins associated with the mTOR signalling pathway in both men and women following exercise. For example, the phosphorylation status of mTOR and S6K1 increased (Figures 4 & 5) and eEF2 phosphorylation decreased during post-exercise recovery (Figure 6) to a similar extent in both men and women. Our data support the hypothesis that the early muscle protein synthesis response during post-exercise recovery is independent of sex and is not a contributor to human skeletal muscle sexual dimorphism as it pertains to the lower extremities.
The basal rate of mixed leg muscle protein synthesis was not different between young men and women in the current study. This is consistent with our previous publication in which we reported no sex differences in basal phenylalanine kinetics, muscle protein synthesis, muscle protein breakdown, mixed-muscle fractional synthetic rate or phenylalanine net balance across the leg (an indicator of muscle anabolism) in resting young subjects (Fujita et al. 2007). In our previous paper (Fujita et al. 2007) we studied 10 men and 8 women. Since we used nearly identical procedures and stable isotopic tracers in the basal period for both the current study and the previous study (Fujita et al. 2007) we combined all subjects to determine if basal differences existed for mixed muscle protein synthesis when data from a larger number of subjects are analyzed (i.e., N=19 men and N=16 women). Despite the increase in subject number we still find no differences in basal mixed muscle protein synthesis (men: 0.062±0.004%/hour vs. women: 0.064±0.004%/hour; P=0.79). However, a recent paper has identified that young women have higher rates of basal mixed muscle protein synthesis than men (Henderson et al. 2009). In that study the authors measured basal FSR in 30 young men and 32 young women and found a significantly higher (~14%) rate of protein synthesis in young women (Henderson et al. 2009). Unfortunately, the authors used blood as the precursor enrichment (rather than muscle tissue enrichment to calculate FSR) and present raw data values much lower than typically seen in the literature making comparisons somewhat difficult. Moreover, understanding why young women would have higher rates of muscle protein synthesis when men have much higher circulating concentrations of testosterone and larger overall muscle mass further complicates issues related to sex and basal muscle protein synthesis. The authors suggest that women must have higher muscle protein breakdown rates to offset the higher rate of synthesis (i.e., women have higher muscle protein turnover rates to replace damaged or old proteins) but as breakdown was not measured no definitive conclusion can be made in this regard. However, several published reports do not support these conclusions and indicate that sex does not affect basal rates of muscle protein synthesis (Balagopal et al. 1997, Jahn et al. 1999, Yarasheski et al. 1999, Parise et al. 2001, Short et al. 2004, Fujita et al. 2007, Smith et al. 2009), muscle protein breakdown (Fujita et al. 2007) or whole body protein breakdown (Volpi et al. 1998) in young men and women. In any event, the potential influence of sex on basal rates of leg muscle protein synthesis and breakdown in young subjects warrants further investigation.
The stimulation of muscle protein synthesis following the acute bout of high-intensity resistance exercise was also similar between young men and women (Figure 1) and would argue against a sexual dimorphism in response to an acute anabolic stimulus. However, our study focused on the early post-exercise recovery period and as such we cannot rule out the possibility that a longer time course post-exercise or resistance exercise training may result in the emergence of sexual dimorphism for rates of muscle protein synthesis or that nutrient status (our subjects were fasting throughout the study) may potentially influence the anabolic response. However, our acute data support previous reports showing that the relative increase in muscle hypertrophy is similar between young men and women following several weeks of resistance exercise training (Abe et al. 2000, Hubal et al. 2005, Kosek et al. 2006) and together appear to suggest that acute changes are potentially predictive of the end result. On the other hand, nutrition is also a potent stimulator of muscle protein synthesis, however, Smith et al. (2009) have recently provided evidence that the muscle protein synthesis response to nutrition (amino acids and insulin) is also similar in young men and women. Therefore, it appears that anabolic stimuli such as resistance exercise and nutrition produce similar increases in muscle protein synthesis in young men and women.
Our cell signalling data supports the muscle protein synthesis data (i.e., no sex differences at rest or during early post-exercise recovery) and is in agreement with our previous findings (Dreyer et al. 2006, Dreyer et al. 2008). Resistance exercise stimulates muscle protein synthesis during the post-exercise recovery period in association with increased phosphorylation of mTOR (Figure 4) and its downstream effector S6K1 (Figure 5) which is indicative of enhanced translation initiation. In addition, eEF2 becomes dephosphorylated (Figure 6) during post-exercise recovery, which is indicative of enhanced translation elongation. In contrast, we have consistently shown that resistance exercise does not alter 4E-BP1 phosphorylation during early post-exercise recovery (Dreyer et al. 2006, Dreyer et al. 2008) and in the current study we found similar results (data not shown). Our phosphorylation data is also consistent with what others have shown following resistance exercise although we report larger increases in mTOR phosphorylation and eEF2 dephosphorylation. This is most likely due to differences in when the biopsy was obtained in relation to exercise, exercise volume and intensity, and nutritional status (Eliasson et al. 2006, Deldique et al. 2008, Hulmi et al. 2009).
As mentioned previously, the activation of the mTOR signalling pathway is a primary mechanism by which resistance exercise stimulates translation initiation, elongation and the rate of muscle protein synthesis in humans since an mTOR inhibitor (rapamycin) given prior to exercise can significantly blunt the contraction-induced activation of key components of the mTOR signalling pathway and muscle protein synthesis (Drummond et al. 2009). The mechanism for how mTOR is activated following resistance exercise is not known, however, Akt is an upstream positive regulator of mTOR. In the current study, the phosphorylation of Akt transiently increases during the first hour of recovery and then returns to baseline levels at 2 hours post-exercise (Figure 3). Therefore, it is unlikely that Akt phosphorylation is the primary regulator of enhanced mTOR signalling following resistance exercise since the time courses for Akt phosphorylation and mTOR/S6K1 phosphorylation are different. In fact, a recent paper would support this notion as O’Neil et al. (2009) have shown that muscle contraction can stimulate mTOR signalling independent of Akt. The authors of that paper provide evidence for a mechanical mechanism which activates mTOR signalling and involves the release of phosphatidic acid, an activator of mTOR (O’Neil et al. 2009).
In the current study, the men had significantly greater leg lean mass and significantly less leg fat mass than women despite having similar total leg volume and whole body fat mass (Table 1). To account for differences in leg composition our protocol was standardized to help ensure that each subject completed 10 sets of 10 repetitions beginning at 70% of their 1RM. We found that the average load lifted, as a percentage of their 1RM, for the entire exercise session was 67% and 66% for men and women, respectively. The absolute total weight lifted showed a trend to be greater in the men vs. the women (Table 1), however, the total load lifted relative to total bilateral leg lean mass demonstrated a reverse trend with relative load being slightly higher in the women. In any event, these findings are consistent with the blood glucose, lactate and pH data shown in (Table 2). For example, femoral vein lactate concentrations increased to a large extent in both groups during exercise but more so in the young men. Blood pH decreased and glucose increased during exercise in both groups but tended to decrease more in the men. These slight differences in blood metabolites can most likely be attributed to the larger amount of working leg muscle mass in the men, however, it does appear that on a relative scale the two groups were working at a similar exercise intensity which is consistent with our leg muscle protein synthesis and mTOR signalling data.
The data presented in the current paper supports the conclusion that sexual dimorphism of muscle protein synthesis at rest or following an acute bout of resistance exercise does not exist in young human subjects. However, it should be acknowledged that two recent papers have found differences in muscle protein synthesis between older men and women (Smith et al. 2008, Henderson et al. 2009). In the paper by Smith et al. (2008), the authors report that baseline mixed leg muscle protein synthesis in older (65–80 years of age) men (N=13) and women (N=16) was 30% higher (P=0.02) in the older women. The authors also measured some signalling proteins and found no baseline differences in the phosphorylation status of Akt, S6K1, eIF4E, or 4E-BP1. However, they do report that the eEF2 phosphorylation was 40% less in the older women indicating that translation elongation was more activated (Smith et al. 2008). In addition, following feeding muscle protein synthesis was only increased in the older men and not in the older women (Smith et al. 2008). It is somewhat difficult to compare this study to the data in the present paper as it was conducted in older men and women who had BMI’s of 36±1 and 38±2, respectively (Smith et al. 2008). It is unknown whether obesity may be a confounding variable. The second paper by Henderson et al. (2009) expands the results of the former paper to healthy older and younger men and women. They also included data from 87 older men and 57 older women in the basal postprandial state after 3 days of a weight maintaining diet. They reported that whole body protein synthesis and muscle fractional synthesis rates were lower in the men than in women across the lifespan and this difference was independent of BMI (Henderson et al. 2009). Therefore, although it appears unlikely that sex differences exist for rates of muscle protein synthesis in younger subjects (Fujita et al. 2007, Smith et al. 2009), the data from these two papers suggest that sexual dimorphism in muscle protein synthesis may develop during aging. Future studies across the lifespan are needed to help clearly define potential age-associated differences in muscle protein metabolism and how factors such as sex, nutrient status (i.e., pre and post-prandial) and exercise potentially influence synthesis and breakdown rates and what signalling pathways are involved.
In summary our study shows that muscle protein synthesis and mTOR signalling is increased to a similar extent in young men and women during early recovery following a single bout of high-intensity resistance exercise. We conclude that the resistance exercise induced increase in early post-exercise anabolic signalling and leg muscle protein synthesis is independent of sex and appears to not be playing a role in the sexual dimorphism of young adult human skeletal muscle.
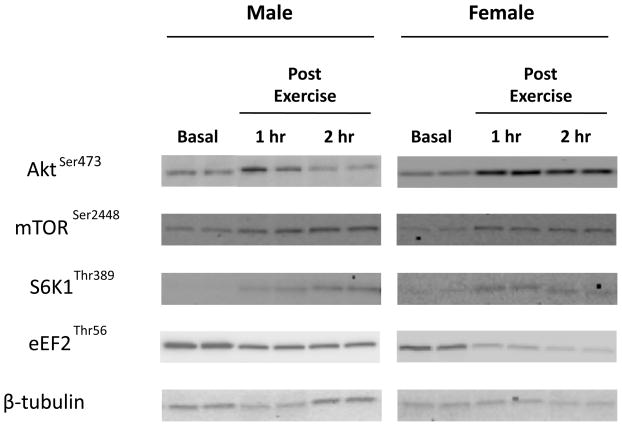
Figure 2. Representative images of immunoblots.
Male and female representative leg muscle biopsy samples at Baseline, and at the 1st and 2nd hour of post-exercise recovery.
Acknowledgments
We wish to thank the nurses and personnel of the General Clinical Research Center of the University of Texas Medical Branch for their help with the conduct of the clinical portion of this study. We would also like to thank Ming-Qian Zheng and Shelly Medina for technical assistance.
This study was supported by grant R01 AR049877 from the National Institute for Arthritis and Musculoskeletal and Skin Diseases, grant S10 RR16650 from the Shared Instrumentation Grant Program, and grant M01 RR00073 from the General Clinical Research Branch, National Center for Research Resources, National Institutes of Health, and National Institute on Aging grant P30 AG024832. Dr. Dreyer was supported by grant H133P040003 from the National Institute on Disability and Rehabilitation Research, Department of Education and E.L. Glynn was supported by a NIH predoctoral training grant 5T32HD007539.
Footnotes
Conflict of Interest
The authors have no conflict of interest.
References
- Abe T, DeHoyos DV, Pollock ML, Garzarella L. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur J Appl Physiol. 2000;81:174–180. doi: 10.1007/s004210050027. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol. 1997;273:790–800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:1172–1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol. 1995;268:E514–E520. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom. 1992;6:421–424. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- Deldique L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M. Decrease in Akt/PKB signalling in human skeletal muscle by resistance exercise. Eur J Appl Physiol. 2008;104:57–65. doi: 10.1007/s00421-008-0786-7. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392–E400. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587:1535–1546. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson J, Elfegoun T, Nilson J, Kohnke R, Ekblom B, Blomstrand E. Maximal lengthening contractions increase p70 S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. Am J Physiol Endocrinol Metab. 2006;291:E1197–E1205. doi: 10.1152/ajpendo.00141.2006. [DOI] [PubMed] [Google Scholar]
- Fujita S, Rasmussen BB, Bell JA, Cadenas JG, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Am J Physiol Endocrinol Metab. 2007;292:E77–E83. doi: 10.1152/ajpendo.00173.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Volpi E, Rasmussen BB. Essential amino acid and carbohydrate ingestion before resistance exercise does not enhance postexercise muscle protein synthesis. J Appl Physiol. 2009;106:1730–1739. doi: 10.1152/japplphysiol.90395.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson GC, Dhatariya K, Ford GC, Klaus KA, Basu R, Rizza RA, Jensen MD, Khosla S, O’Brien P, Nair KS. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J. 2009;23:631–41. doi: 10.1096/fj.08-117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Seip RL, Clarkson PM. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc. 2005;37:964–972. [PubMed] [Google Scholar]
- Hulmi JJ, Tannerstedt J, Selanne H, Kainulainen H, Kovanen V, Mero AA. Resistance exercise with whey protein ingestion affects mTOR signaling pathway and myostatin in men. J Appl Physiol. 2009;106:1720–1729. doi: 10.1152/japplphysiol.00087.2009. [DOI] [PubMed] [Google Scholar]
- Jahn LA, Barrett EJ, Genco ML, Wei L, Spraggins TA, Fryburg DA. Tissue composition affects measures of postabsorptive human skeletal muscle metabolism: comparison across genders. J Clin Endocrinol Metab. 1999;84:1007–1010. doi: 10.1210/jcem.84.3.5522. [DOI] [PubMed] [Google Scholar]
- Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol. 2006;101:531–544. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interisano SA, Yarasheski KE. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol. 1995;20:480–486. doi: 10.1139/h95-038. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil TK, Duffy LR, Frey JW, Hornberger TA. The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. J Physiol. 2009;587:3691–3701. doi: 10.1113/jphysiol.2009.173609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise G, Mihic S, MacLennan D, Yarasheski KE, Tarnopolsky MA. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J Appl Physiol. 2001;91:1041–1047. doi: 10.1152/jappl.2001.91.3.1041. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol. 1997;273:99–107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88:386–392. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286:E92–E101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- Smith GI, Atherton P, Villareal DT, Frimel TN, Rankin D, Rennie MJ, Mittendorfer B. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65–80 year old men and women. PLoS ONE. 2008;3:e1875. doi: 10.1371/journal.pone.0001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GI, Atherton PJ, Reeds DN, Mohammed BS, Jaffrey H, Rankin D, Rennie MJ, Mittendorfer B. No major sex differences in muscle protein synthesis rates in the postabsorptive state and during hyperinsulinemia-hyperaminoacidemia in middle-aged adults. J Appl Physiol. 2009 Jul 30; doi: 10.1152/japplphysiol.00348.2009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando AA. Testoserone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E820–E826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- Volpi E, Lucidi P, Bolli GB, Santeusanio F, De Feo P. Gender differences in basal protein kinetics in young adults. J Clin Endocrinol Metab. 1998;83:4363–4367. doi: 10.1210/jcem.83.12.5330. [DOI] [PubMed] [Google Scholar]
- Wolfe RR. Radioactive and stable isotope tracers in biomedicine: principles and practice of kinetic analysis. Wiley-Liss; New York: 1992. [Google Scholar]
- Yarasheski KE, Pak-Loduca J, Hasten DL, Obert KA, Brown MB, Sinacore DR. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men >/=76 yr old. Am J Physiol. 1999;277:118–125. doi: 10.1152/ajpendo.1999.277.1.E118. [DOI] [PubMed] [Google Scholar]