Abstract
The ability of the opportunistic fungal pathogen Candida albicans to form filaments has been strongly linked to its capacity to cause disease in humans. We previously described the construction of a strain in which filamentation can be modulated both in vitro and in vivo by placing one copy of the NRG1 gene under the control of a tetracycline-regulatable promoter. To further characterize the role of NRG1 in controlling filamentous growth, and in an attempt to determine whether NRG1 downregulation is a requirement for filamentation per se, or is only necessary under certain environmental conditions, we have conducted an analysis of the growth of the tet-NRG1 strain under a variety of in vitro conditions. Through overexpression of NRG1, we were able to block filamentation of C. albicans in both liquid media and on solid media. Filamentation in response to the low-oxygen environment of embedded growth was also inhibited. In all of these conditions, normal filamentation could be restored by down regulating expression from the tet-NRG1 allele. Interestingly, although elevated NRG1 levels were able to inhibit the formation of true hyphae in response to a wide range of environmental stimuli, elevated NRG1 expression did not affect the formation of pseudohyphae on nitrogen-limiting synthetic low ammonia dextrose (SLAD) medium. This work further illustrates the key role played by NRG1 in the control of filamentation and suggests that, although NRG1 repression plays a key role in regulating true hyphal growth, it apparently does not regulate pseudohyphal growth in the same fashion.
Keywords: Candida albicans, Filamentation, NRG1, Hyphae, Pseudohyphae
Introduction
Candida albicans is a commensal organism of humans, but is also an opportunistic fungal pathogen, capable of causing serious disease. C. albicans infections are most often associated with immuno-compromised patients such as those with HIV/AIDS, transplant recipients and chemotherapy patients. Mortality rates from C. albicans systemic infections range from 30 to 50% [1, 2].
C. albicans can grow as yeast cells, pseudohyphae and true hyphae, and the ability to filament has been linked with the disease potential of this species. Filamentation is required for the formation of biofilms; these complex structures exhibit increased resistance to antifungal drugs and are important for C. albicans infections arising from implanted medical devices such as catheters. Further, strains that are locked either in the yeast form [3-5] or in the filamentous form [6, 7] of growth are avirulent in the murine model of systemic candidiasis.
Filamentation is controlled by a complex network of signalling pathways, including the mitogen-activated protein kinase (MAPK) and cyclic AMP/protein kinase A pathways [8]. Although some components of these pathways have been identified, many aspects of the signalling process, including the initial signal transduction elements and the potential crosstalk between the different pathways remain unknown. Different environmental stimuli act to control filamentation through a number of different positive and negative factors at the base of these pathways. Expression of genes such as EFG1, CPH1, RIM101 and CZF1 promotes filamentation whereas expression of other genes including RFG1, NRG1 and TUP1 represses filamentous growth [9].
A key repressor of filamentation is the DNA binding protein Nrg1p, which acts in concert with the global transcriptional repressor Tup1p to suppress hypha formation. Elevated NRG1 expression is known to repress the expression of a number of hypha-specific genes [10-14] while downregulation of NRG1 is associated with the ability to form filaments [10, 13, 14]. In addition, putative Nrg1p response element (NRE) sequences have been identified in the upstream promoter regions of several hypha-specific genes including ALS3 [15] and HWP1 [16]. NRG1 has also been identified as playing a key role in the regulation of chlamydospore formation in both Candida dubliniensis and C. albicans [17, 18].
We previously described the construction of a novel C. albicans strain in which one copy of NRG1 was placed under the control of a tetracycline-regulatable promoter [5]. In the absence of the tetracycline analogue doxycycline, this strain produces elevated levels of NRG1 and is unable to filament, but in the presence of doxycycline forms true hyphae when grown under filament-inducing conditions. This tet-NRG1 strain, when kept in the yeast form, is avirulent in the murine model of systemic candidiasis, even though the yeast cells are able to escape the bloodstream and penetrate the organs as efficiently as hyphal forms [5]. This strain is proving to be a valuable tool both in dissecting the role of NRG1 in the control of filamentation in C. albicans and examining the host response to the yeast and hyphal forms. In an attempt to determine whether NRG1 downregulation is a requirement for filamentation per se, or is only necessary under certain specific environmental conditions, we conducted an analysis of the response of the tet-NRG1 strain grown under a variety of in vitro conditions.
Methods
Strains and Media
The yeast strains used in the present study were the wild-type strain CAF2-1 URA3/ura3::imm434 [19], the transactivator-containing strain THE1 ade2::hisG/ade2::hisG ura3::imm434/ura3::imm434 ENO1/eno1:: ENO1-tetR-ScHAP4AD-3×HA-ADE2 [20], the tet-NRG1 strain SSY50-B (derived from THE1) ade2::-hisG/ade2::hisG ura3::imm434/ura3::imm434 ENO1/eno1::ENO1-tetR-ScHAP4AD-3×HA-ADE2 NRG1/nrg1::tetO-NRG1-URA3 [5] and the Δtup1 and Δnrg1 null strains BCa2-10 ura3::imm434/ura3::imm434 tup1:: hisG/tup1::p405-URA3 and Bca23-3 ura3::imm434/ura3::imm434 nrg1/nrg1::URA3 iro1::imm434/iro1::imm434, respectively [10] (derived from strain CAI4 [19]). These strains were routinely maintained as −80°C glycerol freezer stocks and retrieved on yeast extract-peptone-dextrose (YPD) medium as required. Expression from the tet-NRG1 allele was abolished through the addition of 20 μlg ml−1 doxycycline to the growth medium as needed.
RNA Isolation and Northern Blot Analysis
To verify the DOX regulation of the modified tet-NRG1 allele, the wild-type CAF2-1 and modified strains were grown for 5 h in GlcNAc Medium or RPMI-1640 at 37°C. Total RNA was isolated using a previously published bead beater protocol [21], then separated through formaldehyde-containing agarose gels transferred to a nytran membrane (Whatmann) and subjected to Northern blot analysis as previously described [22]. The probe has the PCR product (NRGB), comprising the sequence −33 to +446 relative to the ATG start codon of the NRG1 open reading frame previously described [5] and labelled with 32P-dCTP using Ready-To-Go DNA labelling beads (GE Healthcare).
Liquid Assays
For filamentation assays in liquid media, strains were grown overnight in YPD at 25°C and diluted 1:20 into fresh RPMI-1640 supplemented with L-glutamine and buffered with morpholinepropanesulfonic acid (Angus Buffers and Chemicals), GlcNAc Medium [23], or Medium 199 (M199) buffered to pH 4.0 or pH 8.0 (Invitrogen) and incubated with shaking at 37°C. Samples were taken from these cultures at various time points, and their morphology was evaluated microscopically using a 40× objective lens and photographed using a digital camera.
Agar Invasion Assay
For the invasion assay, strains were streaked on YPD plates and incubated at 30°C for 48 h. The plates were then photographed with a digital camera both prior to and after surface cells were removed by gentle washing under running water.
Embedded Assays
Strains were grown in YPD overnight at 25°C, washed in sterile phosphate-buffered saline (PBS) and counted using a haemocytometer. Approximately 250 cells were mixed with molten YPD agar and poured onto YPD plates which were subsequently incubated at 30°C for 3 days. Embedded colonies were examined and photographed using a GL9-280 Stereo Zoom microscope (Jenco) equipped with a digital camera.
Spider Medium
For filamentation assays on solid Spider medium [24], strains were grown in YPD overnight at 25°C, washed in sterile PBS, counted using a haemocytometer and resuspended to a final concentration of 5 × 106 cells ml−1. Aliquots of 2 μl (1 × 104 cells) were spotted onto Spider plates and the plates incubated at 37°C for 7 days. Colonies were examined and photographed using a GL9-280 Stereo Zoom microscope (Jenco) equipped with a digital camera. Cells removed from the colony were photographed using the 10× objective lens of a Micromaster Inverted Microscope equipped with a digital camera (Fisher Scientific).
SLAD Medium
For filamentation assays on SLAD medium [25], strains were grown in YPD overnight at 25°C, washed in sterile PBS and counted using a haemocytometer. Approximately 200 cells were spread onto SLAD plates. These plates were then incubated at 25°C for 7 days and colonies photographed using a digital camera. Cells removed from the colony were photographed using the 10× objective lens of a Micromaster Inverted Microscope equipped with a digital camera (Fisher Scientific).
Results and Discussion
Deletion of NRG1 has previously demonstrated the involvement of Nrg1p in the negative regulation of hypha-specific gene expression and its requirement in the control of filamentous growth [10, 14]. We have sought to further understand the role of Nrg1p in regulating filamentation by constructing an engineered strain in which we can control NRG1 expression. Using this novel strain, we previously demonstrated the ability to regulate C. albicans virulence in a murine model of disseminated candidiasis through the modulation of NRG1 expression [5]. Many in vitro conditions are known to stimulate filament formation, but which of these is the predominant driver of hyphal development in vivo remains unknown. These filament-inducing stimuli are transmitted down their associated signalling pathways [8, 9]. Each of these different pathways can be differentially stimulated by growing the fungus in several specific media. In an attempt to determine whether Nrg1p plays a preferential role in regulating any of the pathways, we present an in vitro characterization of the growth of the tet-NRG1 strain in a variety of conditions which stimulate filamentation through distinct mechanisms.
Effect of NRG1 Overexpression on C. albicans Morphology in Liquid Media
We have previously shown that elevated expression of NRG1 is able to inhibit filamentation when the cells are grown in YPD+ serum at 37°C [5]. To examine whether this inhibition was specific to a particular filamentation signal or a more general regulation, the tet-NRG1 strain was grown in liquid media known to induce filamentation in C. albicans both in the presence and in the absence of doxycycline, and its growth was compared to that of the wild-type strain CAF2-1. Unlike the Dnrg1 null strain which is constitutively filamentous [10, 14], the tet-NRG1 strain grows as round free yeast cells in liquid YPD at 25°C and 30°C in the absence (elevated NRG1 levels) or presence (reduced NRG1 levels) of doxycycline (data not shown). Thus, even when expression from the tet-NRG1 allele has been abolished, expression of NRG1 from the remaining native copy of the gene is sufficient to prevent filamentous growth under yeast-growth conditions.
Growth in cell culture medium RPMI-1640 at 37°C stimulates formation of true hyphae in wild-type strains, and these conditions are typically used for in vitro biofilm formation on polystyrene surfaces [26]. When grown in RPMI-1640 medium for 3 h, the wild-type strain CAF2-1 grew, as expected, as long filaments in both the presence and absence of doxycycline (Fig. 1a). The tet-NRG1 strain grown in the presence of doxycycline also grew as filaments. However, in the absence of doxycycline (elevated NRG1 expression) the tet-NRG1 strain grew almost exclusively as yeast cells (Fig. 1a).
Fig. 1.
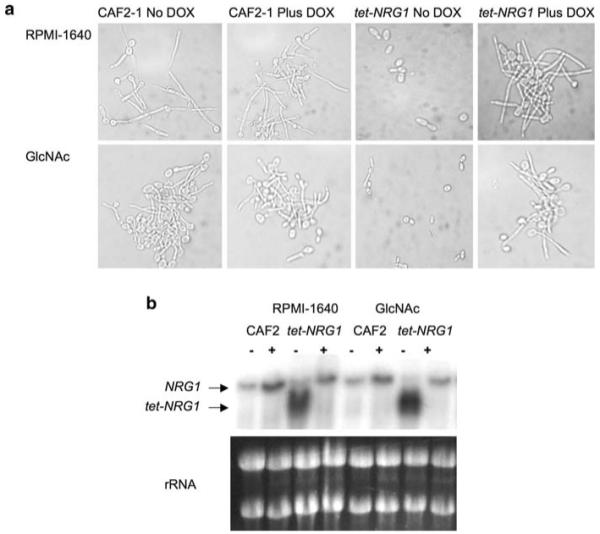
Growth in filament-inducing liquid media. a Samples were taken from cultures grown in RPMI-1640 or GlcNAc medium at 37°C for 5 h in the presence or absence of doxycycline, and their morphology was examined microscopically. b Total RNA was prepared from the cultures in (a) and subjected to Northern blot analysis to determine NRG1 expression levels. As seen in our previous study [5], the CAF2-1 wild-type strain contains one hybridizing band while the tet-NRG1 strain contains two hybridizing bands: one identical in size to the wild-type allele and a second, smaller band whose expression is entirely dependent on the presence or absence of doxycycline. Ethidium bromide-stained rRNA bands are shown to confirm equal loading of all samples
Growth in liquid medium containing the amino sugar N-acetylglucosamine (GlcNAc) also stimulates C. albicans filamentation [23]. Cells were grown for 5 h in GlcNAc medium at 37°C and examined microscopically. Nearly all (>95%) of the CAF2-1 cells were filamentous, as were the cells of the tet-NRG1 strain grown in the presence of doxycycline. In the absence of doxycycline, more than 90% of the tet-NRG1 cells remained in the yeast form (Fig. 1a). To verify that NRG1 transcription from the tet-NRG1 allele was being properly modulated in these hypha-inducing media, as we previously demonstrated in YPD+ serum at 37°C [5], RNA was extracted and subjected to Northern blot analysis. In both RPMI-1640 and GlcNAc medium, transcription from the modified allele was entirely dependent on the presence or absence of doxycycline, and expression was higher than that from the wild-type alleles in the control strain CAF2-1 (Fig. 1b).
Elevated NRG1 expression was sufficient to inhibit filamentation in liquid culture using cell culture medium RPMI-1640 or GlcNAc medium (Fig. 1) which are common conditions used by a number of investigators to induce filamentation in liquid cultures [9, 23, 27-30]. A role for Nrg1p regulation of filamentation in RPMI-1640 and GlcNAc media has not previously been demonstrated.
NRG1 Involvement in Alkaline pH-Driven Morphogenesis
The different host environments inhabited by C. albicans can vary widely in pH, especially the GI tract which ranges from the highly acidic stomach environment to the alkaline intestine. It has also been shown that the ability of C. albicans to respond to changes in pH is required for pathogenesis [32]. Filamentation is triggered in response to a shift from acidic growth conditions to a neutral or alkaline environment with signals related to pH transmitted through the conserved RIM101-mediated pathway [31-33]. While in Saccharomyces cerevisiae the transcription factor Rim101p acts through NRG1, this is not the case in C. albicans where a Δrim101 null strain fails to filament in response to neutral pH [34], but the Δnrg1 null strain is still able to form true hyphae under these conditions [14]. To further assess the role of NRG1 expression in regulating C. albicans filamentation via the pH induction pathway, the tet-NRG1 and CAF2-1 strains were grown in liquid M199 at pH 4 or pH 8. When grown for 5 h in M199 pH 4, cultures of both strains consisted of free-floating yeast cells or short chains of yeast cells (Fig. 2). In M199 at pH 8, CAF2-1 grew almost exclusively as filaments with very few free yeast cells observed irrespective of the presence of doxycycline (Fig. 2). Conversely, the tet-NRG1 strain filamented normally only in the presence of doxycycline. Although Rim101p does not appear to regulate alkaline responses through Nrg1p in C. albicans, elevating NRG1 expression was able to block the filamentation response to increased/alkaline pH. This indicates that Nrg1p functions in opposition to and downstream of the Rim101p pH response pathway.
Fig. 2.
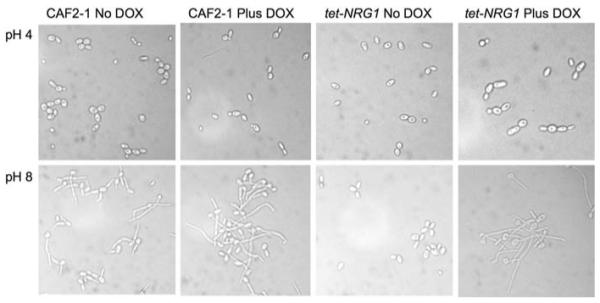
Induction of filamentation in response to neutral pH. Samples were taken from cultures grown for 5 h at 37°C in M199 buffered to either pH 4 or pH 8, in the presence or absence of doxycycline, and their morphology was examined microscopically
Overexpression of NRG1 Blocks Hypha Formation on Spider Medium
Spider medium is one of the strongest stimulators of filamentous growth in C. albicans [24]. It has previously been described that constitutively expressing NRG1 using the ACT1 promoter rescues the constitutively filamentous phenotype of the Δnrg1 null strain on both YPD and Spider medium [10]. We wanted to test whether a regulatable promoter could also control filamentation on Spider medium. When CAF2-1 was grown on Spider plates, highly wrinkled colonies were formed, indicating the presence of filamentous cells (Fig. 3). Material removed from one of the colonies and examined microscopically confirmed that the colony consisted almost entirely of clumps of filamentous cells. In the presence of doxycycline, the tet-NRG1 strain also formed highly wrinkled colonies consisting primarily of filamentous cells. In the absence of doxycycline, however, the tet-NRG1 strain formed flat, ruffled colonies. When a portion of one of these colonies was examined microscopically, it was found to be composed almost exclusively of yeast cells (Fig. 3). Thus, alterations in control of NRG1 expression were able to influence filamentation on solid Spider medium.
Fig. 3.
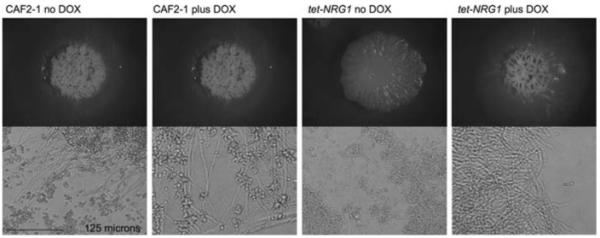
Growth on solid Spider medium. Cells from an overnight culture were washed in sterile PBS, counted and spotted onto solid Spider medium in the presence or absence of doxycycline and incubated at 37°C for 7 days. The morphology of the colonies and of cells taken from those colonies was examined microscopically
Regulating NRG1 Expression Modulates Agar Invasion
To examine whether Nrg1p also had an impact on filamentation and invasion, various strains were streaked onto YPD medium with and without doxycycline and incubated at 30°C for 2 days (Fig. 4a). After gentle washing, the constitutively filamentous Δtup1 and Δnrg1 null strains remained attached to the plate (Fig. 4b). Although most of the CAF2-1 cells washed off, some had invaded the agar. The behaviour of these three strains was not affected by the presence or absence of doxycycline. However, with respect to the tet-NRG1 strain, invasion of the agar to similar extents as the CAF2-1 wild-type strain was seen only in the presence of doxycycline. In the absence of doxycycline, the tet-NRG1 cells failed to invade the agar and were completely washed off the surface. We have previously demonstrated that a Δefg1 null strain, while impaired in its ability to filament, was nonetheless able to successfully transmigrate across an extracellular matrix-based substrate [35]. Similarly, the tet-NRG1 strain has been shown to successfully escape the bloodstream and invade tissues in mice even when grown in the yeast form [5]. In contrast, the results presented here indicate that although yeast cells of the tet-NRG1 strain are able to escape the bloodstream, they are severely impaired in their capacity to invade an agar plate.
Fig. 4.
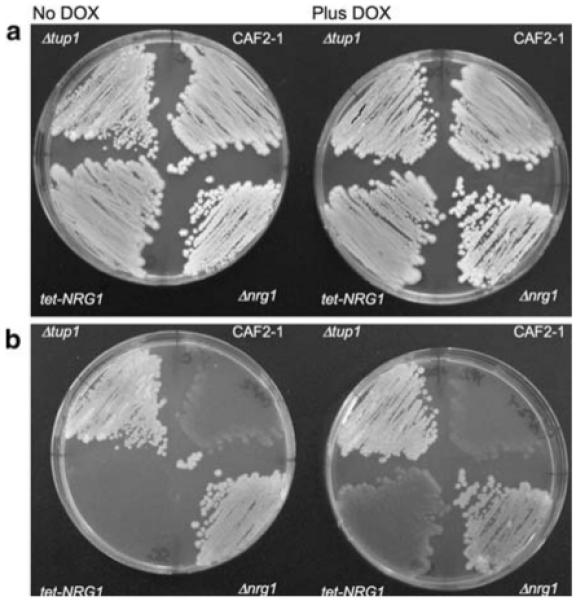
Invasion of solid medium. The wild-type CAF2-1, Δtup1 null, Δnrg1 null and the tet-NRG1 strains were streaked onto YPD agar and incubated at 30°C. Plates were photographed after 2 days growth (a). The plates were subsequently washed under running water and photographed again (b). Cells that had not invaded the solid medium washed away while those cells that had invaded remained
NRG1 Overexpression Affects Embedded Growth Morphology
Growth of C. albicans cells embedded in solid medium is also known to stimulate filamentation. Studies of the transcriptional regulator CZF1 have helped to characterize this response to growth under the increased physical pressure and low-oxygen environment encountered by C. albicans during growth in embedded conditions [36, 37]. The transcription factor Czf1p is required for filamentous growth under embedded conditions, while EFG1, which normally promotes filamentation, appears to play a role in the repression of filamentation genes in these low-oxygen conditions [38]. The function of NRG1 in regulating filamentous growth under embedded conditions has not previously been described. Wild-type CAF2-1 cells embedded in the solid medium began to filament after 48-h growth, and by 72 h all of the cells had formed filamentous colonies (Fig. 5). When the tet-NRG1 strain was grown in the presence of doxycycline, it too formed filamentous colonies after 48 h and all colonies were filamentous after 72 h. However, unlike the wild-type strain which formed colonies with several large filamentous projections, the tet-NRG1 colonies formed many thin filamentous projections giving the colonies a fuzzy appearance. This suggests that there may be a haploinsufficiency phenotype associated with the tet-NRG1 strain which is effectively heterozygous for NRG1 (on a transcriptional level) when grown in the presence of doxycycline. This is supported by the observation that the tet-NRG1 parent strain THE1 displays the same phenotype on this medium as the CAF2-1 strain (data not shown). Conversely, when the tet-NRG1 strain was grown embedded in medium lacking doxycycline, all the colonies formed after 48-h growth were smooth and after 72 h only very few colonies (<5%) showed evidence of filamentation, demonstrating a role for Nrg1p regulation in the control of the C. albicans response to the embedded/microaerophilic conditions. While the transcriptional regulator Efg1p switches from promoting to repressing filamentation when switched from aerobic to low-oxygen-embedded conditions, the observation that the tet-NRG1 strain colonies remained smooth when NRG1 expression was high but filamented readily when NRG1 levels were low (Fig. 5), suggests that Nrg1p functions to suppress CZF1-mediated transcription directly rather than through its repression of Efg1p function. The lack of filamentation when NRG1 levels are kept high in a low-oxygen environment surrounded by a solid matrix is reminiscent of the yeast cells observed in organs such as the kidney during a murine infection with the tet-NRG1 strain in the absence of doxycycline [5].
Fig. 5.
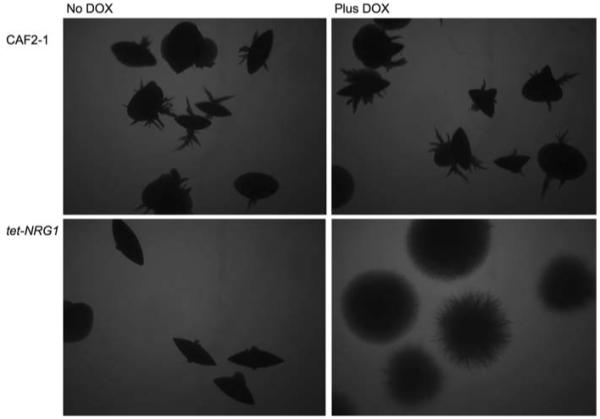
Growth under embedded conditions. Cells from an overnight culture were washed in sterile PBS, counted and were embedded in molten YPD agar in the presence or absence of doxycycline and incubated for 72 h at 30°C. The morphology of the colonies formed in the medium was examined microscopically
Elevated NRG1 Expression does not Inhibit Pseudohyphal Formation
Many of the regulators for hyphal growth in C. albicans are conserved in S. cerevisiae, but whereas S. cerevisiae filamentous growth occurs only as pseudohyphal cells, C. albicans is able to form both pseudohyphae and true hyphae. Although Park and Morschhauser demonstrated that NRG1 was required for C. albicans hypha formation on SLAD when grown at elevated temperatures [39], this medium was originally designed to stimulate pseudohyphal formation at low temperatures [25]. When the wild-type CAF2-1 strain was grown on SLAD medium at 25°C for 7 days, it formed colonies that were generally smooth with some short, ruffled projections and some long individual filaments (Fig. 6). Similarly, the tet-NRG1 strain showed a halo of filamentous growth around a central smooth colony when grown in the presence of doxycycline, although the length of the extended filaments was increased compared to those see in the wild-type strain (note scale bars Fig. 6). Strikingly, the filamentous phenotype was also observed when this strain was grown in the absence of doxycycline. Although the tet-NRG1 strain lacked the longer filaments when NRG1 was highly expressed, it was still able to form colonies with a halo of pseudohyphal growth (Fig. 6). The presence of pseudohyphal cells was confirmed by removing some cells from the colonies and examining them microscopically (Fig. 6). Unlike all the other conditions tested, elevated NRG1 expression was not sufficient to block filamentation on SLAD medium, suggesting that formation of pseudohyphae may not be regulated by Nrg1p. Interestingly, it has been observed previously that the Δcek1 null mutant was unable to form true hyphae on SLAD medium, but was still able to form pseudohyphae [40], indicating that blocking hyphal induction, either by impairing the MAP-kinase signalling pathway or through increased NRG1 expression, fails to block pseudohyphal formation on SLAD medium. Taken together, these results suggest that there are important differences between the regulation of hypha and pseudohypha formation in C. albicans, even though many of the genes controlling filamentation are conserved between S. cerevisiae and C. albicans.
Fig. 6.
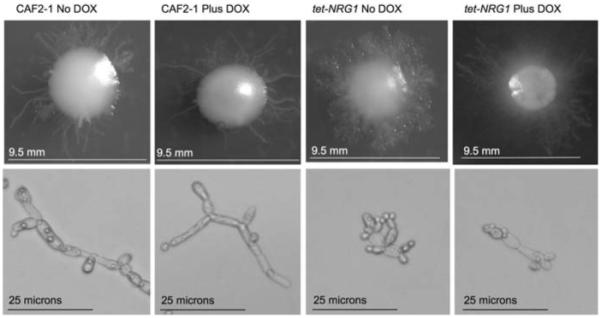
Growth on solid SLAD medium. Cells from an overnight culture were washed in sterile PBS and spread onto solid SLAD medium in the presence of absence of doxycycline and incubated at 25°C for 7 days. The morphology of the colonies (upper panels) and of cells removed from these colonies (lower panels) was examined microscopically
Summary
C. albicans is a common commensal organism of humans and under the right conditions can become a successful pathogen. It can be found in several different environments within the host, including the oral and vaginal cavities, the gastrointestinal tract, the bloodstream, and deep within internal organs. These locations vary greatly in environmental conditions including oxygen levels, pH, physical stress and nutrient availability. The ability of C. albicans to grow in this variety of conditions indicates a capacity to adapt its growth in response to these differing external stimuli [8, 41]. Filamentation is controlled by a complex network of regulatory pathways that converge onto specific transcriptional regulators. Modulation of the expression of the negative regulator, NRG1, is sufficient to control the ability to filament and to cause disease in the murine model of disseminated candidiasis, and is here demonstrated to be able to control the ability to filament in response to a variety of specific environmental signals in vitro. Elevated NRG1 expression was able to block filamentation in liquid media such as RPMI-1640 and YPD containing serum [5] or media containing GlcNAc and was also able to regulate the invasion of solid medium.
The specific environmental conditions used here were designed to examine the involvement of Nrg1p in blocking filamentation stimulated through the activity of the four major regulatory transcription factors. Increased NRG1 expression blocked filamentation on solid Spider medium, which induces filamentation in a Cph1p dependent fashion. NRG1 overexpression was also able to block the filamentation response to increased pH by the Rim101p-mediated pathway and also filamentation in response to low oxygen, and embedded conditions mediated by the transcription factors Czf1p and Efg1p. Taken together with our previously published observations using the tet-NRG1 strain in vivo, it is clear that NRG1 is of fundamental importance for regulating the filamentation response in C. albicans and that this response can be controlled through modulation of NRG1 expression. Perhaps most significantly, the ability to form pseudohyphae on nitrogen-limiting SLAD medium was not impaired by elevated NRG1 levels and is so far the sole filamentation response that could not be blocked in this strain. This suggests that while regulation of NRG1 expression is pivotal to the induction of true hyphae by a wide range of environmental conditions, it does not play such a key role in pseudohyphal formation.
Acknowledgments
We thank Professor A.D Johnson (UCSF) for supplying the Δtup1 (Bca2-10) and Δnrg1 (BCa23-3) null strains used in this study. The work presented here was funded in part by National Institutes of Health Grant award RO1 A1063256 from the National Institute of Allergy and Infectious Diseases to SPS.
References
- 1.Viudes A, Peman J, Canton E, Ubeda P, Lopez-Ribot JL, Gobernado M. Candidemia at a tertiary-care hospital: epidemiology, treatment, clinical outcome and risk factors for death. Eur J Clin Microbiol Infect Dis. 2002;21:767–74. doi: 10.1007/s10096-002-0822-1. [DOI] [PubMed] [Google Scholar]
- 2.Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med. 1988;148:2642–5. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]
- 3.Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–49. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 4.Stoldt VR, Sonneborn A, Leuker CE, Ernst JF. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982–91. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell. 2003;2:1053–60. doi: 10.1128/EC.2.5.1053-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun BR, Head WS, Wang MX, Johnson AD. Identification and characterization of TUP1-regulated genes in Candida albicans. Genetics. 2000;156:31–44. doi: 10.1093/genetics/156.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun BR, Johnson AD. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–9. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- 8.Biswas S, Van Dijck P, Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev. 2007;71:348–76. doi: 10.1128/MMBR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berman J, Sudbery PE. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat Rev Genet. 2002;3:918–30. doi: 10.1038/nrg948. [DOI] [PubMed] [Google Scholar]
- 10.Braun BR, Kadosh D, Johnson AD. NRG1, a repressor of filamentous growth in C.albicans, is down-regulated during filament induction. EMBO J. 2001;20:4753–61. doi: 10.1093/emboj/20.17.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Sanchez S, Mavor AL, Russell CL, Argimon S, Dennison P, Enjalbert B, et al. Global roles of Ssn6 in Tup1- and Nrg1-dependent gene regulation in the fungal pathogen, Candida albicans. Mol Biol Cell. 2005;16:2913–25. doi: 10.1091/mbc.E05-01-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadosh D, Johnson AD. Induction of the Candida albicans filamentous growth program by relief of transcriptional repression: a genome-wide analysis. Mol Biol Cell. 2005;16:2903–12. doi: 10.1091/mbc.E05-01-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murad AM, d’Enfert C, Gaillardin C, Tournu H, Tekaia F, Talibi D, et al. Transcript profiling in Candida albicans reveals new cellular functions for the transcriptional repressors CaTup1, CaMig1 and CaNrg1. Mol Microbiol. 2001;42:981–93. doi: 10.1046/j.1365-2958.2001.02713.x. [DOI] [PubMed] [Google Scholar]
- 14.Murad AM, Leng P, Straffon M, Wishart J, Macaskill S, MacCallum D, et al. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 2001;20:4742–52. doi: 10.1093/emboj/20.17.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Argimon S, Wishart JA, Leng R, Macaskill S, Mavor A, Alexandris T, et al. Developmental regulation of an adhesin gene during cellular morphogenesis in the fungal pathogen Candida albicans. Eukaryot Cell. 2007;6:682–92. doi: 10.1128/EC.00340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S, Wolyniak MJ, Staab JF, Sundstrom P. A 368-base-pair cis-acting HWP1 promoter region, HCR, of Candida albicans confers hypha-specific gene regulation and binds architectural transcription factors Nhp6 and Gcf1p. Eukaryot Cell. 2007;6:693–709. doi: 10.1128/EC.00341-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staib P, Morschhauser J. Differential expression of the NRG1 repressor controls species-specific regulation of chlamydospore development in Candida albicans and Candida dubliniensis. Mol Microbiol. 2005;55:637–52. doi: 10.1111/j.1365-2958.2004.04414.x. [DOI] [PubMed] [Google Scholar]
- 18.Moran GP, MacCallum DM, Spiering MJ, Coleman DC, Sullivan DJ. Differential regulation of the transcriptional repressor NRG1 accounts for altered host-cell interactions in Candida albicans and Candida dubliniensis. Mol Microbiol. 2007;66:915–29. doi: 10.1111/j.1365-2958.2007.05965.x. [DOI] [PubMed] [Google Scholar]
- 19.Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–28. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakayama H, Mio T, Nagahashi S, Kokado M, Arisawa M, Aoki Y. Tetracycline-regulatable system to tightly control gene expression in the pathogenic fungus Candida albicans. Infect Immun. 2000;68:6712–9. doi: 10.1128/iai.68.12.6712-6719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramage G, Saville SP, Wickes BL, Lopez-Ribot JL. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002;68:5459–63. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984;81:1991–5. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubbard MJ, Sullivan PA, Shepherd MG. Morphological studies of N-acetylglucosamine induced germ tube formation by Candida albicans. Can J Microbiol. 1985;31:696–701. doi: 10.1139/m85-132. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Kohler J, Fink GR. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–6. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- 25.Gimeno CJ, Ljungdahl PO, Styles CA, Fink GR. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68:1077–90. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 26.Ramage G, Vandewalle K, Wickes BL, Lopez-Ribot JL. Characteristics of biofilm formation by Candida albicans. Rev Iberoam Micol. 2001;18:163–70. [PubMed] [Google Scholar]
- 27.Chaffin WL, Lopez-Ribot JL, Casanova M, Gozalbo D, Martinez JP. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol Mol Biol Rev. 1998;62:130–80. doi: 10.1128/mmbr.62.1.130-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molero G, Diez-Orejas R, Navarro-Garcia F, Monteoliva L, Pla J, Gil C, et al. Candida albicans: genetics, dimorphism and pathogenicity. Int Microbiol. 1998;1:95–106. [PubMed] [Google Scholar]
- 29.Lopez-Ribot JL, Casanova M, Martinez JP, Sentandreu R. Characterization of cell wall proteins of yeast and hydrophobic mycelial cells of Candida albicans. Infect Immun. 1991;59:2324–32. doi: 10.1128/iai.59.7.2324-2332.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramage G, VandeWalle K, Lopez-Ribot JL, Wickes BL. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol Lett. 2002;214:95–100. doi: 10.1111/j.1574-6968.2002.tb11330.x. [DOI] [PubMed] [Google Scholar]
- 31.Davis D, Wilson RB, Mitchell AP. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol Cell Biol. 2000;20:971–8. doi: 10.1128/mcb.20.3.971-978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis D. Adaptation to environmental pH in Candida albicans and its relation to pathogenesis. Curr Genet. 2003;44:1–7. doi: 10.1007/s00294-003-0415-2. [DOI] [PubMed] [Google Scholar]
- 33.Ramon AM, Porta A, Fonzi WA. Effect of environmental pH on morphological development of Candida albicans is mediated via the PacC-related transcription factor encoded by PRR2. J Bacteriol. 1999;181:7524–30. doi: 10.1128/jb.181.24.7524-7530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bensen ES, Martin SJ, Li M, Berman J, Davis DA. Transcriptional profiling in Candida albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol Microbiol. 2004;54:1335–51. doi: 10.1111/j.1365-2958.2004.04350.x. [DOI] [PubMed] [Google Scholar]
- 35.Saville SP, Thomas DP, Lopez Ribot JL. A role for Efg1p in Candida albicans interactions with extracellular matrices. FEMS Microbiol Lett. 2006;256:151–8. doi: 10.1111/j.1574-6968.2006.00109.x. [DOI] [PubMed] [Google Scholar]
- 36.Brown DH, Jr, Giusani AD, Chen X, Kumamoto CA. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Mol Microbiol. 1999;34:651–62. doi: 10.1046/j.1365-2958.1999.01619.x. [DOI] [PubMed] [Google Scholar]
- 37.Vinces MD, Haas C, Kumamoto CA. Expression of the Candida albicans morphogenesis regulator gene CZF1 and its regulation by Efg1p and Czf1p. Eukaryot Cell. 2006;5:825–35. doi: 10.1128/EC.5.5.825-835.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giusani AD, Vinces M, Kumamoto CA. Invasive filamentous growth of Candida albicans is promoted by Czf1p-dependent relief of Efg1p-mediated repression. Genetics. 2002;160:1749–53. doi: 10.1093/genetics/160.4.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park YN, Morschhauser J. Tetracycline-inducible gene expression and gene deletion in Candida albicans. Eukaryot Cell. 2005;4:1328–42. doi: 10.1128/EC.4.8.1328-1342.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Csank C, Schroppel K, Leberer E, Harcus D, Mohamed O, Meloche S, et al. Roles of the Candida albicans mitogen activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect Immun. 1998;66:2713–21. doi: 10.1128/iai.66.6.2713-2721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cottier F, Muhlschlegel FA. Sensing the environment: response of Candida albicans to the X factor. FEMS Microbiol Lett. 2009;295:1–9. doi: 10.1111/j.1574-6968.2009.01564.x. [DOI] [PubMed] [Google Scholar]


