Abstract
The resin-based dental composites commonly used in restorations result in more plaque accumulation than other materials. Bacterial biofilm growth contributes to secondary caries and failure of resin-based dental composites. Methods to inhibit biofilm growth on dental composites have been sought for several decades. It is demonstrated here that zinc oxide nanoparticles (ZnO-NPs) blended at 10% (w/w) fraction into dental composites display antimicrobial activity and reduce growth of bacterial biofilms by roughly 80% for a single-species model dental biofilm. Antibacterial effectiveness of ZnO-NPs was assessed against Streptococcus sobrinus ATCC 27352 grown both planktonically and as biofilms on composites. Direct contact inhibition was observed by scanning electron microscopy and confocal laser scanning microscopy while biofilm formation was quantified by viable counts. An 80% reduction in bacterial counts was observed with 10% ZnO-NP-containing composites compared with their unmodified counterpart, indicating a statistically significant suppression of biofilm growth. Although, 20% of the bacterial population survived and could form a biofilm layer again, 10% ZnO-NP-containing composites maintained at least some inhibitory activity even after the third generation of biofilm growth. Microscopy demonstrated continuous biofilm formation for unmodified composites after one day growth, but only sparsely distributed biofilms formed on 10% ZnO-NP-containing composites. The minimum inhibitory concentration of ZnO-NPs suspended in S. sobrinus planktonic culture was 50 μg/ml. 10% ZnO-NP-containing composites qualitatively showed less biofilm after one day anaerobic growth of a three-species initial colonizer biofilm after when compared to unmodified composites, but did not significantly reduce growth after three days.
Keywords: antimicrobial, nanomaterials/nanophase, dental/craniofacial material, ZnO, biofilm
1. Introduction
Polymer resin/inorganic filler-based dental composites are widely used due to their aesthetic superiority as fillings, strong bonding ability as luting cements, and mechanical reinforcement in restorative procedures. However, in vitro1,2 and in vivo studies3,4 have reported that resin composites result in more plaque accumulation than either other restorative materials or dental hard tissues such as enamel.5 In vitro enamel demineralization via dental biofilm accumulation has been demonstrated around different resin restorations.6 Accumulation adjacent to the restoration margins may lead to secondary caries in vivo and finally shorten the serving life of composite restoration.7
One of several methods to inhibit biofilm formation on resin composites incorporates antimicrobial agents into their composition. The slow release of antibiotics and biocides added to dental composites can be used to inhibit or kill oral biofilms that contribute to caries and cause composite degradation.8 For example, composites with chlorhexidine successfully inhibited microbial growth, but were only effective over the short term due to this antibiotics high solubility.9 The addition of chlorhexidine and many other biocides in concentrations sufficient to inhibit biofilm growth via slow release leaves voids in dental composites which unfavorably alter their mechanical properties.8,10 Antimicrobial agents can also be immobilized into the resin fraction of the composite or can be used as filler particles, depending on the agents’ physical and chemical properties. Antimicrobial agents such as silver11 or silver ion-implanted fillers have also been used in composites without inducing catastrophic changes in mechanical properties. However, the antimicrobial effect of silver ions implanted into SiO2 lasts for only a short period12 and discoloration of the composite resin was found to be a significant problem.13 It has been argued that insoluble, white, or colorless fillers with long-lasting antimicrobial properties may be the preferred path to longer lasting dental composites.2
Metal oxides powders such as silica, zirconia, and alumina have long been used to increase the strength of restorative materials.14,15 Zinc oxide (ZnO) powders have also been incorporated as opaque reinforcing fillers into resin composites.16 However, ZnO powders also display antimicrobial properties; ZnO and several other oxide powders have been specifically shown to kill several oral microbes known to contribute to caries.17 Smaller particles of ZnO nanoparticles (NPs) have been found to be more effective than larger particles against both gram negative and gram positive bacteria.18,19 However, the antimicrobial behavior ZnO-NPs incorporated into dental composites has not been widely reported.
This work examines the inhibition of Streptococcus sobrinus biofilms and planktonic cultures by ZnO-NPs blended commercial composites. S. sobrinus is one of the microorganisms known to contribute to caries development and was chosen for this study due to its superior adherence to tooth surfaces compared to S. mutans.20 S. sobrinus isolates also display higher resistance to chlorhexidine antibiotic than S. mutans isolates,21 making S. sobrinus a more rigorous test of antimicrobial activity.
A three-species biofilm of Streptococcus oralis ATCC 10557, Streptococcus gordonii ATCC 10558, and Actinomyces naeslundii ATCC 19039 was also used here to evaluate antimicrobial activity of modified and unmodified composites. These three co-aggregating microbes are amongst the first colonizers on tooth surfaces,22 which has lead to their application as a model of multi-species dental biofilms.23
2. Materials and Methods
2.1. Inorganic antibacterial agents and selection of ZnO-NPs
Uncoated, 40 – 100 nm size zinc oxide nanoparticles (ZnO-NPs) of elongated shape and zincite crystal phase (hexagonal, NanoGard™, >99.0% purity, Lot L27Q023, AlfaAesar, Ward Hill, MA, USA) were chosen over two other types of ZnO-NPs for blending into composites, as described further below. 70 nm elongated ZnO-NPs of zincite crystal phase coated 2% by weight with either nonpolar or polar coatings (NanoTek™ C1 and C2, >99.0% purity, Lots C20R048 and G27R029, respectively, AlfaAesar) were also evaluated to determine the relative antimicrobial effectiveness of the coatings.
Two known antimicrobial materials were used as references: titanium dioxide nanoparticles (TiO2-NPs) having a nominal average size of 30 nm (NanoTek™ titanium dioxide powder, Batch T90511-03, Nanophase, Burr Ridge, IL, USA), and coarse silver nitrate powder (AgNO3 Crystal, Lot AD-8085-43, Fisher Scientific, Rochester, NY, USA).
2.2. Preparation of bacterial suspensions
Liquid culture of Streptococcus sobrinus ATCC 27352 was grown in 40 μg/mL Bacitracin (Bacillus licheniformis, Fluka, Buchs, Switzerland) containing brain heart infusion (BHI) broth (Difco, Detroit, MI) shaken at 300 rpm for two days, at 37°C. Bacterial turbidity was optically adjusted after centrifuging the liquid culture at 3180 g for 15 min.
2.3. Determining MIC and MBC for ZnO-NPs
Prior to the investigation of biofilm inhibition potential of experimental composites, the antimicrobial activity of ZnO-NPs was evaluated against liquid cultures of S. sobrinus ATCC 27352 bacteria. The minimal inhibitory concentration (MIC) of uncoated ZnO-NPs was evaluated using the broth dilution method. A wide concentration range of ZnO-NPs, between 1 – 1400 μg/mL, was examined with five triplicate experiments were completed at different time intervals for selected concentrations. Standard 15 mL Falcon tubes having 5 mL BHI media were each inoculated with 50 μL of adjusted bacterial culture (final ~25×105 CFU in 5 mL media). Tubes were shaken overnight at 300 rpm and 37°C.
2.4. Preparation of composites
Descriptions and abbreviated designations of the different groups of composites with and without ZnO-NPs as well as the control samples are given in Table 1. A universal microhybrid commercial composite (AElite™ All-Purpose Body, Lot 800007590, Bisco Inc., Schaumburg, IL, USA) was used as obtained and also blended with the inorganic antibacterial agents in the combinations described in Table 1. A commercial universal nanofilled composite (Filtek™ Supreme Plus Universal Restorative, Yellow Translucent Shade, Lot CY, 3M ESPE, St. Paul, MN, USA) was used as a reference to compare with the nanoparticle blended experimental composites. A composite mixer (SpeedMixer™, FlackTek Inc., Landrum, SC, USA) was used to blend the ZnO-NPs, TiO2-NPs, and AgNO3 powders into the composites.
Table 1.
Designations and descriptions dental composites examined along with experiments performed on each sample type and statistical significance of results
| Designation | Composite Description | Experiments Performed | Statistical Significance of Results |
|---|---|---|---|
| Ag control | Negative control with 1% (w/w) AgNO3 in microhybrid composite |
Used in all experiments except kinetic measurement of bacterial growth |
No bacterial growth or significantly less biofilm (p=0.003) when compared to other groups |
| 10% ZnO-NP | 10% uncoated ZnO-NPs blended into microhybrid |
Used in all experiments | Significant biofilm inhibition when compared to other groups except Ag control |
| 5% ZnO-NP | 5% uncoated ZnO-NPs in microhybrid |
Used in all experiments | Significant biofilm inhibition when compared to unmodified (p=0.025) |
| 1% ZnO-NP | 1% uncoated ZnO-NPs blended into microhybrid |
Used in all experiments | No inhibition in bacterial growth or in biofilm |
| 1% polar ZnO-NP | Polar ZnO-NPs blended at 1% into microhybrid |
Used in biofilm inhibition study | No inhibition in bacterial growth and biofilm, similar to that 1% ZnO-NPs (p>0.05) |
|
1% nonpolar
ZnO-NP |
Nonpolar coated ZnO- NPs blended at 1% into microhybrid |
Used in biofilm inhibition study | No biofilm inhibition. Instead, significantly increased biofilm growth |
| unmodified | Unmodified commercial microhybrid composite without any NPs or Ag |
Used in all experiments | Neither inhibition in bacterial growth nor in biofilm |
|
unmodified
nanofilled |
Unmodified commercial nanofilled composite without any NPs or Ag |
Used in main biofilm inhibition study and in preliminary studies comparing ZnO-NP to TiO2-NP incorporation |
Neither inhibition in bacterial growth nor in biofilm |
|
10% ZnO-NP-
nanofilled |
10% (w/w) uncoated ZnO-NPs blended into nanofilled |
Used in preliminary studies comparing ZnO-NPs to TiO2- NPs |
Significant biofilm inhibition when compared to nanofilled (p=0.045), and 10% TiO2- NP-nanofilled (p=0.021) |
|
10% TiO2-NP-
nanofilled |
10% (w/w) TiO2-NP blended into nanofilled |
Used in preliminary studies comparing ZnO-NPs to TiO2- NPs |
No significant biofilm inhibition when compared to unmodified nanofilled (p>0.05) |
2.5. Determining biofilm inhibition of composites and quantifying cell counts
The plaque inhibition potential of ZnO-NP-composites was evaluated by biofilm inhibition studies using a biofilm model previously demonstrated for testing prospective anti-plaque agents.24 Viable counts were recorded at the third day of biofilm growth. Composite discs (n=12, 100±3 mg) were visible light (470 nm) cured for 10 minutes (Triad™ 2000™ Visible Light Cure System, Dentsply, Milford, DE, USA), then polished. Composite discs were prepared between two glass slides in standard ring-shaped molds, enabling smooth surfaces for top and bottom areas of the discs. The composite discs were polished using a commercial system (superfine-grit discs, Sof-Lex disc system, 3M ESPE). Three day biofilms were generated on composite discs using 24-well plates. Each well was inoculated with 150 μL (~108 CFU/mL) adjusted bacterial inoculum. Biofilms were grown at 37°C and media (2 mL BHI containing 0.5% sucrose) were changed every 24 hours. At the end of the third day, each composite disc was rinsed with PBS, then vortexed in PBS with 3 mm glass beads. Next, 10 mL PBS was homogenized at 16,000 ± 400 rpm to disagglomerate the biofilm cells. Serial dilutions were made using 100 μL of the homogenized biofilm resuspension, then BHI agar plates were inoculated with the resuspension. After 48 hours, colony forming units (CFUs) were counted visually, scaled by dilution factors, then groups were statistically compared to each other. Two samples per group were used each time, and the experiment was repeated six times under identical conditions.
2.6. Biofilm inhibition on composite discs aged by previous biofilm growth
S. sobrinus biofilms were generated on the same discs for three cycles of three days of growth each. The same methodology described above was used to grow biofilms on composite discs, except that discs were separately sonicated in doubly-distilled water for 15 minutes between each cycle to remove loosely adsorbed proteins and biofilm matrix residues. Viable counts were recorded at the third day of biofilm growth for each cycle, two samples per group were used, and the experiment was repeated once using a second set of fresh composite discs under identical conditions.
2.7. Three-species biofilm inhibition on composite discs
Inoculum for the cultivation of three-species biofilms was prepared based on the procedure reported by Stewart and coworkers.23 Separate liquid cultures of Streptococcus oralis ATCC 10557, Streptococcus gordonii ATCC 10558, and Actinomyces naeslundii ATCC 19039 were grown from frozen stocks of bacterial cells in BHI for 48 hours at 37°C under anaerobic conditions (GasPak EZ anaerobe pouch system, Becton Dickinson, Sparks, MA, USA). The absorbance at 600 nm of all bacterial suspensions was adjusted to 0.05 prior to preparing inoculum, and 150 μL from each culture was used to inoculate the composite disc in each well of 24-well plates. Next, wells were filled with 1.55 mL of TSB containing 0.5% sucrose medium, and three-species biofilms were allowed to grow in a stationary fashion for three days at 37°C, under anaerobic conditions. TSB containing 0.5% sucrose medium was replaced every 24 hours. Viable counts were recorded at the third day of biofilm growth as described before. However, BHI agar plates were incubated anaerobically.
2.8. Direct contact inhibition of composites evaluated by scanning electron microscopy and confocal laser scanning microscopy
Bacterial attachment and early biofilm formation on ZnO-NP-composites were demonstrated by direct contact inhibition studies. Composite discs were inoculated with 10 μL (~107 CFU) bacterial solution without additional nutrients, incubated for 1 h at 37 °C, then wells of the 24-well plate were filled with 750 μL BHI broth without sucrose and allowed to culture for an additional 23 h. One hour fixation was then performed in 2.5% glutaraldehyde and 0.1 M sodium cacodylate pH 7.4 buffer (Electron Microscopy Sciences, Lot 080116, PA, USA). Dehydration was completed in a gradient ethanol series. Samples were visualized with scanning electron microscopy (S-3000N, Hitachi Sciences Systems, Hitachinaka, Japan) at 10 - 13 keV beam energies.
Samples for confocal laser scanning microscopy (CLSM) were prepared as described above without an additional dehydration step. Instead, staining was performed using blue-fluorescent nucleic acid stain DAPI (4′,6-diamidino-2-phenylindole dihydrochloride, Molecular Probes, Invitrogen, Carlsbad, CA, USA) and samples were visualized by CLSM (LSM 510 META, Carl Zeiss MicroImaging, Jena, Germany) connected to a long pass, 380 nm cut-off filter.
2.9. Preparation of composite-lined 96-well plates and kinetic measurement of bacterial growth
Bacteriostatic activity of ZnO-NP-composites against planktonic bacterial culture was evaluated by kinetic measurement of bacterial growth in composite containing wells, which recorded per hour absorbance measurements of liquid culture that came into contact with the composites. Side walls of the two 96-well flat bottom plates (96 well suspension culture plate, Greiner Bio-One North America Inc., Monroe, NC, USA) were coated evenly with composite resin (30±3 mg), and cured for 10 min. Each well of the plate containing 330 μL BHI was inoculated with 20 μL (~107 CFU/mL) adjusted bacterial culture. The inoculated plate was placed over a shaker to induce initial bacterial growth, and shaken at 150 rpm for 6 h at 37°C. Next, the plate was placed into a plate reader (Victor3 V 1420 Multilabel Counter, Perkin Elmer, Oak Brook, IL, USA) set to 37°C. Absorbance measurements were taken at 490 nm at one hour intervals for 12 h, after shaking of the plate for 15 s.
2.10. Disc diffusion test
Antibacterial activity of ZnO-NP-composites via solubility and diffusion of Zn2+ from ZnO-NPs was tested using disc diffusion tests. 7 mm diam. composite discs (n=4) were placed on BHI agar plates which were inoculated with a 200 μL bacterial solution (~200 CFU-taken from 104 serial dilutions of adjusted bacterial culture (~107 CFU/mL). Plates were incubated for 48 hours and inhibition zones around composite discs were detected optically.
2.11. Antibacterial properties of eluted components
Antibacterial activity of eluted components from ZnO-NP-composites was also evaluated. Identical composite discs (n=3 for each group, 100±3 mg) were placed into a tube containing 5 mL BHI media. At three, 15, and 30 days, BHI media were aspirated and put into 15 mL plastic tubes. Tubes were inoculated with 50 μL bacterial culture (final ~25×105 CFU in 5 mL media) and shaken for 24 hours at 300 rpm at 37°C.
2.12. Statistical evaluation of data
Statistical evaluation was performed through a multistep process. Normalization of the data was evaluated using Q-Q plots. If the data was not distributed normally, as in the cases of biofilm inhibition potential of dental composites and kinetic measurement of bacterial growth, then nonparametric tests were used. Comparison of independent, but abnormally distributed, groups was evaluated by the Kruskal-Wallis test. If the result was smaller than 0.05, then the Mann-Whitney test was used for paired comparisons. For dependent but abnormally distributed cases, the Wilcoxon Signed Ranks test for paired comparisons was used to compare the groups. Antibacterial variance in eluants was evaluated in this manner at three sequential time intervals.
3. Results
3.1. MIC and MBC of uncoated ZnO-NPs
The antibacterial effect of ZnO-NPs against S. sobrinus planktonic growth was verified by two established measures: minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). The broth dilution method determined the MIC of uncoated ZnO-NPs to be 50 μg/mL, sufficient to justify evaluation of their incorporation into composites.
However, bactericidal activity given by MBC is more relevant than bacteriostatic activity given by MIC for evaluation of antimicrobial activity in dental composites. Therefore, the MBC was also determined as the minimum concentration of ZnO-NPs that resulted in no observable bacterial growth on the agar plate. MBC was found for ZnO-NPs when a 100 μL aliquot from the tubes appeared without turbidity, for concentrations above the MIC value (50 μg/mL) spread over BHI agar plates. No colonies were seen at a MBC of 150 μg/mL of ZnO-NPs.
3.2. Biofilm inhibition potential of composites
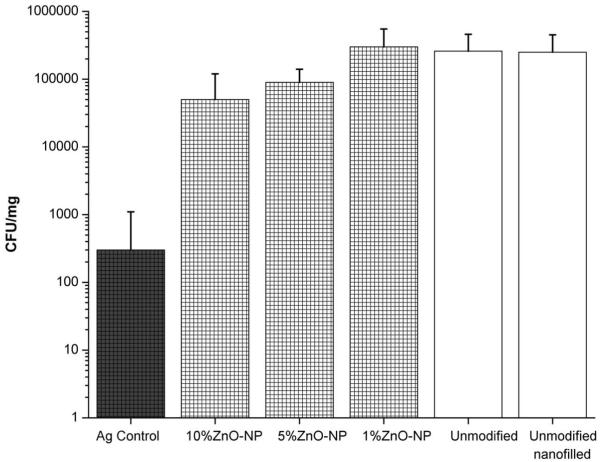
Mature biofilms were cultured on commercial composites with and without added ZnO-NPs, then viable cell counts were recorded for these biofilms. Counts of viable bacteria after three days S. sobrinus biofilm growth are shown in Figure 1 for the following groups of composites: three uncoated ZnO-NP-containing composites at NP concentrations of 1%, 5%, and 10% (by weight) as well three control composites without ZnO-NPs (unmodified, unmodified nanofilled, and Ag control). Table 1 lists abbreviated designations for the composites, full descriptions of the composites, experiments performed with each composite, and statistical significance of the results obtained. Incorporation of 10% ZnO-NPs reduced biofilm growth by approximately 80% (p=0.000) compared with either their unmodified counterpart or with unmodified nanofilled composites. A smaller, but significant biofilm inhibition in composites with 5% ZnO-NPs was observed compared to their unmodified counterpart (p=0.025). The positive Ag control composite displays a very large ~103 reduction in biofilm growth compared with the samples without ZnO-NPs (p<0.003).
Figure 1.
Viable counts of three day S. sobrinus biofilms on the following composites: Ag control, 10% ZnO-NP, 5% ZnO-NP, 1% ZnO-NP, unmodified, and unmodified nanofilled. See Table 1 for full description of composites.
A preliminary biofilm inhibition study using only nanofilled composites with 10% ZnO-NPs found a significant reduction in biofilm growth compared with its unmodified counterpart (p=0.045), as shown in Figure 2. The biofilm inhibition by 10% ZnO-NP composites was significant compared to 10% TiO2-NP-nanofilled composites (p=0.021). These experiments were performed under standard laboratory fluorescent lighting.
Figure 2.
Viable counts of three day S. sobrinus biofilms on the following composites: unmodified nanofilled, 10% ZnO-NP-nanofilled, and 10% TiO2-NP-nanofilled.
1% polar ZnO-NP composites were not statistically different from either unmodified or unmodified nanofilled composites (p>0.05), as shown in Figure 1. However, 1% nonpolar ZnO-NP composites had statistically larger CFU counts than unmodified composites (p<0.05, data not shown), but 1% polar ZnO-NPs appeared equally effective as uncoated ZnO-NPs when incorporated into composites. However, polar ZnO-NPs were not studied further since the coating agent was not specified. All subsequent experiments were done with uncoated ZnO-NPs.
3.3. Biofilm inhibition on composite discs aged by previous biofilm growth
Tests were also run to determine whether the composites maintained antimicrobial properties after several cycles of biofilm growth, evaluated by scrapping off biofilms and reusing the composites for new biofilm growth. While there was no statistically significant difference between the first and second cycle of growth on 10% ZnO-NP discs (p=0.127), the third cycle demonstrated significantly larger biofilm growth when compared to the second cycle (p=0.015), as shown in Figure 3. However, after the third cycle of biofilm growth, the viable cell counts on 10% ZnO-NP remained lower than observed for their unmodified counterparts (p=0.04). Biofilm growth in both 10% ZnO-NP and unmodified groups were significantly greater than that on the Ag control group (p=0.000).
Figure 3.
Viable cell counts after the third day of S. sobrinus biofilm growth on composite discs after the first, second, and third cycle of subsequent biofilm growth on the same discs. Discs were cleaned between growth cycle to remove loosely adsorbed species (see text).
3.4. Three-species biofilm inhibition potential of composites
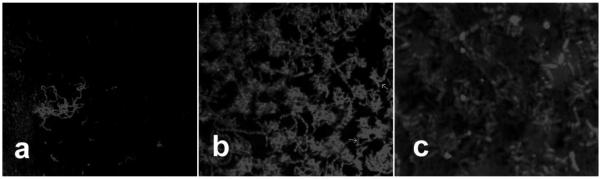
A three-species biofilm of initial colonizer species Streptococcus oralis, Streptococcus gordonii, and Actinomyces naeslundii were cultured anaerobically on unmodified and 10% ZnO-NP composites. Co-aggregation of multiple species after one day of biofilm growth is shown in Figure 4 by confocal laser scanning microscopy (CLSM) after visualization with DAPI staining, qualitatively demonstrating lower biofilm growth. After three days of anaerobic growth, viable cell counts were recorded and are shown in Figure 5. Incorporation of 10% ZnO-NPs did not significantly reduce biofilm anaerobic growth (p=0.117) when compared with the unmodified composite after three days. There was no three-species biofilm growth on the Ag control group (not shown). Growth of three-species biofilms was reduced to only ~10% of its anaerobic value under aerobic conditions on both unmodified and 10% ZnO-NP composite discs, with no significant difference between the two. Only streptococcal viable counts were performed for anaerobic and aerobic conditions, since viable counts on Actinomyces selective agar or BHI agar did not demonstrate CFUs specific to Actinomyces naeslundii. However, A. naeslundii was seen after one day of biofilm growth (as shown in Figure 4b).
Figure 4.
Visualization by confocal laser scanning microscopy of DAPI stained three-species biofilm of Streptococcus oralis, Streptococcus gordonii, and Actinomyces naeslundii cultured anaerobically. Biofilm attachment after one day on: (a) 10% ZnO-NP and (b) unmodified composite, (c) magnified view of biofilm on unmodified composite (2400× magnification).
Figure 5.
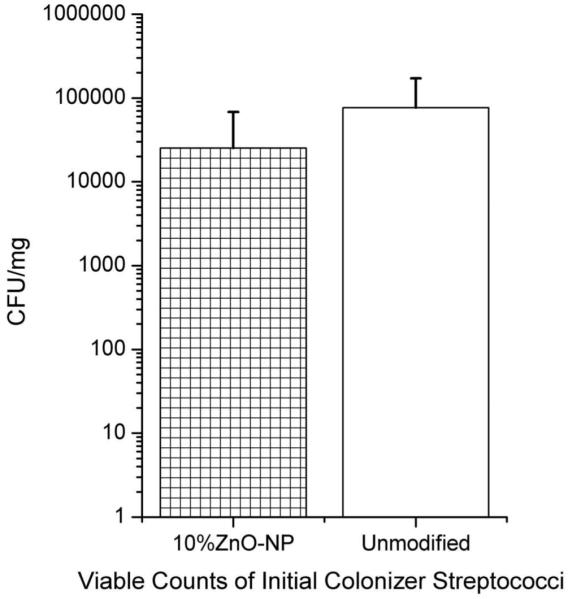
Counts of viable streptococci after three days of anaerobic three-species biofilm growth. No attempts were made to distinguish two different species of streptococci: S. gordonii and S. oralis counts were plotted together.
3.5. Biofilm inhibition by microscopy
Cell adherence and growth patterns of biofilms were observed on experimental composites. S. sobrinus attachment after one day of growth was qualitatively higher on unmodified compared to 10% ZnO-NP-composites, as shown in Figure 6 by SEM. Higher biofilm coverage is also shown on the unmodified composite after one day, as shown in Figure 7 by CLSM. Both SEM and CLSM of the 10% ZnO-NP-composite showed sparsely distributed microbes and less dense biofilm formation compared with the unmodified composites, which allowed formation of a continuous biofilm layer. Neither SEM nor CLSM showed any cell attachment with Ag control composites after one day (data not shown).
Figure 6.
S. sobrinus biofilm attachment after one day on: (a) 10% ZnO-NP and (b) unmodified composite recorded by scanning electron microscopy (5000× magnification).
Figure 7.
Visualization of DAPI stained S. sobrinus biofilm by confocal laser scanning microscopy. Biofilm attachment after one day on: (a) 10% ZnO-NP and (b) unmodified composite, (c) low magnification of biofilm on unmodified composite (120× magnification).
3.6. Kinetic measurement of bacterial growth
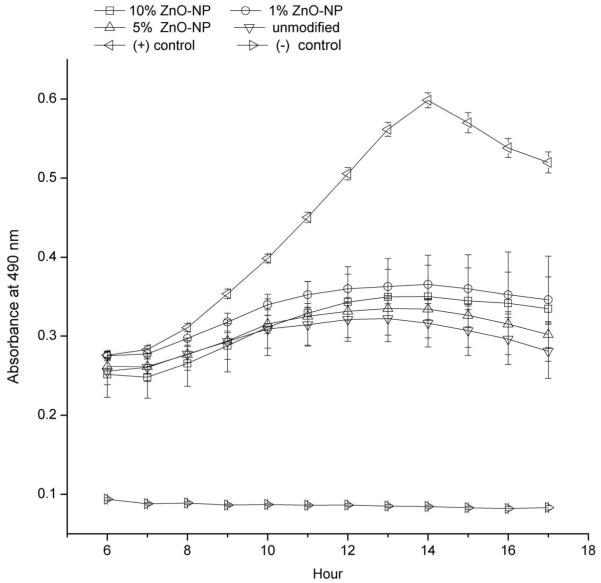
Inhibition of planktonic growth of S. sobrinus by contact with ZnO-NP-composites was evaluated by the kinetic measurement test, where composites were held in contact with planktonic cells. There was no statistically significant difference between the composite groups in composite-lined plates at the 14th hour (shown in Figure 8). However, all composite groups were significantly different than the positive and negative control groups (p=0.000). The Ag control composites gave false absorbance readings and were excluded. The negative control was empty wells with only BHI media while the positive control was empty wells with both media and bacteria.
Figure 8.
Kinetic measurement of bacterial growth in 96-well plates lined with the following composites recorded by optical absorbance at 490 nm: 10% ZnO-NP, 5% ZnO-NP, 1% ZnO-NP, and unmodified. Empty wells with media either with cells as positive control (+) and without cells as negative (−) control were also examined.
3.7. Disc diffusion test
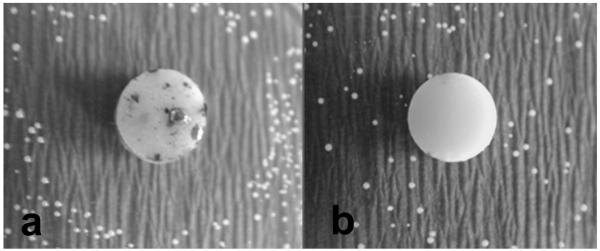
The antibacterial effect by dissolution and/or diffusion of Zn2+ from the ZnO-NPs blended in experimental composites was investigated by the disk diffusion test. Only Ag control group showed an inhibition zone of ~5 ± 0.5 mm, as shown in Figure 9. There were no inhibition zones around the other composite samples, including those containing ZnO-NPs. Furthermore, colonies were observed at the bottom of all composite discs except for the Ag control.
Figure 9.
Digital photograph of disk diffusion test of (a) Ag control and (b) 10% ZnO-NP composites growth on BHI agar for 48 hours.
3.8. Antibacterial properties of eluted components
Any residual antibacterial effect of eluted Zn2+ from ZnO-NP-composites was evaluated by growing cells in the eluted media. The Kruskal-Wallis test showed no difference in bacterial growth among groups at the third (p=0.193), 15th (p=0.280), and 30th day (p=0.409) of culture. The absence of bacterial contamination after all growth periods was verified by optical microscopy. There were also no growth differences between the media from the corresponding groups for the third, 15th, and 30th days of growth (p>0.05, Wilcoxon Signed Ranks test, data not shown).
4. Discussion
Composites blended with ZnO-NPs were found to moderately inhibit S. sobrinus biofilm formation for periods of three days compared with unmodified composites. Specifically, a single three day cycle of biofilm growth on 10% ZnO-NP-composites was only ~20% of that on unmodified, unmodified nanofilled, or 10% TiO2-NP-composites. There was no statistically significant difference between biofilm formation on commercial nanofilled and universal microhybrid composites. 10% ZnO-NP composites used for multiple cycles of biofilm growth were not as effective as their first time use, but biofilm growth on them was still significantly less than commercial composites.
10% ZnO-NP composites did not significantly inhibit the three-species biofilm of initial colonizer bacteria grown anaerobically for three days, although qualitative reduction was observed by microscopy after one day of growth. However, growth of the three-species biofilms under aerobic conditions greatly reduced biofilm growth on both unmodified and 10% ZnO-NP composite discs. Given the possible role of atmospheric oxygen in the antimicrobial mechanism of ZnO-NPs (see below),25 a different multi-species biofilm that thrives in an aerobic environment would be a better test.
ZnO-NPs were effective against planktonic cultures of S. sobrinus at concentrations as low as 50 μg/mL. Higher minimum inhibitory concentrations (MIC) for ZnO-NPs were determined against different microorganisms, but these studies used different types of ZnO-NPs. The MIC was 80 μg/mL against S. aureus for 8 nm ZnO-NPs; 1.2 mg/mL against S. aureus for 50 – 70 nm ZnO-NPs;19 and 500 μg/mL against S. mutans for 125 nm ZnO-NPs.26 The MBC for 13 nm ZnO-NPs was 81 μg/mL against S. aureus,27 but 150 μg/mL for the 40 – 100 nm ZnO-NPs used here against S. sobrinus. Apart from differences in the microorganisms studied, these results confirm that smaller ZnO-NPs are generally more effective against microorganisms than larger particles, as it appears antibacterial activity depends on the total contact surface area.18,19,28
Nanofilled universal composites have been widely used for direct anterior restorations and provide desired polishing results29 with a good clinical retention time.30 However, nanofilled commercial composites containing non-aggregated, 75 nm silica nanofillers showed the same level of bacterial growth compared with unmodified microhybrid composites, even though the former had a high, 72.5% fraction of nanoparticles. Furthermore, prior results that found SiO2-NPs displayed five to ten times lower antibacterial effect than ZnO-NPs, depending on the microorganism type.18 Therefore, fillers containing ZnO-NPs in addition to silica nanofillers could be an advantageous approach for plaque inhibition.
Bacterial growth in the presence of composites demonstrated that even 10% ZnO-NPs only moderately inhibited biofilm growth while 1% AgNO3 containing composite discs showed inhibition of ~103. Furthermore, the Ag-composite disks displayed a 5 ± 0.5 mm inhibition zone not observed for any of the other composites, regardless of ZnO-NPs. However, silver containing compounds are poorly suited to aesthetic dental applications as they discolor the composite.13
It is worth considering the relative efficacy of ZnO-NPs in composites compared with other antimicrobial strategies. 10% ZnO-NP-composites demonstrated less biofilm formation when compared to unmodified commercial composites. Previous studies evaluated the amount of plaque accumulation and found a 50% decrease in the turbidity of resuspended biofilms grown on composites containing an antimicrobial organic monomer.31 However, turbidity measurements are limited as they count both dead and vital bacteria present together in a biofilm layer.24 The present study evaluates the biofilm inhibition effect of composites by ranking only the number of the live bacterial cells at the end of the third day. A previous study using pooled human saliva culture found that four day old biofilms on 9% chlorhexidine containing composites were revived, and viable counts were determined.9 After three days of biofilm growth on 10% ZnO-NP-composites, roughly similar biofilm inhibition was observed here (Figure 1). It therefore appears that 10% ZnO-NP-composites are as effective as 9% chlorhexidine containing composites. However, ZnO-NP composites should not lose their antibacterial effect over time due to lower release rates or suffer from poorer mechanical properties by the formation of voids after leaching, both problems with chlorhexidine and other traditional antibiotics.8 ZnO-NPs are insoluble and can be used as filler particles either in additional to or instead of nanosilica fillers currently in use to reinforce the composite. However, ZnO-NPs are not as antimicrobial as other strategies currently under examination, such as the cationic-polymeric nanoparticles in composites that exhibited no turbidity in kinetic bacterial growth tests with S. mutans.32
One mechanism proposed to explain the antimicrobial properties of ZnO-NPs is that they generate active oxygen species such as H2O2 which inhibit growth of planktonic microbes. The antimicrobial properties of TiO2 and some other metal oxide nanoparticles in the presence of ultraviolet light have been attributed to their production of active oxygen species.28 TiO2-NPs are apparently unable to inhibit bacterial growth significantly in the absence of light. Like TiO2-NPs, ZnO-NPs can be photocatalytic under ultraviolet light leading to the production of antimicrobial active oxygen species.33
However, none of the ZnO-NPs results on S. sobrinus reported above were recorded under exposure to any light activation beyond ambient fluorescent laboratory lighting conditions and the effects of illumination were not otherwise examined here. Furthermore, antimicrobial activity of ZnO-NPs has been previously observed under dark conditions.33,34 An alternate mechanism for the antimicrobial activity of ZnO-NPs (even in the dark) has been proposed in which halogens adsorbed on the nanoparticle surfaces behave as oxidizers, imparting bactericidal activity.25 Such a mechanism would be enhanced in an aerobic environment.
Another potential antimicrobial mechanism of ZnO-NP occurs via the leaching of Zn2+ into the growth media. Toxicological mechanisms of zinc ions play an important role in biofilm inhibition by inhibiting the active transport and metabolism of sugars35 as well as disrupting enzyme systems of dental biofilms by displacing magnesium ions essential for enzymatic activity of the plaque.36 Zinc can also reduce acid production by Mutans streptococci biofilms, which includes both S. mutans and S. sobrinus, due to its ability to inhibit glucosyltransferase activity, thus impeding decalcification in vitro37,38 and in vivo.39 Zinc ions were found to inhibit growth at a minimum 8 mg/mL concentration against S. sobrinus.40 Zinc sulfate (3000 μ/mL) was used to inhibit the biofilm of K. pneumoniae, and resulted in a 90% decrease in viable counts.41 When compared to high concentrations of regular zinc powders, use of the ZnO-NPs at relatively low concentrations (150 μ/mL) caused a 99.9% decrease in viable counts of planktonic bacteria. However, Zn2+ is required for the growth of some biofilms, as demonstrated by the prevention of S. epidermidis and methicillin-resistant S. aureus biofilm formation by Zn2+ removal.42 The potential of zinc to assist the growth of other colonizer bacteria on the tooth argues for further studies with other microbes.
There were no inhibition zones around samples containing ZnO-NPs. Bacterial inhibition zones can only be created by diffusion of antimicrobial material and only the Ag control composites demonstrated inhibition zones due to the solubility of Ag+. The insolubility of the ZnO-NPs prevented diffusion of a sufficient amount of Zn2+ to the surrounding environment to impart an observable antibiotic effect, rendering the NPs ineffective for non-contact biofilm inhibition. Furthermore, media containing components eluted from the composite resins did not exhibit any antibacterial behavior at periods up to one month, implying that there was insufficient release of Zn2+ from ZnO-NPs to activate this mechanism.
Acknowledgements
We thank Gerald L. Gasper for assistance with the biofilm studies and Peter J. Koin for assistance with the dental composites. We also thank Bisco Inc. and 3M ESPE for providing commercial composites and Bisco Inc. for permitting use of their facilities to prepare the composite materials. This work was supported by the National Institute of Dental and Craniofacial Research via grant DE-007979. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Dental and Craniofacial Research or the National Institutes of Health.
References
- 1.Zalkind MM, Keisar O, Ever-Hadani P, Grinberg R, Sela MN. Accumulation of Streptococcus mutans on light-cured composites and amalgam: an in vitro study. J Esthet Dent. 1998;10:187–190. doi: 10.1111/j.1708-8240.1998.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 2.Beyth N, Domb AJ, Weiss E. An in vitro quantitative antibacterial analysis of amalgam and composite resins. J Dent. 2007;35:201–206. doi: 10.1016/j.jdent.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Günyakti N, Gür G, Misirligil A. In vivo adhesion of Streptococcus mutans on amalgam and composite restorative materials. Ankara Univ Hekim Fak Derg. 1990;17:83–86. [PubMed] [Google Scholar]
- 4.Auschill TM, Arweiler NB, Brecx M, Reich E, Sculean A, Netuschil L. The effect of dental restorative materials on dental biofilm. Eur J Oral Sci. 2002;110:48–53. doi: 10.1046/j.0909-8836.2001.101160.x. [DOI] [PubMed] [Google Scholar]
- 5.Hahn R, Weiger R, Netuschil L, Brüch M. Microbial accumulation and vitality on different restorative materials. Dent Mater. 1993;9:312–316. doi: 10.1016/0109-5641(93)90049-v. [DOI] [PubMed] [Google Scholar]
- 6.Sousa RP, Zanin IC, Lima JP, Vasconcelos SM, Melo MA, Beltrão HC, Rodrigues LK. In situ effects of restorative materials on dental biofilm and enamel demineralisation. J Dent. 2009;37:44–51. doi: 10.1016/j.jdent.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Burke FJ, Crisp RJ, Bell TJ, Healy A, Mark B, McBirnie R, Osborne-Smith KL. One-year retrospective clinical evaluation of hybrid composite restorations placed in United Kingdom general practices. Quintess Inter. 2001;32:293–298. [PubMed] [Google Scholar]
- 8.Imazato S, Ebi N, Takahashi Y, Kaneko T, Ebisu S, Russell RRB. Antibacterial activity of bactericide-immobilized filler for resin-based restoratives. Biomater. 2003;24:3605–3609. doi: 10.1016/s0142-9612(03)00217-5. [DOI] [PubMed] [Google Scholar]
- 9.Leung D, Spratt DA, Pratten J, Gulabivala K, Mordan NJ, Young AM. Chlorhexidine-releasing methacrylate dental composite materials. Biomater. 2005;26:7145–7153. doi: 10.1016/j.biomaterials.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Jedrychowski JR, Caputo AA, Kerper S. Antibacterial and mechanical properties of restorative materials combined with chlorhexidines. J Oral Rehab. 1983;10:373–381. doi: 10.1111/j.1365-2842.1983.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 11.Kawashita M, Tsuneyama S, Miyaji F, Kokubo T, Kozuka H, Yamamoto K. Antibacterial silver-containing silica glass prepared by sol-gel method. Biomater. 2000;21:393–398. doi: 10.1016/s0142-9612(99)00201-x. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto K, Ohashi S, Aono M, Kokubo T, Yamada I, Yamauchi J. Antibacterial activity of silver ions implanted in SiO2 filler on oral streptococci. Dent Mater. 1996;12:227–229. doi: 10.1016/s0109-5641(96)80027-3. [DOI] [PubMed] [Google Scholar]
- 13.Syafiuddin T, Hisamitsu H, Toko T, Igarashi T, Goto N, Fujishima A, Miyazaki T. In vitro inhibition of caries around a resin composite restoration containing antibacterial filler. Biomater. 1997;18:1051–1057. doi: 10.1016/s0142-9612(97)88072-6. [DOI] [PubMed] [Google Scholar]
- 14.Ferracane JL. Current trends in dental composites. Crit Rev Oral Biol Med. 1995;6:302–318. doi: 10.1177/10454411950060040301. [DOI] [PubMed] [Google Scholar]
- 15.Kelley JR, Nishimura I, Campbell SD. Ceramics in dentistry: Historical roots and current perspectives. J Prosthet Dent. 1996;75:18–32. doi: 10.1016/s0022-3913(96)90413-8. [DOI] [PubMed] [Google Scholar]
- 16.Bowen RL, Cleek GW. A new series of X-ray-opaque reinforcing fillers for composite materials. J Dent Res. 1972;51:177–182. doi: 10.1177/00220345720510011301. [DOI] [PubMed] [Google Scholar]
- 17.Fang M, Chen J-H, Xu X-L, Yang P-H, Hildebrand HF. Antibacterial activities of inorganic agents on six bacteria associated with oral infections by two susceptibility tests. Inter J Antimicrob Agents. 2006;27:513–517. doi: 10.1016/j.ijantimicag.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Adams LK, Lyon DY, McIntosh A, Alvarez PJ. Comparative toxicity of nano-scale TiO2, SiO2 and ZnO water suspensions. Water Sci Technol. 2006;54:327–334. doi: 10.2166/wst.2006.891. [DOI] [PubMed] [Google Scholar]
- 19.Jones N, Ray B, Ranjit KD, Manna AC. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett. 2008;279:71–76. doi: 10.1111/j.1574-6968.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 20.de Soet JJ, van Loveren C, Lammens AJ, Pavicić MJ, Homburg CH, ten Cate JM, de Graaff J. Differences in cariogenicity between fresh isolates of Streptococcus sobrinus and Streptococcus mutans. Caries Res. 1991;25:116–122. doi: 10.1159/000261353. [DOI] [PubMed] [Google Scholar]
- 21.Grönroos L, Mättö J, Saarela M, Luoma AR, Luoma H, Jousimies-Somer H, Pyhälä L, Asikainen S, Alaluusua S. Chlorhexidine susceptibilities of mutans streptococcal serotypes and ribotypes. Antimicrob Agents Chemother. 1995;39:894–898. doi: 10.1128/aac.39.4.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ. Communication among oral bacteria. Microbiol Mol Biol Rev. 2002;66:486–505. doi: 10.1128/MMBR.66.3.486-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takenaka S, Trivedi HM, Corbin A, Pitts B, Stewart PS. Direct visualization of spatial and temporal patterns of antimicrobial action within model oral biofilms. Appl Environ Microbiol. 2008;74:1869–1875. doi: 10.1128/AEM.02218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guggenheim B, Giertsen E, Schupbach P, Shapiro S. Validation of an in vitro biofilm model of supragingival plaque. J Dent Res. 2001;80:363–370. doi: 10.1177/00220345010800011201. [DOI] [PubMed] [Google Scholar]
- 25.Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ. Metal oxide nanoparticles as bactericidal agents. Langmuir. 2002;18:6679–6686. [Google Scholar]
- 26.Hernández-Sierra JF, Ruiz F, Pena DC, Martínez-Gutiérrez F, Martínez AE, Guillén Ade J, Tapia-Pérez H, Castañón GM. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedic. 2008;4:237–240. doi: 10.1016/j.nano.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Reddy KM, Feris K, Bell J, Wingett DG, Hanley C, Punnoose A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl Phys Lett. 2007;90:213902. doi: 10.1063/1.2742324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sunada K, Watanabe T, Hashimoto K. Bactericidal activity of copper-deposited TiO2 thin film under weak UV light illumination. Environ Sci Technol. 2003;37:4785–4789. doi: 10.1021/es034106g. [DOI] [PubMed] [Google Scholar]
- 29.Mitra SB, Wu D, Holmes BN. An application of nanotechnology in advanced dental materials. J Amer Dent Assoc. 2003;134:1382–1390. doi: 10.14219/jada.archive.2003.0054. [DOI] [PubMed] [Google Scholar]
- 30.Türkün LS, Celik EU. Noncarious class V lesions restored with a polyacid modified resin composite and a nanocomposite: a two-year clinical trial. J Adhes Dent. 2008;10:399–405. [PubMed] [Google Scholar]
- 31.Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR. Incorporation of bacterial inhibitor into resin composite. J Dent Res. 1994;73:1437–1443. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 32.Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomater. 2006;27:3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Zhou G, Li Y, Xiao W, Zhang L, Zuo Y, Xue J, Jansen JA. Synthesis, characterization, and antibacterial activities of a novel nanohydroxyapatite/zinc oxide complex. J Biomed Mater Res A. 2008;85:929–937. doi: 10.1002/jbm.a.31527. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Jiang Y, Ding Y, Povey M, York D. Investigation into the antibacterial behavior of suspensions of ZnO nanoparticles (ZnO nanofluids) J Nanopart Res. 2007;9(9):479–489. [Google Scholar]
- 35.Devulapalle KS, Mooser G. Subsite specificity of the active site of glucosyltransferases from Streptococcus sobrinus. J Biol Chem. 1994;269:11967–11971. [PubMed] [Google Scholar]
- 36.Paunio IK. The effect of certain cations and anions on the alkaline phosphomonoesterase activities in human dental plaque. Acta Odontol Scand. 1970;28:399–415. doi: 10.3109/00016357009032043. [DOI] [PubMed] [Google Scholar]
- 37.Wunder D, Bowen W. Action of agents on glucosyltransferases from Streptococcus mutans in solution and adsorbed to experimental pellicle. Arch Oral Biol. 1999;44:203–214. doi: 10.1016/s0003-9969(98)00129-0. [DOI] [PubMed] [Google Scholar]
- 38.He G, Pearce EI, Sissons CH. Inhibitory effect of ZnCl(2) on glycolysis in human oral microbes. Arch Oral Biol. 2002;47:117–129. doi: 10.1016/s0003-9969(01)00093-0. [DOI] [PubMed] [Google Scholar]
- 39.Giertsen E, Scheie AA, Rölla G. Inhibition of plaque formation and plaque acidogenicity by zinc and chlorhexidine combinations. Scand J Dent Res. 1988;96:541–550. doi: 10.1111/j.1600-0722.1988.tb01594.x. [DOI] [PubMed] [Google Scholar]
- 40.Eisenberg AD, Young DA, Fan-Hsu J, Spitz LM. Interactions of sanguinarine and zinc on oral streptococci and Actinomyces species. Caries Res. 1991;25:185–190. doi: 10.1159/000261365. [DOI] [PubMed] [Google Scholar]
- 41.LeChevallier MW, Cawthon CD, Lee RG. Inactivation of biofilm bacteria. Appl Environ Microbiol. 1988;54:2492–2499. doi: 10.1128/aem.54.10.2492-2499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conrady DG, Brescia CC, Horii K, Weiss AA, Hassett DJ, Herr AB. A zinc-dependent adhesion module is responsible for intercellular adhesion in staphylococcal biofilms. Proc Nat Acad Sci USA. 2008;105:19456–19461. doi: 10.1073/pnas.0807717105. [DOI] [PMC free article] [PubMed] [Google Scholar]