Abstract
Purpose
Oncofertility, an emerging discipline at the intersection of cancer and fertility, strives to give cancer patients options when they are confronting potential infertility as a consequence of cancer treatment. Fertility preservation decisions must be made before treatment begins, adding stress to the decision-making process.
Methods
Healthcare providers need to be aware of the intricacies involved in oncofertility decision making, and the often tight time line that patients face when making these decisions. Cancer patient’s perspectives may also change, as the dual burden of a cancer diagnosis and potential infertility can cause great flux in emotions.
Results
A provider-facing decision tree was created to enhance patient decision-making capacities and outline the multiple potential intervention points.
Conclusions
Decision trees, which highlight the important decision points during which providers can approach patients, can be a useful tool to help providers in counseling patients on fertility preservation.
Keywords: Fertility preservation, Cancer, Decision-making, Decision tree
Introducing oncofertility in the clinical context
A recent case at Northwestern University demonstrates why it is so imperative to approach cancer patients about fertility preservation multiple times and through multiple care providers. A 36 year old, high level executive and married woman presented with breast cancer to her physician. An elite athlete, the patient had been more focused on her athletic goals than on her family goals before her diagnosis. In fact, her initial concerns about the diagnosis were centered around her training and return to competition. The patient was approached by her oncologist about fertility preservation and after a brief discussion with her husband, the two decided that they would not pursue fertility preservation, as they both felt overwhelmed by the cancer diagnosis and felt their energy would be best used to focus on recovery. However, 9 months into her treatment the patient returned with her husband, stating her desire for children and inquiring into whether there were any remaining fertility preservation options. Unfortunately, the patient had already undergone treatment so the damage was already done; the patient remained amenorrheic after treatment and expressed regret at not pursuing fertility preservation prior to treatment. While the oncologist acted responsibly in approaching this patient about fertility preservation before treatment, this patient could have benefitted immensely from multiple discussions with a variety of care providers. Had another healthcare provider broached the subject with her following her initial conversation, perhaps she would have spent more time thinking about the consequences and weighing her options. The complexity of fertility preservation decision-making demands that patients receive counseling on this subject at multiple points in their cancer care trajectory, and by multiple different care providers
Interdisciplinary work through a critical lens
The field of oncofertility was founded on the principles of interdisciplinary work. Since oncofertility spans the disciplines of oncology and fertility, progress in this field has required bridging two distinct disciplines that are not accustomed to working together. Further, the complexities behind decision-making, the immaturity of the technology, and the nature of reproductive medicine itself create implicit ethical and legal considerations. Thus, a diverse team of scholars has been mobilized to try to make an intractable problem (fertility preservation for cancer patients) tractable [1–10].
The advantages of interdisciplinary teamwork are routinely touted, presenting collaborative approaches as the ideal mechanism to drive progress and innovation [8]. In the context of science, interdisciplinary teamwork is often necessary, as academic science and medicine creates specialties by nature. Scientists are trained in one area, becoming experts in one specific niche. In order to create larger projects and accommodate larger visions, team work is often the only viable option. In the case of oncofertility, not only did fertility merge with oncology, but scholars in ethics and humanities collaborated with lawyers and economists, creating a synthetic environment to grow human follicles within the laboratory. These relationships and their resulting accomplishments represent tangible metrics of the value of interdisciplinary work, and continue to drive progress within the field of oncofertility [1].
The downside of interdisciplinary team work is founded in these same, specialized silos that allow for such specific expertise. As described by Wuchty, “teams may bring greater collective knowledge and effort, but they are known to experience social network and coordination losses that make them underperform individuals even in highly complex tasks” [11]. In the context of fertility preservation for cancer patients, patients are navigated through an immense system of specialists, with one person in charge of cancer diagnosis, another for fertility, a different for cancer treatment, and so on. As patients are expedited through this network, they become the responsibility of a collective system, rather than a specific individual. In this way, certain aspects of care may become lost, as one provider assumes the patient has already received care from another colleague in the treatment chain.
As an emerging specialty in the clinical care environment, oncofertility is particularly susceptible to this phenomenon of getting “lost in the treatment trajectory”. As demonstrated in the aforementioned clinical case, even if the oncologist presents fertility preservation to the patient, the patient can still benefit from multiple discussions at varying points in the clinical care trajectory. It is well-known that patient decision-making is not a straightforward process, and perspectives, wishes, wants and needs changes as patients undergo their cancer diagnosis and treatment. By approaching patients at multiple points throughout the trajectory of care, and by approaching them through different specialties, healthcare providers can empower patients to make their own autonomous decision. There are three mechanisms of action we have employed to combat ‘loss’ in a multidisciplinary, non-traditional system; a patient navigator who is at the intersection of all disciplines, a patient/provider web resource (myoncofertility.org) and decision trees [12, 13]. We refer the reader to other resources to learn more about patient navigation and myoncofertility and will focus here on the third mechanism, decision trees with multiple points of intervention.
The decision tree: a provider-facing resource
Medical decision-making is a complicated process in which patients must weigh compatible or competing interests, including doctor’s advice or attitudes about fertility interventions, the expectations of their family members and the relative probability of success. It has been observed that decision makers have a tendency to make decisions more on the basis of intuition than a rational weighing of outcomes and probabilities, with this phenomenon exemplified in the clinical setting when patients need to make intricate decisions with a limited amount of time and resources [14]. Optimal methods for treatment decision-making in multidisciplinary teams are currently not established [15]. However, research indicates that a systematic approach to the provision of patient information—a process that involves assessment of the patient’s information needs, the provision of information that meets those needs and the assessment of how well the information provided has been understood and how satisfied the patients are with the information they have received—is the best approach to facilitate decision-making among cancer patients [16]. Uncertainty among patients during the decision-making process is both multifaceted and changing; effective communication by healthcare professionals provides reassurance, knowledge, and understanding [17].
Various decision tools have been developed to help ease the burden of these critical patient choices. One such tool is a decision tree, which is a pictorial illustration of all plausible relationships, alternatives, and outcomes in a given decision. Decision trees can help patients accurately weigh the outcomes associated with a given decision, and lead to more informed clinical judgments [14]. These schematics describe the path of patient decision-making, portraying the consequences of each separate decision, and the multiple ways in which a patient may arrive at the final outcome. Healthcare decision-making demands tradeoffs between risks and benefits, and decision trees reflect these tradeoffs in their structure [18]. Such tools are often utilized among patients, but retain value for the population of healthcare providers as well. Decision trees can help individuals to think through and discuss treatment choices from various perspectives, and can be used to help providers communicate in a systematic and logical way [19]. The American Society of Clinical Oncology recently developed a decision flow diagram that provides guidance to oncologists on initial discussions about the possibility of treatment-related infertility [5]. The flow diagram, which outlines three steps in the decision-making process as faced by both male and female cancer patients, is accompanied by a table that describes key points that should be included when discussing fertility preservation options with patients.
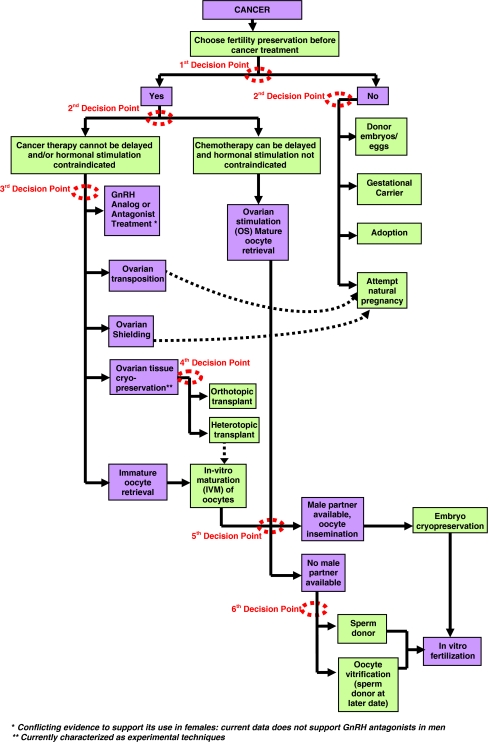
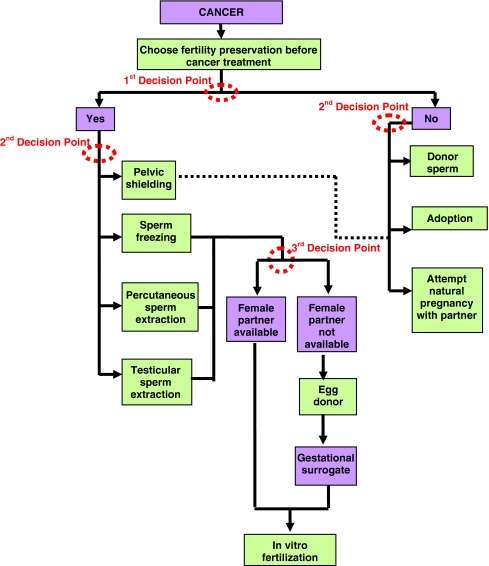
An interdisciplinary team of researchers at Northwestern University expanded upon this existing ASCO flow diagram to create a decision tree for providers to use when counseling cancer patients on their fertility preservation options. The team of oncologists, surgeons, and social workers constructed the tool as a practical guide for the diverse audience of healthcare providers who are in the position to counsel cancer patients regarding fertility preservation. Two schematics were created, one for women, and one for men, to capture the unique aspects and options for these distinct groups. These two tools lay out the diverse, and often overwhelming, options currently available for men and women in a single space, with special attention paid to each “decision point” in which the patient can change the course of their trajectory. The decision tree for female cancer patients is shown in Fig. 1 and the decision tree for male patients is shown in Fig. 2. These trees expand upon other excellent decision-making resources that provide roadmaps for navigating patients with cancer [20, 21].
Fig. 1.
Female fertility preservation decision tree. The patient may attempt natural pregnancy after appropriate treatment or disease specific waiting period, and subsequent to the completion of her cancer therapy and in consultation with her treating physician. No disease specific waiting period for females has been determined. If attempting natural pregnancy, preimplantation diagnosis may also be recommended depending on cancer type and treatment regimen [21]
Fig. 2.
Male fertility preservation decision tree. The patient may attempt natural pregnancy after appropriate treatment or disease specific waiting period, and subsequent to the completion of his cancer therapy and in consultation with his treating physician, approximately 2 years [1, 22, 23]. If attempting natural pregnancy, preimplantation diagnosis may also be recommended depending on cancer type and treatment regimen [21]
Decision trees as a way to enhance interdisciplinary work in the clinical setting
Interdisciplinary team work has the potential to offer great benefits for researchers and patients alike, creating novel treatments and providing specialized, expert care. This approach is not without disadvantages, however, as this large-system structure creates vulnerabilities at the patient level. Patients can easily become “lost” in the system as they are absorbed as a collective responsibility instead of an individual responsibility, with meaningful health discussions overlooked because provider assume that someone else on the chain of care has already addressed the patient. In this way, the perceived strength of the shared system can actually lead to weakening and underperformance at the level of individual healthcare providers.
Mobilizing the already time-pressed network of clinical care providers to think outside of their silos and broaden their discussions with patients is no small task, but carries a great reward. Patients need time to digest and process information, particularly when they are facing both a cancer diagnosis and a threat of potential infertility. These patients also need to hear their options from multiple voices, reinforcing their knowledge base and expanding their opportunities for autonomous decision-making.
Decision trees tailored to healthcare providers provide an ideal platform to convey the importance of each provider’s position within the overall spectrum of care. By visually mapping out the sequence of events that a patient must undergo, and pointing awareness towards each decision point, providers can gain a better understanding of when they can approach patients and at which moments patients may demand extra attention. Decision trees have long been used for patients, but they are exemplary tools for healthcare providers as well. The current network of specialized care providers, as well as their many shared patients, could benefit immensely from the dissemination of provider-focused decision trees.
Footnotes
Funding
Supported by a grant from the National Institutes of Health (UL1RR024926, RL1HD058296)
Capsule Fertility preservation decision-making among cancer patients is a complicated process. Provider-focused decision trees are a useful tool in supporting patient fertility preservation decision-making.
References
- 1.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360(9):902–11. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, Telfer EE, Woodruff TK, Zelinski MB. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010. [DOI] [PMC free article] [PubMed]
- 3.West ER, Zelinksi MB, Kondapalli LA, Gracia C, Chang J, Coutifaris C, Critser J, Stouffer RL, Shea LD, Woodruff TK. Preserving female fertility following cancer treatment: current options and future possibilities. Pediatr Blood Cancer. 2009;53(2):289–95. doi: 10.1002/pbc.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieman CL, Kinahan KE, Yount SE, Rosenbloom SK, Yost KJ, Hahn EA, Volpe T, Dilley KJ, Zoloth L, Woodruff TK. Fertility preservation and adolescent cancer patients: lessons from adult survivors of childhood cancer and their parents. [DOI] [PMC free article] [PubMed]
- 5.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 6.Woodruff TK. The emergence of a new interdiscipline: oncofertility. Cancer Treat Res. 2007;138:3011. doi: 10.1007/978-0-387-72293-1_1. [DOI] [PubMed] [Google Scholar]
- 7.Zoloth L, Backhus L, Woodruff T. Waiting to be born: the ethical implications of the generation of “NUBorn” and “NUAge” mice from pre-pubertal ovarian tissue. Am J Bioeth. 2008;8(6):9. doi: 10.1080/15265160802248294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guimerà R, Uzzi B, Spiro J, Amaral LA. Team assembly mechanisms determine collaboration network structure and team performance. Science. 2005;308(5722):697–702. doi: 10.1126/science.1106340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolin G, Roberts DE, Rodriguez LM, Woodruff TK. Medical hope, legal pitfalls: potential legal issues in the emerging field of oncofertility. Santa Clara Law Rev. 2009;49(3):673–716. doi: 10.1007/978-1-4419-6518-9_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campo-Englestein L. Consistency in insurance coverage for iatrogenic conditions resulting from cancer treatment including fertility preservation. J Clin Oncol. 2010; epub ahead of print. [DOI] [PMC free article] [PubMed]
- 11.Wutchy S, Jones BF, Uzzi B. The increasing dominance of teams in production of knowledge. Science. 2007;36(5827):1036–9. doi: 10.1126/science.1136099. [DOI] [PubMed] [Google Scholar]
- 12.American Brain Tumor Association. Tips for Living and Coping. Available at: http://oncofertility.northwestern.edu/files/oncofertility-and-fertility-preservation/tips-living-and-coping. Accessed February 13, 2010.
- 13.www.myoncofertility.org
- 14.Aleem IS, Jalal H, Aleem IS, Sheikh AA, Bhandari M. Clinical decision analysis: incorporating the evidence with patient preferences. Patient Prefer Adherence. 2009;3:21–4. doi: 10.2147/ppa.s4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidger J, Murdoch J, Donovan JL, Blazeby JM. Clinical decision-making in a multidisciplinary gynaecological cancer team: a qualitative study. BJORG: An International Journal of Obstetrics & Gynecology, 116(4):511–-17. [DOI] [PubMed]
- 16.Sainio C, Eriksson E, Lauir S. Patient participation in decision making about care: the cancer patient’s point of view. Cancer Nurs. 2001;24(3):172–9. doi: 10.1097/00002820-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Nanton V, Docherty A, Meystre C, Dale J. Finding a pathway: Information and uncertainty along the prostate cancer patient journey. Br J Health Psychol. 14(3):437–58. [DOI] [PubMed]
- 18.Detsky AS, Naglie G, Krahn MD, Redelmeier DA, Naimark D. Primer on medical decision analysis: part 2—building a tree. Med Decis Making. 1997;17:126–35. doi: 10.1177/0272989X9701700202. [DOI] [PubMed] [Google Scholar]
- 19.Hunink MGM. In search of tools to aid logical thinking and communicating about medical decision making. Med Decis Making. 2001;21:267. doi: 10.1177/0272989X0102100402. [DOI] [PubMed] [Google Scholar]
- 20.Chian RC, Huang JY, Gilbert L, Son WY, Holzer H, Cui SJ, Buckett WM, Tulandi T, Tan SL. Obstetric outcomes following vitrification of in vitro and in vivo matured oocytes. Fertil Steril. 2009;91(6):2391–8. doi: 10.1016/j.fertnstert.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Quinn GP, Vadaparampil ST, Jacobsen PB, Knapp C, Keefe DL, Bell GE. Frozen hope: fertility preservation for women with cancer. J Midwifery Women’s Health. 2010;55(2):175–80. doi: 10.1016/j.jmwh.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Tempst HG, Ko E, Chan P, Robaire B, Rademaker A, Martin RH. Sperm aneuploidy frequencies analysed before and after chemotherapy in testicular cancer and Hodgkin’s lymphoma patients. Hum Reprod. 2008;23:251–8. doi: 10.1093/humrep/dem389. [DOI] [PubMed] [Google Scholar]
- 23.Stahl O, Eberhard J, Jepson K, et al. Sperm DNA intergrity in testicular cancer patients. Human Reprod. 2006;21:3199–205. doi: 10.1093/humrep/del292. [DOI] [PubMed] [Google Scholar]