Abstract
The primary causes of age related changes in mitochondrial metabolism are not known. The goal of this study is to document the influence of naturally occurring mtDNA variation on age dependent changes in mitochondrial respiration, hydrogen peroxide (H2O2) generation and antioxidant defenses in the fly Drosophila simulans. Possible changes include an increase in rates of reactive oxygen species production with age and/or an age dependent decrease in antioxidant response. For this study we have used flies harboring distinct siII and siIII mtDNA types. Previously we have shown that males harboring siII mtDNA had higher rates 30 of mitochondrial H2O2 production from complex III at 11 d compared to males with the siIII mtDNA type. Here, we corroborate those results and show that Drosophila harboring the siII and siIII mtDNA types exhibit significantly different patterns of pro-oxidant and antioxidant activities as they age. Flies harboring siII mtDNA had higher rates of mitochondrial H2O2 production and manganese superoxide dismutase activity at 11 and 18 d of age than siIII mtDNA 35 harboring flies. Copper-zinc superoxide dismutase activity increased from 11 to 25 d in siII flies while the accumulation of oxidized glutathione did not change between 11 and 25 d. In contrast, siIII harboring flies showed an age dependent increase in H2O2 production, reaching higher production rates on day 25 than that observed in siII flies. Copper-zinc superoxide dismutase activities did not change between 11 and 25 d while the oxidized glutathione accumulation increased with age. The results show antioxidant levels correlate with pro-oxidant levels in siII but not siIII flies. These results demonstrate our ability to correlate mtDNA variation with differences in whole mitochondrial physiology and individual complex biochemistry.
Keywords: mtDNA, SOD, catalase, glutathione, respiration, H2O2 production
1. Introduction
Understanding age-related changes in the structure and functioning of mitochondria is critical for elucidation of the molecular basis of aging and for better management of aging and age-related diseases. Mitochondria are not only the major metabolic energy supplier, but are also the main intracellular source and target of reactive oxygen species (ROS) generated by the respiratory chain. Mitochondrial metabolism may be influenced by (i) mitochondrial DNA (mtDNA) encoded genes, (ii) nuclear encoded genes that produce proteins imported into the mitochondrion, and/or (iii) interactions between mtDNA and nuclear encoded proteins (mitonuclear interactions). The goal of this study is to document the influence of naturally occurring mtDNA variation on age dependent changes in mitochondrial respiration, ROS generation and antioxidant defenses.
The free radical theory of aging implicates oxidative molecular damage as an underlying cause of age related changes in metabolism (Harman, 1956). Such oxidative damage is believed to arise from an imbalance between metabolically generated ROS and anti-oxidative defenses. In flies, respiratory chain complexes I and III are thought to be the major sites of ROS production in mitochondria (Chen et al., 2003; Miwa et al., 2003). Complex I releases superoxide anions towards the matrix side whereas complex III along with glycerol 3-phosphate dehydrogenase releases superoxide on either side of the inner mitochondrial membrane. Superoxide, enzymatically or non-enzymatically, dismutates to hydrogen peroxide (H2O2) and both reactive oxygen species may damage DNA, lipids and biomembranes. The anti-oxidative defenses consist primarily of antioxidant enzymes including superoxide dismutase (SOD) and catalase and non enzymatic anti-oxidants such as glutathione.
Here we examine levels of pro-oxidants and anti-oxidants at 11, 18 and 25 d of age. Currently, it is not clear whether it is the rate of ROS production, status of antioxidants and/or their relative concentrations that are the major determinants of age related changes in mitochondrial metabolism. Sohal et al. (1990a) evaluated antioxidant defenses and prooxidant generation in three tissues of Sprague-Dawley rats and in short-lived insects. These authors suggested that pro-oxidant generation was more crucial than antioxidant levels as possible longevity determinants. However, maximum life span potential (MLSP) is correlated with antioxidant levels in liver tissues of six different mammalian species. Sohal et al.(1990b) compared antioxidant defenses in species that ranged from 3.5 to 30 years in MLSP and found that SOD and catalase activities were positively correlated with MLSP. In contrast, glutathione concentration was negatively correlated with MLSP.
The utility of Drosophila melanogaster as a model organism for studying aging has been firmly established (Partridge et al., 2005; Tatar, 2007; Jafari et al., 2006). The same five complexes of the electron transport chain are present in flies and humans and the same 13 mtDNA protein coding genes are found in both taxa (Anderson et al., 1981; Clary, 1985). Further, it is known that key molecular and metabolic pathways in aging are shared by flies and mammals (Partridge and Gems, 2006). Not all pathways are shared (Lehtinen et al., 2006), but this validates the use of Drosophila as a model system. A potential disadvantage of employing D. melanogaster is that it’s mtDNA exhibits the statistical signature of a recent selective sweep (Ballard and Kreitman, 1994). In addition to reducing variation this process has the potential to disrupt co-adapted gene complexes by the process of genetic hitchhiking (Maynard Smith, 1974; Kaplan, 1989). As a consequence of the low mtDNA variation in D. melanogaster we propose D. simulans as a parallel model for studying the role of mtDNA in aging (Ballard, 2005).
D. simulans harbors three distinct mtDNA haplogroups (siI, -II and -III) (Baba-Aïssa et al., 1988; Satta et al., 1987; Solignac et al., 1986). Flies with siII mtDNA are globally most widespread and co-occur with siIII flies in Tanzania, Kenya, Madagascar, and Reunion Island. The siI harboring flies are restricted to Indian/Pacific Islands and have not been found with those harboring any other mtDNA type. Flies harboring the distinct mtDNA types cannot be distinguished morphologically, they mate randomly in the laboratory, and there is no evidence of any nuclear subdivision at 16 nuclear encoded loci tested to date (Ballard, 2000a; Ballard et al., 2002; Dean et al., 2003; Ballard et al., 2007). Within D. simulans the mtDNA haplogroups differ by about 3%. Flies, harboring siI mtDNA are evolutionarily most divergent from those harboring siII and siIII mtDNA. In this study we quantify broad-brush differences among flies harboring divergent siII and siIII harboring flies that were collected together in Kenya. One potential limitation of D. simulans as a model is that the mtDNA variation among these two haplogroups is greater than that observed within most species including humans (Horai et al., 1995; Krings et al., 1999; Ballard, 2000a, 2000b).
We investigate age related changes in mitochondrial metabolism in flies harboring siII or siIII mtDNA. Multiple strategies can be employed to determine if mtDNA influences mitochondrial bioenergetics and organismal fitness in Drosophila. De Stordeur et al.(1989) used the microinjection approach to assay the frequencies of foreign injected mtDNA. They demonstrated that the three mitotypes have unequal ability to out compete the host mtDNA type (siII>-III>-I). Ballard and James (2004) showed the same rank order of mtDNA fitness in laboratory perturbation cages. James and Ballard (2003) used the reciprocal introgression approach to show differences in three life-history traits. Katewa and Ballard (2007), and this study, include eight D. simulans fly lines collected from Nairobi, Kenya. Both studies make the assumption that random mating in nature has effectively randomized the nuclear genome. This assumption is supported by two observations. First, siII/-III mtDNA heteroplasmy rates of up to 10% have been found in D. simulans collected in Africa and Reunion Island (Ballard, 2000a; Ballard et al., 2002; Dean et al., 2003; Matsuura et al., 1991; Satta et al., 1988). Second, no fixed nucleotide differences in nine nuclear encoded mitochondrial complex IV genes, and four isoforms, have been detected. Using the same flies as used in this study Ballard et al (2007) sequenced six mtDNA and 13 nuclear encoded genes (9 subunits and the four known isoforms of complex IV of the electron transport chain). In siII or siIII harboring flies there were seven nonsynonymous (four in ND2, two in COII and one in ATP8) and 108 synonymous fixed mtDNA differences. In contrast, nuclear genes showed no fixed amino acid or synonymous differences between siII or siIII harboring flies. These data show population genetic subdivision in mtDNA genes but not in any nuclear encoded complex IV genes. However, as over 1,000 proteins are imported into the mitochondrion and are essential for mitochondrial metabolism it remains plausible that specific mitochondrial-nuclear gene complexes exist.
Here, we extend Katewa and Ballard (2007) and take a top down approach by measuring state 3 and state 4 respiration of mitochondria respiring on NAD- and FAD-linked substrates at three ages to assess the efficiency of the electron transport chain (Hafner et al., 1990). Next, we target interactions between pro-oxidants and antioxidants at the same ages. Generally, males harboring siII mtDNA show higher rates of mitochondrial H2O2 production, higher SOD activities while the accumulation of oxidized glutathione (GSSG) does not change. On the other hand siIII flies show lower rates of mitochondrial H2O2 production, lower SOD activities and GSSG accumulation. The results show that the antioxidants SOD and GSSG correlate with prooxidant levels in siII but not in siIII mtDNA harboring flies.
2. Materials and methods
2.1 Chemicals
All chemicals were of reagent grade and purchased from Sigma (St. Louis, MO, U.S.A.), unless noted otherwise.
2.2 Fly lines
We study the same four siII and four siIII isofemale lines as Katewa and Ballard (2007). These lines were randomly selected from 20 isofemale lines that were collected in Nairobi (Kenya) during November 2004. In the lab, fly lines were maintained at constant density, temperature (23 °C), humidity (50 % RH), diurnal cycle (12 hr Light:12 hr Dark) and diet (Carolina Biological instant Drosophila media). Parents of the experimental flies were released into population cages and allowed to acclimate for 2 to 3 d. In all cases these adults were less than two weeks old (Hercus and Hoffmann, 2000). Ovi-position resources (Petri dish with solidified agar based medium containing 4 % agar and 10 % molasses) were provided for 4 hr periods to collect cohorts of eggs. Eggs were harvested, washed and suspended in a small volume of 1 ✕ PBS (Clancy and Kennington, 2001) and 15 µl of this suspension containing about 200 eggs were added to instant Drosophila media. Four day old non-virgin flies were shorted by sex on ice and males were maintained in demography cages for the duration of the study. Food was replaced every 2–3 d in the demography cages.
Immediately prior to each biochemical analysis an equal number of 11, 18 or 25 d old males from each of the four independently maintained lines that harbored siII mtDNA were pooled. Similarly, an equal number of males from the four lines that harbored siIII mtDNA were combined. The pooling strategy minimized individual differences caused by the nuclear genome of a particular fly line and enabled us to detect more general differences that are due to the mtDNA in-of-itself and/or of fixed nuclear differences that interact with each mtDNA haplogroup (Ballard, 2000b; Ballard et al., 2002; Dean and Ballard, 2004). Pooling of flies may lead to a biased result if flies were of unequal size and contributed disproportionately to any subsequent assay or genetic subdivision. Katewa and Ballard (2007) tested whether the size of each line could bias their results using the same fly lines we studied. If we assume thorax weight is proportional to whole fly weight each fly line contributed between 24–27 % to each biochemical assay. Ballard et al. (2007) sequenced over 5,500bp of the mitochondrial genome to investigate genetic variation among the fly lines. We observed only one nonsynonymous change within the four siII fly lines and one nonsynonymous change within the four siIII lines.
2.3 Isolation of intact mitochondria
For each flight muscle mitochondrial preparation we included 80 thoraces of male D. simulans aged 11, 18 or 25 d. We did not assay flies less than 11 d because Sohal (1975) reported that Drosophila show variation in both numbers and size of mitochondria in the first week of life. We did not assay flies older than 25 d because Melvin and Ballard (2006) failed to isolate mitochondria with respiratory control ratios (RCR) of more than 3 (for glycerol 3-phosphate) from 32 d old flies using the same protocol we employ here. RCR was calculated by dividing the state 3 rate by the state 4 rates.
All mitochondrial isolation steps were performed on ice or at 4 °C. Briefly, thoraces were separated from heads and abdomens and homogenized in 200 µl of isolation buffer (Van den Bergh, 1967) with 0.3 % (w/v) bovine serum albumin (BSA) in a 1.5 ml micro-centrifuge tube. Homogenate was filtered by pipetting into 1 cc tuberculin syringe (BD, New Jersey, USA), followed by 300 µl of isolation buffer and then gently forced through the gauze into a clean 1.5 ml micro-centrifuge tube. Filtered homogenate was centrifuged at 150 × g for 3 min (Eppendorf centrifuge 5804 R, Hamburg, Germany) at 4 °C. The supernatant was passed through one layer of gauze and recentrifuged at 9000 × g for 10 min. The supernatant was discarded and the pellet was carefully washed with 200 µl of isolation buffer and then resuspended in 40 µl of the same. Protein content of each sample was determined by using a Bio-Rad DC protein assay kit (Hercules, CA). All assays using whole mitochondria were conducted within 3 h of isolation.
2.4 Mitochondrial respiration
To measure the efficiency of the electron transport chain we used a top down approach (Hafner et al., 1990). A 3 ml Clark-type oxygen electrode, maintained at 25 °C, was used to measure respiration rates from mitochondria isolated from thoraces (Rank Brothers, Cambridge, England). The system was connected to a Powerlab data acquisition and analysis system (ADInstuments, Colorado Springs, CO), assuming 479 nmol O / ml at air saturation (Katewa and Ballard, 2007; Miwa et al., 2003). Mitochondria (about 0.4 mg/ml) were incubated in assay medium (120 mM KCl, 5 mM KH2PO4, 3 mM Hepes, 1 mM EGTA, 1 mM MgCl2, and 0.2 % BSA, pH 7.2) supplemented with a mixture of 10 mM sodium pyruvate and 10 mM proline (complex I) or 20 mM glycerol 3-phosphate (complex III) (Miwa et al., 2003). State 3 rates were measured after the addition of 0.2–0.4 mM ADP. State 4 rates were measured after the addition of oligomycin (1 µg / ml). All isolations with an RCR of less than 5.0 when pyruvate and proline was the substrate were excluded. There were 0, 2, and 1 excluded on days 11, 18 and 25 for siII flies and 1, 1, and 1 excluded on days 11, 18 and 25 for siIII flies. Respiration measurement with each substrate was carried out in duplicate from each mitochondrial preparation and then averaged. The respiration assays were repeated on four to six independent mitochondrial preparations.
2.5 Mitochondrial H2O2 production
The H2O2 produced in mitochondria comes from dismutation of mitochondrial superoxide. Assays were performed using an Amplex Red H2O2/Peroxidase Assay kit (Molecular 215 Probes, USA). Reaction of Amplex Red reagent with H2O2 in the presence of horseradish peroxidase produces the oxidation product, resorufin, which has an absorption maximum at 560 nm.
Standard reactions contained 5–15 µg mitochondrial protein, 10 µM acetylated cytochrome c, 2 µM Rotenone (ROT), 30U/ml SOD, 10 mM sodium pyruvate and 10 mM 220 proline (for complex I) or 20 mM glycerol 3-phosphate (glycerol 3-phosphate dehydrogenase and complex III) and the respiration buffer (Katewa et al., 2006). In all studies, reactions containing known concentrations of H2O2 were used to construct a standard curve and run in each assay plate. H2O2 production rate was calculated from the initial increase in absorbance at 560 nm measured every 2 min for 1 h using a Molecular Devices, SpectraMax Plus spectrophotometer and SoftMAX Pro software (Molecular Devices Corp., Sunnyvale, CA) at 25 °C and is expressed as rate of H2O2 production per mg of mitochondrial protein. H2O2 production measurement was carried out in duplicate for each mitochondrial preparation and then averaged. The assay was repeated on six independent mitochondrial preparations.
2.6 Antioxidants enzyme assays
In flies, the major anti-oxidative enzymes are SOD and catalase. We have used 20 male thoraces to obtain each homogenate preparation (Mocket et al., 1999). Six such independent homogenate preparations were used for the antioxidant assays. The thoraces were homogenized in 50 mM potassium phosphate buffer (pH 7.4). Homogenate was centrifuged at 500 × g for 10 mins and the clear supernatant was used for assays of SOD and catalase. Samples for catalase were diluted 1:1 with 50 mM potassium phosphate buffer (pH 7.4) containing 0.2% triton-X 100.
Total superoxide dismutase was assayed with the SOD assay kit from Sigma (St. Louis, MO, U.S.A.). The inhibition curves for pure bovine erythrocyte Cu-Zn superoxide dismutase purchased from Sigma (St. Louis, MO, U.S.A.) are shown in supplementary Fig. S.1. The plots show that the assay is linear from 0.1 U/ml to 10 U/ml of SOD. The fly thorax homogenates were diluted to obtain an inhibition in the linear range. All assays were done in triplicate following the manufacturer’s directions and then averaged. To separate Mn SOD activity we used 5mM potassium cyanide. Cu-Zn SOD was then calculated by subtracting Mn SOD from total SOD activity.
Catalase was assayed using an Amplex Red Catalase Assay kit (Molecular probe, USA). Activities are expressed as units/mg protein of the homogenate.
2.7 Glutathione content measurements
Levels of reduced glutathione (GSH) indicate the antioxidant status, whereas levels of GSSG are indicative of oxidative stress (Agarwal and Sohal, 1994; Rebrin and Sohal, 2006; Sohal and Dubey, 1994). We determined GSH and GSSG levels from thorax homogenates. Briefly, 20 thoraces were homogenized in 800 µl of 5% sulfosalicylic acid followed by a centrifugation for 15 min at 12000 ✕ g to obtain one preparation. GSH and GSSG were measured in the supernatant (Allen et al., 2000; Baker et al., 1990) and the measurements were done in duplicate from each homogenate preparation and then averaged. The assays were repeated on six independent homogenate preparations. For GSH the samples were further diluted 1:20 in 5% sulfosalicylic acid. For GSSG, 7.5 µl of 2-vinylpyridine was added per 100 µl of undiluted supernatant. The sulfosalicylic acid in assay tubes was neutralized by 14–16 µl of 1:4 diluted tri-ethanolamine. Finally, 125 µl of DTNB assay buffer (final concentrations in the assay were NADPH, 221.3 µM; DTNB, 462.6 µM; Glutathione reductase 10.6–12.3 U/ml; sodium phosphate buffer 92.7 mM; EDTA 4.1 mM) was added to 25 µl of supernatant. The plate was shaken for 15 seconds and then the absorbance was read on microplate reader at 412 nm for 5 min at 20 s intervals.
We also assayed GSH and GSSG levels in whole fly homogenates (Mockett et al., 1999; Rebrin et al., 2004; Rebrin and Sohal, 2006). A potential problem with assaying GSH and GSSG from thoraces is that the time lag between chopping the first fly and putting the thorax in sulfosalicylic acid may reduce the observed concentration of thiols (Anderson, 1985). We have no expectation that the time lag will differ between flies harboring distinct mtDNA types but tissue specific differences between thoraces and whole flies may occur (Katewa et al. 2006).
2.8 Statistical analysis
Data are expressed in graph as means ± S.E.M of four to six individual samples measured in duplicate or in triplicate. We employed two-way ANOVA’s to determine statistical differences between the two mtDNA types and three age points (JMP, 1995). F and p values are reported in the text. To illustrate differences among mtDNA types and between days we employed the post-hoc students ‘t” test (significance p<0.05).
3. Results
3.1 Mitochondrial respiration
We quantified the bioenergetic performance of the entire electron transport chain with complex I substrates pyruvate and proline. With complex I substrates, state 3 respiration rates were lower in siII mitochondria compared to siIII mitochondria (Fig. 1A). ANOVA showed a significant effect of mtDNA (F1,22 = 23.48, p <0.001) but not of day (F2,22 = 1.25, p =0.30) nor their interaction (F2,22 = 0.02, p =0.98). State 4 respiration rates were lower in siII flies and were highest on day 11 in both haplogroups (Fig. 1B). ANOVA showed a significant effect of mtDNA (F1,20 = 5.78, p =0.026) and day (F2,20 = 8.93, p =0.002) but not their interaction (F2,20 = 0.06, p =0.94). RCR values did not show a significant influence of mtDNA haplogroup (F1,20 = 1.50, p =0.23), day (F2,20 = 3.21, p >0.05) or their interaction (F2,20 = 0.66, p =0.53, respectively).
Fig. 1.
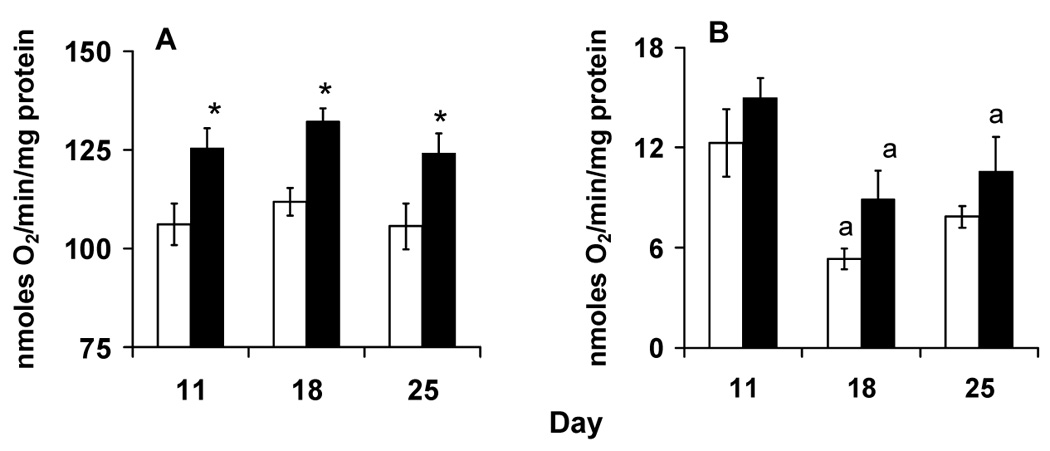
Respiration rates of flight muscle mitochondria isolated from of flies harboring siII and siIII mtDNA type with pyruvate and proline as substrate. A. Flies with siIII mtDNA showed higher state 3 respiration. B. State 4 respiration rates are higher in siIII flies and are highest on day 11 in both groups. Bars show mean ± S.E.M of four to six independent mitochondrial preparations. Mean values were significantly different (P< 0.05, post-hoc students ‘t” test) from *: siII mtDNA, a: day 11(within mtDNA type).
The performance of complexes III-V may be assayed by using glycerol 3- phosphate as substrate for respiration. These data and summary statistics are presented in supplementary Fig. S.2. Briefly, siII flies had lower state 3 respiration rates on all days assayed. There was a significant effect of fly age but not mtDNA on state 4 respiration rates.
3.2 Mitochondrial H2O2 production
In flies, about 80% of ROS is produced from complex III and glycerol 3-phosphate dehydrogenase while 20% is produced from complex I (Katewa and Ballard, 2007; Melvin and Ballard, 2006; Miwa et al., 2003). We assayed H2O2 production using complex III and complex I based substrates. We present the glycerol 3-phosphate substrate data in Fig. 2. When glycerol 3-phosphate was used in the presence of rotenone, siII flies showed similar levels of ROS production on days 11, 18 and 25 (Fig. 2). In contrast, siIII flies exhibited an age dependent increase in mitochondrial H2O2 production. Two-way ANOVA shows a significant effect of mtDNA (F1,30 = 21.04, p <0.001), day (F2,30 = 53.55, p <0.001) and their interaction (F2,30 = 51.56, p <0.001). For complex I based substrates, H2O2 production showed no clear trend (supplementary Fig. S.3).
Fig. 2.
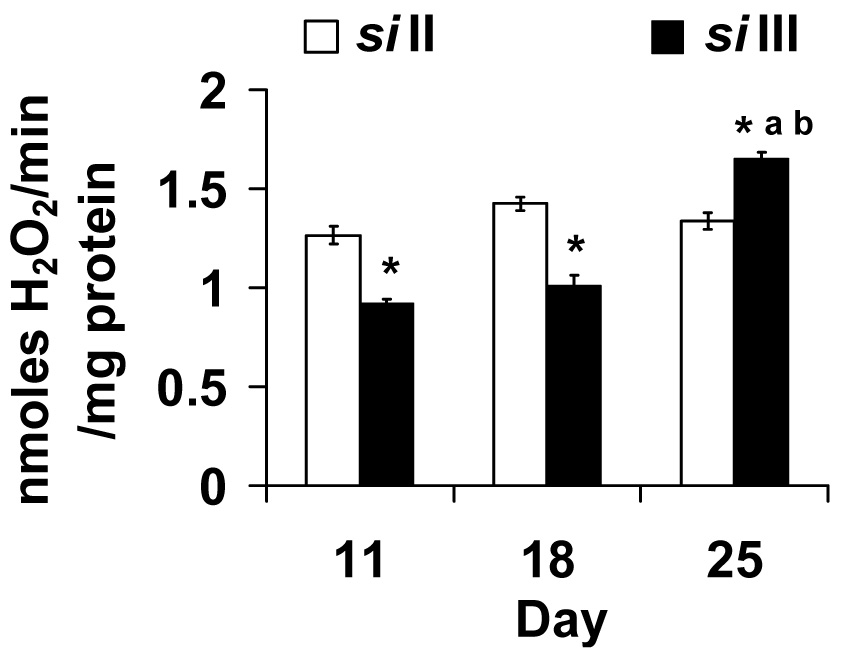
The pattern of hydrogen peroxide (H2O2) production rate differs among flies that harbor siII or siIII mitochondria when glycerol 3 phosphate was used as the substrate. Flies with siIII showed an age dependent increase in rate of H2O2 production whereas siII harboring flies showed no age related change. Bars show mean ± S.E.M of four to six independent mitochondrial preparations. Mean values were significantly different (P< 0.05, post-hoc students ‘t” test) from *: siII mtDNA, a: day 11(within mtDNA type), b: day 18 (within mtDNA type).
3.3 Antioxidant enzymes assays
In flies, Mn SOD is the primary defense of mitochondria against the highly reactive superoxide produced inside the mitochondria. Mn SOD activities were higher in siII flies at day 11 and day 18 but were similar to siIII flies at day 25 (Fig. 3). Peak activity occurred on day 18 in both mtDNA groups. Two-way ANOVA showed a significant effect of mtDNA (F1,26 = 22.14, p <0.001), day (F2,26 = 32.69, p <0.001) and their interaction (F2,26 = 5.74, p <0.01).
Fig. 3.
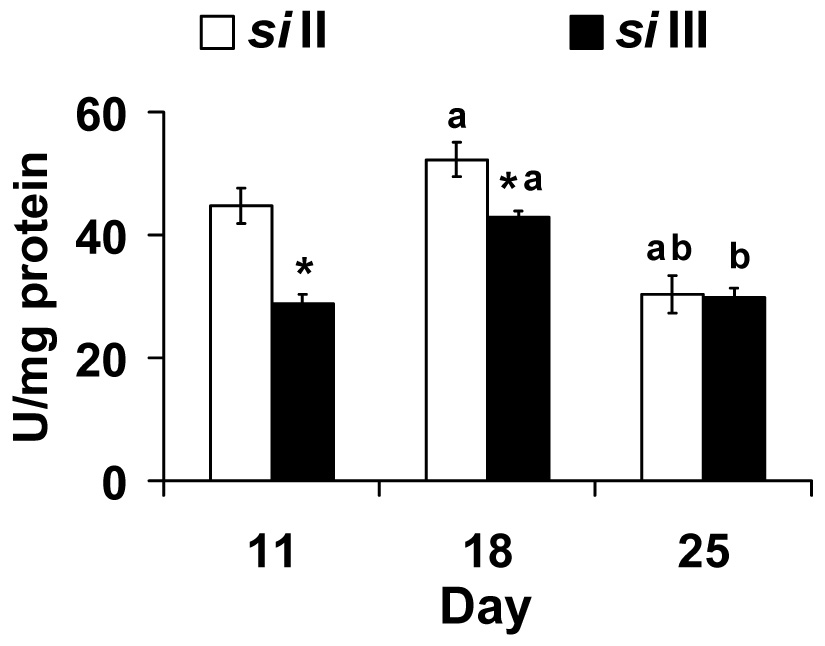
Manganese superoxide dismutase activity (U/mg protein) is highest in siII mtDNA harboring flies at day 11 and 18. Bars show mean ± S.E.M of four to six independent homogenate preparations for each mtDNA type (siII and siIII). Mean values were significantly different (P< 0.05, post-hoc students ‘t” test) from *: siII mtDNA, a: day 11(within mtDNA type), b: day 18 (within mtDNA type).
Cu-Zn SOD is present in the intermembrane space in mitochondria and in the cytosols of all eukaryotic cells. Activity of Cu-Zn SOD increased with age in siII but not siIII flies (Fig. 4). Two-way ANOVA showed a significant effect of mtDNA (F1,26 = 15.94, p <0.001), day (F2,26 = 10.90, p <0.001) and their interaction (F2,26 = 5.06, p <0.014).
Fig. 4.
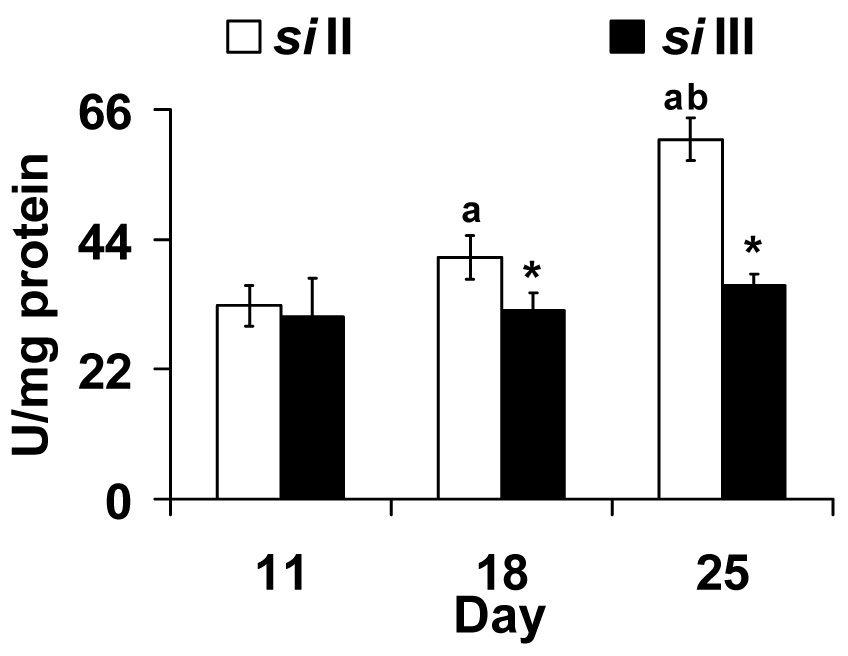
Copper-Zinc superoxide dismutase activity (U/mg protein) increases with age in flies harboring siII mtDNA. Bars show mean ± S.E.M of four to six independent homogenate preparations for each mtDNA type (siII and siIII). Mean values were significantly different (P< 0.05, post-hoc students ‘t” test) from *: siII mtDNA, a: day 11(within mtDNA type), b: day 18 (within mtDNA type).
MtDNA haplogroup does not influence catalase activity and peak activity is seen in both groups at 18 d of age (Fig. 5). Two-way ANOVA shows a significant effect of day (F2,30 = 21.56, p <0.001) but not of mtDNA (F1,30 = 0.83, p <0.37) nor their interaction (F1,30 = 0.27, p <0.76).
Fig. 5.
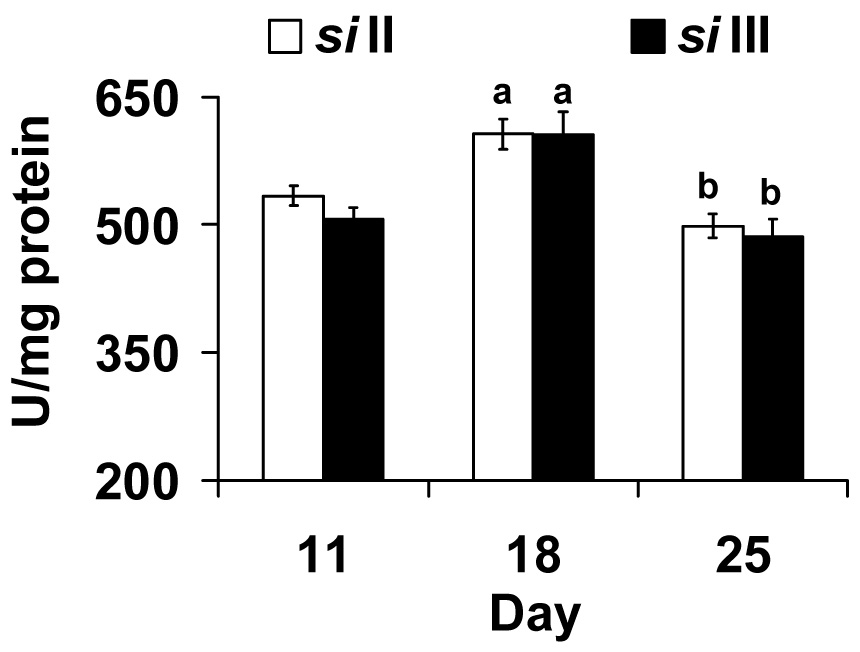
Catalase activity in both siII and siIII mtDNA groups peaks at day 18. Bars show mean ± S.E.M of four to six independent homogenate. Mean values were significantly different (P< 0.05, post-hoc students ‘t” test) from; a: day 11(within mtDNA type), b: day 18 (within mtDNA type).
3.4 Glutathione content measurements
Levels of GSSG are indicative of oxidative stress while GSH measures antioxidant status. In the thorax only homogenates GSSG levels were lower in siII mtDNA harboring flies at all ages (Fig. 6). Two-way ANOVA indicates a significant mtDNA effect (F1,27 = 20.96, p <0.001) but no significant effect of day (F2,27 = 1.83, p =0.179) or their interaction (F2,27 = 1.316, p =0.285). To further investigate the mtDNA effect we conducted a students ‘t’ post hoc contrasts comparing day 11 and 25 for siIII flies. This analysis shows that GSSG levels were significantly higher on day 25 (p < 0.05). Neither siII nor siIII flies showed any change in GSH content with age. ANOVA shows no effect of mtDNA (F1,26 = 2.22, p =0.15), day (F2,26 = 2.32, p =0.12) or their interaction (F2,26 = 0.52, p =0.60).
Fig. 6.
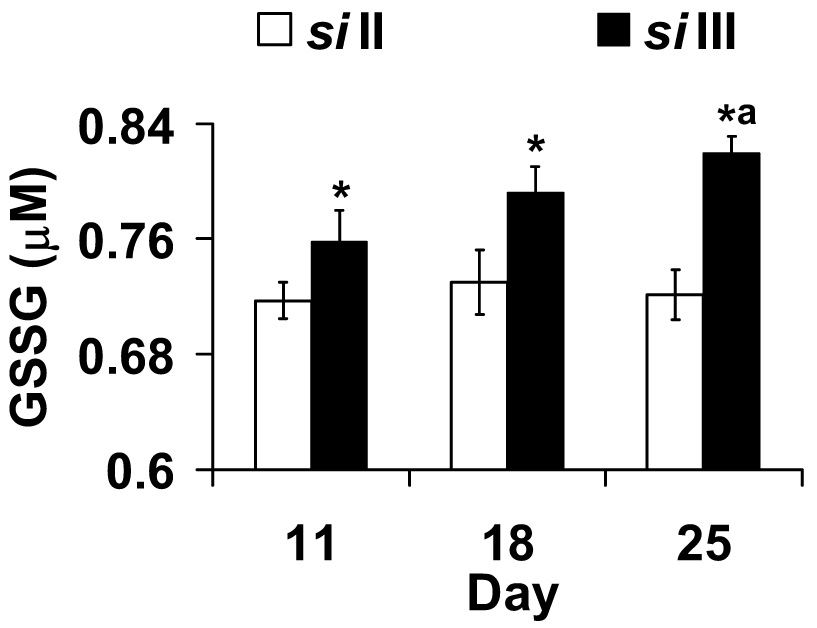
Flies harboring siIII mtDNA have higher oxidized glutathione (GSSG) content in thorax homogenates at all age groups. Bars show mean ± S.E.M of four to six independent homogenate preparations for each mtDNA type (siII and siIII). Mean values were significantly different (P< 0.05, post-hoc students ‘t” test) from *: siII mtDNA, a: day 11(within mtDNA type).
We measured GSSG and GSH in whole fly homogenates because we were concerned that the processing of thorax samples could bias our results. As expected, GSSG and GSH levels were generally higher in whole flies than thoraces but the data show a difference between mtDNA types and tissues (supplementary Figs S.4 and S.5). Consistent with the thorax only data GSSG levels were significantly lower in siII harboring flies at all the three age time points (supplementary Fig. S.4). In contrast to data from thoraces GSH levels from whole flies tended to decrease with age (supplementary Fig. S.5). We suggest that the observed difference between GSH levels from thoraces and whole flies is a tissue effect that warrants further study. A decrease in the GSH contents with age have been reported in whole fly homogenates for D. melanogaster (Mockett et al., 1999; Rebrin et al., 2004; Rebrin and Sohal, 2006).
4. Discussion
There is debate as to whether age related changes in mitochondrial metabolism are determined by rates of ROS production, antioxidant status and/or their relative concentrations (Harman, 1956; Sohal et al., 1990a; Sohal et al., 1990b). Here, we show that flies harboring siII and siIII mtDNA types exhibit significantly different patterns of pro-oxidant and antioxidant activities as they age and antioxidant activities correlate with pro-oxidant levels in siII but not in siIII mtDNA harboring flies.
H2O2 production does not increase with age in all Drosophila studies (Ballard, 2005; Ferguson et al., 2005; Katewa et al., 2006; Miwa et al., 2004; Ross, 2000; Sohal et al., 1995a; Sohal et al., 1995b). Like the majority of studies the rates of H2O2 production increased with age in siIII flies (Ballard, 2005; Ferguson et al., 2005; Katewa and Ballard, 2007; Ross, 2000; Sohal et al., 1995b). In contrast, siII flies showed no change in rate of H2O2 production over the range tested. It is possible that this is a true biological affect. Miwa et al.(2004) did not observe an increase in mitochondrial ROS production with age in D. melanogaster that were either calorie restricted or overexpressing mitochondrial adenine nucleotide translocase. Alternatively, the fact that rates of H2O2 production did not increase in siII flies may be a reflection of the limited duration of the study. Sohal et al.(1995a) showed that the age dependent increase in the rate of mitochondrial H2O2 production was higher between 32 – 58 d than 11 – 32 d. Another possibility is that the age dependent decline in cytochrome c oxidase may be different in the two flies. Cytochrome c oxidase is known to influence ROS production (Ferguson et al., 2005) and we have shown that it is higher in siIII than siII flies on days 4 and 11 (Ballard et al., 2007; Katewa and Ballard, 2007).
We observe that SOD activities were higher in siII but not siIII flies. Flies with siII mtDNA had high Mn SOD activity at 11 and 18 days of age. The rates of H2O2 production were also high at the same ages. The siII flies also showed an age dependent increase in Cu-Zn SOD activity. This result suggests that level of ROS production influences the SOD level and that Mn SOD activity responds earlier than Cu-Zn SOD activity. One possible explanation for this result is that higher levels of ROS are produced towards the mitochondrial matrix (Katewa and Ballard, 2007; Miwa et al., 2003). Our favored explanation for the different activities of Mn SOD and Cu-Zn SOD in siII and siIII flies is that activities of SOD are modulated by various factors including ROS levels (Franco et al., 1999). Further studies are required to directly test SOD mRNA’s expression levels at different ages. The significance of superoxide anion radical levels in influencing lifespan has been shown in studies of transgenic animals where overexpression of SOD has been shown to cause a net increase in lifespan (Mockett et al., 2003; Orr and Sohal, 1992; Sun et al., 2002; Sun et al., 1999). We find no support for the alternative hypothesis that there is subdivision in nuclear encoded SOD loci associated with the mtDNA haplogroup. We examined data from the D. simulans MD106 and MD199 fly lines that harbor siII and siIII mtDNA respectively (Ballard, 2000a; Grumbling and Strelets, 2006) and found no amino acid differences in the Mn SOD or Cu-Zn SOD genes.
We observed that flies harboring siIII mtDNA had higher levels of GSSG and were more oxidatively stressed. We propose that the higher GSSG levels resulted from the age dependent increase in rates of H2O2 production. Hyperoxia, which increases the rates of mitochondrial generation of ROS (Turrens et al., 1982a; Turrens et al., 1982b) and accelerates the accrual of macromolecular oxidative damage to tissues (Agarwal and Sohal, 1994; Sohal and Dubey, 1994), results in an age dependent increase of GSSG levels in D. melanogaster (Rebrin and Sohal, 2006). In D. simulans, however, high GSSG levels may not influence organismal longevity (Fig. 6 and supplementary Fig. S.4). Ballard et al. (submitted) detected small differences in the mean survival of these same siII and siIII fly lines and James and Ballard (2003) used the reciprocal introgression approach to standardize the nuclear genome of three fly lines and showed that males harboring siII and siIII mtDNA did not differ in mortality rates.
Flies harboring siIII mtDNA had higher state 3 and 4 respiration rates on all days assayed (Fig. 1 and supplementary Fig. S.2). This implies that siIII males are more bioenergetically efficient than those with siII mtDNA. This result is in conflict with the observations that siII flies are more successful than siIII flies in nature and in laboratory studies (Dean et al., 2003; Ballard and James, 2004; Satta et al., 1988). If the efficiency of mitochondrial metabolism is related to organismal success there are at least six potential reasons for the observed conflict. First, the efficiency of mitochondrial metabolism may be low in very young siIII flies. Fig. 1A shows state 3 respiration increases from 11 d to 18 d in siIII but is constant in siII. Second, we assayed only males because we expected less variability between replicates (Wigby and Chapman, 2005). MtDNA is maternally inherited and slightly deleterious mutations can accumulate in the mtDNA of males such that “good” mitochondria in males are “bad” in females (Rand et al., 2001). Third, slightly deleterious mutations may have accumulated in flies harboring siII mtDNA in Africa but may not be accumulating in regions outside of Africa because of positive selection. Dean et al. (2003) reported three populations of D. simulans siII in eastern Africa that showed a higher amount of mtDNA polymorphism than all previous descriptions of this species. Fourth, the allocation and rate of utilization of mitochondrial substrates by siII and siIII may be differentially influenced by dietary protein and carbohydrate. We employed one organismal diet and two sets of mitochondrial substrates that may not reflect what flies consume in nature (Raubenheimer and Simpson, 1993, 1997, 2003). Fifth, we assayed mitochondria at 25 °C. Flies harboring siIII are restricted to eastern Africa while siII flies are globally distributed and 25 °C may be closer to optimality for the former than the latter flies. Sixth, mitochondrial bioenergetic efficiency determined from thoraces may have no influence on the distribution and abundance of whole organisms in nature. Rather, bioenergetic efficiency of other tissues or of larvae may be more important. Additional studies are required to determine if metabolic efficiency changes with tissue, age, gender, diet, temperature and life history stage.
These results demonstrate our ability to correlate mtDNA variation with differences in whole mitochondrial physiology and individual complex biochemistry. This report, combined with previous studies, quantifies genetic subdivision and establishes broad-brush metabolic differences among flies harboring siII and siIII mtDNA (Ballard and Kreitman 1994; Ballard, 2000a; Ballard, et al., 2002; Ballard et al., 2007; Katewa and Ballard, 2007). Fixed mtDNA encoded amino acid differences among haplogroups exist in each mitochondrial complex. In contrast, no genetic subdivision associated with the mtDNA has been found in 16 nuclear genes suggesting the differences seen in mitochondrial metabolism could be due to the variations in the genes coded by mtDNA. Sequencing the three mtDNA encoded COX genes and 9 nuclear encoded (plus the four known isoforms) COX genes supports this suggestion. Fixed amino acid differences between flies harboring siII and siIII mtDNA are seen only in the COII mtDNA encoded gene. One nonsynonymous change occurs in a loop towards the intermembrane space and causes an isoleucine to threonine replacement. The second change causes an isoleucine to valine replacement in a β8–strand of the cupredoxin fold towards the intermembrane space (Williams et al. 1999). We suggest these amino acid changes are involved in age dependent modification of cytochrome c oxidase activity. We have observed that cytochrome c oxidase activity declined more quickly in siIII than siII harboring flies and ROS production increases with age in siIII but not siII flies (Ballard et al. 2007; Katewa and Ballard 2007; Fig. 2). Ferguson et al. (2005) have shown that cytochrome c oxidase activity declines with age and a decrease in cytochrome c oxidase may cause an increase in ROS production. A limitation of this hypothesis and of comparing siII and siIII flies is that it is not possible to pinpoint which amino acid causes a specific change. Amino acid changes in complex IV, in subunit assembly or in another complex may affect cytochrome c oxidase activity (Jesina et al., 2004; Varlamov et al., 2002; Williams et al. 2004). Future studies, will contrast flies harboring siII mtDNA. The genetic variation within siII flies from east Africa is equivalent to that seen within humans (Ballard, 2000a, Ballard et al., 2007).
Supplementary Material
Fig. S.1. Inhibition curves for Bovine Cu-Zn superoxide dismutase. The curves are linear between concentration of 0.1 to 10 U/ml.
Fig. S.2. Respiration by isolated mitochondria from flight muscles when glycerol 3 phosphate was used as the substrate.
Fig. S.3. The rate of hydrogen peroxide production from flight muscles with pyruvate and proline as substrate showed a mixed pattern with age.
Fig. S.4. Oxidized glutathione content in whole fly homogenates is lower in siII flies and is highest on day 18.
Fig. S.5. Flies harboring the siII and siIII flies showed an age dependent decrease in the reduced glutathione contents in whole fly homogenates but declines more rapidly in siII flies.
Acknowledgements
We would like to acknowledge Prof. Sarjeet Gill and two anonymous reviewers for giving useful comments on an earlier version of the manuscript. We would also like to thank Rich Melvin for discussions and Ines Ricafuente (UNSW) for fly maintenance. Funding for this study is provided by National Science Foundation Grant DEB-0444766, and National Institutes of Health RO1 grant GM067862-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Agarwal S, Sohal RS. DNA oxidative damage and life expectancy in houseflies. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12332–12335. doi: 10.1073/pnas.91.25.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S, Shea JM, Felmet T, Gadra J, Dehn PF. A kinetic microassay for glutathione in cells plated on 96-well microtiter plates. Methods Cell. Sci. 2000;22:305–312. doi: 10.1023/a:1017585308255. [DOI] [PubMed] [Google Scholar]
- Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Baba-Aïssa F, Solignac M, Dennebouy N, David JR. Mitochondrial DNA variability in Drosophila simulans: quasi absence of polymorphism within each of the three cytoplasmic races. Heredity. 1988;61:419–426. doi: 10.1038/hdy.1988.133. [DOI] [PubMed] [Google Scholar]
- Baker MA, Cerniglia GJ, Zaman A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal. Biochem. 1990;190:360–365. doi: 10.1016/0003-2697(90)90208-q. [DOI] [PubMed] [Google Scholar]
- Ballard JWO. Comparative genomics of mitochondrial DNA in Drosophila simulans. J. Mol. Evol. 2000a;51:64–75. doi: 10.1007/s002390010067. [DOI] [PubMed] [Google Scholar]
- Ballard JWO. Comparative genomics of mitochondrial DNA in members of the Drosophila melanogaster subgroup. J. Mol. Evol. 2000b;51:48–63. doi: 10.1007/s002390010066. [DOI] [PubMed] [Google Scholar]
- Ballard JWO. Drosophila simulans as a novel model for studying mitochondrial metabolism and aging. Exp. Gerontol. 2005;40:763–773. doi: 10.1016/j.exger.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Ballard JWO, Chernoff B, James AC. Divergence of mitochondrial DNA is not corroborated by nuclear DNA, morphology, or behavior in Drosophila simulans. Evolution. 2002;56:527–545. doi: 10.1111/j.0014-3820.2002.tb01364.x. [DOI] [PubMed] [Google Scholar]
- Ballard JWO, James AC. Differential fitness of mitochondrial DNA in perturbation cage studies correlates with global abundance and population history in Drosophila simulans. Proc. Biol. Sci. 2004;271:1197–1201. doi: 10.1098/rspb.2004.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard JWO, Kreitman M. Unraveling selection in the mitochondrial genome of Drosophila. Genetics. 1994;138:757–772. doi: 10.1093/genetics/138.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard JWO, Melvin RG, Katewa SD, Mass K. Mitochondrial DNA variation causes measurable differences in life history traits and mitochondrial metabolism in Drosophila simulans. Evolution. 2007 doi: 10.1111/j.1558-5646.2007.00133.x. (in press) [DOI] [PubMed] [Google Scholar]
- Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J. Biol. Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Kennington WJ. A simple method to achieve consistent larval density in culture bottles. Drosoph. Inf. Serv. 2001;84:168–169. [Google Scholar]
- Clary DO, Wolstenhome DR. The mitochondrial DNA molecule of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J. Mol. Evol. 1985;22:252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- de Stordeur E, Solignac M, Monnerot M, Mounolou JC. The generation of transplasmic Drosophila simulans by cytoplasmic injection: effects of segregation and selection on the perpetuation of mitochondrial DNA heteroplasmy. Mol. Gen. Genet. 1989;220:127–132. doi: 10.1007/BF00260866. [DOI] [PubMed] [Google Scholar]
- Dean MD, Ballard JWO. Linking phylogenetics with population genetics to reconstruct the geographic origin of a species. Mol. Phylogenet. Evol. 2004;32:998–1009. doi: 10.1016/j.ympev.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Dean MD, Ballard KJ, Glass A, Ballard JWO. Influence of two Wolbachia strains on population structure of east African Drosophila simulans. Genetics. 2003;65:1959–1969. doi: 10.1093/genetics/165.4.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M, Mockett RJ, Shen Y, Orr WC, Sohal RS. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem. J. 2005;390:501–511. doi: 10.1042/BJ20042130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco AA, Odom RS, Rando TA. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radic. Biol. Med. 1999;27:1122–1132. doi: 10.1016/s0891-5849(99)00166-5. [DOI] [PubMed] [Google Scholar]
- Grumbling G, Strelets V. FlyBase: anatomical data, images and queries. Nucleic Acids Res. 2006;34:D484–D488. doi: 10.1093/nar/gkj068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner RP, Brown GC, Brand MD. Analysis of the control of respiration rate, phosphorylation rate, proton leak rate and protonmotive force in isolated mitochondria using the 'top-down' approach of metabolic control theory. Eur. J. Biochem. 1990;188:313–319. doi: 10.1111/j.1432-1033.1990.tb15405.x. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: A theory based on free radical radiation chemistry. J. Gerentol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hercus MJ, Hoffmann AA. Maternal and grandmaternal age influence offspring fitness in Drosophila. Proc. R. Soc. Lond. B. 2000;267:2105–2110. doi: 10.1098/rspb.2000.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horai S, Hayasaka K, Kondo R, Tsugane K, Takahata N. Recent African origin of modern humans revealed by complete sequences of hominoid mitochondrial DNAs. Proc. Natl. Acad. Sci. U.S.A. 1995;92:532–536. doi: 10.1073/pnas.92.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari M, Long AD, Mueller LD, Rose MR. The pharmacology of ageing in Drosophila. Curr. Drug Targets. 2006;7:1479–1483. doi: 10.2174/1389450110607011479. [DOI] [PubMed] [Google Scholar]
- James AC, Ballard JWO. Mitochondrial genotype affects fitness in Drosophila simulans. Genetics. 2003;164:187–194. doi: 10.1093/genetics/164.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesina P, Tesarova M, Fornuskova D, Vojtiskova A, Pecina P, Kaplanova V, Hansikova H, Zeman J, Houstek J. Diminished synthesis of subunit a (ATP6) and altered function of ATP synthase and cytochrome c oxidase due to the mtDNA 2 bp microdeletion of TA at positions 9205 and 9206. Biochem. J. 2004;383:561–571. doi: 10.1042/BJ20040407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JMP. JMP Statistics and Graphics Guide. Cary, NC: SAS Institute Inc.; 1995. [Google Scholar]
- Kaplan NL, Hudson RR, Langley CH. The "hitchhiking effect" revisited. Genetics. 1989;123:887–899. doi: 10.1093/genetics/123.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katewa SD, Ballard JWO. Sympatric Drosophila simulans flies with distinct mtDNA show difference in mitochondrial respiration and electron transport. Insect Biochem. Mol. Biol. 2007;37:213–222. doi: 10.1016/j.ibmb.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Katewa SD, Melvin RG, Ballard JWO. Comparison of methods and body parts for estimation of hydrogen peroxide production by isolated mitochondria from Drosophila simulans. Drosoph. Inf. Serv. 2006 (in press) [Google Scholar]
- Krings M, Geisert H, Schmitz RW, Krainitzki H, Paabo S. DNA sequence of the mitochondrial hypervariable region II from the neandertal type specimen. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5581–5585. doi: 10.1073/pnas.96.10.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Matsuura ET, Fukuda H, Chigusa SI. Mitochondrial DNA heteroplasmy maintained in natural populations of Drosophila simulans in Reunion. Genet. Res. 1991;57:123–126. doi: 10.1017/s0016672300029189. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J, Haigh J. The hitchhiking effect of a favourable gene. Genet. Res. 1974;23:23–35. [PubMed] [Google Scholar]
- Melvin RG, Ballard JWO. Intraspecific variation in survival and mitochondrial oxidative phosphorylation in wild caught Drosophila simulans. Aging Cell. 2006;5:225–233. doi: 10.1111/j.1474-9726.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- Miwa S, Riyahi K, Partridge L, Brand MD. Lack of correlation between mitochondrial reactive oxygen species production and life span in Drosophila. Ann. N. Y. Acad. Sci. 2004;1019:388–391. doi: 10.1196/annals.1297.069. [DOI] [PubMed] [Google Scholar]
- Miwa S, St-Pierre J, Partridge L, Brand MD. Superoxide and hydrogen peroxide production by Drosophila mitochondria. Free. Radic. Biol. Med. 2003;35:938–948. doi: 10.1016/s0891-5849(03)00464-7. [DOI] [PubMed] [Google Scholar]
- Mockett RJ, Bayne AC, Kwong LK, Orr WC, Sohal RS. Ectopic expression of catalase in Drosophila mitochondria increases stress resistance but not longevity. Free Radic. Biol. Med. 2003;34:207–217. doi: 10.1016/s0891-5849(02)01190-5. [DOI] [PubMed] [Google Scholar]
- Mockett RJ, Sohal RS, Orr WC. Overexpression of glutathione reductase extends survival in transgenic Drosophila melanogaster under hyperoxia but not normoxia. FASEB J. 1999;13:1733–1742. doi: 10.1096/fasebj.13.13.1733. [DOI] [PubMed] [Google Scholar]
- Orr WC, Sohal RS. The effects of catalase gene overexpression on life span and resistance to oxidative stress in transgenic Drosophila melanogaster. Arch. Biochem. Biophys. 1992;297:35–41. doi: 10.1016/0003-9861(92)90637-c. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D. Beyond the evolutionary theory of ageing, from functional genomics to evo-gero. Trends Ecol. Evol. 2006;21:334–340. doi: 10.1016/j.tree.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Partridge L, Piper W, Mair W. Dietary restriction in Drosophila. Mech. Ageing Devel. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Rand DM, Clark AG, Kann LM. Sexually antagonistic cytonuclear fitness interactions in Drosophila melanogaster. Genetics. 2001;159:173–187. doi: 10.1093/genetics/159.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raubenheimer D, Simpson SJ. The geometry of compensatory feeding in the locust. Anim. Behav. 1993;45:953–964. [Google Scholar]
- Raubenheimer D, Simpson SJ. Integrative models of nutrient balancing: application to insects and vertebrates. Nutr. Res. Rev. 1997;10:151–179. doi: 10.1079/NRR19970009. [DOI] [PubMed] [Google Scholar]
- Raubenheimer D, Simpson SJ. Nutrient balancing in grasshoppers: behavioural and physiological correlates of dietary breadth. J. Exp. Biol. 2003;206:1669–1681. doi: 10.1242/jeb.00336. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Bayne AC, Mockett RJ, Orr WC, Sohal RS. Free aminothiols, glutathione redox state and protein mixed disulphides in aging Drosophila melanogaster. Biochem J. 2004;382:131–136. doi: 10.1042/BJ20040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Sohal RS. Comparison between the effects of aging and hyperoxia on glutathione redox state and protein mixed disulfides in Drosophila melanogaster. Mech. Ageing Dev. 2006;127:869–874. doi: 10.1016/j.mad.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RE. Age-specific decrease in aerobic efficiency associated with increase in oxygen free radical production in Drosophila melanogaster. J. Insect. Physiol. 2000;46:1477–1480. doi: 10.1016/s0022-1910(00)00072-x. [DOI] [PubMed] [Google Scholar]
- Satta Y, Ishiwa H, Chigusa SI. Analysis of nucleotide substitutions of mitochondrial DNAs in Drosophila melanogaster and its sibling species. Mol. Biol. Evol. 1987;4:638–650. doi: 10.1093/oxfordjournals.molbev.a040464. [DOI] [PubMed] [Google Scholar]
- Satta Y, Toyohara N, Ohtaka C, Tatsuno Y, Watanabe K, Matsuura ET, Chigusa SI, Takahata N. Dubious maternal inheritance of mitochondrial DNA in D. simulans and evolution of D. mauritiana. Genetical Res. 1988;52:1–6. [Google Scholar]
- Sohal RD. Mitochondrial changes in flight muscles of normal and flightless Drosophila melanogaster with age. J. Morphol. 1975;145:337–353. doi: 10.1002/jmor.1051450307. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Agarwal A, Agarwal S, Orr W. Simultaneous overexpression of copper and zinc containing superoxide dismutase and catalase retards age-related oxidative damage and increases metabolic potential in Drosophila melanogaster. J. Biol. Chem. 1995a;270:15671–15674. doi: 10.1074/jbc.270.26.15671. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Arnold LA, Sohal BH. Age-related changes in antioxidant enzymes and prooxidant generation in tissues of the rat with special to parameters in two insect species. Free Radic. Biol. Med. 1990a;9:495–500. doi: 10.1016/0891-5849(90)90127-5. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Dubey A. Mitochondrial oxidative damage, hydrogen peroxide release, and aging. Free Radic. Biol. Med. 1994;16:621–626. doi: 10.1016/0891-5849(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Sohal BH, Brunk UT. Relationship between antioxidant defenses and longevity in different mammalian species. Mech. Ageing Dev. 1990b;53:217–227. doi: 10.1016/0047-6374(90)90040-m. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Sohal BH, Orr WC. Mitochondrial superoxide and hydrogen peroxide generation, protein oxidative damage, and longevity in different species of flies. Free Radic. Biol. Med. 1995b;19:499–504. doi: 10.1016/0891-5849(95)00037-x. [DOI] [PubMed] [Google Scholar]
- Solignac M, Monnerot M, Mounolou JC. Mitochondrial DNA evolution in the melanogaster species subgroup of Drosophila. J. Mol. Evol. 1986;23:31–40. doi: 10.1007/BF02100996. [DOI] [PubMed] [Google Scholar]
- Sun J, Folk D, Bradley TJ, Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase transgene extends the lifespan of adult Drosophila melanogaster. Genetics. 2002;161:661–672. doi: 10.1093/genetics/161.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LV, Babaratsas A, Savakis C, O'Neill SL, Bourtzis K. Gene Organization of the dnaA Region of Wolbachia. J. Bacteriol. 1999;181:4708–4710. doi: 10.1128/jb.181.15.4708-4710.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M. Diet restriction in Drosophila melanogaster. Design and analysis. Interdiscip. Top. Gerontol. 2007;35:115–136. doi: 10.1159/000096559. [DOI] [PubMed] [Google Scholar]
- Turrens JF, Freeman BA, Crapo JD. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch. Biochem. Biophys. 1982a;217:411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- Turrens JF, Freeman BA, Levitt JG, Crapo JD. The effect of hyperoxia on superoxide production by lung submitochondrial particles. Arch. Biochem. Biophys. 1982b;217:401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]
- Van den Bergh SG. Insect mitochondria. In: Estabrook RW, Pullman ME, editors. Methods Enzymol. Vol. 10. New York and London: Academic Press; 1967. pp. 117–122. [Google Scholar]
- Varlamov DA, Kudin AP, Vielhaber S, Schroder R, Sassen R, Becker A, Kunz D, Haug K, Rebstock J, Heils A, Elger CE, Kunz WS. Metabolic consequences of a novel missense mutation of the mtDNA CO I gene. Hum. Mol. Genet. 2002;11:1797–1805. doi: 10.1093/hmg/11.16.1797. [DOI] [PubMed] [Google Scholar]
- Wigby S, Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 2005;15:316–321. doi: 10.1016/j.cub.2005.01.051. [DOI] [PubMed] [Google Scholar]
- Williams PA, Blackburn NJ, Sanders D, Bellamy H, Stura EA, Fee JA, McRee DE. The CuA domain of Thermus thermophilus ba3-type cytochrome c oxidase at 1.6 A resolution. Nat. Struct. Biol. 1999;6:509–516. doi: 10.1038/9274. [DOI] [PubMed] [Google Scholar]
- Williams SL, Valnot I, Rustin P, Taanman JW. Cytochrome c oxidase subassemblies in fibroblast cultures from patients carrying mutations in COX10, SCO1, or SURF1. J. Biol. Chem. 2004;279:7462–7469. doi: 10.1074/jbc.M309232200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S.1. Inhibition curves for Bovine Cu-Zn superoxide dismutase. The curves are linear between concentration of 0.1 to 10 U/ml.
Fig. S.2. Respiration by isolated mitochondria from flight muscles when glycerol 3 phosphate was used as the substrate.
Fig. S.3. The rate of hydrogen peroxide production from flight muscles with pyruvate and proline as substrate showed a mixed pattern with age.
Fig. S.4. Oxidized glutathione content in whole fly homogenates is lower in siII flies and is highest on day 18.
Fig. S.5. Flies harboring the siII and siIII flies showed an age dependent decrease in the reduced glutathione contents in whole fly homogenates but declines more rapidly in siII flies.


