Abstract
Head group analogues of the antibacterial and antiparasitic drug Nitazoxanide (NTZ) are presented. A library of 39 analogues was synthesized and assayed for their ability to suppress growth of Helicobacter pylori, Campylobacter jejuni, Clostridium difficile and inhibit NTZ target pyruvate:ferredoxin oxidoreductase (PFOR). Two head groups assayed recapitulated NTZ activity and possessed improved activity over their 2-amino-5-nitrothiazole counterparts, demonstrating that head group modification is a viable route for the synthesis of NTZ-related antibacterial analogues.
Nitazoxanide 1 (NTZ – Fig. 1) is a broad-spectrum FDA approved drug utilized for the treatment of anaerobic intestinal parasites Giardia lamblia and Cryptosporidium parvum but is efficacious in the treatment of other anaerobic bacteria and parasites residing in the human gut.1-4 NTZ has also been shown to have activity against microbial biofilms, rotavirus, influenza A/B/HIN1, hepatitis B/C and Mycobacterium tuberculosis.5-10 Although structurally similar to other nitro-drugs like metronidazole 2 (Fig. 1), the 5-nitro group on the thiazole ring of NTZ is not metabolically reduced. As a result, nitro reduction is not responsible for NTZ activity as is the case for most nitro-containing drugs.3, 11, 12 Resistance to NTZ has not been observed clinically or through in vitro generation methods which further supports a different mechanism of action (MoA).13
Figure 1.
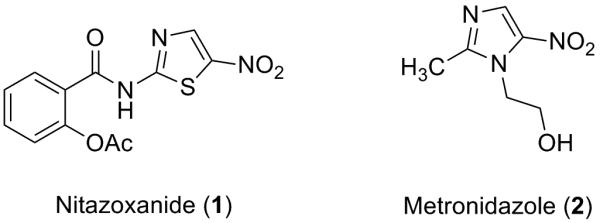
Nitro heterocyclic drugs.
The MoA for NTZ has been elucidated to occur through the anion of NTZ. The NTZ anion abstracts a proton from the thiamine pyrophosphate vitamin cofactor of the essential enzyme pyruvate:ferredoxin oxidoreductase (PFOR), thereby inhibiting production of acetyl-Coenzyme A and CO2 necessary for energy metabolism.14, 15 PFOR is present in all strictly anaerobic bacteria, anaerobic parasites and ε-proteobacteria (Helicobacter pylori and Campylobacter jejuni), while mammals and eubacteria oxidize pyruvate by the NTZ insensitive pyruvate dehydrogenase (PDH) (Fig. 2).14-19 Therefore, PFOR of H. pylori, C. jejuni, and Clostridium difficile is a logical small-molecule drug target and we postulated that modification of the 2-amino-5-nitrothiazole portion of NTZ would potentiate analogue activity.
Figure 2.
Pyruvate:ferredoxin oxidoreductase (PFOR) enzymatic reaction. PFOR catalyzes the oxidative decarboxylation of pyruvate producing Acetyl-CoA and CO2 requiring the electron acceptors ferredoxin (Fd) or flavodoxin (Fld). Oxidation of Fd/Fld is accomplished by NADP oxidases or hydrogenases, respectively. Solid arrows indicate the forward reaction; hollow arrows indicate reverse reaction.
The key interaction responsible for biological activity is believed to occur between NTZ and the TPP vitamin co-factor (Fig. 3).14 NTZ was shown to completely prevent the production of both acetyl-CoA and CO2 indicating that inhibition occurs very early in the catalytic cycle. In this system only the anion of NTZ is biologically active and becomes inactive upon protonation. Additionally, NTZ is not chemically modified during the enzymatic reaction and is only protonated, allowing NTZ to be utilized again after equilibration.14 NMR analysis revealed four resonance structures existing simultaneously for the anion involving the nitro, N2′ and N3 nitrogens and the amide oxygen.14 The amide (pKa 6.18) anion (Fig. 3) or the other resonance forms are predicted to interact with the N4′ of the pyrimidine ring and prevent pyruvate binding. NTZ has a Ki of ~5 × 10−6 M with PFOR which is roughly two orders of magnitude better than pyruvate (Km = ~3 × 10−4 M) providing evidence that NTZ is a competitive inhibitor of PFOR.14
Figure 3.
Pyruvate binding to activated vitamin co-factor thiamine pyrophosphate (TPP) which then is consumed in the production of acetyl-CoA. NTZ is thought to bind to TPP in an analogous fashion to pyruvate and prevent its binding thereby inhibiting the enzymatic reaction.
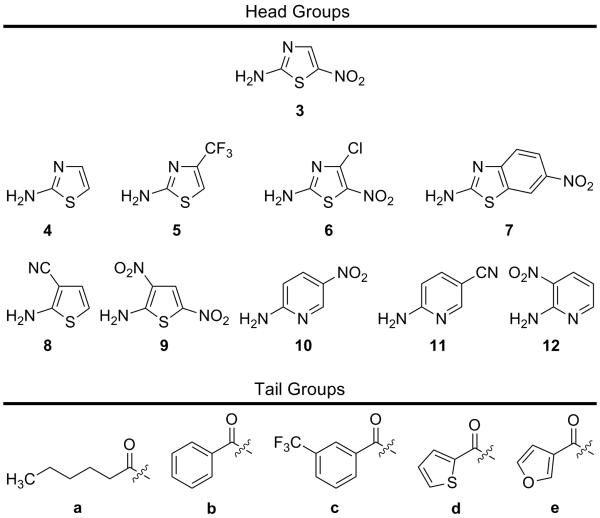
Owing to the structural simplicity of NTZ we postulated that modifications of the benzene ring moiety (tail region) and thiazole (head group) could yield increased antibacterial properties. Previous research in this area has determined that the 2-amino-5-nitrothiazole ring 3 is well suited for activity yet has been problematic to replace or modify to obtain increased activity. In an effort to further test this conclusion, we aimed to synthesize a small library of NTZ-head group analogues based on five simple amide tail region analogues. Through the synthesis of a library of ~100 NTZ-tail region analogues, five diverse tail regions were chosen to screen new head groups for comparison against NTZ and the 2-amino-5-nitrothiazole 3 analogues, respectively (Fig. 4).
Figure 4.
Composition of library separated as head group and tail groups.
The head groups to be tested were mainly comprised of electron-withdrawing substituted heterocycles in hopes of mimicking either the electronic or spatial characteristics of 3. Previous work by Rossignol, Hemphill and Appelbaum has clearly shown that the 5-nitro group is necessary for activity and its removal and modification tend to reduce activity against PFOR organisms but may increase activity against other organisms or viruses.4, 20-22 Unsubstituted 2-aminothiazole 4 was planned as a control head group that should possess no activity while the other head groups in question had not been previously reported.
All of the head groups were available through commercial sources except for 6 which was synthesized as previously reported (Fig. 4).23, 24 Synthesis of the NTZ-head group analogue library was accomplished through amide bond coupling of the acid chloride/carboxylic acid of tail regions a-e with the respective head group 3-12 (Fig. 4). As most of the head groups are strongly electron withdrawing, nucleophilicity of the amine head group was reduced resulting in low to moderate yields (supplementary data). Following synthesis, the head group library was subjected to in vitro minimum inhibitory concentration (MIC) testing against H. pylori, C. jejuni and C. difficile followed by in vitro direct PFOR enzyme inhibition.
H. pylori is a microaerophilic gram-negative bacterium that resides in the gastric mucosa. It has been linked to duodenal and peptic ulcer disease and a risk factor for gastric cancer.25 C. jejuni is also a microaerophilic gram-negative bacterium and causes severe lower gastrointestinal infections in mammals.26 C. difficile is a gram-positive bacterium that naturally resides in gut flora.27, 28 Many broad-spectrum antibiotics deplete natural floral enabling C. difficile to dominate and produce severe enterocolitis. Recently, C. difficile infections have become more challenging due to increased antibiotic resistance worsened by increased recurrence rates of infection.27 Current therapies to treat these infections are problematic and new drugs acting through different MoAs are necessary for continued treatment.
All library members were screened for activity against H. pylori and C. jejuni using a standard dilution procedure to determine MIC values. E. coli MIC screening was also incorporated to preclude activity against PDH utilizing organisms. Selected analogues were then further tested for their ability to inhibit C. difficile and directly inhibit the PFOR enzyme. Activity for selected active analogues, their respective 3a-e counterparts and NTZ (1) is summarized in Table 1. Activity for all analogues synthesized is summarized in Supplemental Table S1.
Table 1.
Selected NTZ-head group Analogue Activity
| MIC’s (μM) | ||||
|---|---|---|---|---|
| Analogue | H. pylori | C. jejuni | C. difficile | PFOR Inhibition [Drug] = 40 μM (%) |
| Nitazoxanide (1) | 13.0 | 39.1 | 0.8 | 54 ± 7 |
| 3a | 0.5 | 2.1 | 8.2 | 70 |
| 3b | 1.3 | 3.0 | 32.1 | 53 ± 8 |
| 3c | 3.5 | 4.7 | 0.4 | 47 |
| 3d | 2.9 | 2.9 | 1.0 | 50 |
| 3e | 0.8 | 12.5 | 2.1 | 56 |
| 6a | 0.9 | 3.6 | 28.8 | 63a |
| 6b | 8.8 | 7.0 | >28.2 | 59 |
| 6c | 5.7 | 34.1 | >22.7 | 27 ± 2 |
| 6d | 6.9 | 13.8 | >13.8 | 51 |
| 9a | 2.6 | 0.9 | 27.8 | 80 ± 8a |
| 9b | 1.3 | 0.9 | 27.3 | 55 |
| 9c | 0.5 | 2.1 | 4.2 | 47 |
| 9d | 2.5 | 1.0 | 20.0 | 77 |
| 9e | 2.6 | 0.4 | 3.5 | 66 ± 13a |
Complex pattern of inhibition with two different rates.
Overall, most head group analogues generated were inactive against the target organisms with high double to triple digit μM MIC values. This result was not unexpected as this trend for inactivity has been reported.20-22 We did note minor activity with 5 and 12 but the activity was not consistent across the tail regions (supplementary data). Although many of the analogues were inactive, we did discover two head groups (6a-d and 9a-e) that possessed notable activity against the target organisms with all tail regions. All library members were also completely inactive against E. coli indicating that PDH is not being targeted (data not shown).
The head group analogues 6a-d are direct derivatives of 3 with 6 bearing an additional 4-chloro substituent. The 4-chloro substituent imparts both steric and electronic effects on the thiazole, notably the 5-nitro group. A slight reduction in activity for 6 compared to 3 (H. pylori, C. jejuni MIC’s and PFOR) is most likely attributable to the steric interaction with the 5-nitro group forcing the nitro slightly out-of-plane with the thiazole ring, lowering the resonance stability of the amide anion. More pronounced loss of activity for C. difficile for these analogues may be attributed to minor differences in enzyme and binding pocket structure. Further studies to support substitution at the 4-position employing a 4-methyl or 4-bromo substituent are currently under investigation.
Recapitulation of bio-activity compared to 3a-e with 9a-e was unanticipated but appreciated. Dinitrothiophene 9 replaced thiazole 3 and improved activity for all analogues compared to 3 against H. pylori and C. jejuni with comparable activities against C. difficile. Thiophenes 9a-e were subsequently assayed for their ability to directly inhibit PFOR compared to 3a-e, and NTZ. Nearly all of the thiophene analogues 9a-e inhibited PFOR to an equal or greater extent then NTZ or 3a-e respectively. Of note, several of the 9a-e analogues inhibited PFOR at different inhibition rates possibility indicating that nitroreduction via PFOR may be occurring. To test this, thiophenes 9a-e were subjected to an NfsB nitroreductase assay to determine if these analogues were substrates for nitroreduction. Compared to NTZ, analogues 9b-e were only slightly more susceptible to nitroreduction compared to a good substrate (nitrofurazone), however analogue 9a showed higher rates of nitroreduction (supplementary data).
Analogue 9a possessing an increased risk for nitroreduction and inhibition of PFOR through two rates may indicate multiple MoAs that are responsible for the activity of this analogue. For analogues 9b-e, the nitroreduction assay results indicate that the dinitrothiophene analogues are more stable than anticipated and that activity against PFOR utilizing organisms is most likely due to direct PFOR inhibition (like NTZ). We postulate that the double presentation of resonance stabilized nitro-groups for 9a-e is responsible for activity and efforts to synthesize and assay mononitro thiophene derivatives are currently being studied.
Through this study we discovered two unique and active 2-amino-5-nitrothiazole surrogate head groups from a library of 39 analogues. We also identified 2-amino-5-nitrothiazole analogues (3a-e) that displayed better activity than the parent compound NTZ (1) against PFOR utilizing microorganisms. While most of the library members were inactive, head group 6 was discovered to be only slightly less active while analogues of 9 were equipotent or more active compared to NTZ or 3. Direct PFOR enzyme inhibition was also noteworthy for 9 although some activity may be related to nitroreduction activity. Overall we have been able to show that head group modification is a viable route for the synthesis of NTZ-related antibacterial analogues. Further modifications of 6 and 9 are currently under investigation as well as linkage of tail regions with increased activity profiles. Disclosure of the full tail region library and biological activity will be presented in due course.
Supplementary Material
Acknowledgements
This work was supported by U01 grant AI075520 from the National Institute of Allergy and Infectious Diseases to PSH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Data
Complete analogue activity table, full biochemical and chemical experimental procedures, spectral data for all new compounds and representative spectra. This material is available free of charge via the Internet at xxxx.
References
- 1.Gilles HM, Hoffman PS. Trends Parasitol. 2002;18:95. doi: 10.1016/s1471-4922(01)02205-x. [DOI] [PubMed] [Google Scholar]
- 2.Ortiz JJ, Chegne NL, Gargala G, Favennec L. Trans. R. Soc. Trop. Med. Hyg. 2002;96:193. doi: 10.1016/s0035-9203(02)90301-9. [DOI] [PubMed] [Google Scholar]
- 3.Anderson VR, Curran MP. Drugs. 2007;67:1947. doi: 10.2165/00003495-200767130-00015. [DOI] [PubMed] [Google Scholar]
- 4.Pankuch GA, Appelbaum PC. Antimicrob. Agents Chemother. 2006;50:1112. doi: 10.1128/AAC.50.3.1112-1117.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossignol J-F, Abu-Zekry M, Hussein A, Santoro MG. The Lancet. 2006;368:124. doi: 10.1016/S0140-6736(06)68852-1. [DOI] [PubMed] [Google Scholar]
- 6.Shamir ER, Warthan M, Brown SP, Nataro JP, Guerrant RL, Hoffman PS. Antimicrob. Agents Chemother. 2010;54:1526. doi: 10.1128/AAC.01279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tchouaffi-Nana F, Ballard TE, Cary CH, Macdonald TL, Sifri CD, Hoffman PS. Antimicrob. Agents Chemother. 2010 doi: 10.1128/AAC.00901-09. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossignol J. F. o., La Frazia S, Chiappa L, Ciucci A, Santoro MG. J. Biol. Chem. 2009;284:29798. doi: 10.1074/jbc.M109.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossignol J-F, Elfert A, El-Gohary Y, Keeffe EB. Gastroenterology. 2009;136:856. doi: 10.1053/j.gastro.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 10.de Carvalho LPS, Lin G, Jiang X, Nathan C. J. Med. Chem. 2009;52:5789. doi: 10.1021/jm9010719. [DOI] [PubMed] [Google Scholar]
- 11.Broekhuysen J, Stockis A, Lins RL, De Graeve J, Rossignol JF. Int. J. Clin. Pharmacol. Ther. 2000;38:387. doi: 10.5414/cpp38387. [DOI] [PubMed] [Google Scholar]
- 12.Olekhnovich IN, Goodwin A, Hoffman PS. FEBS J. 2009;276:3354. doi: 10.1111/j.1742-4658.2009.07060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Megraud F, Occhialini A, Rossignol JF. Antimicrob. Agents Chemother. 1998;42:2836. doi: 10.1128/aac.42.11.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman PS, Sisson G, Croxen MA, Welch K, Harman WD, Cremades N, Morash MG. Antimicrob. Agents Chemother. 2007;51:868. doi: 10.1128/AAC.01159-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sisson G, Goodwin A, Raudonikiene A, Hughes NJ, Mukhopadhyay AK, Berg DE, Hoffman PS. Antimicrob. Agents Chemother. 2002;46:2116. doi: 10.1128/AAC.46.7.2116-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cremades N, Bueno M, Toja M, Sancho J. Biophysical Chemistry. 2005;115:267. doi: 10.1016/j.bpc.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 17.Evans MC, Buchanan BB, Arnon DI. Proc. Natl. Acad. Sci. U. S. A. 1966;55:928. doi: 10.1073/pnas.55.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman PS, Goodman TG. J. Bacteriol. 1982;150:319. doi: 10.1128/jb.150.1.319-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kletzin A, Adams MW. J. Bacteriol. 1996;178:248. doi: 10.1128/jb.178.1.248-257.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gargala G, Le Goff L, Ballet J-J, Favennec L, Stachulski AV, Rossignol J-F. Antimicrob. Agents Chemother. 2010 doi: 10.1128/AAC.00614-09. DOI:10.1128/AAC.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stadelmann B, Scholl S, Muller J, Hemphill A. J. Antimicrob. Chemother. 2010;65:512. doi: 10.1093/jac/dkp490. [DOI] [PubMed] [Google Scholar]
- 22.Rossignol JF. 20080097106 US. 2008
- 23.Beck G, Reinecke P, Paulus W, Schmitt H-G. 4698357 US. 1987
- 24.Beck G, Dutzmann S, Brandes W, Paulus W. 5071865 US. 1991
- 25.Fuccio L, Zagari RM, Minardi ME, Bazzoli F. Aliment. Pharmacol. Ther. 2007;25:133. doi: 10.1111/j.1365-2036.2006.03183.x. [DOI] [PubMed] [Google Scholar]
- 26.Young KT, Davis LM, DiRita VJ. Nat. Rev. Micro. 2007;5:665. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- 27.Kelly CP, LaMont JT. N. Engl. J. Med. 2008;359:1932. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 28.Mylonakis E, Ryan ET, Calderwood SB. Arch. Intern. Med. 2001;161:525. doi: 10.1001/archinte.161.4.525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.