Abstract
Over the past several decades our understanding of Alzheimer’s disease (AD) has seen an evolution from the dichotomous concept of normal versus AD in the dementia state to a more accurate and complete appreciation of AD as a progressive disorder with clinical, biological, and pathological features occurring along a continuum from normal to end-stage disease. Integrating our understanding of the relationships and interplay between the clinical, biological, and pathological features of AD may allow the identification of AD at even preclinical, completely asymptomatic stages of the disease. This review attempts to summarize the clinical stages of AD in terms of epidemiology, historical evolution of disease stage diagnoses, cognitive/neuropsychologic features, psychiatric/behavioral manifestations, and functional decline in the context of our developing understanding of the biological processes responsible for the pathogenesis of AD described in detail in the accompanying articles.
Keywords: Alzheimer’s disease, mild cognitive impairment, Preclinical AD, clinical features
Evolution of the conceptual framework for staging the clinical phenotype of Alzheimer’s disease
Over the past several decades our understanding of the clinical features of Alzheimer’s disease (AD) has evolved from the dichotomous concept of normal versus AD to a more accurate and complete appreciation of AD as a progressive disorder with clinical features occurring along the entire cognitive continuum from normal to end-stage disease.[1–4] The clinical features of AD are a manifestation of the underlying severity and neuroanatomic involvement of specific brain regions and circuits that are affected by the biological processes responsible for neuronal dysfunction and death that characterize AD.[1, 5–10] The pathological evolution of AD is described in detail in this issue (see section on neuropathology by WRM), however a brief overview of the neuroanatomic progression of AD here provides the framework to understand the progression of clinical signs and symptoms of AD across the cognitive, behavioral, and functional continuum that characterizes AD. AD, like many neurodegenerative diseases, starts focally and spreads outward, eventually consuming the entire brain in the end-stage of disease.[8, 9, 11, 12] While recent antemortem imaging data have called into question the direct relationship between extent of pathology (as determined by neurofibrillary tangle and senile plaque burden) and anatomic involvement in certain areas such as the precuneus (as evidenced by atrophy on MRI and hypometabolism on PET)[13], several recent reports reinforce the association of extent of pathology with the clinical progression of dementia, especially the extent of neurofibrillary tangles in cortical regions. [14, 15] Conflicting studies suggest that coexistent or mixed pathological disease states and age of patients at onset of dementia confound the association between the pathological hallmarks of AD and clinical phenotype. [8, 16–22] Resolution of such disparate data is an important focus for future work in the field of AD. The ultimate definition of anatomic disease progression is clearly in evolution, as is our concept of the clinical phenotype of AD along the continuum from normal cognition to end-stage dementia. This review conceptualizes clinical AD in a biological sense through comparison with the neuroanatomic involvement of the pathological hallmarks of AD (neurofibrillary tangles and senile plaques), but clearly acknowledges that this may be an oversimplified view that will require further elucidation and perhaps evolution as the field progresses.
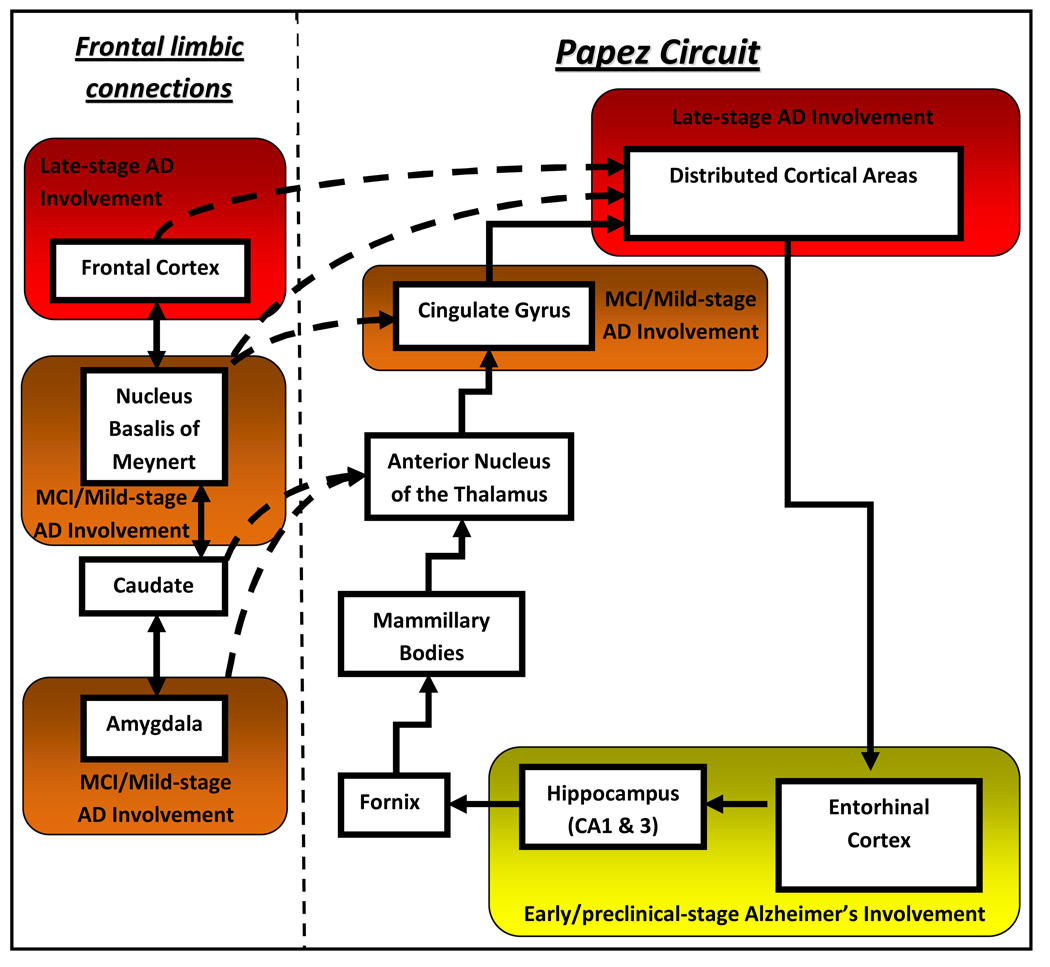
Neuronal degeneration and dysfunction in AD begin in the limbic circuitry of medial temporal lobe structures including the entorhinal cortex and hippocampus.[8, 9, 11, 12] These structures are critical components of the Papez circuit (Figure 1) involved in the creation and consolidation of short term memory processes.[23] Further neuroanatomic spread inferiorly and posteriorly in the temporal lobes includes brain structures involved in semantic memory (Figure 2).[1, 5, 6, 8, 9, 11, 12] Anterior progression along limbic pathways leads to involvement of subcortical structures including the cholinergic projections from the nucleus basalis of Meynert that diffusely activate the neocortex (Figure 1).[24] Involvement of the amygdala, anterior cingulate, and other limbic structures in the basal forebrain leads to the many neuropsychiatric and behavioral features of AD.[25, 26] Eventually neuronal dysfunction and death engulf the entire cerebrum with characteristic sparing of primary motor and sensory areas in the frontal and parietal regions.[5, 8, 9, 11, 12] This biological spread of disease precedes and causes the evolving clinical phenotype seen in AD, from normal cognition to end-stage disease.
Figure 1.
Simplified schematic of the Papez circuit. Early Alzheimer’s disease (AD) pathological changes start in the entorhinal cortex, spreading through the perforant pathway to involve the CA1 & 3 hippocampal subfields leading to anterograde amnesia. Later involvement includes pathological spread into the cingulate cortex and eventually the entire neocortex leading to both short-term and long-term memory impairment (retrograde amnesia). Limbic systems involved in emotion, behavior, arousal, and cortical activation including the amygdala and nucleus basalis of Meynert converge on this pathway and are heavily involved in the early stages of biological AD. This simplified schematic does not imply a linear or regimented progression for all cases of AD, but rather a generalization schema for understanding the typical progression of disease.
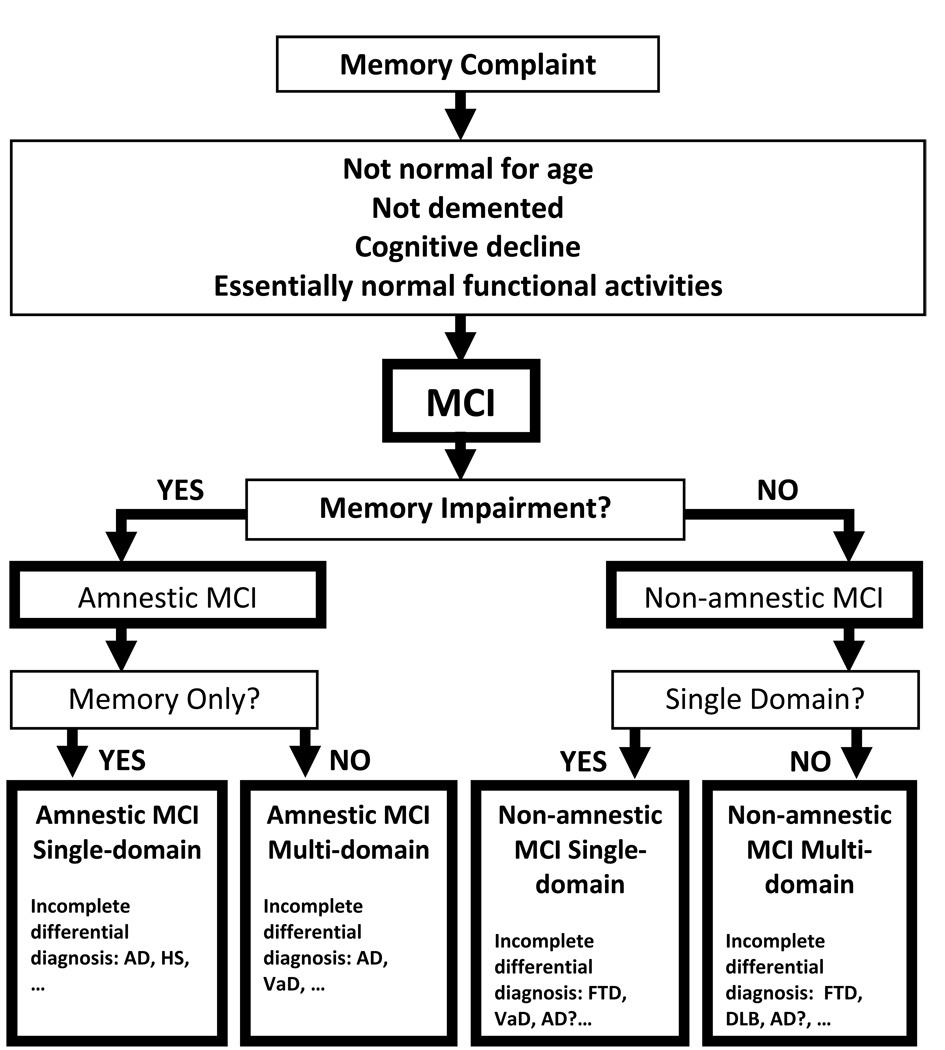
Figure 2.
Diagrammatic algorithm for the diagnosis of MCI subtypes proposed by the 2nd International Working Group on MCI [68, 75]. Subgroup classification may allow for the formulation of a differential diagnosis for the cognitive profile characteristic of each group that remains unproved at present. Abbreviations: MCI, mild cognitive impairment; AD, Alzheimer’s disease; HS, hippocampal sclerosis; VaD, vascular dementia; FTD, frontotemporal dementia; DLB, dementia with Lewy bodies.
Much of what has been learned regarding the clinical changes that occur along the continuum from normal to end-stage AD has come from longitudinal studies that recruited subjects with normal cognition and followed them with serial evaluations to the end-stage of disease and subsequent autopsy following death. Neuropathological confirmation of disease is important as many studies have demonstrated diagnostic accuracy (percent correct diagnoses) for even fulminate AD at 80–90% in tertiary care centers specializing in AD.[27, 28] Others have reported specificity of diagnosis lower than 65% in select populations with high prevalence of cerebrovascular disease (CVD).[29] Coexistent pathological heterogeneity below a given subjective threshold for acknowledging mixed AD-CVD, as well as the lack of standardized pathological criteria for the diagnosis of and many combinations of pathological features of CVD, may confound this issue and lead to further diagnostic inaccuracy. Such diagnostic confusion is often the result of distinct neurodegenerative disease processes such as hippocampal sclerosis, vascular dementia (VaD), Lewy body disease (DLB), or frontotemporal dementia (FTD) involving the same neuroanatomic areas of the brain that are affected in AD.[7, 8, 10, 30]
Any conceptual model of AD must consider the mixed pathological phenotypes, age-dependent decreases in AD pathological burden associated with clinical dementia, and the potential influence of asymmetric atrophy and pathology in medial temporal lobe structures in the biological development and clinical expression of AD. As commented on above, the extent and neuroanatomic distribution of such confounds may greatly influence the clinical phenotype of disease and may play significant roles in decline across all stages of the cognitive continuum of AD discussed herein.
The presence of coexistent CVD, LBD, and TDP-43 positive inclusions in AD is widely appreciated by all in the field and can be documented in 38% to nearly 100% of subjects studied in different cohorts with specific demographic, clinical, and genetic exposures. Dementia states associated with mixed pathological phenotypes can give rise to a multitude of clinical phenotypic variants that appear to be age-dependent and possibly influenced by other demographic (gender, education, socioeconomic status, etc.), clinical (hypertension, hyperlipidemia, history of stroke or cardiac disease, etc.), and genetic factors (ApoE status). [8, 16–19] Some of these factors strengthen and others lessen the association of the clinical signs and symptoms of AD with the classical pathological hallmarks of AD (neurofibrillary tangles and neuritic plaques). Of these varied comorbid pathological disease states, “silent” CVD including periventricular leukoariosis, microvascular infarcts, and arteriosclerotic disease, has been suggested by some to be a major contributor to dementia, above AD pathological features.[31–34] Such heterogeneity would be expected to substantially increase the variance in clinical presentation of AD as a result of neuroanatomic involvement of areas not typically affected in AD, but more typically involved in the coexistent non-AD pathologic entities. Despite such confounds, the clinical phenotype of AD is remarkably robust and, despite the underlying pathological heterogeneity, a relatively consistent progression of disease is seen.
Several studies have demonstrated that advancing age decreases the association of AD pathology with the clinical phenotype of dementia and AD in the oldest old.[20–22] The decreased burden of neocortical amyloid deposition in the subjects studied by Savva et al., 2009 remains unexplained, but it is important to note that these pathological findings relate to extent of AD pathology rather than localization of anatomic involvement or alterations in the clinical phenotype of AD in the oldest old. [22] Reasons for this observed heterogeneity of pathological burden in the oldest old with AD could include an increasing prevalence of mixed pathological features as described above, sensory or physical impairments distinct from AD processes (visual, auditory, somatosensory, or motor impairments from glaucoma, macular degeneration, cataracts, hearing impairment, neuropathy, arthritis, or other systemic disease), or from an as of yet unidentified cause of neuronal senescence or loss, synaptic dysfunction, and age-related cortical atrophy as postulated by Savva et al. 2009.[22] Consideration of such age-related comorbidities and heterogeneity in the pathologic substrate of dementia is important for understanding the progressive evolution of the clinical phenotype of AD and its relationship to true biological AD. Further work in this area is clearly needed to define a more accurate and complete understanding of the complex processes that can coalesce to create the clinical phenotype of AD especially in the oldest old.
Recent studies using structural MRI volumetrics have begun to highlight the potential importance of asymmetric medial temporal lobe atrophy in the development of AD.[35–43] Most of these studies have demonstrated the presence of hippocampal asymmetry early in the disease course, with the observation of asymmetry lessening with disease progression.[35–37, 40–43] Left greater than right hippocampal volumes can be seen in many normal controls, with early increases in the rate of left greater than right hippocampal atrophy in MCI and early AD stages.[35–37, 40–43] The rate of atrophy begins to equalize as the AD patient progresses through the moderate to severe stages of disease. The clinical significance of asymmetric medial temporal lobe atrophy are largely unexplored, but one recent study demonstrated increased impairment in visual memory tasks with right greater than left hippocampal asymmetry. [36, 39, 41] Regardless of the question of asymmetry in mesial temporal lobe atrophy in AD, it is clear from many existing imaging and pathological studies medial temporal lobe atrophy may be the most agreed upon predictor of the presence of biological AD in a demented person. Such biological evidence of AD has been included in the proposed new criteria for AD described below attesting to the importance of this structural correlate of biological disease in the clinical diagnosis of AD.[44]
Many clinico-pathological studies show that biological AD can evolve atypically, leading to heterogeneity in clinical phenotypes that diverge from the typical signs, symptoms, and patterns of clinical progression.[45–49] Several patterns of disease have been characterized and are often referred to as focal cortical variants of AD. The visual variant of AD, also termed the Heidenhein variant, posterior cortical degeneration or atrophy, is characterized by a unique clinical phenotype reflecting the underlying pathological involvement of parietal-occipital and or parietal-temporal association cortices.[49–51] The clinical phenotype of primary progressive aphasia, fluent as well as nonfluent types, is often a manifestation of underlying AD pathologic involvement of neuroanatomic regions involved in language processing or production in the dominant hemisphere.[49, 52–56] Likewise, cases have been described where the clinical phenotypes of FTD and corticobasal degeneration are related to asymmetric or symmetric AD pathology in the frontal, temporal, or parietal lobes.[57–60] Such atypical presentations of AD have been well characterized, and while recognition of their existence is important, they are relatively rare phenotypes of disease and will not be discussed further. Most commonly, AD presents as a consistent pattern of evolving clinical signs and symptoms that will be the focus of this review. The characteristic clinical signs and symptoms of fulminate AD are embodied in the NINCDS-ADRDA criteria for AD adopted in 1986 (Table 1).[61]
Table 1.
NINCDS-ADRDA criteria for Alzheimer’s disease. [61]
| Diagnosis | Criteria |
|---|---|
| Unlikely Alzheimer’s Disease |
Dementia with sudden onset, focal neurologic signs, seizures, or gait disturbance early in the illness |
| Possible Alzheimer’s Disease |
Dementia with atypical onset, presentation, or progression without known etiology, absence of co-morbid disease capable of producing dementia |
| Probable Alzheimer’s Disease |
Dementia established by clinical and neuropsychological examination. Cognitive impairments are progressive and present in two or more areas of cognition. Onset of deficits between 40 and 90 years of age and absence of other diseases capable of producing dementia. |
| Definite Alzheimer’s Disease |
Criteria for probable Alzheimer's disease have been met and there is histopathologic evidence of AD from autopsy or biopsy. |
Historical evolution of the diagnosis of AD and its predementia disease stages
Recognition that the artificial threshold for diagnosis of AD inherent in the NINCDS-ADRDA criteria did not capture the slowly progressive nature of the underlying disease process in AD led to many early attempts to further describe and characterize the intervening clinical evolution of the signs and symptoms of AD.
The development of diagnostic criteria for age-associated memory impairment (AAMI) was the first attempt.[62] This diagnosis relied on the combination of both a nondementia state, characterized by a lack of functional impairment, and objective evidence of low or poor performance on neuropsychological test measures of memory, defined as 1 standard deviation below the mean for nondemented controls of any age. Analysis of age-based normative test data quickly revealed that this diagnostic designation suffered from two distinct flaws. First, it failed to account for normal, age-related change in test profiles. Second, it encompassed all individuals scoring at the low end of the normal distribution, irrespective of whether they were exhibiting an exaggerated slope of decline or change in cognitive skills that might reflect an underlying degenerative process (as incorporated in the current 2nd International Working Group on the diagnosis of MCI criteria. See below and in Figure 2).[63] The diagnostic criteria lacked utility because normal persons with lifelong, static poor performance could not be differentiated from those in the early stages of decline due to AD or other degenerative disease.[63]
Refinement of these criteria led to the development of age-associated cognitive decline (AACD).[64] This diagnostic definition included the use of age- and education-adjusted normative test scores overcoming one of the major confounds of AAMI.[64] While AACD narrowed the focus of detection, it still did not account for lifelong, static poor performers, again relying on absolute cutoffs of 1 standard deviation below the mean. Further refinement was clearly needed to reliably identify persons suffering from the earliest clinical features of AD.
Out of these efforts emerged the diagnosis of mild cognitive impairment (MCI) in the early 1990s based on scores of 3 or greater on the Global Deterioration Scale.[65, 66] This diagnosis and its utility to accurately predict future development of AD were reworked and eventually popularized by the seminal work of Petersen and colleagues at the Mayo clinic in 1999.[67] The original diagnostic criteria for MCI are presented in Table 2. The focus of MCI remained on the detection of early memory impairment, but now included necessary symptomatic decline by history. These criteria proved to have a diagnostic accuracy of approximately 85% to predict the development of a future dementing state.[67] Rates of conversion from MCI to dementia were 15% per year compared to previous estimates of 1–2% per year for the normal aged population, demonstrating the practical utility of such formalized criteria for the detection of prodromal dementia.[67]
Table 2.
| MCI Criteria |
|---|
| Memory complaint, preferably corroborated by an informant |
| Objective memory impairment |
| Normal general cognitive function |
| Intact activities of daily living |
| Not demented |
Despite these encouraging results, many studies applying various permutations of the MCI diagnosis have questioned the diagnostic utility of MCI in the detection of prodromal dementia states.[68] Reversion or stability rates as high as 70% have been reported.[69, 70] Understanding these differences and the presumed heterogeneity of MCI has spurred research in this area over the last decade.[68] Studies investigating the neuropathological features of MCI and eventual outcomes again support the heterogeneity that has called into question the utility of these diagnostic criteria.[30, 71, 72] Despite these caveats, the utility of this diagnosis was proved through its use in the multicenter Alzheimer’s Disease Cooperative Study group collaborative trial with Pfizer investigating the effects of donepezil on rates of conversion from MCI to dementia.[73, 74] While the overall trial failed to meet its primary outcome measures and was considered negative, the strict operational definition used in this study across centers demonstrated the utility of MCI to identify an intermediate clinical phenotype between normal and AD states as well as prediction of conversion from MCI to AD at a rate of 16% per year.[73, 74]
The 2nd International Working Group on the diagnosis of MCI, convened in 2005, further expanded this diagnosis to include the assessment and inclusion of nonmemory domain cognitive impairment.[68, 75] These refined criteria have been utilized as a conceptual framework to identify specific prodromal disease states (Figure 2). These diagnostic criteria involve the classification of subjects with early cognitive decline into four groups based on the presence or absence of memory domain involvement and the involvement of single or multiple domains and have been adopted by the National Alzheimer’s Coordinating Center (NACC, available online at http://www.alz.washington.edu/) and the Alzheimer’s Disease Neuroimaging Initiative (ADNI, available online at http://www.loni.ucla.edu/ADNI/).[68, 75] In this conceptual model, prodromal AD (MCI) may be represented by either single or multiple domain amnestic MCI. The development of multiple domain involvement has been suggested to represent the evolution of the clinical phenotype of prodromal AD reflective of the underlying pathological spread of AD.[68, 75]
Concurrent with the development of the diagnosis of MCI to detect predementia AD, scales for staging AD were developed including the Global Deterioration Scale and the Clinical Dementia Rating scale (CDR).[66, 76] The CDR is the most widely used instrument for outlining ordinal stages of AD as mild (CDR 1), moderate (CDR 2), or severe (CDR 3) and provide a conceptual framework for classifying the clinical phenotype of AD along the cognitive continuum after the development of overt dementia.[76] Like the original dichotomous diagnosis of normal versus AD embodied by the NINCDS-ADRDA criteria, these stages represent arbitrary cutoffs that do not clearly reflect the slowly progressive nature of the disease along the continuum of cognitive and functional decline. Nonetheless, they serve as a useful mechanism for describing points along the continuum of AD that allow clinicians and researchers to communicate effectively, guiding both therapeutic interventions and clinical research activities. This staging has also proved practical for the layperson, allowing preparation for and expectation of current and future clinical signs and symptoms of AD.
One recent international conference addressed the issues of a cognitive continuum reflecting the underlying biologic process of AD and proposed revised criteria for the diagnosis of AD given our enhanced understanding of the disease process and advancements in the area of biomarkers for AD (Table 3).[44] These criteria rely on a combination of both early clinical signs of anamnesis and biological evidence for a degenerative process consistent with AD through CSF, MRI, SPECT, or PET imaging.[44] Adoption of such criteria would be a step forward in our recognition of AD as a biological process that underlies the later development of the clinical signs and symptoms of AD meeting DSM-IV and NINCDS-ADRDA criteria for AD, and may allow both earlier identification and therapeutic intervention. Technologic advances and scientific discoveries over the last 23 years may now render the NINCDS-ADRDA criteria potentially obsolete as clinicians and researchers learn to more accurately diagnosis biologic AD at the first signs of clinical decline. Further work will hopefully yield reliable biomarkers of disease that may allow preclinical detection of disease, leading again to further revisions of the diagnostic criteria for AD.
Table 3.
Proposed “new” criteria for a diagnosis of Alzheimer’s disease (AD) that does not rely on functional decline seen in the later stages of disease, but rather utilizes supportive imaging and cerebrospinal fluid analysis to identify both the clinical and biological processes reflective of AD in its earliest stages.[44]
| New, proposed criteria for Alzheimer’s disease | ||
|---|---|---|
| Probable AD: A plus one or more supportive features B, C, D, or E | ||
| Core diagnostic criteria | ||
| A. Presence of an early and significant episodic memory impairment that includes the following features: | ||
| 1. Gradual and progressive change in memory function reported by patients or informants over more than 6 months | ||
| 2. Objective evidence of impaired episodic memory on testing: this generally consists of recall deficit that does not improve or does not normalize with cueing or recognition testing and after effective encoding of information has been previously controlled |
||
| 3. The episodic memory impairment can be isolated or associated with other cognitive changes at the onset of AD or as AD advances |
||
| Supportive features | ||
| B. Presence of medial temporal lobe atrophy | ||
| • Volume loss of hippocampi, entorhinal cortex, amygdala evidenced on MRI with qualitative ratings using visual scoring (referenced to well characterized population with age norms) or quantitative volumetry of regions of interest (referenced to well characterized population with age norms) |
||
| C. Abnormal cerebrospinal fluid biomarker | ||
| • Low amyloid β1–42 concentrations, increased total tau concentrations, or increased phospho-tau concentrations, or combinations of the three |
||
| • Other well validated markers to be discovered in the future | ||
| D. Specific pattern on functional neuroimaging with PET | ||
| • Reduced glucose metabolism in bilateral temporal parietal regions | ||
| • Other well validated ligands, including those that foreseeably will emerge such as Pittsburg compound B or FDDNP | ||
| E. Proven AD autosomal dominant mutation within the immediate family | ||
| Exclusion criteria | ||
| History: | ||
| • Sudden onset | ||
| • Early occurrence of the following symptoms: gait disturbances, seizures, behavioral changes | ||
| Clinical features: | ||
| • Focal neurological features including hemiparesis, sensory loss, visual field deficits | ||
| • Early extrapyramidal signs | ||
| Other medical disorders severe enough to account for memory and related symptoms: | ||
| • Non-AD dementia | ||
| • Major depression | ||
| • Cerebrovascular disease | ||
| • Toxic and metabolic abnormalities, all of which may require specific investigations | ||
| • MRI FLAIR or T2 signal abnormalities in the medial temporal lobe that are consistent with infectious or vascular insults | ||
| Criteria for definite AD | ||
| AD is considered definite if the following are present: | ||
| • Both clinical and histopathological (brain biopsy or autopsy) evidence of the disease, as required by the NIA-Reagan criteria for the postmortem diagnosis of AD; criteria must both be present |
||
| • Both clinical and genetic evidence (mutation on chromosome 1, 14, or 21) of AD; criteria must both be present | ||
Recent pathological data from longitudinal cohorts of normal subjects and data derived from studies investigating structural and molecular imaging, CSF and serum biomarkers have demonstrated the existence of biological AD in asymptomatic individuals with completely normal cognition.[77–87] The concept of preclinical AD (pAD) has emerged as a diagnostic dilemma and no consensus on diagnostic criteria for this stage of AD has been reached. The presence of biological AD in the absence of clinical signs and symptoms likely reflects both extent of pathological burden as well as “cognitive reserve”. [88] Cognitive reserve is hypothesized to entail the existence or development of compensatory mechanisms that maintain the output of neural activity in affected neuronal circuits in AD.[88] Thus, persons with pAD can maintain normal neurological, cognitive, and behavioral function despite the presence of fulminate biological AD. The development of accurate antemortem biomarkers of AD is rapidly expanding our appreciation of this entity, however persons with pAD will remain undiagnosed until a biomarker isavailable that has high diagnostic accuracy sufficient enough to replace autopsy confirmation of the disease..
It is clear from the discussion above that pAD, MCI, mild, moderate, and severe AD represent arbitrary thresholds or cutoffs for the development of operational criteria categorizing points along the cognitive continuum that reflect the underlying development of biological AD. It is important to realize that the true development and progression of the clinical signs and symptoms of AD do not involve step-wise decline along these diagnostic stages of disease, but rather a gradual evolution of the clinical phenotype of AD. Nonetheless, to provide a conceptual framework for discussion, the evolution of the clinical signs and symptoms of AD across the cognitive continuum will be discussed in the context of pAD, MCI, mild, moderate, and severe AD. Accompanying manuscripts detailing genetic, imaging (MRI, SPECT, PET), CSF, neuropathology, Aβ, synaptic changes, oxidative stress, inflammation, mitochondrial function, and the role of heavy metals in AD progression from preclinical to end-stage disease are presented in this edition of the Journal of Alzheimer Disease and will not be discussed further in this review.
Epidemiology
Overview
AD is the most common cause of degenerative dementia in the population age 65 and older and is also a common cause of dementia in those under 65. [89–95] The current NINCDS-ADRDA criteria for AD include individuals with clinical onset as young as 40 years of age.[61] Many epidemiological studies of AD have been conducted in populations from around the world, demonstrating that AD is a disease that crosses all ethnic, cultural, and geographical boundaries. [89–99] While age at disease onset may vary between genetically and culturally distinct populations, the disease course and clinical features are invariant. A comparison of incidence and prevalence rates across the stages of progression of AD could reveal three possible patterns: 1) Equality suggesting that the progression is linear and independent of confounding effects; 2) Increasing rates suggesting that some individuals may escape detection at earlier stages, progress rapidly through them, or that clinical signs and symptoms appear abruptly once critical thresholds for pathological burden exceed cognitive reserve; or 3) Decreasing rates suggesting the comorbid impact of factors influencing survival, inclusion of persons with degenerative disease other than AD in MCI, or an aborted disease process in certain individuals with pAD.
Preclinical Alzheimer’s disease
No true epidemiologic studies of pAD exist. Such studies would require a population-based enrollment and longitudinal evaluation of subjects with normal cognition that eventually come to autopsy. The costs of such an endeavor would be astronomical and the feasibility would be questionable even if financial concerns were negotiated. Barriers to autopsy recruitment exist that would also preclude such studies. Potential selection bias in subjects recruited to autopsy series is inherent in all existing studies of pAD to date. The development of diagnostically accurate biomarkers of disease in the antemortem period may eventually overcome these obstacles.
Several groups have attempted to overcome these obstacles through retrospective analysis of subjects that eventually develop the signs and symptoms of clinical AD years before the diagnosis was made. The Canadian Study of Health and Aging (CSHA) used such an approach to demonstrate that early changes in both category fluency and Trailmaking test part B are predictive of incipient dementia 5 years prior to diagnosis.[86, 87] Such conclusions are problematic for several reasons. First, the logic of using clinical decline to predict further clinical decline is circular and self fulfilling. Second, if the decline is detected clinically then it cannot, by definition, represent a “preclinical” state. Instead it represents an arbitrary shift in the threshold for detecting clinical change. Last, it cannot evaluate the potential for subjects not meeting thresholds for clinical detection to have true underlying biological and pathological AD. By definition, pAD represents biological AD without any clinical evidence of such processes.
At present, the best data available come from frequency measurement in selected autopsy cohorts of subjects that died with normal cognition.[71, 79, 81, 85, 100–107] While selection bias is inherent in these studies, at present the only reliable method to detect pAD relies on autopsy verification of AD. Other caveats include time from last evaluation to death and the rigor with which evidence for cognitive decline was evaluated antemortem. The reported frequency of fulminate pathological AD in series that have documented rigorous clinical evaluation and limited times from last evaluation to death (typically less than one year on average) ranges from 10–20%.[71, 79, 81, 85, 100–107] Data on incidence cannot be derived currently based on pathological evaluation. The future development and validation of accurate diagnostic biomarkers for AD that can be efficiently applied to true epidemiologic cohorts are needed to provide additional data on incidence rates for pAD.
Mild cognitive impairment
Epidemiologic studies have examined the incidence and prevalence of MCI with disparate findings.[69, 108–112] The wide variances in estimates may reflect variation in diagnostic criteria and evaluation between studies. Formal operationalization of the diagnostic criteria for MCI has led to more consistent estimates with reduced variability between studies in recent years.[108, 111, 112] The prevalence and incidence of MCI still appear to be highly dependent on age of the population studied consistent with the epidemiologic data on AD and range between 5–40%.[69, 108–112] Incidence rates are typically much lower, ranging from 1–10% per year, reflecting the duration of disease in persons with MCI from onset of diagnosis to conversion or transition to dementia.[69, 108–112]
Alzheimer’s disease
The incidence rate for AD typically ranges from 1–2% per year, but again is highly dependent on age of the population studied. [89–99] Prevalence rates vary with age, but are typically estimated at 15–20% of the population over the age of 65 years, with reports as high as 50% for individuals above the age of 85 years. [89–99] The incidence and prevalence of AD increase with age in an exponential fashion, identifying AD as the most common cause of dementia for those over the age of 65 years.
Summary
From a broad perspective, the frequency, incidence, and prevalence rates of pAD, MCI, and AD are consistent across diagnosis supporting the notion that each diagnosis represents a defined stage in the progression of the same underlying biological disease process that eventually leads to the clinical and pathological diagnoses of AD. These data also attest to the diagnostic accuracy inherent in the construct of MCI.
Neuropsychology
Overview
The current diagnosis of AD strictly depends on identifying memory and other cognitive domain involvements that lead to functional decline and impairment in activities of daily living (ADLs).[61, 113] Neuropsychological testing has played a predominant role in advancing our knowledge and understanding of the clinical sequelae of the underlying disease. Identifying clinical deficits leads to the early predementia diagnosis of MCI, but cannot, by definition, identify pAD. Global cognitive measures such as the Folstein Mini-Mental State Examination (MMSE) and the CDR are widely used to identify the presence and clinical stage of disease for persons suffering from biological AD as well as other degenerative causes for cognitive decline in the general population.[76, 114] Specific neuropsychological test measures have provided further insights into the clinical progression of disease that reflects the underlying neuroanatomic spread of disease in AD that will not be discussed further in this review.
Neuropsychological test scores (both global and specific) overlap between stages of disease, again reflecting the underlying pathological and overt cognitive continuum of decline in AD. The following sections are framed in typical scores for the global test measures MMSE and CDR, yet the overlap requires recognition and acknowledgement. Normal cognition and/or pAD can occur with both MMSE below 27 dependent on baseline abilities, or can coexist with CDR greater than 0 dependent on the cognitive domain(s) involved and the reliability of the informant report. Likewise, persons with MCI may have scores as high as 30 on the MMSE or well into the range typically assigned to the stages of AD depending on the considerations above. The CDR is an instrument that focuses on anamnesis; many persons with MCI may score normally on the CDR yet have considerable impairment even to the point of dementia.
The weaknesses of these global assessment measures have spurred the search for more specific and definitive clinical measures and pushed forward the search for more definitive biomarkers of AD. Other global measures that are commonly used to gauge the presence and severity of AD include the GDS, Alzheimer’s Disease Cooperative Study Cognitive Test Battery (ADAS-Cog), and Mattis Dementia Rating scale.[66, 115, 116] These measures may have a ceiling effect for normals, pAD, and even MCI subjects. Specific neuropsychological test measures that are widely used include components of the WAIS (digit-span, digit-symbol, logical memory), category fluency (animals and vegetables), Trailmaking A & B and are included in the newly adopted Unified Data Set (UDS) by the NACC.[117] The establishment of a universal test battery, used for not only NACC centers but also as part of the ADNI will hopefully allow for more successful crosstalk between centers and the ability to pool data for much larger numbers of subjects than any one center could obtain independently. Although the approach has not been validated, it holds much promise for moving AD research forward.
While the development of the aforementioned global cognitive measures and UDS has helped standardize assessment in the field, it is apparent to many that the test measures themselves may lack both sensitivity and specificity to identify AD in its early (MCI) and prodromal (pAD) states. The use of supraspan word list tasks for memory assessment, such as the Rey and California verbal learning tests, may increase the sensitivity of detection thresholds for amnestic MCI.[118] Newer developments, such as contextual learning and recall paradigms, are in their infancy but hold much promise in enhancing early detection of specific disease states such as AD in its predementia forms. Full discussion of such advances in neuropsychological test paradigms is beyond the scope of this review.
Preclinical Alzheimer’s disease (CDR 0, MMSE 27–30)
Again, by definition, pAD represents a biological state of disease that is present before sufficient neuronal damage and dysfunction begins to affect overt or domain-specific cognitive function. As such, pAD is characterized by normal CDR 0 and MMSE scores 27–30. Yet, these global measures do not fully assess the full range of cognitive function that could identify an early disease state. Studies investigating the neuropsychological identification of pAD have exclusively relied on longitudinal collection of test data from subjects enrolled as normal that later developed sufficient cognitive decline to meet criteria for AD.
The CSHA, discussed above, retrospectively evaluated neuropsychological test measures that might predict future conversion to AD and found several neuropsychological test measures that were significant predictors of cognitive decline five years before the onset of dementia.[86, 87] This study did not utilize criteria for MCI, but rather relied on the cutoff of clinical dementia for comparison. The average duration of MCI estimated through conversion rates to dementia is 4–5 years, suggesting that this and similar studies may be detecting the appearance of incident MCI rather than true pAD.[67, 68, 73–75]
Rush University investigated pAD in subjects that came to autopsy with a diagnosis of normal cognition proximal to death and found that almost 20% met the pathological criteria for AD.[101] Retrospective analysis of their neuropsychological test performance demonstrated significant differences in only delayed recall tasks between subjects with pathological AD autopsy findings and those with normal autopsy findings, suggesting that memory decline may be present, albeit subtly, in persons with pAD before sufficient cognitive decline to warrant the diagnosis of either MCI or dementia.[101] Early pathological involvement of the entorhinal cortex and hippocampus through connections of the perforant pathway would suggest early changes in delayed recall as the first clinical manifestations of underlying biological AD. Yet, such findings are subject to scrutiny as they rely on the application of cut-off scores or subjective impressions of examining neuropsychologists in determining significance of findings. It could be argued that if decline was present and identifiable clinically, then these subjects did not truly represent pAD, but rather reflect an artificial threshold for the detection of cognitive decline.
Mild cognitive impairment (CDR 0.5, MMSE 25–30)
The diagnosis of MCI is dependent on the first objective signs of clinically detectable cognitive decline.[67, 68, 75, 119] Global test measures fall in the normal range such as the MMSE at 27–30, yet if all three points are lost in the delayed recall potion the test is definitively abnormal using normative standards for individual test items. While global measures are impractical in early stages of disease, specific patterns of deficits could be diagnostic if evaluated. CDR scores are typically 0.5 reflecting identified memory impairment on the Blessed portion of the CDR worksheet and the informant or study partner attestation as to the presence of a memory impairment.[68, 71, 75, 76] Sensitive neuropsychological test measures can detect underlying biological AD at the earliest stages that represent MCI.[68, 75] The sensitivity of such diagnostic tests and the threshold for detecting impairment differ widely between neuropsychological test measures and may be at least partially responsible for the heterogeneity of MCI reported in the literature. Central to the theme of this review is the discussion and elucidation of a clinical cognitive continuum that reflects the underlying progression of pathological AD. Arbitrary cutoffs for AD or MCI represent artificial constructs that help us define disease state, but may lack cohesion or uniformity when applied universally unless stringent criteria for the diagnosis of MCI are held to.[68, 75]
Understanding MCI as a continuum from the earliest objective clinical signs and symptoms of AD to the end-stage of MCI occurring just before functional decline is imperative for a rational interpretation of data from the many disparate studies of MCI published over the last decade. The realization that MCI may present with impairments in varied cognitive domains has not only spurred investigation into prodromal or predementia disease states of non-AD degenerative dementias, but has also planted the seed to explore the progression of MCI as a continuous process that leads to progressive and accumulative cognitive impairments that characterize this predementia state of AD.[68, 75, 120, 121]
The MCI stage of AD includes impairments in the cognitive domains of attention, executive function, language, visuospatial function, among others.[68, 75, 122] Recent data suggest that MCI presents with single-domain amnestic involvement, but rapidly includes additional impairments in attention/executive function that reflectspread into frontal areas, as well as language impairments first manifest as semantic deficits that reflect temporal lobe dysfunction, and visuospatial deficits that reflect dysfunction of parieto- and temporo-occipital association areas.[123] This pattern of clinical progression parallels the neuroanatomic involvement of the underlying biological disease process in AD.[8, 11, 12] The precise pattern of clinical progression across neuropsychological test measures is not well-defined, but clearly demonstrates progression along a continuum as well as some degree of heterogeneity that may be inherent in the expression of biological AD between individuals. Variation in progression may reflect the influence of underlying genetic or environmental risks for the development and progression of AD. Further work in this area investigating the relative contributions of putative genetic and environmental risk factors is needed to fully understand the clinical and neuroanatomic progression of biological AD in MCI.
MCI may best be understood by a continuum, but also as an ordinal continuum, as is AD. Mild MCI may manifest as isolated anamnesia.[123] Moderate MCI may begin to include a single domain of either attention/executive, language, or visuospatial dysfunction in addition to the amnesia inherent in earlier stages. Severe or late-stage MCI may involve several or all of these domains before functional impairment sufficient to meet criteria for AD is realized. As objective measures of functional decline are largely experimental and not universally applied, the distinction between late-stage MCI and early stage dementia remains a “gray-zone”.
Mild Alzheimer’s disease (CDR 0.5–1, MMSE 21–26)
Mild AD is characterized by documented impairment in neuropsychological function in memory and at least one other cognitive domain by NINDCS-ADRDA criteria.[61] The MMSE score typically drops to 21–26, reflecting the involvement of multiple nonmemory domains. Informants detail considerable interference with functional activities as the result of the cognitive decline. Involvement of attentional and executive domains is almost universal at this stage. Language testing may show differential impairment of category over semantic fluency, attesting to the preferential neuroanatomic involvement of anterior temporal lobe over frontal lobe structures. Visuospatial involvement manifests early in this stage and continues to progress throughout the disease course.
Moderate Alzheimer’s disease (CDR 2, MMSE 14–20)
As the person with AD progresses along the cognitive continuum, the MMSE score drops to the range of 14–20 and CDR scores fall to 2. This decline reflects widespread involvement of neocortical regions that manifests through impairment on all cognitive measures. Essentially no cognitive domain is spared at this stage of disease, although compensatory mechanisms for skills reliant on long term memory may remain intact leading to some degree of functional independence in scattered activities.
Severe Alzheimer’s disease (CDR 3, MMSE 0–13)
The severe stage of AD is characterized pathologically by widespread involvement of all neocortical regions reflected in severe disturbance of cognitive function across all domains. While this may also reflect a parallel development of coexistent pathological processes such as CVD, DLB, or age-related brain atrophy, the clinical phenotype is invariant and that of late stage disease. In this stage, the MMSE score drops below 14 and affected persons may be disoriented to place and time and begin to have trouble identifying close relatives. The CDR progresses to a score of 3 and essentially all independent activity is lost. Neuropsychological test measures such as the Severe Impairment Battery (SIB) have been developed to allow evaluation of cognitive function in this severe stage of AD.[124] The SIB is useful as subjects continue to progress to the point of complete disorientation to self, long after the MMSE and CDR have resulted in an absolute floor effect. [124] As the disease progresses, continence becomes a major issue as the frontal micturation and bowel control centers are overwhelmed with AD pathology. The disease progresses until the entire neocortex is subsumed, the MMSE reaches zero and the individual is completely mute and dependent.
Summary
Cognitive decline throughout the preclinical and clinical stages of AD can be carefully and precisely monitored using a variety of instruments in addition to those described above. The progressive involvement of neuroanatomic areas is reflected in the gradual involvement of cognitive domains other than memory. The severity of pathological AD in brain areas involved in cognitive functions as memory, attention, executive function, language, visuospatial function and praxis is mirrored by clinical deterioration that can be objectively evaluated and provide a reliable methodology for estimating the neuroanatomic extent and severity of the underlying biological disease process. These clinico-pathological associations, however, begin to lose their validity with the development of coexistent pathological features such as CVD in the aging population. [125] Careful clinical and radiological evaluation is needed to ensure that features associated with the severe stage of AD are not representative of coexistent CVD, DLB, or other diseases.
Psychiatric and behavioral disturbances
Overview
The neuroanatomic involvement of prefrontal subcortical limbic regions is an early feature of pathological AD.[8, 11, 12] Reflecting this is the early, often prodromal appearance of neuropsychiatric and behavioral alterations that become more fulminate and problematic as AD progresses.[126] Depression, anxiety, irritability, aggressiveness, apathy, euphoria, sleep and appetite disturbances, motor restlessness, hallucinations, delusions, and paranoia are typical features of fulminate AD that are routinely assessed in Alzheimer’s Disease Centers using the informant based Neuropsychiatric Inventory (NPI).[127] These features can be the presenting signs of an impending dementia years before cognitive decline becomes apparent and are often the most difficult to deal with from the perspective of the caregiver, friends, family, and clinicians of persons with AD at any stage.
Preclinical Alzheimer’s disease
The emergence of de novo neuropsychiatric symptoms of depression and anxiety can appear years before even a diagnosis of MCI is made. One recent study from the Mayo Clinic showed that both were significant predictors of impending cognitive decline in the elderly.[126] Almost 2/3 of persons over the age of 65 with new onset depression or anxiety developed MCI or dementia in a subsequent 12 year period. The de novo development of these symptoms in persons over the age of 65 should immediately prompt an evaluation for an underlying dementia. Apathy is also a common precursor and should alert the caregiver, friends, family, and clinicians to the possibility offutureAD. [128] Other more developed or severe neuropsychiatric features are not commonly seen in the early stages of biological AD, but do become more prevalent as the disease progresses and neuronal dysfunction and death involve more extensive areas of the brain.[129] Earlier appearance of psychotic or behavioral symptoms such as overt, well-formed hallucinations, delusions, obsessions, compulsions, personality changes, and socially inappropriate behaviors should raise the possibility of alternate non-AD causes such as DLB and FTD.[129–135]
Mild cognitive impairment
Depression and anxiety are increasingly prevalent at this stage of disease.[136] Other psychiatric and behavioral symptoms begin to appear and can include delusions and paranoia often related to beliefs that others have stolen or moved objects.[136] and reflect the underlying primary involvement of the Papez circuitry and short term memory dysfunction.[23] Irritability and agitation can ensue as persons in this stage of AD try to make sense of their world and maintain their construct of reality that is progressively fragmenting. Motor restlessness may be an expression of underlying anxiety. Overt, well-formed hallucinations are rare at this stage of disease and again should prompt a consideration of non-AD causes for decline. [129–135] Mild sleep disturbances may begin to emerge with disruption of the normal circadian rhythm. Exaggeration of basic personality traits is also often seen, e.g., the needy becoming needier, the hostile becoming more aggressive, etc. These behavioral and psychiatric alterations reflect the spread of pathology into frontal and limbic areas involved in regulation and suppression of inappropriate behaviors. Overall social graces are maintained and neuropsychiatric symptoms other than depression, anxiety, and irritability are usually not major issues in MCI. Data from the Cardiovascular Health Study based on the NPI demonstrate significant elevations of overall scores that fall midway between subjects with normal cognition and those with AD.[137]
Mild Alzheimer’s disease
The development of functional decline sufficient to meet DSM criteria for dementia and the subsequent diagnosis of AD by NINCDS/ADRDA criteria is accompanied by a concomitant increase in the extent and severity of neuropsychiatric and behavioral symptoms.[129, 137] Depression, anxiety, irritability are now accompanied by more widespread evidence of sleep disturbance, more prominent features of delusions and paranoia, the emergence of motor restlessness in some, severe apathy, and social withdrawal. These features are associated with progressive involvement of widespread frontal lobe regions, and increasing severity of disease in previously affected limbic regions. Overt hallucinations are still rarely seen in this early stage of disease, but by now most cortical regions are showing changes of AD.[8, 11, 12] Circadian disturbances are poorly understood in AD, but could reflect either early neuronal dysfunction in hypothalamic or brain stem nuclei involved in sleep (which have been poorly investigated), or altered signaling to these areas from AD-involved cortical areas. Personality and behavioral changes beyond the above mentioned are rare as the person with AD is able to maintain a sense of self throughout this stage of disease.
Moderate Alzheimer’s disease
As the disease progresses in severity in affected limbic and neocortical regions, the psychiatric and behavioral features seen in early stages progress. Frank hallucinations begin to emerge and delusions become more integrated into the construct of reality. [129, 137] Motor restlessness, often manifested through picking or scratching behaviors, can lead to recurrent superficial skin infections. Social inappropriateness can become problematic and lead to social isolation for persons with AD and their immediate circle of caregivers, friends, and family. The symptoms become more difficult and resistant to treat and begin to emerge as the primary issues of concern for all involved in the care of the person with AD.
Severe Alzheimer’s disease
In the final stages of AD, the construct of reality is completely fragmented. Familiarity with family, friends, and caregivers exists only in the implicit, rather than explicit state. Worsening hallucinations and behavioral agitation, for those that experience these symptoms, soon give way to progressive apathy. Engagement in all activities eventually ceases and the person with AD becomes bedridden, and the outcome is inevitably death.
Summary
Psychiatric and behavioral disturbances can be seen across the spectrum of clinical phenotypes of ADand are not merely reactive, but instead reflect the progressive neuroanatomic involvement of key structures including limbic areas affected by AD. While psychiatric manifestations of AD such as depression and anxiety can precede the development of cognitive decline in AD, early onset hallucinations, delusions, or severe behavioral disturbances should alert the examining clinician to the possible presence of a non-AD degenerative dementia such as DLB or the behavioral variant of FTD. As these individual disease states progress, the distinction provided by the early occurrence of such symptoms begins to blur. The severe stage of dementia, irrespective of biological cause, almost universally includes profound psychiatric and behavioral disturbances.
Functional decline
Overview
Impairment of daily social, educational, or occupational functioning is essential for a clinical diagnosis of AD.[61, 113] Indeed, by the endstage of disease, functional impairment in all activities is affected resulting in complete dependency. The primary importance of functional decline in establishing the cutoff for transition from multidomain amnestic MCI to early AD cannot be understated. This is the sole criterion distinguishing these stages of disease.[61, 68, 75, 113, 119] In routine clinical practice, the level of functional impairment is determined by the examining clinician and is purely subjective. Additionally the assessment of functional impairment is often confounded by societal and lifestyle demands as well as the development of comorbid medical conditions. Comorbid medical conditions that can affect daily function in the elderly are numerous, ranging from physical limitations related to musculoskeletal causes such as severe arthritis and sarcopenia, to visual loss related to glaucoma and macular degeneration. Distinguishing functional decline caused by comorbidities from that of AD can challenge even the most experienced clinician. Early attempts aimed at developing quantitative measures to assess functional impairment in those with AD are underway and may provide tools that will allow a more uniform and consistent assessment of functional decline in AD across the cognitive continuum.[138–141]
Preclinical Alzheimer’s disease
By definition, persons with pAD are fully functional and will not be discussed further in this section.
Mild cognitive impairment
By definition, persons with MCI should not be experiencing considerable functional decline. Any functional impairment experienced must be below the clinical threshold to warrant a diagnosis of dementia by DSM criteria, otherwise, the appropriate diagnosis would be AD.[61, 68, 75, 113, 119] Nonetheless, subtle changes in functional performance have been documented across studies in persons with MCI, typically involving complex skills dependent on short term memory functions that reflect the early biological involvement of medial temporal lobe limbic structures in AD.[113, 140, 141] Persons with MCI more commonly struggle with, but are able to successfully accomplish, functional activities relating to their social, educational, and occupational activities. For example, an executive secretary finds that she is able to complete her tasks appropriately, but that a normal 8 hour workday has now transformed into a 12 hour struggle. She is not objectively functionally impaired in ultimate accomplishments, but the increasing time required to complete her routine tasks could be considered a functional impairment. The judgment call on behalf of the examining clinician is subjective. For many in the field, this arbitrary distinction is less meaningful than the realization that she suffers from an underlying biological disease process known as AD. The biological diagnosis in this case was supported by structural MRI showing medial temporal lobe atrophy, PET with the characteristic signature of AD, and CSF analysis demonstrating elevated total/phospho-tau and reduced β-amyloid levels characteristic of biological AD. The clinician’s call influences the patient/caregiver preparation for the ensuing difficulties she is likely to experience, influences the choice of therapeutic intervention and the likelihood that her prescription insurance will cover any prescribed medication, and further influences her ability to engage in clinical trials of potential disease-modifying agents that are being developed. Clearly the differentiation of MCI from early AD deserves a more objective discriminant.
Recent work in the field has focused on developing an objective measure of functional impairment that may aid clinicians in making diagnostic distinctions with far reaching consequences. Assessments of monetary and financial abilities have been quantified and provide an objective measure of functional abilities.[141] While such developments hold promise for the future, normative data are lacking and assessment of decline from one individual to the next based on cross sectional evaluation is unhelpful. More work is needed in this area if researchers and clinicians are to continue to rely on clinical rather than biological diagnostic criteria in the diagnosis of the underlying biological disease state of AD.
Mild Alzheimer’s disease
Major functional decline in the most advanced independent ADLs is first seen at this stage of disease. [138–141] Difficulties with complex financial management precede and often predict the future development of difficulties with simple monetary exchanges.[141] Occupational impairment almost universally leads to retirement, disability, or loss of job through firing of persons with AD at this stage. Driving may be impaired and the operation of telephones, other appliances, and electronic gadgetry may be affected.[142–146] Yet, most of the more basic ADLs may be entirely intact. Shopping, cooking, cleaning, simple hobbies, and bathing, dressing, feeding and continence may be preserved. Measures of global cognitive decline such as the CDR and GDS rely on identifying functional changes in staging the disease process.[66, 76] Others such as the MMSE and Mattis Dementia Rating Scale have been highly correlated with stages of functional decline.[114, 115] The American Academy of Neurology Practice Parameter on driving for persons with dementia uses a CDR global score of 1 to indicate the need to cease driving, whereas a scale score of 0.5 with a diagnosis of AD is considered potentially safe.[144] Such global cognitive assessment instruments may be useful at the stages of mild/moderate/severe AD in assessing functional decline independent of performance, but this is clearly not the case in MCI.
Moderate Alzheimer’s disease
As persons with AD progress along the cognitive continuum, they develop increasing difficulties with simple ADLs. At the moderate stage of AD, cooking, cleaning, operation of complex electrical appliances and simple finances become impaired. Supervision is absolutely necessary for medication administration to avert potential health care crises. Assistance is often needed to prompt persons with moderate AD to engage in routine hygiene and grooming activities, although they may still be able to perform such activities independently to some degree. Clothes for the day often need to be laid out and assistance with dressing in the proper sequence may be required. Feeding behaviors often devolve, and at this stage the person with AD often regresses to the use of a single eating utensil or even a reliance on finger foods in the later stages of disease. Twenty-four hour supervision is almost universally necessary, although some degree of independence in basic ADLs can still be seen.
Severe Alzheimer’s disease
As persons approach and enter the stage of severe AD characterized by MMSE<14 and CDR 3, the underlying AD pathology, coexistent pathological features, and or age related atrophy have almost completely engulfed the brain and they become essentially entirely reliant on others for almost all of their daily care. Casual conversation and physical abilities may be preserved as one enters the severe stage of AD, but as the disease progresses complete dependence ensues and all meaningful function essentially ends. At this point in the continuum of AD, affected persons become bedridden and death ultimately ensues from comorbid or other medical conditions.
Summary
The arbitrary cutoff of predementia (MCI) from dementia (AD) based on functional impairment may hold little meaning unless more rigorous methods for the assessment of subtle functional impairment that are both valid and reliable are developed. While functional impairment and decline remain the most critical issues affecting the lives of those stricken with biological AD, once they progress to the early stages of MCI, it has little practical use in identifying pAD using current assessment measures. The recognition of pAD remains dependent on the development of preclinical biological markers of disease, at least for the time being.
Conclusions
The continuum of AD, from pAD in subjects with normal cognition, through MCI, and the stages of AD manifests in a predictable pattern of progressive cognitive, psychiatric, behavioral, and functional clinical impairment reflecting the underlying neuroanatomic involvement and extent of the biological disease process. Clinical signs and symptoms allow accurate identification of the underlying disease process in the more advanced stages of mild, moderate, and severe AD but not in diagnosing or monitoring disease progression in pAD and are questionable in MCI without supportive biological evidence. A reliable surrogate marker or combination of markers for biological AD with high diagnostic validity is essential as researchers and clinicians push back the diagnosis of AD to encompass pAD, allowing earlier therapeutic intervention and the possibility of curing the disease before the first subtle clinical signs and symptoms of AD can be detected. These advances are all extremely exciting, and the wealth of biological targets and number of compounds undergoing current evaluation are reasons for cautious hope that a cure for biological AD is on the horizon.
Acknowledgements
First we gratefully acknowledge the many subjects, caregivers, friends, and families of persons with AD that have engaged in clinical research activities that have allowed such discoveries to be made. Without your selfless engagement, such advances would not be possible. Second we acknowledge the many researchers and clinicians in the field that have not been cited in this manuscript. Your contributions have been critical in leading us to our current investigations and search for disease-modifying therapies for AD. Dr. Jicha is supported by funding from the NIH/NIA 1 P30 AG028383 & 2R01AG019241-06A2, NIH LRP 1 L30 AG032934-01, Alzheimer’s Association NIRG-07-59967, and the Sanders-Brown Foundation. Dr Jicha has also received research support for clinical trial activities from NIH/NIA ADCS U01AG010483, Pfizer Inc., Elan Pharmaceuticals, and Baxter Inc.
References
- 1.Gron G, Riepe MW. Neural basis for the cognitive continuum in episodic memory from health to Alzheimer disease. Am J Geriatr Psychiatry. 2004;12:648–652. doi: 10.1176/appi.ajgp.12.6.648. [DOI] [PubMed] [Google Scholar]
- 2.Hachinski V. Shifts in thinking about dementia. Jama. 2008;300:2172–2173. doi: 10.1001/jama.2008.525. [DOI] [PubMed] [Google Scholar]
- 3.Liddell BJ, Paul RH, Arns M, Gordon N, Kukla M, Rowe D, Cooper N, Moyle J, Williams LM. Rates of decline distinguish Alzheimer's disease and mild cognitive impairment relative to normal aging: integrating cognition and brain function. J Integr Neurosci. 2007;6:141–174. doi: 10.1142/s0219635207001374. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer's disease. Neurologia. 2000;15:93–101. [PubMed] [Google Scholar]
- 5.Gomez-Isla T, Hyman BT. Neuropathological changes in normal aging, minimal cognitive impairment, and Alzheimer's disease. In: Petersen RC, editor. Mild Cognitive Impairment: Aging to Alzheimer's Disease. New York: Oxford University Press, Inc; 2003. [Google Scholar]
- 6.Haroutunian V, Perl DP, Purohit DP, Marin D, Khan K, Lantz M, Davis KL, Mohs RC. Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer disease. Arch Neurol. 1998;55:1185–1191. doi: 10.1001/archneur.55.9.1185. [DOI] [PubMed] [Google Scholar]
- 7.Korf ES, Wahlund LO, Visser PJ, Scheltens P. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology. 2004;63:94–100. doi: 10.1212/01.wnl.0000133114.92694.93. [DOI] [PubMed] [Google Scholar]
- 8.Markesbery WR. Neuropathology of Dementing Disorders. London: Arnold; 1998. [Google Scholar]
- 9.Riley KP, Snowdon DA, Markesbery WR. Alzheimer's neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 10.Smith CD, Malcein M, Meurer K, Schmitt FA, Markesbery WR, Pettigrew LC. MRI temporal lobe volume measures and neuropsychologic function in Alzheimer's disease. J Neuroimaging. 1999;9:2–9. doi: 10.1111/jon1999912. [DOI] [PubMed] [Google Scholar]
- 11.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, del Tredici K, Braak E. Spectrum of Pathology. In: Petersen RC, editor. Mild Cognitive Impairment: Aging to Alzheimer's Disease. New York: Oxford University Press, Inc; 2003. pp. 149–189. [Google Scholar]
- 13.Nelson PT, Abner EL, Scheff SW, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Patel E, Markesbery WR. Alzheimer's-type neuropathology in the precuneus is not increased relative to other areas of neocortex across a range of cognitive impairment. Neurosci Lett. 2009;450:336–339. doi: 10.1016/j.neulet.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Davis DG, Poduska JW, Patel E, Mendiondo MS, Markesbery WR. Modeling the Association between 43 Different Clinical and Pathological Variables and the Severity of Cognitive Impairment in a Large Autopsy Cohort of Elderly Persons. Brain Pathol. 2008 doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, Abner EL, Markesbery WR. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles "do count" when staging disease severity. J Neuropathol Exp Neurol. 2007;66:1136–1146. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown DF, Dababo MA, Bigio EH, Risser RC, Eagan KP, Hladik CL, White CL., 3rd Neuropathologic evidence that the Lewy body variant of Alzheimer disease represents coexistence of Alzheimer disease and idiopathic Parkinson disease. J Neuropathol Exp Neurol. 1998;57:39–46. doi: 10.1097/00005072-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Hu WT, Josephs KA, Knopman DS, Boeve BF, Dickson DW, Petersen RC, Parisi JE. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol. 2008;116:215–220. doi: 10.1007/s00401-008-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 19.Zekry D, Hauw JJ, Gold G. Mixed dementia: epidemiology, diagnosis, and treatment. J Am Geriatr Soc. 2002;50:1431–1438. doi: 10.1046/j.1532-5415.2002.50367.x. [DOI] [PubMed] [Google Scholar]
- 20.Haroutunian V, Schnaider-Beeri M, Schmeidler J, Wysocki M, Purohit DP, Perl DP, Libow LS, Lesser GT, Maroukian M, Grossman HT. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol. 2008;65:1211–1217. doi: 10.1001/archneur.65.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prohovnik I, Perl DP, Davis KL, Libow L, Lesser G, Haroutunian V. Dissociation of neuropathology from severity of dementia in late-onset Alzheimer disease. Neurology. 2006;66:49–55. doi: 10.1212/01.wnl.0000191298.68045.50. [DOI] [PubMed] [Google Scholar]
- 22.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 23.Triarhou LC. Centenary of Christfried Jakob's discovery of the visceral brain: an unheeded precedence in affective neuroscience. Neurosci Biobehav Rev. 2008;32:984–1000. doi: 10.1016/j.neubiorev.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Mufson EJ, Ginsberg SD, Ikonomovic MD, DeKosky ST. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J Chem Neuroanat. 2003;26:233–242. doi: 10.1016/s0891-0618(03)00068-1. [DOI] [PubMed] [Google Scholar]
- 25.Cummings JL. The neuroanatomy of depression. J Clin Psychiatry. 1993;54 Suppl:14–20. [PubMed] [Google Scholar]
- 26.Smith GS, Kramer E, Ma Y, Kingsley P, Dhawan V, Chaly T, Eidelberg D. The functional neuroanatomy of geriatric depression. Int J Geriatr Psychiatry. 2009 doi: 10.1002/gps.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kazee AM, Eskin TA, Lapham LW, Gabriel KR, McDaniel KD, Hamill RW. Clinicopathologic correlates in Alzheimer disease: assessment of clinical and pathologic diagnostic criteria. Alzheimer Dis Assoc Disord. 1993;7:152–164. doi: 10.1097/00002093-199307030-00004. [DOI] [PubMed] [Google Scholar]
- 28.Klatka LA, Schiffer RB, Powers JM, Kazee AM. Incorrect diagnosis of Alzheimer's disease. A clinicopathologic study. Arch Neurol. 1996;53:35–42. doi: 10.1001/archneur.1996.00550010045015. [DOI] [PubMed] [Google Scholar]
- 29.Petrovitch H, White LR, Ross GW, Steinhorn SC, Li CY, Masaki KH, Davis DG, Nelson J, Hardman J, Curb JD, Blanchette PL, Launer LJ, Yano K, Markesbery WR. Accuracy of clinical criteria for AD in the Honolulu-Asia Aging Study, a population-based study. Neurology. 2001;57:226–234. doi: 10.1212/wnl.57.2.226. [DOI] [PubMed] [Google Scholar]
- 30.Jicha GA, Parisi JE, Dickson DW, Johnson K, Cha R, Ivnik RJ, Tangalos EG, Boeve BF, Knopman DS, Braak H, Petersen RC. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006;63:674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 31.Misra C, Fan Y, Davatzikos C. Baseline and longitudinal patterns of brain atrophy in MCI patients, and their use in prediction of short-term conversion to AD: results from ADNI. Neuroimage. 2009;44:1415–1422. doi: 10.1016/j.neuroimage.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61:1531–1534. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- 33.Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 34.Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes J, Scahill RI, Schott JM, Frost C, Rossor MN, Fox NC. Does Alzheimer's disease affect hippocampal asymmetry? Evidence from a cross-sectional and longitudinal volumetric MRI study. Dement Geriatr Cogn Disord. 2005;19:338–344. doi: 10.1159/000084560. [DOI] [PubMed] [Google Scholar]
- 36.Bigler ED, Tate DF, Miller MJ, Rice SA, Hessel CD, Earl HD, Tschanz JT, Plassman B, Welsh-Bohmer KA. Dementia, asymmetry of temporal lobe structures, and apolipoprotein E genotype: relationships to cerebral atrophy and neuropsychological impairment. J Int Neuropsychol Soc. 2002;8:925–933. doi: 10.1017/s1355617702870072. [DOI] [PubMed] [Google Scholar]
- 37.Geroldi C, Laakso MP, DeCarli C, Beltramello A, Bianchetti A, Soininen H, Trabucchi M, Frisoni GB. Apolipoprotein E genotype and hippocampal asymmetry in Alzheimer's disease: a volumetric MRI study. J Neurol Neurosurg Psychiatry. 2000;68:93–96. doi: 10.1136/jnnp.68.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Likeman M, Anderson VM, Stevens JM, Waldman AD, Godbolt AK, Frost C, Rossor MN, Fox NC. Visual assessment of atrophy on magnetic resonance imaging in the diagnosis of pathologically confirmed young-onset dementias. Arch Neurol. 2005;62:1410–1415. doi: 10.1001/archneur.62.9.1410. [DOI] [PubMed] [Google Scholar]
- 39.Moossy J, Zubenko GS, Martinez AJ, Rao GR, Kopp U, Hanin I. Lateralization of brain morphologic and cholinergic abnormalities in Alzheimer's disease. Arch Neurol. 1989;46:639–642. doi: 10.1001/archneur.1989.00520420059023. [DOI] [PubMed] [Google Scholar]
- 40.Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: Meta-analyses of MRI studies. Hippocampus. 2009 doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- 41.Soininen HS, Partanen K, Pitkanen A, Vainio P, Hanninen T, Hallikainen M, Koivisto K, Riekkinen PJ., Sr Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: correlation to visual and verbal memory. Neurology. 1994;44:1660–1668. doi: 10.1212/wnl.44.9.1660. [DOI] [PubMed] [Google Scholar]
- 42.Wolf H, Grunwald M, Kruggel F, Riedel-Heller SG, Angerhofer S, Hojjatoleslami A, Hensel A, Arendt T, Gertz H. Hippocampal volume discriminates between normal cognition; questionable and mild dementia in the elderly. Neurobiol Aging. 2001;22:177–186. doi: 10.1016/s0197-4580(00)00238-4. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y, Valentino DJ, Scher AI, Dinov I, White LR, Thompson PM, Launer LJ, Toga AW. Age effects on hippocampal structural changes in old men: the HAAS. Neuroimage. 2008;40:1003–1015. doi: 10.1016/j.neuroimage.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O'Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 45.Jackson GR, Owsley C. Visual dysfunction, neurodegenerative diseases, and aging. Neurol Clin. 2003;21:709–728. doi: 10.1016/s0733-8619(02)00107-x. [DOI] [PubMed] [Google Scholar]
- 46.Karlstrom H, Brooks WS, Kwok JB, Broe GA, Kril JJ, McCann H, Halliday GM, Schofield PR. Variable phenotype of Alzheimer's disease with spastic paraparesis. J Neurochem. 2008;104:573–583. doi: 10.1111/j.1471-4159.2007.05038.x. [DOI] [PubMed] [Google Scholar]
- 47.Petersen RC. Clinical subtypes of Alzheimer's disease. Dement Geriatr Cogn Disord. 1998;9 Suppl 3:16–24. doi: 10.1159/000051199. [DOI] [PubMed] [Google Scholar]
- 48.Storey E, Slavin MJ, Kinsella GJ. Patterns of cognitive impairment in Alzheimer's disease: assessment and differential diagnosis. Front Biosci. 2002;7:e155–e184. doi: 10.2741/A914. [DOI] [PubMed] [Google Scholar]
- 49.Kramer JH, Miller BL. Alzheimer's disease and its focal variants. Semin Neurol. 2000;20:447–454. doi: 10.1055/s-2000-13177. [DOI] [PubMed] [Google Scholar]
- 50.Renner JA, Burns JM, Hou CE, McKeel DW, Jr, Storandt M, Morris JC. Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology. 2004;63:1175–1180. doi: 10.1212/01.wnl.0000140290.80962.bf. [DOI] [PubMed] [Google Scholar]
- 51.Tang-Wai DF, Graff-Radford NR, Boeve BF, Dickson DW, Parisi JE, Crook R, Caselli RJ, Knopman DS, Petersen RC. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63:1168–1174. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- 52.Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, Hodges JR. Focal cortical presentations of Alzheimer's disease. Brain. 2007;130:2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- 53.Galton CJ, Patterson K, Xuereb JH, Hodges JR. Atypical and typical presentations of Alzheimer's disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;123(Pt 3):484–498. doi: 10.1093/brain/123.3.484. [DOI] [PubMed] [Google Scholar]
- 54.Kertesz A, Davidson W, McCabe P, Takagi K, Munoz D. Primary progressive aphasia: diagnosis, varieties, evolution. J Int Neuropsychol Soc. 2003;9:710–719. doi: 10.1017/S1355617703950041. [DOI] [PubMed] [Google Scholar]
- 55.Mesulam M. Primary progressive aphasia pathology. Ann Neurol. 2008;63:124–125. doi: 10.1002/ana.20940. [DOI] [PubMed] [Google Scholar]
- 56.Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, Weintraub S, Bigio EH. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128:1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- 58.Mott RT, Dickson DW, Trojanowski JQ, Zhukareva V, Lee VM, Forman M, Van Deerlin V, Ervin JF, Wang DS, Schmechel DE, Hulette CM. Neuropathologic, biochemical, and molecular characterization of the frontotemporal dementias. J Neuropathol Exp Neurol. 2005;64:420–428. doi: 10.1093/jnen/64.5.420. [DOI] [PubMed] [Google Scholar]
- 59.Spina S, Murrell JR, Huey ED, Wassermann EM, Pietrini P, Grafman J, Ghetti B. Corticobasal syndrome associated with the A9D Progranulin mutation. J Neuropathol Exp Neurol. 2007;66:892–900. doi: 10.1097/nen.0b013e3181567873. [DOI] [PubMed] [Google Scholar]
- 60.Tolnay M, Probst A. The neuropathological spectrum of neurodegenerative tauopathies. IUBMB Life. 2003;55:299–305. doi: 10.1080/1521654032000114348. [DOI] [PubMed] [Google Scholar]
- 61.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 62.Crook T, Bartus RT, Ferris SH, Whitehouse P, Cohen GD, Gershon S. Ageassociated memory impairment: proposed diagnositc criteria and measures of clinical change-report of a National Institute of Mental Health Work Group. Developmental Neuropsychology. 1986;2:261–276. [Google Scholar]
- 63.Smith G, Ivnik RJ, Petersen RC, Malec JF, Kokmen E, Tangalos E. Age-associated memory impairment diagnoses: problems of reliability and concerns for terminology. Psychol Aging. 1991;6:551–558. doi: 10.1037//0882-7974.6.4.551. [DOI] [PubMed] [Google Scholar]
- 64.Levy R. Aging-associated cognitive decline. Working Party of the International Psychogeriatric Association in collaboration with the World Health Organization. Int Psychogeriatr. 1994;6:63–68. [PubMed] [Google Scholar]
- 65.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41:1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 66.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 67.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 68.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 69.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 70.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 71.Morris JC, Price AL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. J Mol Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 72.Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, Jicha GA, Ivnik RJ, Smith GE, Tangalos EG, Braak H, Kokmen E. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 73.Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR, Jr, Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R, van Dyck CH, Mintzer J, Zamrini EY, Cahn-Weiner D, Thal LJ. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 74.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and Donepezil for the Treatment of Mild Cognitive Impairment. N Engl J Med. 2005 doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 75.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 76.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 77.Braskie MN, Klunder AD, Hayashi KM, Protas H, Kepe V, Miller KJ, Huang SC, Barrio JR, Ercoli LM, Siddarth P, Satyamurthy N, Liu J, Toga AW, Bookheimer SY, Small GW, Thompson PM. Plaque and tangle imaging and cognition in normal aging and Alzheimer's disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chong MS, Sahadevan S. Preclinical Alzheimer's disease: diagnosis and prediction of progression. Lancet Neurol. 2005;4:576–579. doi: 10.1016/S1474-4422(05)70168-X. [DOI] [PubMed] [Google Scholar]
- 79.Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58:376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 80.de Leon MJ, Mosconi L, Blennow K, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Tsui W, Saint Louis LA, Sobanska L, Brys M, Li Y, Rich K, Rinne J, Rusinek H. Imaging and CSF studies in the preclinical diagnosis of Alzheimer's disease. Ann N Y Acad Sci. 2007;1097:114–145. doi: 10.1196/annals.1379.012. [DOI] [PubMed] [Google Scholar]
- 81.Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in "normal" aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 82.Jobst KA, Barnetson LP, Shepstone BJ. Accurate prediction of histologically confirmed Alzheimer's disease and the differential diagnosis of dementia: the use of NINCDS-ADRDA and DSM-III-R criteria, SPECT, X-ray CT, and APO E4 medial temporal lobe dementias. The Oxford Project to Investigate Memory and Aging. Int Psychogeriatr. 1997;9 Suppl 1:191–222. discussion 247–152. [PubMed] [Google Scholar]
- 83.Johnson KA, Jones K, Holman BL, Becker JA, Spiers PA, Satlin A, Albert MS. Preclinical prediction of Alzheimer's disease using SPECT. Neurology. 1998;50:1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- 84.Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 85.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. "Preclinical" AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 86.Tierney MC, Szalai JP, Snow WG, Fisher RH, Nores A, Nadon G, Dunn E, St George-Hyslop PH. Prediction of probable Alzheimer's disease in memory-impaired patients: A prospective longitudinal study. Neurology. 1996;46:661–665. doi: 10.1212/wnl.46.3.661. [DOI] [PubMed] [Google Scholar]
- 87.Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64:1853–1859. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- 88.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 89.Alloul K, Sauriol L, Kennedy W, Laurier C, Tessier G, Novosel S, Contandriopoulos A. Alzheimer's disease: a review of the disease, its epidemiology and economic impact. Arch Gerontol Geriatr. 1998;27:189–221. doi: 10.1016/s0167-4943(98)00116-2. [DOI] [PubMed] [Google Scholar]
- 90.Fitzpatrick AL, Kuller LH, Ives DG, Lopez OL, Jagust W, Breitner JC, Jones B, Lyketsos C, Dulberg C. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 91.Jalbert JJ, Daiello LA, Lapane KL. Dementia of the Alzheimer type. Epidemiol Rev. 2008;30:15–34. doi: 10.1093/epirev/mxn008. [DOI] [PubMed] [Google Scholar]
- 92.Minati L, Edginton T, Bruzzone MG, Giaccone G. Current Concepts in Alzheimer's Disease: A Multidisciplinary Review. Am J Alzheimers Dis Other Demen. 2008 doi: 10.1177/1533317508328602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanders S, Morano C. Alzheimer's disease and related dementias. J Gerontol Soc Work. 2008;50 Suppl 1:191–214. doi: 10.1080/01634370802137900. [DOI] [PubMed] [Google Scholar]
- 94.Wang XP, Ding HL. Alzheimer's disease: epidemiology, genetics, and beyond. Neurosci Bull. 2008;24:105–109. doi: 10.1007/s12264-008-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ziegler-Graham K, Brookmeyer R, Johnson E, Arrighi HM. Worldwide variation in the doubling time of Alzheimer's disease incidence rates. Alzheimers Dement. 2008;4:316–323. doi: 10.1016/j.jalz.2008.05.2479. [DOI] [PubMed] [Google Scholar]
- 96.Dong MJ, Peng B, Lin XT, Zhao J, Zhou YR, Wang RH. The prevalence of dementia in the People's Republic of China: a systematic analysis of 1980–2004 studies. Age Ageing. 2007;36:619–624. doi: 10.1093/ageing/afm128. [DOI] [PubMed] [Google Scholar]
- 97.Froehlich TE, Bogardus ST, Jr, Inouye SK. Dementia and race: are there differences between African Americans and Caucasians? J Am Geriatr Soc. 2001;49:477–484. doi: 10.1046/j.1532-5415.2001.49096.x. [DOI] [PubMed] [Google Scholar]
- 98.Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, Luchsinger JA, Ogunniyi A, Perry EK, Potocnik F, Prince M, Stewart R, Wimo A, Zhang ZX, Antuono P. Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shafqat S. Alzheimer disease therapeutics: perspectives from the developing world. J Alzheimers Dis. 2008;15:285–287. doi: 10.3233/jad-2008-15211. [DOI] [PubMed] [Google Scholar]
- 100.Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology. 1992;42:1681–1688. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- 101.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 102.Bouras C, Hof PR, Morrison JH. Neurofibrillary tangle densities in the hippocampal formation in a non-demented population define subgroups of patients with differential early pathologic changes. Neurosci Lett. 1993;153:131–135. doi: 10.1016/0304-3940(93)90305-5. [DOI] [PubMed] [Google Scholar]
- 103.Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13:179–189. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- 104.Hof PR, Glannakopoulos P, Bouras C. The neuropathological changes associated with normal brain aging. Histol Histopathol. 1996;11:1075–1088. [PubMed] [Google Scholar]
- 105.Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 106.Troncoso JC, Martin LJ, Dal Forno G, Kawas CH. Neuropathology in controls and demented subjects from the Baltimore Longitudinal Study of Aging. Neurobiol Aging. 1996;17:365–371. doi: 10.1016/0197-4580(96)00028-0. [DOI] [PubMed] [Google Scholar]
- 107.Villemagne VL, Pike KE, Darby D, Maruff P, Savage G, Ng S, Ackermann U, Cowie TF, Currie J, Chan SG, Jones G, Tochon-Danguy H, O'Keefe G, Masters CL, Rowe CC. Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer's disease. Neuropsychologia. 2008;46:1688–1697. doi: 10.1016/j.neuropsychologia.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 108.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 109.Graham JE, Rockwood K, Beattie BL, Eastwood R, Gauthier S, Tuokko H, McDowell I. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 110.Larrieu S, Letenneur L, Orgogozo JM, Fabrigoule C, Amieva H, Le Carret N, Barberger-Gateau P, Dartigues JF. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 111.Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 112.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Diagnostic and Statistical Manual of Mental Disorders. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 114.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 115.Mattis S. Dementia Rating Scale: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 116.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 117.Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 118.Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- 119.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 120.de Mendonca A, Ribeiro F, Guerreiro M, Garcia C. Frontotemporal mild cognitive impairment. J Alzheimers Dis. 2004;6:1–9. doi: 10.3233/jad-2004-6101. [DOI] [PubMed] [Google Scholar]
- 121.Jicha GA, Schmitt FA, Abner E, Nelson PT, Cooper GE, Smith CD, Markesbery WR. Prodromal clinical manifestations of neuropathologically confirmed Lewy body disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nelson AP, O'Connor MG. Mild cognitive impairment: a neuropsychological perspective. CNS Spectr. 2008;13:56–64. doi: 10.1017/s1092852900016163. [DOI] [PubMed] [Google Scholar]
- 123.Jicha GA, Abner E, Schmitt FA, Cooper GE, Stiles N, Hamon R, Carr S, Smith CD, Markesbery WR. Clinical features of mild cognitive impairment differ in the research and tertiary clinic settings. Dement Geriatr Cogn Disord. 2008;26:187–192. doi: 10.1159/000151635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saxton J, Swihart AA. Neuropsychological assessment of the severely impaired elderly patient. Clin Geriatr Med. 1989;5:531–543. [PubMed] [Google Scholar]
- 125.Jicha GA, Parisi JE, Dickson DW, Cha RH, Johnson KA, Smith GE, Boeve BF, Petersen RC, Knopman DS. Age and apoE associations with complex pathologic features in Alzheimer's disease. J Neurol Sci. 2008;273:34–39. doi: 10.1016/j.jns.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Geda YE, Knopman DS, Mrazek DA, Jicha GA, Smith GE, Negash S, Boeve BF, Ivnik RJ, Petersen RC, Pankratz VS, Rocca WA. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol. 2006;63:435–440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 127.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 128.Bartolini M, Coccia M, Luzzi S, Provinciali L, Ceravolo MG. Motivational symptoms of depression mask preclinical Alzheimer's disease in elderly subjects. Dement Geriatr Cogn Disord. 2005;19:31–36. doi: 10.1159/000080968. [DOI] [PubMed] [Google Scholar]
- 129.Cummings JL. Dementia syndromes: neurobehavioral and neuropsychiatric features. J Clin Psychiatry. 1987;48 Suppl:3–8. [PubMed] [Google Scholar]
- 130.Filley CM. Neuropsychiatric features of Lewy body disease. Brain Cogn. 1995;28:229–239. doi: 10.1006/brcg.1995.1254. [DOI] [PubMed] [Google Scholar]
- 131.Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer's disease. J Neural Transm Suppl. 1996;47:103–123. doi: 10.1007/978-3-7091-6892-9_6. [DOI] [PubMed] [Google Scholar]
- 132.Harciarek M, Jodzio K. Neuropsychological differences between frontotemporal dementia and Alzheimer's disease: a review. Neuropsychol Rev. 2005;15:131–145. doi: 10.1007/s11065-005-7093-4. [DOI] [PubMed] [Google Scholar]
- 133.Hooten WM, Lyketsos CG. Differentiating Alzheimer's disease and frontotemporal dementia: receiver operator characteristic curve analysis of four rating scales. Dement Geriatr Cogn Disord. 1998;9:164–174. doi: 10.1159/000017042. [DOI] [PubMed] [Google Scholar]
- 134.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 135.Weisman D, McKeith I. Dementia with Lewy bodies. Semin Neurol. 2007;27:42–47. doi: 10.1055/s-2006-956754. [DOI] [PubMed] [Google Scholar]
- 136.Apostolova LG, Cummings JL. Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord. 2008;25:115–126. doi: 10.1159/000112509. [DOI] [PubMed] [Google Scholar]
- 137.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. Jama. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 138.Desai AK, Grossberg GT, Sheth DN. Activities of daily living in patients with dementia: clinical relevance, methods of assessment and effects of treatment. CNS Drugs. 2004;18:853–875. doi: 10.2165/00023210-200418130-00003. [DOI] [PubMed] [Google Scholar]
- 139.Patterson MB, Mack JL, Neundorfer MM, Martin RJ, Smyth KA, Whitehouse PJ. Assessment of functional ability in Alzheimer disease: a review and a preliminary report on the Cleveland Scale for Activities of Daily Living. Alzheimer Dis Assoc Disord. 1992;6:145–163. [PubMed] [Google Scholar]
- 140.Galasko D, Bennett DA, Sano M, Marson D, Kaye J, Edland SD. ADCS Prevention Instrument Project: assessment of instrumental activities of daily living for community-dwelling elderly individuals in dementia prevention clinical trials. Alzheimer Dis Assoc Disord. 2006;20:S152–S169. doi: 10.1097/01.wad.0000213873.25053.2b. [DOI] [PubMed] [Google Scholar]
- 141.Griffith HR, Belue K, Sicola A, Krzywanski S, Zamrini E, Harrell L, Marson DC. Impaired financial abilities in mild cognitive impairment: a direct assessment approach. Neurology. 2003;60:449–457. doi: 10.1212/wnl.60.3.449. [DOI] [PubMed] [Google Scholar]
- 142.Adler G. Driving and dementia: dilemmas and decisions. Geriatrics. 1997;52 Suppl 2:S26–S29. [PubMed] [Google Scholar]
- 143.Dawson JD, Anderson SW, Uc EY, Dastrup E, Rizzo M. Predictors of driving safety in early Alzheimer disease. Neurology. 2009;72:521–527. doi: 10.1212/01.wnl.0000341931.35870.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dubinsky RM, Stein AC, Lyons K. Practice parameter: risk of driving and Alzheimer's disease (an evidence-based review): report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2000;54:2205–2211. doi: 10.1212/wnl.54.12.2205. [DOI] [PubMed] [Google Scholar]
- 145.Foley DJ, Masaki KH, Ross GW, White LR. Driving cessation in older men with incident dementia. J Am Geriatr Soc. 2000;48:928–930. doi: 10.1111/j.1532-5415.2000.tb06889.x. [DOI] [PubMed] [Google Scholar]
- 146.Ott BR, Anthony D, Papandonatos GD, D'Abreu A, Burock J, Curtin A, Wu CK, Morris JC. Clinician assessment of the driving competence of patients with dementia. J Am Geriatr Soc. 2005;53:829–833. doi: 10.1111/j.1532-5415.2005.53265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]