Abstract
Purpose
This study evaluates the effect of a sequential culture system on the follicular development of sheep lamb ovaries, aiming to establish an available in vitro culture system for ovarian culture.
Methods
Lamb ovarian cortical fragments were cultured on a steel mesh with a nitrocellulose membrane pre-coated by type1 collagen. Several culture media were used for the determinations, specifically, a control medium (α-MEM), a constant medium (control medium supplemented with 75 ng/mL human recombinant EGF, 200 mIU/mL sheep FSH, 100 ng/mL human recombinant GDF-9, and 100 ng/mL human recombinant bFGF), and a sequential medium (control medium supplemented with sequential growth factors added on different days). Ovarian tissues, both fresh and cultured, were processed for histological and apoptotic assays, while spent culture media were processed for hormone assays.
Results
It was found that the growth of lamb primordial follicles can be initiated during culture in vitro. Compared to the control medium, sequential culture medium significantly increased the percentage of secondary follicles in cultures, while the follicle and oocyte diameters of primary and secondary follicles were also observed to increase in this medium. The constant medium was found to increase the number and diameter of secondary follicles only 18 days after culture. After this same period of time, some normal antral follicles were found in the sequential medium, while a few abnormal antral-like follicles were found in the control medium. Moreover, sequential medium appeared to significantly increase estradiol and inhibin production, especially 10–18 days after culture. The highest percentage of normal follicles and the lowest apoptotic cell rates were observed in the sequential medium, suggesting that a sequential addition style of culture can improve follicle and tissue viability.
Conclusions
The sequential addition of FSH, EGF, GDF-9, and bFGF can stimulate primordial follicle transmittal into the later development stages, even as far as the antral stage, improve the survival rate of follicles, and maintain follicular viability.
Keywords: In vitro culture, Sequential medium, Lamb ovary, Hormone, Growth factor, Follicle
Introduction
A large number of quiescent primordial follicles, which represent the reproductive potential of females, are present in mammalian ovarian tissues. However, this number is known to be of a certain amount and is non-renewable; it also depletes gradually in a female’s reproductive life. Thus, identifying how to utilize these follicles to improve reproductive potential is a considerably interesting topic.
Of the methods available, culture of ovarian follicles in vitro is an option that could provide a large potential oocyte source to assist reproductive techniques. To date, however, there is little knowledge about the factors controlling the initiation of primordial follicles. Similarly, the various influences on the growth factors in ovarian follicle development have not been very uniform among different species. Studies have demonstrated the involvement of follicle-stimulating hormone (FSH) [1–3], growth and differentiation factor 9 (GDF-9) [4–7], basic fibroblast growth factor (bFGF) [8–10], insulin [11], and epidermal growth factor (EGF) [12–14] in early follicular development. Other factors that were incorporated in the present study are based on these references.
Generally, the model for studying follicular development in vitro involves the culture of ovarian cortex slices or isolated follicles in vitro. The culture of isolated follicles in vitro from an ovarian cortex slice culture, a two-step culture system, was also reported by Telfer et al. [15]. An in vivo model, which grafted cattle ovarian pieces into the chorioallantoic membrane (CAM) of chick embryos, was reported by Cushman et al. [16]. In ovarian cortex slice study models, it has been reported that membranes coated with an extracellular matrix can stimulate moderate follicle survival in organ cultures [17, 18]. Given this knowledge, the culture system used in this study involved a stainless steel mesh with a nitrocellulose membrane pre-coated with collagen.
We designed a sequential culture medium by which different concentrations of FSH, GDF-9, EGF, and bFGF were added during different culture days. The culture cycle of the sequential addition spanned 18 days. The oestrous cycle of sheep spans about 17 days and are interested in the in vitro follicular development in an oestrous cycle. As such, selecting a culture cycle of 18 days seems reasonable. Histological and TUNEL analyses were performed on the cultures to evaluate the development potency of primordial follicles, follicle morphology, and follicle viability. Estradiol, progesterone, and inhibin levels were also measured to evaluate endocrine functions and tissue viability during culture.
Materials and methods
Source of the ovaries
Female lamb (n = 6, 3–6 months old) ovarian tissues (n = 12) were obtained from a local abattoir and transported to the laboratory in phosphate buffered solution (PBS) at 27–32°C within 2 h of acquisition. In the laboratory, the ovaries were rinsed once with 70% alcohol (5–10 s) under a laminar flow hood, and then rinsed twice with PBS supplemented with 5% bovine serum albumen (BSA) and 50 IU/mL antibioticin. The ovaries were then stripped off with their surrounding fatty tissue and ligaments and cut in half. Next, the cortex was dissected from the medullar tissue and cut into 0.5–1 mm3 pieces using a scalpel under a steromicroscope, after which fine slices (n = 26) were selected. Two to three pieces from each ovary were immediately fixed with 4% PBS-paraformaldehyde; these were used as the non-cultured control. The other slices (n = 24) were randomly divided and used in in vitro culture procedures. Unless otherwise stated, all media and culture reagents were obtained from Sigma Chemical Corp. (USA).
Experimental protocol
The fine ovarian cortex tissue pieces were rinsed three times in α-MEM medium and cultured in a steel mesh culture system. Briefly, prior to the culture, the steel grid was cut into an approximate size of 25 mm × 25 mm. The four angles were bended to about 4 mm in height to form a scaffold. The grid was soaked for 24 h in 75% alcohol, and then dried for 8 h at 160°C. During the culture proper, the surface of the scaffold was confirmed to be horizontal. The scaffold was placed in a culture dish and a similarly sized nitrocellulose membrane pre-coated with type І collagen (3 mg/mL) was put onto the scaffold. Four fine slices of ovarian cortical tissue were placed on each nitrocellulose membrane at a distance of about 5 mm from each other. About 20 μL of the culture medium was dropped onto each slice. Finally, more culture medium was added into the dish until the surface of the culture medium minimally submerged the cortical slices (parallel to the surface of the nitrocellulose membrane). The control medium was composed of α-MEM medium supplemented with glutamine (2 mM), pyruvate (3 mM), BSA (3 mg/mL), ITS (insulin 10 μg/mL, transferrin 6.25 μg/mL, and selenium 6.25 ng/mL), penicillin G (75 μg/mL), and streptomycin (50 μg/mL). For the experimental cultures, constant and sequential culture media were used. The constant medium was composed of the control medium supplemented with 75 ng/mL human recombinant EGF(Sigma Chemical Corp., USA), 200 m IU/mL sheep FSH(Invitrogen, USA), 100 ng/mL human recombinant GDF-9(Invitrogen, USA), and 100 ng/mL human recombinant bFGF(Invitrogen, USA). The composition of the sequential culture medium tested in the present study is shown in Table 1. Ovarian cortical pieces were cultured at 38.6°C in humidified air with 5% carbon dioxide (CO2) for 18 days. Media were changed every two days. Every six days during the culture period, two cortical strips from each culture medium were removed and fixed in 4% PBS-paraformaldehyde for histological and immunohistochemistry analyses (TUNEL).
Table 1.
Composition of sequential culture system
| Day of culture | Composition | |||
|---|---|---|---|---|
| EGF | FSH | GDF-9 | bFGF | |
| ng/mL | mIU/mL | ng/mL | ng/mL | |
| 2 | 25 | 50 | 200 | 150 |
| 4 | 50 | 50 | 200 | 150 |
| 6 | 50 | 100 | 150 | 100 |
| 8 | 75 | 200 | 150 | 100 |
| 10 | 75 | 300 | 100 | 100 |
| 12 | 100 | 400 | 100 | 50 |
| 14 | 100 | 500 | 50 | 50 |
| 16 | 150 | 500 | 25 | 25 |
| 18 | 150 | 500 | 0 | 0 |
Histological analyses
Ovarian cortical tissue fragments were fixed in 4% PBS-paraformaldehyde for 8–24 h at 0, 6, 12, and 18 days after culture in the three culture media. Pieces of ovarian tissues were embedded in paraffin wax, serially sectioned at 4 μm, and then stained with haematoxylin and esosin. Follicular development stages and their survival were determined microscopically at every tenth section via a microscope using 400 × magnification.
Follicles classified [19] as primordial (one layer of flattened and/or small cuboidal somatic cells around the oocyte) or developing follicles were further categorized according to the following: (1) primary follicles with a single layer of large cuboidal granulosa cells around the oocyte; (2) secondary follicles with two or more complete layers of granulosa cells without antrum formation; and (3) antral follicles where the oocyte is encapsulated by a multi-layer of granulosa cells and an antrum has formed. In addition, these follicles were further classified as (i) histologically normal follicles (i.e., follicles spherical in shape with an even distribution of granulosa cells, intact theca, and spherical oocytes) and (ii) degenerated follicles (i.e., follicles exhibiting esosinophilia of the ooplasm, contraction and clumping of the chromatin, and wrinkling of the nuclear membrane). To avoid counting a follicle more than once, only follicles with visible oocyte nuclei were counted. Overall, 180 follicles were evaluated for each medium and culture period (30 follicles per treatment × 6 repetitions=180 follicles).
To evaluate the rates of follicle activation and growth, only histologically normal follicles were observed. In addition, for each follicle category, oocyte and follicle diameters were measured by a computer-driven image analysis program (Nikon NIS-Elements 3.0, 2008). Each follicle was measured in two dimensions; the two measurements were averaged.
TUNEL assay
Paraformaldehyde-fixed ovarian sections were deparaffinized, rehydrated, and incubated with 20 μg/mL proteinase k in 10 mM Tris-HCl for 20 min (pH 7.4 at 37°C). After washing thrice with PBS (5 min per wash), the slides were incubated with 3% hydrogen peroxide for 20 min, followed by a three-time rinse with PBS. The slides were then stained for identification of apoptotic cells via the TUNEL method using an in situ cell death detection kit (KeyGEN, China; CAT number: KGA7022). Briefly, the slides were incubated with 50 μL terminal deoxynucleotidyl transferase (TdT) enzyme reaction mixture (45 μL equilibration buffer, 1 μL Biotin-11-d UTP, 4 μL TdT Enzyme) in a humidified chamber at 37°C for 60 min, followed by a three-time rinse with PBS. The slides were incubated with a streptavidin-HRP work solution in a humidified chamber at 37°C for 30 min. After washing thrice with PBS, the products of the immunoreaction were visualized via incubation with diaminobenzidine. Sections were then counterstained with hematoxylin. Control staining was performed by incubating the sections with a TdT enzyme reaction mixture without the enzyme. A brown nuclei appearance indicates the presence of apoptotic cells, while blue nuclei indicate the presence of viable cells. To evaluate follicle apoptosis, only follicles with a visible nucleolus were counted. To assess stromal cell death, the number of apoptotic and viable cells was counted. The apoptotic rate was calculated as
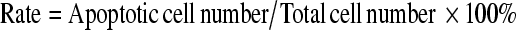 |
Assessment of steroid hormone and inhibin production
The spent medium was collected every 2 days during a cultivation period of 18 days and stored at −20°C for subsequent hormone assays. The levels of estradiol-17β (R&D SYSTEMS, USA, sensitivity = 3.9 pg/mL) and progesterone (R&D SYSTEMS, USA, sensitivity = 0.1 ng/mL) were measured by an enzyme immunoassay modified for the cell culture media. Inhibin was measured with a commercial immunoenzymatic assay using antibodies directed against the α-subunit (R&D SYSTEMS, USA, sensitivity=20 pg/mL).
Statistical analysis
Oocyte and follicular diameters, proportion of follicle stages, and viability and cell apoptotic rates were calculated. An ANOVA, followed by post-hoc testing, was used to evaluate the treatment effects on the abovementioned parameters. Chi-square test was used to compare the production of hormones and inhibin. Prior to analyses, the data were tested for normality and homogeneity of variance. All data are presented as mean ± S.D. P-values of <0.05 were considered significant. All computations were performed using SPSS 13.0 for Windows.
Results
In vitro follicle growth
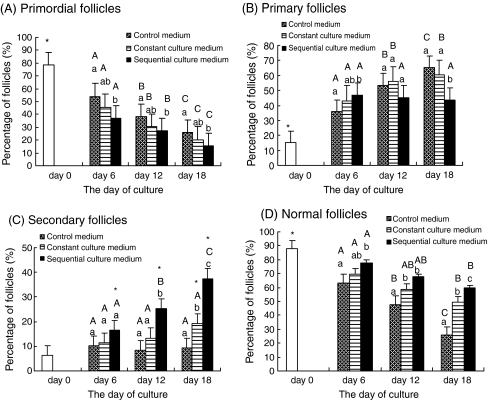
The percentage of primordial and developing follicles in ovarian cortical tissue at 0, 6, 12, and 18 days after culture are shown in Fig. 1a–c. After all cultures in the media were tested, it was observed that the proportion of primordial follicles (Fig. 1a) was reduced significantly, an outcome of a coincident increase in the proportion of developing follicles. Additional progressive reduction in the proportion of primordial follicles was also observed with increasing culture time. Compared to the culture media, the proportion of primordial follicles in the sequential medium was significantly lower vis-à-vis the control medium. The difference in proportions was not significant between the constant medium and control or sequential media.
Fig. 1.
The percentage of a primordial; b primary; c secondary; and d normal follicles at 0, 6, 12, and 18 days after culture. Note: * – Significantly different from non-cultured tissue (P < 0.05). a, b, c – Significantly different between culture media on the same day of culture (P < 0.05). A, B, C – Significantly different between days of culture in the same medium (P < 0.05)
Compared to day 0, the percentage of primary follicles (Fig. 1b) was significantly higher in all culture media after 6, 12, and 18 days of culture in vitro. After 6 days of culture, the percentage of primary follicles in the sequential medium was higher than in the control, but not significantly higher than in the constant medium. After 18 days of culture, this value was lower for the sequential medium than for either the control or constant culture medium. The primary follicle percentage in the sequential medium did not significantly change with increasing culture time, but it was significant increased in both the control and constant media. In terms of the percentage of secondary follicles (Fig. 1c), only the culture in the sequential medium obtained a significant increase relative to the non-cultured tissue, as well as the control and constant media. With increasing culture time, significant increases of the secondary follicles percentage were also observed in the sequential medium.
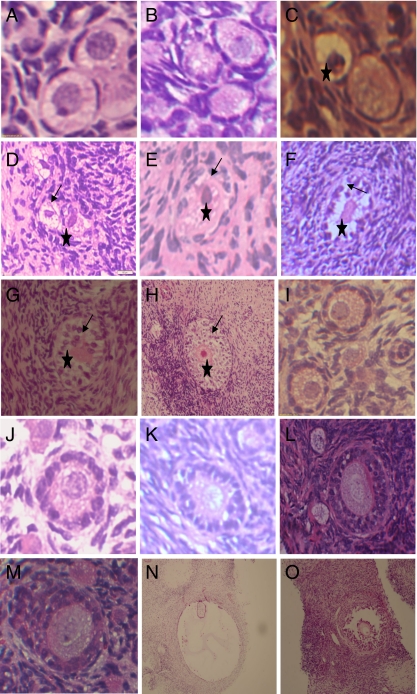
Small antral follicles were observed in all culture media 18 days after culture, although the results obtained no significant meaning in statistical analyses. The number of antral follicles 18 days after culturing in the control, constant, and sequential media were fifteen, five, and three, respectively. More morphologically normal and large-diameter antral follicles were found in the sequential medium (Figs. 2 and 3o), and antrum-like follicles in the control system seemed to have a large empty cavity with little granular cells and degenerated oocyte or no oocyte (Figs. 3n and 4).
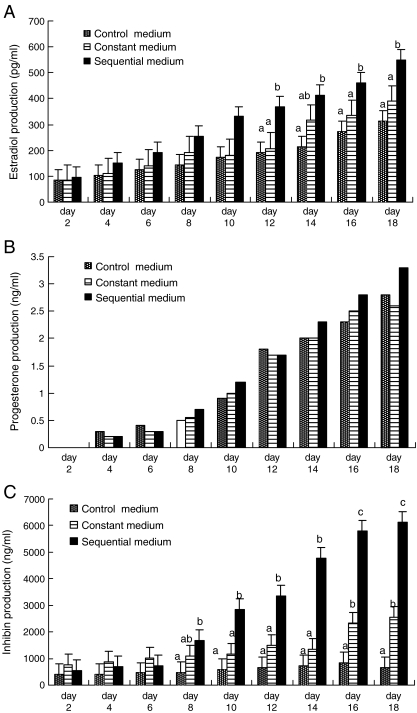
Fig. 2.
The production of a estradiol; b progesterone; and c inhibin in control, constant, and sequential media during culture. Note: a, b, c – Significantly different between culture media on the same day of culture (P < 0.05)
Fig. 3.
Histological section of normal primordial follicles in fresh tissue (3-A) and cultured for 6 days in control (3-B) and sequential medium (3-C); Degenerated primordial follicles cultured for 12 days in control (3-D) and constant (3-E) or sequential (3-C) medium, or degenerate primary follicle cultured for 18 days in constant medium (3-F), or degenerater secondary follicle cultured for 18 days in control (3-G) and sequential medium (3-H); Normal primary follicles cultured for 6 days in control (3-I), and constant (3-J) or sequential medium (3-K) ; Normal secondary follicle cultured for 18 days in constant medium (3-L) or 12 days in sequential medium (3-M); Antral-like follicle cultured for 18 days in control medium (3-N) or antral follicle cultured for 18 days in sequential medium (3-O). Note: Pycnotic nucleus (asterisk) and irregular granulosa cells (arrow); 3-A-M × 400, 3-N, O × 100
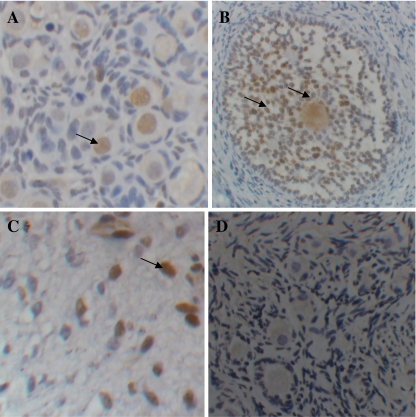
Fig. 4.
TUNEL labeling sections showing a apoptotic positive primordial follicles after 6 days of culture in control medium; b preantral follicles cultured for 18 days in sequential medium; c stromal cells after 18 days of culture in constant medium(C); and d negative control with no TUNEL positive labeling cell. Arrows show apoptotic cells with brown nuclei
After culturing in the sequential and constant media, a significant increase in the diameter for both oocytes and follicles were seen in primary follicles, as compared to the size of their non-cultured counterparts in the control medium (Table 2). The diameters of secondary follicles and oocytes also increased in the two culture media, as compared to the non-cultured tissue. Six days into the culture period, we observed that the diameters of secondary follicles and oocytes in the constant medium were not statistically significantly. After 6, 12, and 18 days of culture, among the culture media studied, tissues cultured in the sequential medium resulted in significantly increased oocyte and follicle diameters for the primary and secondary follicles relative to those in the control medium. Larger follicle and oocyte diameters were also observed in the sequential medium vis-à-vis in the constant medium, while larger follicle and oocyte diameters were observed in the constant medium vis-à-vis in the control medium. Primary follicle and oocyte diameters at 6 and 12 days after culture and secondary follicle and oocyte diameters at 6 days after culture yielded no significant difference between the two culture media. Compared to the findings for Day 6, at 18 days after culture, the sequential medium was also found to have promoted a significant increase in primary oocyte diameters and secondary follicle and oocyte diameters. Similarly, the constant medium promoted only increased secondary follicle and oocyte diameters 18 days after culture.
Table 2.
Mean follicle and oocyte diameters of each follicular stage in the female lamb ovarian cortex in non-cultured tissue and at 6, 12, and 18 days after culture in control, constant, and sequential media
| Day of culture and culture medium | Follicle and oocyte diameter | |||||
|---|---|---|---|---|---|---|
| Primordial (n = 30) | Primary (n = 30) | Secondary (n = 20) | ||||
| Follicle | Oocyte | Follicle | Oocyte | Follicle | Oocyte | |
| 0 day non-cultured | 27.8 ± 0.7 | 25.0 ± 0.8 | 34.1±1.1 | 26.5 ± 0.6 | 54.4±1.6 | 33.7±1.3 |
| After 6 days of culture | ||||||
| Control medium | 27.5 ± 0.4 | 24.5 ± 0.6 | 37.4±1.4a | 28.0±1.1a | 57.6 ± 4.8a | 34.5±2.1a |
| Constant medium | 28.1±0.6 | 25.3 ± 0.4 | 41.1±2.1ab* | 32.0 ± 3.2ab* | 59.1±3.7Aab | 36.4±1.9Aab |
| Sequential medium | 29.2±0.6 | 25.6 ± 0.7 | 45.2±2.3b* | 33.5±2.7Ab* | 63.2±4.3Ab* | 41.6±2.6Ab* |
| After 12 days of culture | ||||||
| Control medium | 27.0±1.0 | 24.8 ± 0.5 | 38.6 ± 0.9a | 28.5±1.5a | 58.6 ± 5.4a | 35.7±1.7a |
| Constant medium | 27.9 ± 0.5 | 25.6 ± 0.8 | 41.5±2.3ab* | 33.7±2.8ab* | 63.3 ± 6.4Aab* | 38.6±2.2Aa* |
| Sequential medium | 28.4 ± 0.7 | 26.0 ± 0.7 | 46.1±2.4b* | 36.6 ± 3.6ABb* | 87.7 ± 8.9Bc* | 44.7 ± 3.6Bb* |
| After 18 days of culture | ||||||
| Control medium | 28.1±0.4 | 25.3 ± 0.9 | 38.0±2.7a | 28.7 ± 0.9a | 61.2±5.3a* | 37.7±2.8a |
| Constant medium | 28.6 ± 0.3 | 26.2±0.7 | 44.2±3.2b* | 34.2±3.5b* | 79.3 ± 7.6Bb* | 42.3 ± 3.8Bb* |
| Sequential medium | 29.1±1.2 | 25.9 ± 0.7 | 49.4 ± 3.9c* | 39.6±1.4Bc* | 102.4±15.7Cc* | 52.5 ± 5.6Cc* |
*Significantly different from non-cultured tissue (P < 0.05)
a, bSignificantly different between culture media on the same day of culture (P < 0.05)
A, B – Significantly different between days of culture in the same medium (P < 0.05)
Assessment of in vitro follicle vitality
Histological analyses showed that normal (Fig. 3a, b, c, i, j, k, l, m) and degenerated follicles (Fig. 3d, e, f, g, h) were found in non-cultured and cultured ovarian cortical slices in all culture media. In the degenerated follicles, shrunken oocytes, pyknotic nuclei, and disorganized granulosa cells were observed. Figure 1d shows the percentage of healthy follicles in the non-culture and cultured ovarian cortical samples at 6, 12, and 18 days after culture in the three media. A significant reduction in the percentage of histologically normal follicles was found (P < 0.05) in all tissue samples cultured in the different media vis-à-vis the non-cultured tissue. The proportion of normal follicles was higher in the sequential medium than in the control and constant media. The difference, however, was not significant between the sequential and constant media at 6 and 12 days after culture. A higher percentage of normal follicles were also observed in the constant medium as compared to that in the control medium 12 and 18 days after culture. Compared to observations during Day 6, the percentages of normal follicles in all culture media were lower at 18 days after culture.
The results of the TUNEL assay are shown in Table 3. In non-cultured tissues, the proportion of TUNEL-positive follicles and stromal cells were 0 and 5.7 ± 1.1, respectively. The lowest proportions of apoptotic follicles and stromal cells were observed in the sequential medium. Compared to the control medium, the sequential and constant media significantly inhibited stromal cells from becoming apoptotic at 18 days after culture. There was no significant difference between the sequential and constant media in terms of proportion of apoptotic cells.
Table 3.
The apoptotic rates (number of apoptotic cells/total cells number) of follicles and stromal cells in non-cultured tissue and tissues at 6, 12, and 18 days after culture
| Culture medium | Apoptotic rate of follicles % | Apoptotic rate of stromal cells % (total stromal cell) | ||||
|---|---|---|---|---|---|---|
| Day of culture | Non-cultured tissue: 0 (0/23) | Non-cultured tissue: 5.7±1.1%(432) | ||||
| Control | Constant | Sequential | Control | Constant | Sequential | |
| 6 day | 7.5(5/67) | 4.1(3/73) | 3.4(2/61) | 13.3 ± 3.2a(571) | 9.4±2.5ab (600) | |
| 12 day | 11.3(6/53) | 6.3(4/63) | 6.7±2.0b(620) | |||
| 5.4(4/74) | 21.4 ± 3.7a*(631) | 12.7 ± 3.1b(628) | ||||
| 18 day | 18.8(8/48) | 7.1(5/70) | 9.5±1.6b(675) | |||
| 5.6(4/72) | 27.5 ± 5.4a *(606) | 17.1±1.8b*(710) | ||||
| 12.4±1.9b(691) | ||||||
*Significantly different from non-cultured tissue (P < 0.05)
a, bSignificantly different between culture media on the same day of culture (P < 0.05)
Analysis of hormones and inhibin production
The levels of estradiol, progesterone, and inhibin increased constantly over the 18-day culture period in all culture media (Figs. 2a–c). The differences in estradiol concentration were significant between the sequential and control media at 12, 14, 16, and 18 days after culture (P < 0.05). For progesterone production, no significant difference was found among the three culture media during the 18-day period, although production in the sequential medium was higher than in any of the other media. The highest concentration of inhibin was observed in the sequential medium at 8–18 days of culture. On Day 7, however, inhibin production in the constant medium appeared to be higher than in any of the other media.
Discussion
In this study, we demonstrate an efficient sequential culture medium for the culture of lamb ovarian cortical tissues in vitro, and confirmed its benefits to the growth of lamb ovarian follicles. To our best knowledge, this offers a novel description on the sequential addition of various growth factors, including FSH, EGF, GDF-9, and bFGF, into lamb ovarian cortical tissue cultures in vitro.
A decrease in the proportion of primordial follicles was found in all culture media, which indicates the spontaneous activation of primordial follicle growth in in vitro cultures. This may be due to the richer collection of stimulatory factors in the culture media used [20] or the elimination of inhibitory factors in in vitro models [21–23]. Compared to observations for the control and constant media, the percentage of primary follicles was low in the sequential medium, but its percentage of secondary follicles was higher than in the two other media. One reason may be that the rate of primordial follicle development in the primary stage was equal to the rate of primary follicle development in the secondary or antral stage in the sequential medium.
Results of the histological analyses indicate that sequentially adding FSH, EGF, GDF-9, and bFGF to the culture medium promoted primordial follicle development into its subsequent stages apart from stimulating follicle growth. The constant medium exerted major effects on secondary follicles. Its effects on follicular development were better with the sequential medium than with the constant medium. That is to say, high concentrations of FSH and EGF and lower concentrations of GDF-9 and bFGF may be beneficial for the development of primary follicles. Studies on growth factors have demonstrated that FSH and EGF are not essential for primordial follicle activation [24–26] but are important for latter follicle development [1, 14]. On the other hand, GDF-9 and bFGF can promote the initiation of primordial follicle development and stimulate early preantral follicle growth [5, 6, 8, 27–29]. Hayashi et al. reported that GDF-9 can enhance the effect of FSH on follicular growth [4, 5]. Matos et al. reported that the interaction between FSH and bFGF stimulates the initiation of primordial follicle growth and the subsequent growth of developing follicles [9]. EGF and FSH do not seem to affect follicular growth, but they appear to stimulate increases in follicle diameter by promoting oocyte growth [29]. Cultures with EGF and bFGF do not appear to affect the proportion of developing follicles present [30]. These references suggest that follicular development is not a simple effect of a single factor, but the coordinated effect of several factors. Under certain physiological conditions, the concentration of FSH in the blood changes dynamically. Additionally, the receptors of EGF, GDF-9, and bFGF expression in the ovaries are found to be dependent on the follicle development stage. Ovarian follicle development during this growth phase is a dynamic process. These reports suggest the dynamic interaction of these factors during follicular development.
In the present study, it was found that there were more normal follicles in the sequential medium than in any of the other media. Compared to the control medium, higher percentage of normal follicles in the constant medium was observed. TUNEL assays indicate that follicle and stromal cell apoptosis was inhibited in the sequential medium. These results suggest that factors added to the sequential medium can inhibit cell apoptosis and maintain follicle viability. The effect of FSH and EGF on a follicle is dependent on the stage of follicle development [31]. Reports have also suggested that these factors can improve follicle survival both in vivo and in vitro [13, 26, 32, 33]. FSH induces functional receptors for bFGF in granulosa cells, which then plays a role in the process of differentiation under the influence of FSH [34]. GDF-9 also acts as a survival factor preventing atresia; this progressively occurs with increasing culture time [35]. GDF-9 may rescue follicles from atresia through the initiation of growth and development. A study by Hreinsson et al. shows that treatment with GDF-9 results in a higher proportion of viable human follicles in organ cultures, suggesting a possible role for GDF-9 in follicular survival [36]. In this study, the highest percentage of normal follicles was observed in the sequential medium 6 days after culture where higher concentrations of GDF-9 and bFGF and lower concentrations of FSH and EGF were present. This result may indicate that a higher concentration of GDF-9 and bFGF is more beneficial to the survival of follicles than a lower one.
Estradiol and progesterone are secreted by granulosa or theca cells; they are used to evaluate granulosa cell differentiation and tissue viability. The mRNA for inhibin subunits was expressed specifically by the granulosa cells of healthy developing follicles in sheep [37]. In the current study, at later culture times (10–18 day), a significant increase of estradiol and inhibin production, but not progesterone, were found in the sequential medium, compared to production in either the control or constant medium. This suggests that less luteinized granulosa cells and more differentiated follicle cells were present in the sequential medium. That is to say, ovarian tissue viability, follicular development, and follicle health are better maintained in the sequential medium. FSH stimulates inhibin and estrogen secretion in the ovary [38]. EGF, in combination with FSH, was found to stimulate progesterone secretion in cultured caprine granulosa cells in vitro [39]. In contrast, EGF appeared to inhibit FSH-stimulated estradiol production in rat ovarian cultures [40]. GDF-9 was shown to stimulate granulosa cell proliferation and basal steroidogenesis, but inhibited FSH-stimulated progesterone biosynthesis [41]. In another study, treatment with GDF-9 stimulated dose-dependent increases in inhibin production [42]. It also reported that bFGF can inhibit FSH-stimulated progesterone production[43].The difference between estradiol and inhibin production was not significant at Day 7 in the sequential medium when compared to the control medium. A possible explanation may be that higher concentrations of GDF-9 and bFGF inhibit the effect of FSH on estradiol and inhibin secretion.
In this study, we used a steel mesh as physical support to provide a solid frame for long-term organ culture in vitro. In order to acquire a three-dimensional structural support, a nitrocellulose membrane pre-coated with type І collagen was used. This is a novel approach for ovarian tissue culture. Results indicate that follicles can develop through to the antral stage in this system. Follicular development and types present in the ovary can be monitored in such cultured tissues since the isolated follicle cultures do not present themselves with much complexity. Recent studies have developed 3D models for isolated follicle cultures. In these models, isolated follicles were embedded in collagen or alginate [44, 45]. As such, the follicle structures were well maintained in these systems and follicles were able to grow. The drawback of such isolated follicle cultures, however, is that agents added to the follicle cultures must have a direct, rather than indirect, effect on the follicles. The addition of such agents has resulted in some damage to the follicles during the isolation procedure. Meanwhile, some studies have reported that membranes coated with extracellular matrix (ECM) [17, 18] can improve the growth of many cell types, as well as follicle viability. Telfer et al. recently reported a two-step culture system wherein ovarian tissues at 6 days after culture were subjected to follicle isolation. The follicles isolated were, in turn, cultured for 4 days. The results of such a system suggest the occurrence of antral formation [15]. In the present study, we used a steel mesh with a nitrocellulose membrane rather than a Millicell insert (usually used in organ culture). Results prove that such a membrane can also support follicle growth. Moreover, it is inexpensive and easy to make.
Conclusions
In summary, the results of this study demonstrate that many factors, including GDF-9, FSH, EGF, and bFGF, may exert coordinated effects on the development and growth of primordial follicles. In particular, the sequential addition of these factors offers benefits to follicle and oocyte growth. Findings suggest that the sequential culture medium tested in the study may be the optimal medium for the in vitro culture of lamb ovarian tissue, thus providing a method by which we can successfully harness the reproduction potential of domestic ovarian tissue. Ongoing projects are aimed to improve this culture system, apart from focusing on the effects of the addition of other growth factors and hormones at appropriate times and concentrations, thus imitating the ovarian follicle’s requirements in vivo.
Acknowledgements
This study was supported by the Project of Scientific Support of China (Fund Item No. 2008BADB2B05-10). The authors thank Chen Rong at XinJiang Medical College for the tissue sectioning, slide preparation, and staining.
Footnotes
Capsule
In this study, we show an efficient sequential culture system for the culture of lamb ovarian cortical tissue in vitro. The system was confirmed to have brought about benefits to the growth and development of lamb ovarian follicles in an organ culture.
References
- 1.Nayudu PL, Vitt UA, Tomasi Jorgelina Barrios, Katti Pancharatna, Alfredo Ulloa-Aguirre. Intact follicle culture: what it can tell us about the roles of FSH glycoforms during follicle development. Reprod BioMed Online. 2002;5:240–253. doi: 10.1016/S1472-6483(10)61827-5. [DOI] [PubMed] [Google Scholar]
- 2.Adriaens I, Cortvrindt R, Smitz J. Differential FSH exposure in preantral follicle culture has marked effects on folliculogenesis and oocyte developmental competence. Hum Reprod. 2004;19:398–408. doi: 10.1093/humrep/deh074. [DOI] [PubMed] [Google Scholar]
- 3.Matos MHT, Lima-Verde IB, Luque MCA, Maia JE, Jr, Silva JRV, Celestino JJH, Martins FS, Báo SN, Lucci CM, Figueiredo JR. Essential role of follicle stimulating hormone in the maintenance of caprine preantral follicle viability in vitro. Zygote. 2007;15:173–182. doi: 10.1017/S0967199407004169. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi M, McGee EA, Min G, Klein C, Rose UM, Duin Marcel, Hsueh AJ. Recombinant growth differentiation factor-9 (GDF-9) enhances growth and differentiation of cultured early ovarian follicles. Endocrinology. 1999;140:1236–1244. doi: 10.1210/en.140.3.1236. [DOI] [PubMed] [Google Scholar]
- 5.Vitt UA, Hayashi M, Klein C, Hsueh AJ. Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol Reprod. 2000;62:370–377. doi: 10.1095/biolreprod62.2.370. [DOI] [PubMed] [Google Scholar]
- 6.Hreinsson JG, Scott JE, Rasmussen C, Swahn ML, Hsueh AJ, Hovatta O. Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. [J]. Clin. Endocrinol. Metab. 2002;87:316–321. doi: 10.1210/jc.87.1.316. [DOI] [PubMed] [Google Scholar]
- 7.Martins FS, Celestino JJH, Saraiva MVA, Matos MHT, Bruno JB, Rocha-Junior MC, Lima-Verde IB, Lucci CM, Báo SN, Figueiredo JR. Growth and differentiation factor-9 stimulates activation of goat primordial follicles in vitro and their progression to secondary follicles. Reprod Fertil Dev. 2008;20:916–924. doi: 10.1071/RD08108. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson EE, Skinner MK. Kit ligand and basic fibroblast growth factor interactions in the induction of ovarian primordial to primary follicle transition. Mol Cell Endocrinol. 2004;214:19–25. doi: 10.1016/j.mce.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Matos MH, Lima-Verde IB, Bruno JB, Lopes CA, Martins FS, Santos KD, Rocha RM, Silva JR, Bao SN, Figueiredo JR. Follicle stimulating hormone and fibroblast growth factor-2 interact and promote goat primordial follicle development in vitro. Reprod Fertil Dev. 2007;19:677–684. doi: 10.1071/RD07021. [DOI] [PubMed] [Google Scholar]
- 10.Roni G, Ronit A, Arye E, Carmela F, Shmuel N, Benjamin F. Effects of basic fibroblast growth factor on in vitro development of human ovarian primordial follicles. Fertil Steril. 2009;91:1967–1975. doi: 10.1016/j.fertnstert.2008.04.075. [DOI] [PubMed] [Google Scholar]
- 11.Kezele PR, Nilsson EE, Skinner MK. Insulin but not insulin-like growth factor-1 promotes the primordial to primary follicle transition. Mol Cell Endocrinol. 2002;192:37–43. doi: 10.1016/S0303-7207(02)00114-4. [DOI] [PubMed] [Google Scholar]
- 12.Boland NI, Gosden RG. Effects of epidermal growth factor on the growth and differentiation of cultured mouse ovarian follicles. J Reprod Fertil. 1994;101:369–374. doi: 10.1530/jrf.0.1010369. [DOI] [PubMed] [Google Scholar]
- 13.Wandji SA, Eppig JJ, Fortune JE. FSH and growth factors affect the growth and endocrine function in vitro of granulosa cells of bovine preantral follicles. Theriogenology. 1996;45:817–832. doi: 10.1016/0093-691X(96)00011-8. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biol Reprod. 2000;62:1322–1328. doi: 10.1095/biolreprod62.5.1322. [DOI] [PubMed] [Google Scholar]
- 15.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23(5):1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 16.Cushman RA, Wahl CM, Fortune JE. Bovine ovarian cortical pieces grafted to chick embryonic membranes: a model for studies on the activation of primordial follicles. Hum Reprod. 2002;17:48–54. doi: 10.1093/humrep/17.1.48. [DOI] [PubMed] [Google Scholar]
- 17.Hovatta O, Silye R, Abir R, Krausz T, Winston RM. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12:1032–1036. doi: 10.1093/humrep/12.5.1032. [DOI] [PubMed] [Google Scholar]
- 18.Scott JE, Carlsson IB, Bavister BD, Hovatta O. Human ovarian tissue cultures: extracellular matrix composition, coating density and tissue dimensions. Reprod BioMed. 2004;9(3):287–293. doi: 10.1016/S1472-6483(10)62143-8. [DOI] [PubMed] [Google Scholar]
- 19.Oktay K, Newton H, Mullan J, Gosden RG. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum Reprod. 1998;13:1133–1138. doi: 10.1093/humrep/13.5.1133. [DOI] [PubMed] [Google Scholar]
- 20.Silva JR, Hurk R, Costa SH, Andrade ER, Nunes AP, Ferreira FV, Lobo RN, Figueiredo JR. Survival and growth of goat primordial follicles after in vitro culture of ovarian cortical slices in media containing coconut water. Anim Reprod Sci. 2004;81:273–286. doi: 10.1016/j.anireprosci.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–3337. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- 22.Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–1084. doi: 10.1210/en.143.3.1076. [DOI] [PubMed] [Google Scholar]
- 23.Derrar N, Price CA, Sirard MA. Effect of growth factors and co-culture with ovarian medulla on the activation of primordial follicles in explants of bovine ovarian cortex. Theriogenology. 2000;54:587–598. doi: 10.1016/S0093-691X(00)00374-5. [DOI] [PubMed] [Google Scholar]
- 24.Braw-Tal R, Yossefi S. Studies in vivo and in vitro on the initiation of follicle growth in the bovine ovary. J Reprod Fertil. 1997;109:165–171. doi: 10.1530/jrf.0.1090165. [DOI] [PubMed] [Google Scholar]
- 25.Fortune JE, Kito S, Wandji AS, Srsen V. Activation of bovine and baboon primordial follicles in vitro. Theriogenology. 1998;49:441–449. doi: 10.1016/S0093-691X(97)00416-0. [DOI] [PubMed] [Google Scholar]
- 26.Wright CS, Hovatta O, Margara R, Trew G, Winston RML, Franks S, Hardy K. Effects of follicle-stimulating hormone and serum substitution on the in vitro growth of human ovarian follicles. Hum Reprod. 1999;14:1555–1562. doi: 10.1093/humrep/14.6.1555. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Roy SK. Growth differentiation factor-9 and stem cell factor promote primordial follicle formation in the hamster: modulation by follicle-stimulating hormone. Biol Reprod. 2004;70:577–585. doi: 10.1095/biolreprod.103.023234. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson E, Parrott JA, Skinner MK. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol. 2001;175:123–130. doi: 10.1016/S0303-7207(01)00391-4. [DOI] [PubMed] [Google Scholar]
- 29.Silva JRV, Hurk R, Matos MHT, Santos RR, Pessoa C, Moraes MO, Figueiredo JR. Influences of FSH and EGF on primordial follicles during in vitro culture of caprine ovarian cortical tissue. Theriogenology. 2004;61:1691–1704. doi: 10.1016/j.theriogenology.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Huanmin Zhou, Yong Zhang. Regulation of in vitro growth of preantral follicles by growth factors in goats. Domest Anim Endocrinol. 2005;28:235–242. doi: 10.1016/j.domaniend.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Markstrom E, Svensson E, Shao R, Svanberg B, Billig H. Survival factors regulating ovarian apoptosis-dependence on follicle differentiation. Reproduction. 2002;123:23–30. doi: 10.1530/rep.0.1230023. [DOI] [PubMed] [Google Scholar]
- 32.Cortvrindt R, Smitz J, Steirteghem AC. Assessment of the need for follicle stimulating hormone in early preantral mouse follicle culture in vitro. Hum Reprod. 1997;12:759–768. doi: 10.1093/humrep/12.4.759. [DOI] [PubMed] [Google Scholar]
- 33.Hurk R, Abir R, Telfer EE, Bevers MM. Primate and bovine immature oocytes and follicles as source of fertilizable oocytes. Hum Reprod. 2000;6:457–474. doi: 10.1093/humupd/6.5.457. [DOI] [PubMed] [Google Scholar]
- 34.Shikone T, Yamoto M, Nakano R. Follicle-stimulating hormone induces functional receptors for basic fibroblast growth factor in rat granulosa cells. Endocrinology. 1992;131:1063–1068. doi: 10.1210/en.131.3.1063. [DOI] [PubMed] [Google Scholar]
- 35.Hreinsson JG, Scott JE, Rasmussen C, Swaha ML, Hsueh AJW, Hovatta O. Growth Differentiation Factor-9 Promotes the Growth, Development, and Survival of Human Ovarian Follicles in Organ Culture. J Clin Endocrinol Metab. 2002;87(1):316–321. doi: 10.1210/jc.87.1.316. [DOI] [PubMed] [Google Scholar]
- 36.Rohan RM, Rexroad J, Guthrie HD. Changes in the concentration of MRNAS for the inhibin subunits in ovarian follicles after administration of gonadotropins to progestin-treated ewes. Domest Anim Endocrinol. 1991;8:445–54. doi: 10.1016/0739-7240(91)90013-A. [DOI] [PubMed] [Google Scholar]
- 37.Souza CJH, Campbell BK, McNeilly AS, Baird DT. Effect of bone morphogenetic protein 2 (BMP2) on oestradiol and inhibin A production by sheep granulosa cells, and localization of BMP receptors in the ovary by immunohistochemistry. Reproduction. 2002;123:363–369. doi: 10.1530/rep.0.1230363. [DOI] [PubMed] [Google Scholar]
- 38.Fortune JE. The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Anim Reprod Sci. 2003;78:135–163. doi: 10.1016/S0378-4320(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 39.Behl R, Pandey RS. Effect of epidermal growth factor on steroidogenesis by caprine granulosa cells in culture: interaction with FSH. Small Rumin Res. 2001;40:57–62. doi: 10.1016/S0921-4488(00)00214-5. [DOI] [PubMed] [Google Scholar]
- 40.Wang Haibin, Xia Guoliang, Li Meiling, Guo Yong, Xie Huirong. Effects of Epidermal Growth Factor on the Follicle Development and Estradiol Secretion of Mouse Fetal Ovary Induced by Follicle-Stimulating Hormone in vitro. Journal of Agricultural Biotechnology. 2001;9:37–40. [Google Scholar]
- 41.Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999;13:1035–1048. doi: 10.1210/me.13.6.1035. [DOI] [PubMed] [Google Scholar]
- 42.Roh JS, Bondestam J, Mazerbourg S, Kaivo-Oja N, Groome N, Ritvos O, Hsueh AJW. Growth differentiation factor-9 stimulates inhibin production and activates Smad2 in cultured rat granulosa cells. Endocrinology. 2003;144(1):172–178. doi: 10.1210/en.2002-220618. [DOI] [PubMed] [Google Scholar]
- 43.Vernon RK, Spicer LJ. Effects of basic fibroblast growth factor and heparin on follicle-stimulating hormone-induced steroidogenesis by bovine granulosa cells. J Anim Sci. 1994;72:2696–2702. doi: 10.2527/1994.72102696x. [DOI] [PubMed] [Google Scholar]
- 44.Torrance C, Telfer E, Gosden RG. Quantitative study of the development of isolated mouse pre-antral follicles in collagen gel culture. J Reprod Fertil. 1989;87:367–374. doi: 10.1530/jrf.0.0870367. [DOI] [PubMed] [Google Scholar]
- 45.Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue eng. 2003;9:1013–1021. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]