Abstract
We have shown previously that cruciferous vegetable constituent benzyl isothiocyanate (BITC) suppresses viability of cultured MCF-7 and MDA-MB-231 human breast cancer cells and retards mammary cancer development in MMTV-neu mice by causing apoptosis, but the mechanism of cell death is not fully understood. We now demonstrate that while p53 is dispensable for BITC-induced cell death, proapoptotic response to this promising chemopreventive agent is mediated by suppression of X-linked inhibitor of apoptosis (XIAP) protein expression. The BITC treatment increased levels of total and Ser15 phosphorylated p53 protein in MCF-7 cells, but the proapoptotic response to this agent was maintained even after knockdown of the p53 protein level. Exposure of MCF-7 and MDA-MB-231 cells to BITC resulted in a marked decrease in protein level of XIAP as early as 8-hours post-treatment. Ectopic expression of XIAP conferred statistically significant protection against BITC-mediated cytoplasmic histone-associated apoptotic DNA fragmentation in both cell lines. Moreover, inhibition of MDA-MB-231 cell growth in vivo in female athymic mice by BITC administration correlated with a modest but statistically significant decrease in XIAP protein level in the tumor xenograft. The BITC treatment also resulted in induction as well as nuclear translocation of survivin only in the MCF-7 cells. The BITC-induced apoptosis was modestly but statistically significantly augmented by RNA interference of survivin in MCF-7 cells. In conclusion, the present study provides novel insight into the molecular circuitry of BITC-induced apoptosis to indicate suppression of XIAP expression as a critical mediator of this process.
Keywords: Benzyl Isothiocyanate, Apoptosis, Chemoprevention
Introduction
Despite remarkable progress towards screening efforts and targeted therapies (1,2), breast cancer continues to be a leading cause of mortality among women around the world (3,4). Risk factors implicated in mammary carcinogenesis include family history, Li-Fraumeni syndrome, atypical hyperplasia of the breast, late age at first full-term pregnancy, and early menarche and late menopause (5–7). Because some of these risk factors are not easily modifiable, other strategies to reduce mortality associated with this malignancy are attractive. This objective is partially fulfilled with the advent of selective estrogen-receptor modulators such as tamoxifen and raloxifene, but this approach is ineffective against estrogen receptor negative breast cancers (8–10). Moreover, long-term administration of selective estrogen-receptor modulators inherits risk of severe side effects including increased risk of uterine cancer, thromboembolism, and perimenopausal symptoms (8–10). Therefore, agents that are relatively safe but could offer protection against mammary carcinogenesis regardless of the estrogen receptor status are desirable. Dietary plants have attracted increasing momentum in recent years for the discovery of potential cancer chemopreventive agents (11,12).
Benefit of a diet rich in cruciferous vegetables (e.g., broccoli, watercress, garden cress etc.) as a modifier of the breast cancer risk can be appreciated by the results of population-based case-control studies. A case-control study involving >300 breast cancer cases and matched controls showed an inverse association between urinary levels of isothiocyanates (ITCs) as a biological indicator of cruciferous vegetable intake and the risk of breast cancer (13). Broccoli consumption was shown to be inversely correlated with the risk of mammary cancer in premenopausal women in another population based case-control study (14). Antineoplastic effect of cruciferous vegetables is ascribed to organic ITCs that occur naturally as thioglucoside conjugates (15). Benzyl isothiocyanate (BITC) is one such compound that appears promising for prevention of mammary cancer (16). For example, our own work has revealed that dietary administration of 3 mmol BITC/kg diet inhibits mammary hyperplasia and carcinoma incidence and/or burden in MMTV-neu mice without causing weight loss or any other side effects (16). The BITC-mediated prevention of mammary cancer in MMTV-neu mice correlated with suppression of cellular proliferation, increased apoptosis, and enhanced infiltration of T cells in the carcinoma (16).
Previous studies including those from our laboratory have demonstrated that BITC treatment effectively inhibits growth of cultured human breast cancer cells by causing apoptotic cell death (17–20). Interestingly, a spontaneously immortalized non-tumorigenic human mammary epithelial cell line (MCF-10A) is highly resistant to growth inhibition and apoptosis induction by BITC in comparison with breast cancer cells (17). We also demonstrated that the molecular circuitry of BITC-induced apoptosis in human breast cancer cells entails production of reactive oxygen species due to inhibition of complex III of the mitochondrial respiratory chain leading to c-Jun N-terminal kinase-dependent activation of multidomain proapoptotic protein Bax (20). The present study builds on these observations and identifies molecular determinants of BITC-induced apoptosis downstream of the reactive oxygen species production. Here, we demonstrate that while p53 tumor suppressor is dispensable for BITC-induced cell death, proapoptotic response to this promising dietary chemopreventive agent is mediated by suppression of XIAP protein expression.
Materials and Methods
Reagents
BITC was purchased from LKT Laboratories. The RPMI 1640 medium and Minimum Essential Media were purchased from Cellgro. Antibody against X-linked inhibitor of apoptosis (XIAP) was from BD Biosciences; anti-survivin antibody was from Novus Biologicals; anti-p53 antibody was from Calbiochem; antibodies against cIAP1 and p-p53 (Ser15) were from Cell Signaling Technology; and anti-actin antibody was from Sigma. The p53 and survivin-targeted small interfering RNA (siRNA) were purchased from Santa Cruz Biotechnology. A nonspecific control siRNA was from Qiagen. FuGENE6 transfection reagent and a kit for quantification of cytoplasmic histone-associated apoptotic DNA fragmentation were procured from Roche Applied Sciences.
Cell lines
The MCF-7 and MDA-MB-231 cells were obtained from the American Type Culture Collection and maintained as described by us previously (17,20). Cell line authentication was performed by analysis of known genetic markers or response (e.g., expression of estrogen receptor and p53 and estrogen-responsiveness). Stock solution of BITC was prepared in dimethyl sulfoxide (DMSO) and an equal volume of DMSO (0.1%) was added to the controls.
Western blotting and detection of apoptosis
After treatment with DMSO (control) or the desired concentrations of BITC for specified time intervals, floating and attached cells were collected and lysed as described by us previously (21). Cytosolic and nuclear fractions from control and BITC-treated cells were prepared using a kit from Pierce. Immunoblotting was done as previously described by us (17,20,21). Apoptosis induction was measured by analysis of cytoplasmic histone-associated DNA fragmentation (22).
RNA interference
The MDA-MB-231 (5×104) and MCF-7 cells (1.5×105) were seeded in 6-well plates and transfected at ~50% confluency with 100 nmol/L of p53- or survivin-targeted siRNA using Oligofectamine. Twenty-four hours after transfection, the cells were treated for 24 h with either DMSO (control) or 5 µmol/L BITC. Subsequently, the cells were collected and processed for immunoblotting and measurement for cytoplasmic histone-associated DNA fragmentation.
Ectopic expression of XIAP by transient transfection
The MDA-MB-231 (5×104) and MCF-7 cells (1.5×105) were transiently transfected at ~50% confluency with the empty pcDNA3.1 vector or pcDNA3.1 encoding for XIAP (Addgene) using FuGENE6 transfection reagent. Twenty-four hours after transfection, the cells were treated with DMSO (control) or BITC for specified time period. Cells were collected and processed for immunoblotting and measurement for apoptosis.
Immunohistochemical analysis of XIAP expression in MDA-MB-231 xenografts
We have shown previously that BITC administration retards growth of MDA-MB-231 cells implanted in female athymic mice (23). We used tumor sections from the same study to determine effect of BITC administration on expression of XIAP protein by immunohistochemistry. Briefly, mice were randomized into two groups of five mice per group. The mice were injected intraperitoneally with either vehicle or vehicle containing 7.5 µmol BITC/mouse on Monday-Friday for 2 wk before tumor cell injection (23). Exponentially growing MDA-MB-231 cells were suspended in phosphate-buffered saline (PBS) and mixed in a 1:1 ratio with Matrigel. A 0.1-mL suspension containing 2.5×106 cells was injected subcutaneously on flank of each mouse above the hind limb. A portion of the tumor tissue removed from the control and BITC-treated mice was fixed in 10% neutral-buffered formalin, dehydrated, embedded in paraffin, and sectioned at 4- to 5-µm thickness. Representative tumor sections from control and BITC-treated mice were processed for H&E staining and immunohistochemical analysis of XIAP expression as described previously by us for other proteins (16,24,25). At least four non-overlapping representative images of each tumor section from 5 mice of each group were captured and analyzed using Image ProPlus 5.0 software for quantitation of XIAP expression.
Reverse transcription-PCR
Total RNA from MCF-7 and MDA-MB-231 cells treated for 16 h with DMSO (control) or BITC (2.5 and 5.0 µmol/L) was extracted with the use of the RNeasy kit (Invitrogen). The cDNA was synthesized with the use of 1 µg of total RNA using SuperScript III reverse transcriptase (Invitrogen) with Oligo dT primer. PCR was done with specific primers (survivin forward primer- 5’-AGGACGGCCCTTCTTGGAGG-3’; survivin reverse primer- 5’-CTTTTTATGTTCCTCTATGGGGTC-3’) with the use of the following amplification conditions; 94°C 2 min, 40 cycles at 94°C for 15 s, at 60°C for 20 s, and at 68°C for 15 s. Human GAPDH was used as a control as in our previous studies (26).
Immunocytochemical localization of survivin
The MCF-7 cells were cultured on coverslips, and treated with DMSO (control) or 5 µmol/L BITC for 16 h. The cells were treated with 200 nmol/L MitoTracker Red at 37°C for 30 min to stain mitochondria. After washing with PBS, the cells were fixed with 4% paraformaldehyde and permeabilized using 0.1% Triton X-100. The cells were incubated with anti-survivin antibody overnight at 4°C. The cells were then washed with PBS, incubated with Alexa Fluor 488-conjugated secondary antibody (1:2000 dilution, Molecular Probes) for 1 h at room temperature. After washing, cells were stained with DAPI (10 ng/mL) for 5 min at room temperature. The cells were visualized using a Leica DC300F fluorescence microscope.
Statistical analysis
Each experiment was repeated at least twice with triplicate measurements for quantitative comparisons. Statistical significance of difference in measured variables between control and treated groups was determined by t-test or one-way ANOVA. Difference was considered significant at P<0.05.
Results
p53 tumor suppressor was dispensable in BITC-induced apoptosis
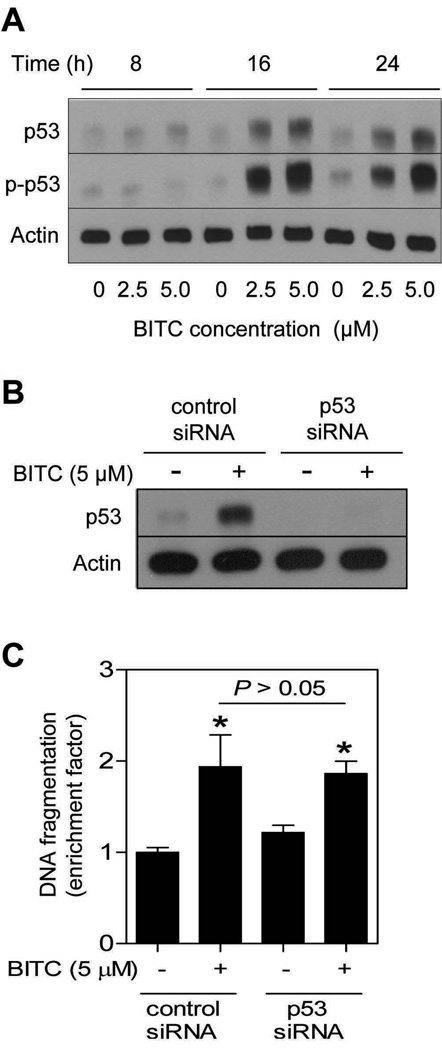
We have shown previously that BITC treatment causes apoptotic cell death in MCF-7 cell line, which expresses wild-type p53 (17). In the present study we used the same cell line to test whether BITC-induced apoptosis was influenced by the p53, which is a well-accepted facilitator of apoptotic cell death by different stimuli (27). As can be seen in Fig. 1A, BITC exposure caused a concentration- and time-dependent increase in the levels of total as well as Ser15 phosphorylated p53. The Ser15 phosphorylation of p53 has been implicated in apoptosis (27). Next, we utilized siRNA technology to directly test possible involvement of p53 in regulation of BITC-induced apoptosis. Transient transfection of MCF-7 cells with a p53-targeted siRNA resulted in complete silencing of the p53 protein expression (Fig. 1B). Moreover, the BITC-mediated induction of p53 protein was abolished in MCF-7 cells transfected with the p53-specific siRNA (Fig. 1B). However, enrichment of cytoplasmic histone-associated apoptotic DNA fragmentation resulting from BITC exposure (5.0 µmol/L for 24 h) over DMSO-treated control was comparable in MCF-7 cells transfected with the control nonspecific siRNA and p53-targeted siRNA (Fig. 1C). Collectively, these results indicated that the BITC-induced apoptosis was not influenced by the p53 status at least in the MCF-7 cell line.
Fig. 1.
A, immunoblotting for total and phospho-(Ser15)-p53 using lysates from MCF-7 cells treated with DMSO (control) or BITC (2.5 and 5.0 µmol/L) for the indicated time periods. B, immunoblotting for p53 using lysates from MCF-7 cells transfected with a control nonspecific siRNA or a p53-targeted siRNA, and treated for 24 h with DMSO (control) or 5.0 µmol/L BITC. C, cytoplasmic histone-associated apoptotic DNA fragmentation in MCF-7 cells transiently transfected with a control nonspecific siRNA or a p53-targeted siRNA, and treated for 24 h with DMSO (control) or 5.0 µmol/L BITC). Columns, mean (n= 3); bars, SD. *Significantly different (P<0.05) compared with nonspecific siRNA transfected cells treated with DMSO by one-way ANOVA followed by Bonferroni’s test. The results were consistent in two independent experiments, and representative data from one such experiment are shown.
BITC treatment down-regulated expression of XIAP protein
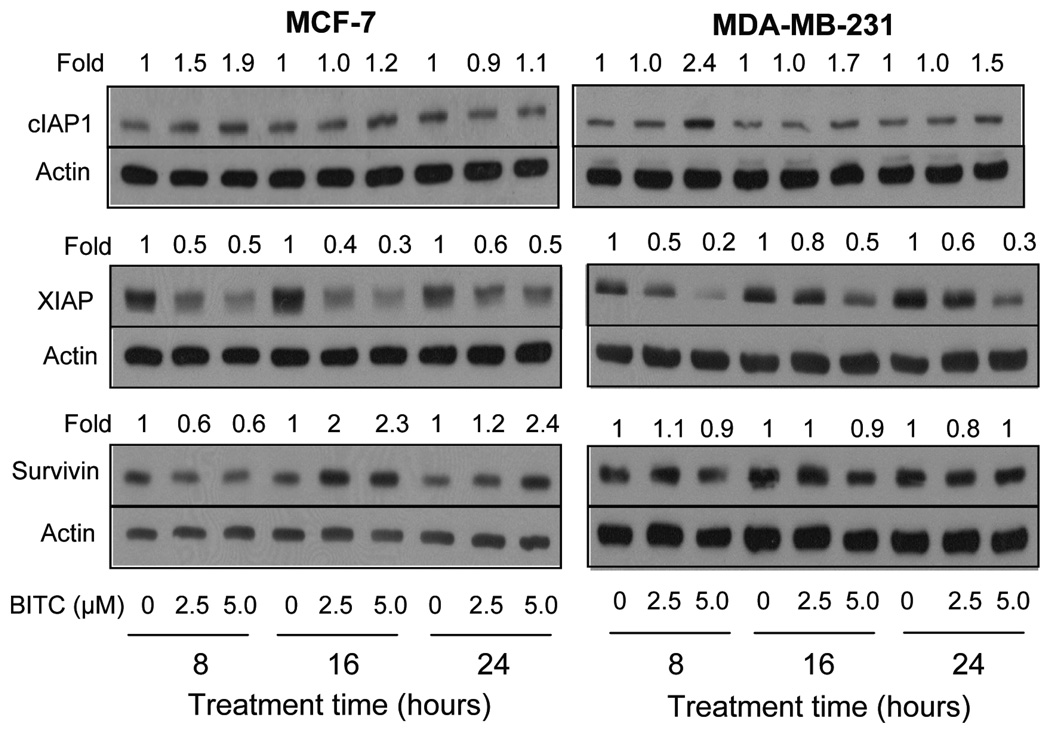
Inhibitor of apoptosis (IAP) proteins, including cIAP1, XIAP and survivin, have emerged as critical regulators of apoptotic cell death (28–30). We raised the question of whether the BITC-induced apoptosis in breast cancer cells was accompanied by alterations in expression of IAP family proteins. Level of cIAP1 protein was increased on BITC treatment in both MCF-7 and MDA-MB-231 cell lines especially at the 5.0 µmol/L concentration, but this effect was not sustainable (Fig. 2). For example, BITC-mediated induction of cIAP1 protein was nearly completely abolished at the 24 h time point in the MCF-7 cell line. The XIAP protein expression was markedly decreased on treatment of MCF-7 and MDA-MB-231 cells with BITC at both 2.5 and 5.0 µmol/L concentrations. The BITC-mediated decline in XIAP protein level was evident as early as 8 h after treatment, and this effect was maintained for the duration of the experiment (24 h post-treatment) in both cell lines (Fig. 2). The BITC treatment elicited a cell-line specific response on survivin protein expression (Fig. 2). The BITC treatment resulted in up-regulation of survivin protein in the MCF-7 cells especially at the 16 and 24 h time points (Fig. 2). The BITC-mediated induction of survivin in MCF-7 cells was preceded by ~40% decrease in its level at the 8 h time point. On the other hand, the BITC treatment did not have an appreciable effect on survivin protein level in the MDA-MB-231 cell line. Collectively, these results indicated that BITC treatment caused a sustained decrease in level of XIAP protein in both cell lines, and caused induction of survivin selectively in the MCF-7 cells.
Fig. 2.
Immunoblotting for cIAP1, XIAP, and survivin using lysates from MCF-7 and MDA-MB-231 cells treated with DMSO (control) or BITC (2.5 or 5.0 µmol/L) for the indicated time periods. Numbers above bands represent densitometric quantitation relative to corresponding DMSO-treated control. Immunoblotting for each protein was performed at least twice using independently prepared lysates. Representative data from one such experiment are shown.
Ectopic expression of XIAP conferred protection against BITC-induced apoptosis
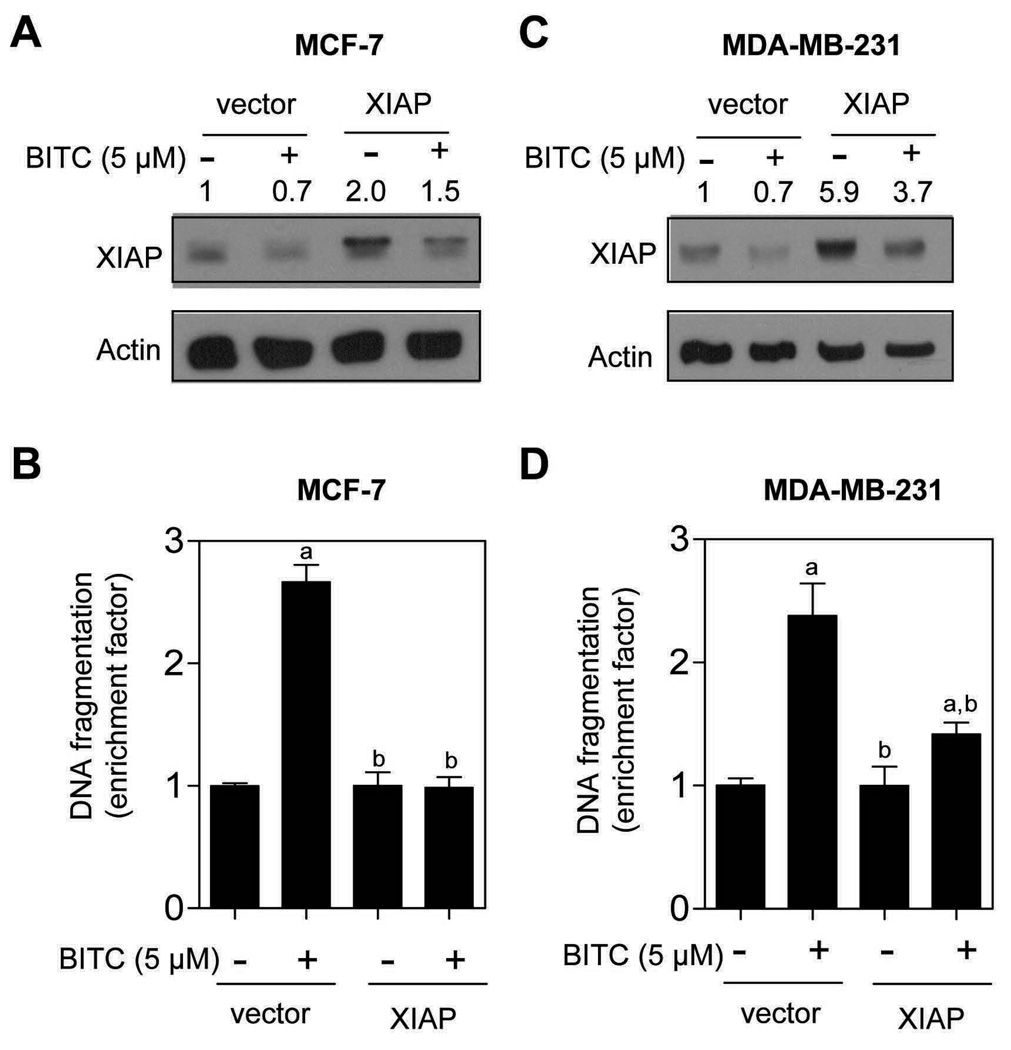
Because BITC treatment exhibited most striking effect on XIAP protein expression in both cell lines (Fig. 2), we designed experiments to determine functional significance of these observations. Transient transfection of MCF-7 cells with a vector encoding for XIAP resulted in about 2-fold increase in its protein level compared with empty-vector transfected cells (Fig. 3A). The BITC treatment (5.0 µmol/L for 24 h) caused a decrease in level of XIAP protein in both empty-vector transfected MCF-7 cells as well as in XIAP overexpressing cells (Fig. 3A). In addition, overexpression of XIAP conferred significant protection against BITC-induced cytoplasmic histone-associated DNA fragmentation in MCF-7 cells (Fig. 3B). Statistically significant inhibition of BITC-induced cytoplasmic histone-associated DNA fragmentation by forced overexpression of XIAP was also observed in the MDA-MB-231 cell line (Fig. 3C,D). These results indicated that XIAP was a target of BITC-induced apoptosis in breast cancer cells.
Fig. 3.
Immunoblotting for XIAP protein using lysates from MCF-7 (A) and MDA-MB-231 cells (C) transiently transfected with empty pcDNA3.1 vector or pcDNA3.1 vector encoding for XIAP, and treated for 24 h with DMSO (control) or 5.0 µmol/L BITC. The numbers above the immunoreactive bands represent change in protein level relative to empty vector-transfected cells treated with DMSO (first lane). Cytoplasmic histone-associated apoptotic DNA fragmentation in MCF-7 (B) and MDA-MB-231 cells (D) transiently transfected with empty pcDNA3.1 vector or vector encoding for XIAP, and treated for 24 h with DMSO (control) or 5.0 µmol/L BITC. Columns, mean (n= 3); bars, SD. Significantly different (P<0.05) compared with empty vector-transfected cells treated with DMSO (a), and empty vector-transfected cells treated with BITC (b) by one-way ANOVA followed by Bonferroni’s test. Comparable results were observed in two independent experiments. Representative data from one such experiment are shown.
BITC-mediated inhibition of MDA-MB-231 cell growth in vivo correlated with suppression of XIAP expression in the tumor xenograft
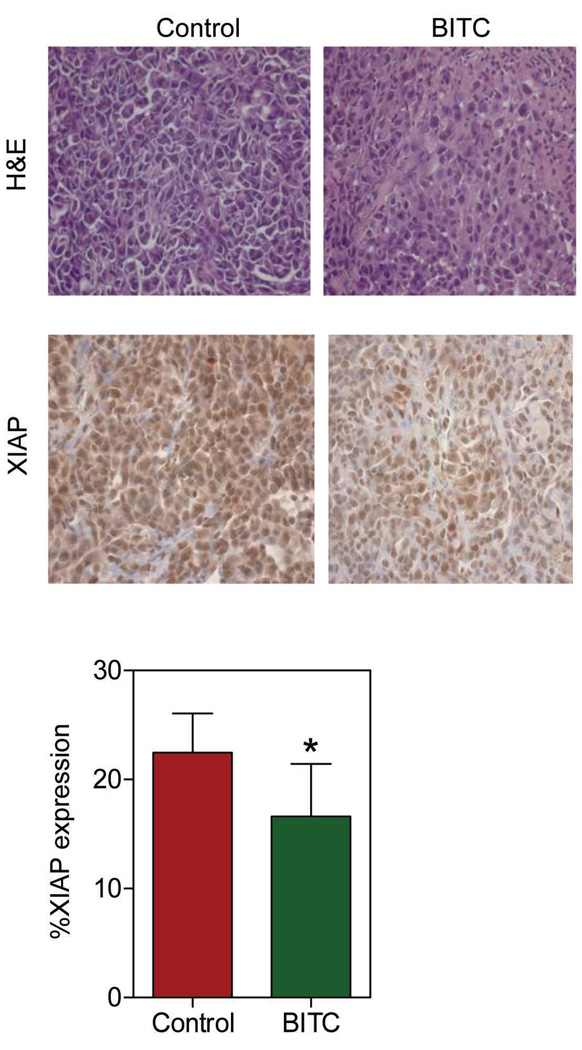
We have shown previously that BITC administration significantly retards growth of MDA-MB-231 cells implanted in female athymic mice without causing weight loss or any other side effects (23). For example, 50 days after tumor cell injection the average tumor volume in vehicle-treated control mice (1581 ± 240 mm3) was approximately 2.5- to 3-fold higher compared with mice intraperitoneally administered with 2.5 and 7.5 µmol BITC, five times per wk (23). In the present study, we used tumor sections from the same experiment to determine if BITC-mediated growth inhibition of MDA-MB-231 cells in vivo was accompanied by suppression of XIAP expression. Fig. 4 depicts H&E staining and immunohistochemical analysis for XIAP expression in representative MDA-MB-231 tumor section of a control mouse and a 7.5 µmol BITC-treated mouse. Tumors from BITC-treated mice exhibited a modest but statistically significant decrease in XIAP protein expression compared with control tumors (Fig. 4). These observations indicated that, similar to cultured cells, BITC administration caused suppression of XIAP protein level in the MDA-MB-231 xenografts in vivo.
Fig. 4.
H&E staining and immunohistochemical analysis for XIAP expression in representative tumor section of a control mouse and a mouse treated with 7.5 µmol BITC (magnification 400X). Bar diagram shows quantitation of XIAP protein expression in tumors from control and BITC-treated mice. At least four randomly selected fields on each tumor section from 5 different mice of each group were scored for XIAP expression. Columns, mean (n= 5); bars, SD. *Significantly different (P<0.05) compared with control by t-test.
BITC treatment caused nuclear translocation of survivin in MCF-7 cells
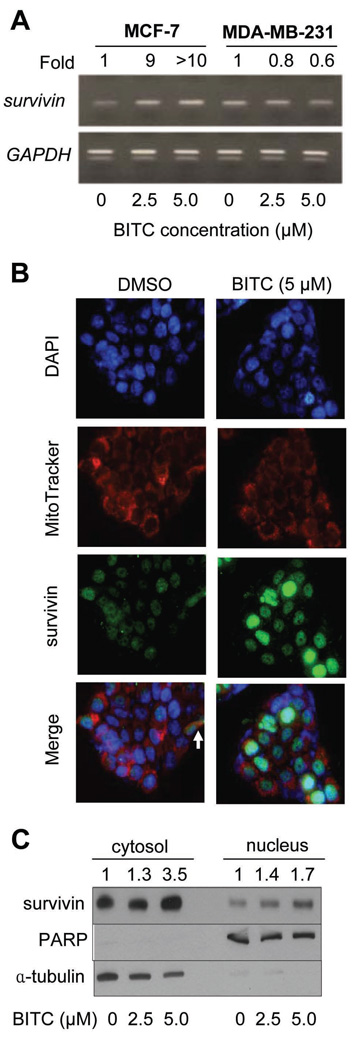
Reverse transcription-PCR was performed to determine if the BITC-mediated induction of survivin in MCF-7 cells (Fig. 2) was due to transcriptional up-regulation. As can be seen in Fig. 5A, exposure of MCF-7 cells to 2.5 and 5.0 µmol/L BITC for 16 h resulted in a marked increase in the levels of survivin mRNA. Consistent with the results of immunoblotting, BITC-mediated increase in survivin mRNA was not observed in the MDA-MB-231 cells (Fig. 5A).
Fig. 5.
A, Reverse transcription-PCR for survivin and GAPDH (loading control) mRNA levels in MCF-7 and MDA-MB-231 cells treated for 16 h with DMSO (control) or the indicated concentrations of BITC. Number above band represents quantitation relative to corresponding DMSO-treated control. B, immunocytochemical analysis for survivin localization in MCF-7 cells following 16 h treatment with DMSO (control) or 5.0 µmol/L BITC. Staining for survivin, mitochondria, and nuclei is indicated by green, red and blue fluorescence, respectively. C, immunoblotting for survivin using isolated cytosolic and nuclear fractions prepared from MCF-7 cells following 16 h treatment with DMSO (control) or the indicated concentrations of BITC. The blots were stripped and reprobed with anti-PARP and anti-α-tubulin antibodies.
Checkpoint kinase 2 (Chk2)-dependent release of survivin from mitochondria has been shown to counteract cell death in tumor cells induced by chemotherapeutic agents (31). We next asked whether BITC treatment affected survivin localization by activating Chk2. This was a strong possibility in light of our previous findings demonstrating activation of Chk2 by sulforaphane, a structural analogue of BITC (32). Initially we explored this possibility by determining the effect of BITC treatment (2.5 and 5.0 µmol/L for 8, 16, and 24 h) on activating phosphorylation of Chk2 (Thr68). Treatment of MCF-7 cells with 2.5 and 5.0 µmol/L BITC resulted in increased Thr68 phosphorylation of Chk2 at 16 and 24 h time points (results not shown). We used immunocytochemistry to determine localization of survivin in control (DMSO, 16 h) and BITC-treated MCF-7 cells (5.0 µmol/L, 16 h). As shown in Fig. 5B, a small fraction of cells exhibited mitochondrial localization of survivin in DMSO-treated control MCF-7 cells (identified by an arrow) as judged by merging of the mitochondria-associated red fluorescence and survivin-associated green fluorescence around DAPI-stained nuclei (blue color). Nuclear localization of survivin was also observed in some DMSO-treated control MCF-7 cells. Interestingly, BITC treatment resulted in a robust increase in nuclear as well as cytosolic levels of survivin in comparison with DMSO-treated control (Fig. 5B). We confirmed these observations by immunoblotting of survivin using isolated cytosolic and nuclear fractions prepared from control (DMSO, 16 h) and BITC-treated MCF-7 cells (5.0 µmol/L, 16 h). In agreement with the results of immunocytochemistry (Fig. 5B), the level of survivin protein was increased in both cytosolic and nuclear fractions on treatment of MCF-7 cells with BITC (Fig. 5C). The blots were stripped and reprobed with anti-poly-(ADP-ribose) polymerase (PARP) and anti-α-tubulin antibodies to normalize for differences in protein loading as well as to rule out cross-contamination of cytosolic and nuclear fractions (Fig. 5C). These results indicated that BITC treatment caused an increase in cytosolic and nuclear levels of survivin in MCF-7 cells.
Survivin knockdown modestly augmented BITC-induced apoptosis in MCF-7 cells
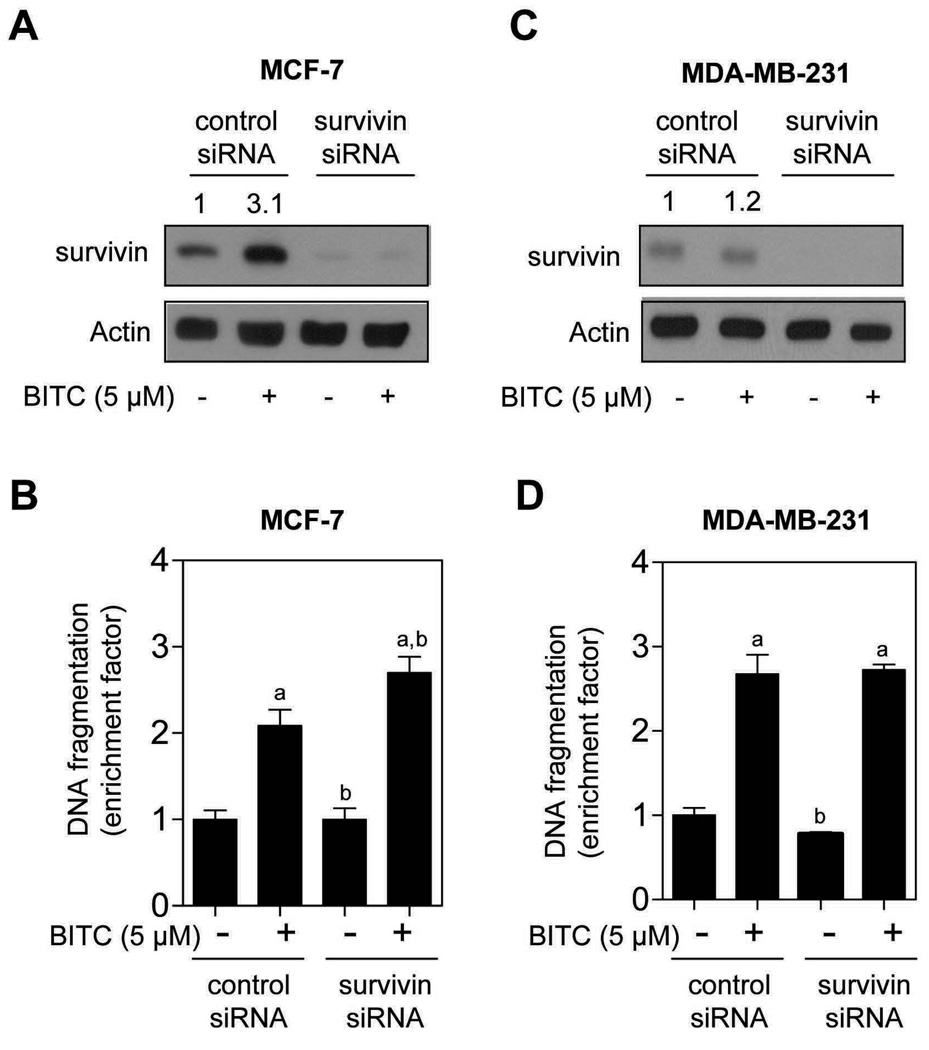
Next, we proceeded to experimentally test functional significance of survivin induction in proapoptotic response to BITC using MCF-7 cells. The MDA-MB-231 cell line was included as a negative control. Similar to untransfected cells (Fig. 2), exposure of nonspecific siRNA transfected MCF-7 cells to 5.0 µmol/L BITC for 24 h resulted in about 3-fold increase in survivin protein level compared with DMSO-treated control (Fig. 6A). The level of survivin protein was decreased by >90% in MCF-7 cells transfected with a survivin-targeted siRNA (Fig. 6A). Moreover, the BITC-mediated induction of survivin protein expression was nearly completely abolished in survivin siRNA transfected cells. As shown in Fig. 6B, the cytoplasmic histone-associated DNA fragmentation enrichment resulting from BITC exposure over DMSO-treated control was modestly but statistically significantly greater in survivin silenced cells in comparison with nonspecific siRNA transfected MCF-7 cells (Fig. 6B). On the other hand, knockdown of survivin protein level (Fig. 6C) did not have an appreciable effect on BITC-induced apoptosis in the MDA-MB-231 cell line (Fig. 6D). We conclude that induction of survivin is marginally cytoprotective against BITC-mediated cell death in the MCF-7 cells.
Fig. 6.
Immunoblotting for survivin using lysates from MCF-7 (A) and MDA-MB-231 cells (C) transiently transfected with a control nonspecific siRNA or a survivin-specific siRNA, and treated with DMSO (control) or 5.0 µmol/L M BITC for 24 h. Cytoplasmic histone-associated DNA fragmentation in MCF-7 (B) and MDA-MB-231 cells (D) transiently transfected with a control nonspecific siRNA or a survivin-specific siRNA, and treated with DMSO (control) or 5.0 µmol/L BITC for 24 h. Columns, mean (n= 3); bars, SD. Significantly different (P<0.05) compared with control nonspecific siRNA transfected cells treated with DMSO (a), and control nonspecific siRNA transfected cells treated with BITC (b) by one-way ANOVA followed by Bonferroni’s test. Results were consistent in two experiments, and representative data from one such experiment are shown.
Discussion
BITC is a highly promising cancer chemopreventive constituent of edible cruciferous vegetables (e.g., garden cress) with inhibitory effect in various chemically-induced rodent cancer models and transgenic mice prone to spontaneous cancer development (15,16,33–35). Recent studies have provided convincing evidence to implicate apoptosis induction as the main mechanism in cancer chemoprevention by BITC (16,35). For example, prevention of mammary cancer development in MMTV-neu mice by dietary administration of BITC correlates with increased apoptosis in carcinoma lesions (16). The main objective of the present study was to gain insight into mechanism of BITC-induced apoptosis, which is not fully understood. Intrinsic value of defining the mechanism of proapoptotic response to BITC can eventually be appreciated during rational design of novel BITC-based preventive interventional regimens. Our initial inquiry focused on possible role of p53 in execution of BITC-induced apoptotic cell death for two main reasons. First, the p53 tumor suppressor was shown to be essential for apoptosis induction by phenethyl isothiocyanate, which is a close structural analogue of BITC (36). Conversely, p53 has been shown to be a negative regulator of apoptosis induction by BITC in human colon CCD-18Co cells (37). The present study reveals that p53 tumor suppressor is dispensable for apoptosis induction by BITC because its knockdown has no influence on proapoptotic response to BITC in MCF-7 cells. Lack of p53 dependence should be viewed as a therapeutic advantage for BITC as loss of function mutation of this tumor suppressor is quite frequent in human cancers (27).
The IAP family of proteins has emerged as critical regulator of apoptosis in response to different stimuli, including death receptor activation, growth factor withdrawal, radiation, and genotoxic insults (30,38,39). The IAPs play important roles in adaptive response to cellular stress, differentiation, motility, and immune response (39). This family of proteins is characterized by the presence of baculovirus IAP repeat (BIR) domains (40). Of the eight IAP members identified to date, XIAP is the best characterized and most potent inhibitor of caspase-3 and -7 (38). Anti-caspase activity of XIAP is attributed to its BIR domains; BIR3 domain inhibits caspase-9 whereas the BIR2 linker region is implicated in inhibition of caspase-3 and -7 (38). Moreover, XIAP overexpression correlates with poor prognosis in some (e.g., childhood acute myelogenous leukemia and bladder cancer), but not all cancers (38,41,42). The present study reveals that BITC exposure decreases protein level of XIAP in breast cancer cells. In addition, the BITC-mediated inhibition of MDA-MB-231 cell growth in vivo is accompanied by suppression of XIAP protein expression in the tumor xenograft (Fig. 4). The BITC-mediated suppression of XIAP expression is not a cell-line specific response because BITC treatment decreases protein level of XIAP in both MCF-7 and MDA-MB-231 cells (Fig. 2). Moreover, RNA interference of XIAP confers statistically significant protection against BITC-mediated DNA fragmentation in MCF-7 as well as in MDA-MB-231 cells (Fig. 3). We have shown previously that the BITC treatment causes proteolytic cleavage (activation) of procaspase-3 and procaspase-9 in MDA-MB-231 cells and pharmacological inhibition of caspase-9 attenuates BITC-mediated apoptosis (17). It is possible that caspase activation by BITC is facilitated by suppression of XIAP protein level.
Precise mechanism by which BITC treatment suppresses expression of XIAP protein is yet to be determined, but several possibilities exist. One such possibility relates to the BITC-mediated proteasomal degradation of XIAP, which is capable of auto-ubiquitination and can be stabilized by inhibition of the proteasome (43,44). It is also plausible that BITC treatment inhibits transcription of XIAP. Finally, BITC-mediated inhibition of XIAP translation is another possibility deserving attention. Previous studies have shown that MDM2 physically interacts with the internal ribosome entry segment (IRES) of the 5’-untranslated region of XIAP, and positively regulates XIAP IRES activity (45). The XIAP IRES-dependent translation is increased in cells transfected with MDM2 (45). However, further studies are needed to systematically explore these possibilities.
Survivin is another IAP family member that contains a single BIR domain and an extended C-terminal helical coiled-coil domain. However, unlike other IAPs, survivin is devoid of the RING-finger domain (28,30,39). Recent studies have pointed towards important roles of survivin in both cell cycle regulation and apoptosis control (46). Subcellular localization of survivin in mitochondria seems important for its anti-apoptotic function (31,46,47). Survivin expression is absent or low in most terminally differentiated normal tissues, but this protein is overexpressed in different tumor types (46,47). Furthermore, survivin overexpression in tumors correlates with clinical pathologic variables of the aggressive disease (46,48) and confers treatment resistance in cancer cells (48). We found that BITC treatment results in transcriptional up-regulation of survivin in MCF-7 cells, but not in the MDA-MB-231 cell line. While the mechanism behind cell-specific transcriptional up-regulation of survivin by BITC treatment is not yet clear, silencing of survivin renders MCF-7 cells modestly more sensitive to BITC-induced apoptosis. We conclude that survivin induction is marginally cytoprotective against BITC-induced apoptosis, and this correlation is cell-specific.
In conclusion, we provide experimental evidence to demonstrate that the BITC-induced apoptosis in human breast cancer cells is independent of p53, but mediated by suppression of XIAP protein level irrespective of the estrogen receptor status. The BITC-mediated suppression of XIAP is also observed in MDA-MB-231 xenograft in vivo. Accordingly, XIAP expression may be a viable biomarker of BITC response.
Acknowledgments
Grant Support: This study was supported in part by the USPHS grants CA129347-03 and CA142604-01, awarded by the National Cancer Institute.
References
- 1.van de Ven SM, Elias SG, van den Bosch MA, Luijten P, Mali WP. Optical imaging of the breast. Cancer Imaging. 2008;8:206–215. doi: 10.1102/1470-7330.2008.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munoz M, Estevez LG, Alvarez I, et al. Evaluation of international treatment guidelines and prognostic tests for the treatment of early breast cancer. Cancer Treat Rev. 2008;34:701–709. doi: 10.1016/j.ctrv.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 4.DeSantis C, Jemal A, Ward E, Thun MJ. Temporal trends in breast cancer mortality by state and race. Cancer Causes Control. 2008;19:537–545. doi: 10.1007/s10552-008-9113-1. [DOI] [PubMed] [Google Scholar]
- 5.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 6.Hulka BS, Stark AT. Breast cancer: cause and prevention. Lancet. 1995;346:883–887. doi: 10.1016/s0140-6736(95)92713-1. [DOI] [PubMed] [Google Scholar]
- 7.Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Annu Rev Public Health. 1996;17:47–67. doi: 10.1146/annurev.pu.17.050196.000403. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 9.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 10.Land SR, Wickerham DL, Costantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2742–2751. doi: 10.1001/jama.295.23.joc60075. [DOI] [PubMed] [Google Scholar]
- 11.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nature Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 12.Stan SD, Kar S, Stoner GD, Singh SV. Bioactive food components and cancer risk reduction. J Cell Biochem. 2007;104:339–356. doi: 10.1002/jcb.21623. [DOI] [PubMed] [Google Scholar]
- 13.Fowke JH, Chung FL, Jin F, et al. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Res. 2003;63:3980–3986. [PubMed] [Google Scholar]
- 14.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134:1134–1138. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 15.Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32:395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- 16.Warin R, Chambers WH, Potter DM, Singh SV. Prevention of mammary carcinogenesis in MMTV-neu mice by cruciferous vegetable constituent benzyl isothiocyanate. Cancer Res. 2009;69:9473–9480. doi: 10.1158/0008-5472.CAN-09-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao D, Vogel V, Singh SV. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol Cancer Ther. 2006;5:2931–2945. doi: 10.1158/1535-7163.MCT-06-0396. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Tang L, Gonzalez V. Selected isothiocyanates rapidly induce growth inhibition of cancer cells. Mol Cancer Ther. 2003;2:1045–1052. [PubMed] [Google Scholar]
- 19.Tseng E, Scott-Ramsay EA, Morris ME. Dietary organic isothiocyanates are cytotoxic in human breast cancer MCF-7 and mammary epithelial MCF-12A cell lines. Exp Biol Med (Maywood) 2004;229:835–842. doi: 10.1177/153537020422900817. [DOI] [PubMed] [Google Scholar]
- 20.Xiao D, Powolny AA, Singh SV. Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger ROS-dependent apoptosis in human breast cancer cells. J Biol Chem. 2008;283:30151–30163. doi: 10.1074/jbc.M802529200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao D, Srivastava SK, Lew KL, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 22.Xiao D, Choi S, Johnson DE, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–5606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 23.Warin R, Xiao D, Arlotti JA, Bommareddy A, Singh SV. Inhibition of human breast cancer xenograft growth by cruciferous vegetable constituent benzyl isothiocyanate. Mol Carcinogenesis. 2010 doi: 10.1002/mc.20600. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh SV, Warin R, Xiao D, et al. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 2009;69:2117–2125. doi: 10.1158/0008-5472.CAN-08-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh SV, Powolny AA, Stan SD, et al. Garlic constituent diallyl trisulfide prevents development of poorly differentiated prostate cancer and pulmonary metastasis multiplicity in TRAMP mice. Cancer Res. 2008;68:9503–9511. doi: 10.1158/0008-5472.CAN-08-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SH, Singh SV. D,L-sulforaphane causes transcriptional repression of androgen receptor in human prostate cancer cells. Mol Cancer Ther. 2009;8:1946–1954. doi: 10.1158/1535-7163.MCT-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 28.LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17:3247–3259. doi: 10.1038/sj.onc.1202569. [DOI] [PubMed] [Google Scholar]
- 29.Deveraux QL, Reed JC. IAP family proteins- suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasula SM, Ashwell JD. IAPs: What’s in a Name? Mol Cell. 2008;30:123–135. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh JC, Dohi T, Raskett CM, Kowalik TF, Altieri DC. Activated checkpoint kinase 2 provides a survival signal for tumor cells. Cancer Res. 2006;66:11576–11579. doi: 10.1158/0008-5472.CAN-06-3095. [DOI] [PubMed] [Google Scholar]
- 32.Singh SV, Herman-Antosiewicz A, Singh AV, et al. Sulforaphane-induced G2/M phase cell cycle arrest involves checkpoint kinase 2 mediated phosphorylation of Cdc25C. J Biol Chem. 2004;279:25813–25822. doi: 10.1074/jbc.M313538200. [DOI] [PubMed] [Google Scholar]
- 33.Wattenberg LW. Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. J Natl Cancer Inst. 1977;58:395–398. doi: 10.1093/jnci/58.2.395. [DOI] [PubMed] [Google Scholar]
- 34.Wattenberg LW. Inhibitory effects of benzyl isothiocyanate administered shortly before diethylnitrosamine or benzo[a]pyrene on pulmonary and forestomach neoplasia in A/J mice. Carcinogenesis. 1987;8:1971–1973. doi: 10.1093/carcin/8.12.1971. [DOI] [PubMed] [Google Scholar]
- 35.Yang YM, Conaway CC, Chiao JW, et al. Inhibition of benzo(a)pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Res. 2002;62:2–7. [PubMed] [Google Scholar]
- 36.Huang C, Ma W-Y, Li J, Hecht SS, Dong Z. Essential role of p53 in phenethyl isothiocyanate-induced apoptosis. Cancer Res. 1998;58:4102–4106. [PubMed] [Google Scholar]
- 37.Miyoshi N, Uchida K, Osawa T, Nakamura Y. Selective cytotoxicity of benzyl isothiocyanate in the proliferating fibroblastoid cells. Int J Cancer. 2007;120:484–492. doi: 10.1002/ijc.22350. [DOI] [PubMed] [Google Scholar]
- 38.Dean EJ, Ranson M, Blackhall F, Dive C. X-linked inhibitor of apoptosis protein as a therapeutic target. Expert Opin Ther Targets. 2007;11:1459–1471. doi: 10.1517/14728222.11.11.1459. [DOI] [PubMed] [Google Scholar]
- 39.Dubrez-Daloz L, Dupoux A, Cartier J. IAPs: more than just inhibitors of apoptosis proteins. Cell Cycle. 2008;7:1036–1046. doi: 10.4161/cc.7.8.5783. [DOI] [PubMed] [Google Scholar]
- 40.Rothe M, Pan MG, Henzel WJ, Ayres TM, Godeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 41.Tamm I, Kornblau SM, Segall H, et al. Expression and prognostic significance of IAP-family genes in human cancer and myeloid leukemias. Clin Cancer Res. 2000;6:1796–1803. [PubMed] [Google Scholar]
- 42.Li M, Song T, Yin ZF, Na Y. XIAP as a prognostic marker of early recurrence of nonmuscular invasive bladder cancer. Chin Med J (Engl.) 2007;120:469–473. [PubMed] [Google Scholar]
- 43.Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science. 2000;288:874–877. doi: 10.1126/science.288.5467.874. [DOI] [PubMed] [Google Scholar]
- 44.Galban S, Duckett CS. XIAP as a ubiquitin ligase in cellular signaling. Cell Death Diff. 2010;17:54–60. doi: 10.1038/cdd.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gu L, Zhu N, Zhang H, Durden DL, Feng Y, Zhou M. Regulation of XIAP translation and induction by MDM2 following irradiation. Cancer Cell. 2009;15:363–375. doi: 10.1016/j.ccr.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18:609–615. doi: 10.1016/j.ceb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 47.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 48.Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy: fulfilled promises and open questions. Carcinogenesis. 2007;28:1133–1139. doi: 10.1093/carcin/bgm047. [DOI] [PubMed] [Google Scholar]