Abstract
Endothelial cells are stimulated by shear stress throughout the vasculature and respond with changes in gene expression and by morphological reorganization. Mechanical sensors of the cell are varied and include cell surface sensors that activate intracellular chemical signaling pathways. Here, possible mechanical sensors of the cell including reorganization of the cytoskeleton and the nucleus are discussed in relation to shear flow. A mutation in the nuclear structural protein lamin A, related to Hutchinson Gilford progeria syndrome, is reviewed specifically since the mutation results in altered nuclear structure and stiffer nuclei; animal models also suggest significantly altered vascular structure. Nuclear and cellular deformation of endothelial cells in response to shear stress provides partial understanding of possible mechanical regulation in the microcirculation. Increasing sophistication of fluid flow simulations inside the vessel is also an emerging area relevant to the microcirculation since visualization in situ is difficult. This integrated approach to study – including medicine, molecular and cell biology, biophysics and engineering – provides a unique understanding of multi-scale interactions in the microcirculation.
Keywords: Nuclear mechanics, Hutchinson Gilford progeria syndrome, computational fluid dynamics (CFD), rheology, lamins
1. Introduction and overview
Studying the effects of shear stress on cell behavior in the microvasculature can be addressed by interdisciplinary, multi-scale experimentation and modeling. Contributions from medicine, cell biology and molecular biology have made great strides; now inclusion of engineering and physics to examine rheology of cellular components and fluid profiles adds needed dimension to this complex problem. The need for an interdisciplinary multi-scale approach to the study of pathologies becomes even more apparent when the boundaries of observation and clinically appropriate models are reached, such as in the microcirculation. Here, we will briefly review the current understanding of cellular response to fluid shear stress related to mechanobiology. We will discuss new directions in cellular study related to nuclear deformation and the role it plays on cell and multicellular response. This is particularly relevant given an aging disorder in which nuclear mechanical properties are altered and clinical manifestations manifest in the cardiovascular system. We will end this review with multiscale engineering techniques useful for both expanding molecular, subcellular and cellular studies to potentially clinically relevant levels as well as adapting in vivo and in vitro information to better understand the microcirculation.
2. Cellular mechanical response to shear stress
The cardiovascular system provides the most relevant system to study cellular response to fluid shear stress due to its widespread clinical implications. Atherosclerotic lesions, that have long been known to lead to cardiovascular disease, preferentially locate at regions of disturbed or unsteady flow and lesions are rarely seen in areas of fully developed laminar flow [11]. These observations lead to the hypothesis that the changes in cellular behavior caused by shear stress protect against the development of atherosclerosis, a phenotype which has been termed "atheroprotective" [22]. In areas of temporally and spatially disturbed flow, cells change shape, expression profiles and become overly mitogenic [31]. In atheroprotective regions, typically experiencing steady laminar flow, endothelial cells release a host of factors that inhibit coagulation, migration of leukocytes, proliferation of smooth muscles, and promote endothelial cell survival while the opposite is true in regions showing atherosclerotic phenomena [15, 16].
Similarly, shear stress plays a vital role in the development of the cardiovascular system. During development, shear stress is particularly high [52] and loss of shear stress from capillaries causes capillary regression and cell apoptosis [76]. Thus cells are responsive to their mechanical environment and respond with a host of chemical and morphological changes. Determining the mechano-sensitive structures in cells and how they are interrelated in chemical pathways in the cell is still an open area of study with many challenges. Force-based experiments are difficult to analyze and the chemo-mechanical mediators are difficult to inhibit and rescue in the same way that traditional chemical factors are studied.
2.1 Mechanotransduction: chemical signaling
In nearly all cell types, force on the cell is able to alter gene expression by chemical factors such as NF-κB activated through chemical pathways induced by strains on the cellular plasma membrane or cytoskeleton through transcriptional activators and repressors [89, 103, 116]. This activation of genes by mechanical forces is a primary example of mechanotransduction. Specifically to endothelial cells, researchers have studied the chemical pathways in cells activated by shear stress including NF-κB [17] and others [65]. In addition to work on specific proteins and signaling pathways suspected of being involved in cellular response to shear stress, DNA chip technology show changes in global gene expression in endothelial cells exposed to shear stress [11, 86, 118].
2.2 Mechanotransduction: mechanobiology
There has been a growing acceptance that cellular activity is modulated by mechanical environment and stress through mechanical means in addition to simple chemical changes in the cell [24]. Force acts in many ways: affecting reaction rates, dislocating bonds, causing the translocation of nuclear factors, stretching the membrane, changing cellular compartment shape, altering polarity and changing cell-cell connections, etc. Below we will discuss the well-established and emerging theories of how force affects cells, termed mechanobiology.
2.2.1 Glycocalyx
Starting from the outside of the cell, the glycocalyx is a thin layer (0.5–3 µm [113]) of proteoglycans, glycosaminoglycans (GAGs) bound to the apical membrane of endothelial cells that is hydrated and mixed with plasma proteins. Interconnection with the cytoskeleton at the cytoskeleton allows for mechanical information to be transmitted into the cell [111]. The glycocalyx is suggested to play an important role in the response of cells to shear stress, particularly in narrow diameter capillaries [113].
2.2.2 Stretch-activated ion channels
Stretch-activated ion channels are present on many types of cells which experience force. Ion-specific stretch activated channels, including sodium [112], potassium [87] and chloride [4], are present on endothelial cells, and blocking these channels has been shown to inhibit shear stress-dependent function. Shear stress-induced increases in membrane tension can alter G-protein cascades or other membrane-linked protein interactions [120].
2.2.3 Cytoskeletal rearrangements
Cell morphology changes significantly when cells are exposed to shear stress, typically involving redistributions of the actin cytoskeleton and focal adhesions [74, 85]. Cells flatten and elongate in the direction of flow [6], and actin filaments typically align into stress fibers parallel to the direction of flow [85]. Significant reorganization of intermediate filament networks is also observed under shear flow suggesting that all structural components of the cell are flow-sensitive [26].
The exact mechanism by which cytoskeletal reorganization is able to affect gene regulation is unclear. Reorganization of stress fibers [122] and actin-binding, stress-sensitive molecules such as filamin [57] may play a role, but the mechanisms in endothelial cells have not been determined. Also, cytoskeletal rearrangements are suggested to influence the cell in many indirect ways, including changing subcellular compartments, reaction surface area, or cell stiffness, which subsequently affects tissue stiffness [26]. The best studied change in cell phenotype associated with shear stress is the change in cell-cell interaction due to the reorganization of the cytoskeleton.
2.2.4 Cell-cell and cell- extracellular matrix connections
PECAM-1 (platelet endothelial cell adhesion molecule; cell-cell adhesion molecule) [37], VE-cadherins (involved in adherens junctions) and β-catenin (anchors adherens to the cytoskeleton) are stimulated by mechanical stress [13]. In addition to cell-cell contacts, cell-contacts with the extracellular matrix are suggested to be important; cells bind the extracellular matrix by means of integrins, which are typically α/β heterocomplexes. Recently, integrin activation including focal adhesion kinase (FAK) [122] has been shown to regulate NF-κB in a shear-stress dependent way in endothelial cells [95].
3. Nuclear mechanical response to shear stress
The nucleus is the largest and stiffest organelle in most mammalian cells, including the endothelial cell, and can influence cell mechanics [12]. This is highlighted in several disease states caused by mutations in nuclear structural proteins which give rise to altered tissue level mechanics in the vasculature and elsewhere [78, 80]. Of particular interest in the vasculature is the premature aging [114] and nuclear stiffening [24] disease Hutchinson Gilford progeria syndrome (HGPS), which will be described in more detail below. Patients with HGPS, which affects proteins within the nucleus, often experience cardiovascular disease [35] suggesting that extracellular fluid flow is related to intracellular stiffness.
Work done to study the effects of shear stress on normal cells also suggests that flow outside the cell impacts nuclear organization. Simulations of cell response to shear stress in a normal endothelial layer suggest that a simple model of minimizing the shape of the nucleus to reduce drag is sufficient to model the morphological changes experienced by cells [50]. This suggests that the nucleus does, in fact, respond to forces over long time scales. Deguchi and colleagues showed that shear stress aligns nuclei from endothelial cells, and the shape is retained even after nuclei are removed from cells [30]. These global arrangements are correlated with a nearly 50% increase in nuclear stiffness [30]. Other studies have shown that shear stress impacts the molecular levels and distributions of the nucleus; structural proteins of the nucleus are upregulated under force and redistributed into a more load-bearing configuration [96].
This redistribution of molecules and force within the nucleus may be related to gene expression. The organization of chromatin domains within the nucleus is related to gene expression [32]. Gross rearrangements of nuclei [30, 46] and subnuclear features [32, 70] are observed in cells exposed to extracellular stress. More directly, studies have shown that mechanically pulling on integrins outside cells transmits force and rearranges nucleoli within the nuclei [70]. While these mechanisms are poorly resolved in endothelial cells under shear, they may be linked to shear-induced gene transcription mechanisms and will be discussed in more detail below.
4. Nuclear structural and mechanical elements
The structural support of the nucleus is the nuclear lamina. The nuclear lamina is a filamentous network of mostly lamin proteins underlying the inner nuclear membrane (INM). Human cells encode three genes for two types of lamins: A-type lamins, alternative splicing of the LMNA gene which produces primarily lamin A and lamin C, and B-type lamins, including lamin B1 and lamin B2, which are encoded by separate genes [66, 123]. B-type lamins are always expressed in all cell types of metazoans [79] and these genes are essential to cell survival [49]. Without A-type lamins, cells are able to survive and proliferate, but at least twelve human diseases result from mutations in LMNA [47].
Lamins can interact with chromatin directly [77, 109], indirectly [42] and via lamin binding proteins [102]. Lamin structures regulate and support protein complexes involved in gene expression [10, 61]; DNA replication, transcription and repair [90]; nuclear positioning [75]; and aging [25, 43]. Loss or alteration of lamin A through disease is associated with heterochromatin loss at the nuclear periphery and alterations in epigenetic modifications regulating heterochromatin at the nuclear interior [104]. Mechanisms of these lamin-dependent regulations are unknown, but since they are responsible for the structural stability of the nucleus, it is suggested that force may be involved.
The nuclear lamins are mechanically stiff proteins that make up a majority of the nuclear envelope and are necessary for the structural integrity of the nucleus. The contribution of nuclear lamins to the stiffness of nuclei is suggested by the following observations: 1) highly fragile nuclei reconstituted from lamin-depleted Xenopus egg extracts [83] and 2) nuclei from mouse lmna-null cells are mechanically weak [61]. In vitro rheology of reconstituted lamin B1 solutions shows lamin filaments to be stiff but elastic [88]. Direct mechanical measurements of Xenopus oocyte nuclei also show the in vivo lamina to be a stiff but elastic network [24].
5. Lamins and disease
The lamina is involved in nuclear mechanics as well as fundamental cellular processes such as chromatin organization, DNA replication and RNA transcription [51]. Abnormalities in the lamina due to mutations in lamins and lamin-associated proteins lead to disease pathology, collectively termed laminopathies [80, 114, 121]. Interestingly, laminopathies manifest in a diverse set of clinical presentations involving a variety of disparate tissues types that depend on the specific mutation involved. There are over 180 known mutations in three genes (LMNA, LMNB1, LMNB2) that result in 13 different laminopathies [114]. Lamina network structure and mechanics play a key role in some of these diseases [24, 61], such that increases and decreases in lamin proteins at the nuclear envelope lead to premature aging and muscular dystrophies, respectively [45]. Lamin concentration and organization are also altered in normal aging processes [25, 47]. In most cases, stress-bearing tissues (such as endothelium, muscle, cartilage, etc.) are most affected by nuclear defects, suggesting that alterations in force transmission may be a pathological factor [45]. The underlying mechanisms of these diseases are not well understood, limiting the development of treatments, cures and preventions.
Nuclear laminopathies and RNAi technologies allow study of nuclear effects of cell responses to force. Loss of lamins and the resulting nuclear softening leads to cardiomyopathy and a muscular dystrophy phenotype in mice [62]. Addition of exogenous lamins often does not alter cellular phenotype because cells compensate by increasing lamin expression. However, the production of mutant lamins like in Hutchison-Gilford progeria syndrome (HGPS) cause an accumulation of lamin filaments and nuclear stiffening that is thought to lead to weakening of vascular tissue. [24]. HGPS shows the most direct connection between lamin mutation, nuclear structure/mechanics and vascular disease, discussed below.
6. Hutchison-Gilford progeria syndrome (HGPS)
HGPS is a devastating form of premature aging which causes systemic issues with tissues. After birth, patients with HGPS experience progressive loss of subcutaneous fat, severe growth retardation, hair loss, bone deformations, osteoporosis, delayed dentition, joint stiffness, hip dislocations and progressive arteriosclerosis [35]. Most of the problems with HGPS are related to load-bearing tissues, and there are no neurological aged-like symptoms associated with the disease. Most patients with HGPS experience severe cardiovascular phenotypes, which is typically the cause of death usually in the early teens [35].
6.1. HGPS and cardiovascular disease
Analysis of various tissue types of HGPS patients suggests that the mutant lamin protein accumulates most dramatically in the nuclei of endothelial and vascular smooth muscle cells. A transgenic animal model for HGPS presents only with cardiovascular pathologies, with mutant protein expression in endothelial and smooth muscle cells and the characteristic nuclear morphological alterations primarily in vascular smooth muscle cells [114]. Over time, smooth muscle cells are lost and replaced by collagen with some calcification [73]. Although no reports have been made on cells of the microvasculature, possibly due to imaging limitations in mouse models, we can speculate what the changes would be with more studies of the molecular, sub-cellular, cellular and tissue level changes associated with the disease.
6.2. Structural and mechanical causes of HGPS
6.2.1 Molecular
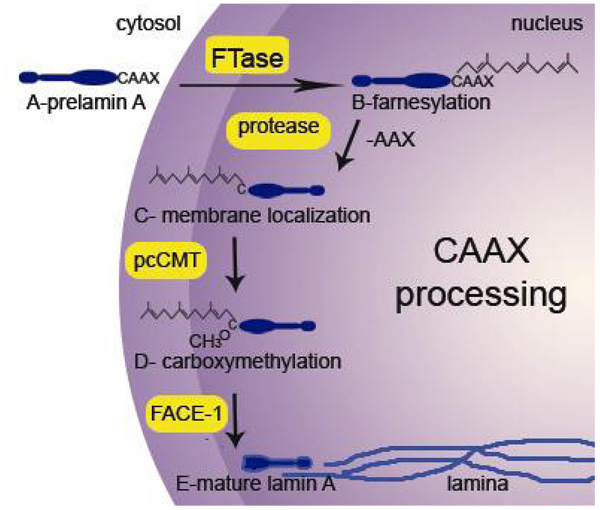
The altered mechanisms of lamin A post-processing in HGPS are of increased interest due to their unique nature and etiology [124]. Normal processing, which is complex for a structural molecule in the cell, is presented in Figure 1. Prenylation is a common posttranslational modification that occurs on a variety of proteins, including Ras proteins, G proteins, lamins and yeast mating factors. Prenylation requires the presence of a CAAX box motif at the C-terminus: the CAAX box consists of cysteine and two aliphatic amino acids, which affect the efficiency of prenylation. The terminal amino acid (X) determines which of the two types of prenylation (farnesylation or geranylgeranylation) can occur [18, 20, 94]. If X is serine, methionine, cysteine, alanine or glutamine, then a farnesyl moiety will be added; however, a leucine or phenylalanine will signal the addition of a geranyl moiety [18]. After the prenylation, the terminal three (AAX) amino acids are cleaved and the newly exposed cysteine residue is carboxyl methylated [125]. Prenylation facilitates proper subcellular localization of proteins, promotes protein-protein and protein-membrane interactions, and regulates other cellular regulatory functions [14, 36, 48, 55, 69, 71]. Lamins A and B contain a carboxy-terminus CAAX box motif with a farnesylated cysteine, which functions to localize these proteins to the inner nuclear membrane [69] and is then carboxymethylated at the membrane [106] (Fig. 1). These modifications have been shown to increase the hydrophobicity of lamin B1 [72], but the farnesyl group is not sufficient for stable association with membranes [54]. Lamin B3b, like the Ras proteins that interact with the plasma membrane, stably associates with the nuclear membrane because of a palmitylation site, a designated cysteine residue near the protein’s association with a membrane for the attachment of a fatty acid chain, and a basic cluster in conjunction with the CAAX motif [54]. Similarly, a farnesylated G-protein was shown to interact with model membranes consisting of phosphatidylethanolamine and phosphatidylserine by positively charged amino acids near the farnesyl group [7]. All of this evidence suggests that hydrophobic modifications of a protein are essential for targeting proteins to the membrane [54] and exhibit an observable physical interaction with membranes [7, 34].
Figure 1. Processing of lamin A.
(A) The lamin A precursor is expressed and transported into the nucleus. Lamin A forms dimers in this process, but is shown as a monomer for simplicity. (B) Once inside the nucleus, farnesyltransferase (FTase) attaches a farnesyl group to the cysteine of the CAAX box at the C-terminus. (C) A protease cleaves the last three amino acids and prelamin A localizes to the inner nuclear membrane. (D) pcCMT carboxymethylates the terminal cysteine. (E) FACE-1 cleaves the last 15 amino acids to produce mature lamin A, which incorporates into the lamin A filaments of the nuclear lamina.
HGPS typically results from a de novo point mutation C608T in exon 11 of LMNA that activates a cryptic splice donor site, resulting in a truncated lamin A protein (called Δ50 lamin A or progerin) that is missing 50 internal amino acids near the carboxy-terminus [27, 35]. As mentioned previously, lamin A contains a CAAX box motif that is farnesylated, cleaved and carboxy-methylated. FACE-1 (ZMPSTE-1 in mice), an endoprotease, trims the lamin A precursor into its mature form [19, 119]. However, the progerin mutant does not undergo this last step in lamin A processing because the FACE-1 cleavage site is located within the deleted region caused by the mutation[35]. As a result, the farnesyl and carboxymethyl groups added during CAAX processing remain, immobilizing progerin at the nuclear membrane and causing significant disruptions to nuclear integrity including changes in nuclear shape, its ability to handle mechanical stress and gene expression [24, 25, 28, 44, 59, 60, 63, 64, 114].
Interestingly, the same progerin mutant is found in cells of unaffected individuals because of sporadic activation of the cryptic splice site [25]. Cells from older individuals contain more progerin proteins than cells from younger individuals [25], suggesting that progerin is not processed like other lamin A proteins within the nucleus, but rather accumulate at the nuclear membrane over time [24, 28].
6.2.2 Nuclear
The retention of CAAX posttranslational lipidations causes progerin proteins to remain associated with the membrane, which then causes the membrane to fragment during during division, resulting in a delayed cytokinesis and mislocalization of nuclear envelope and lamina components in daughter cell nuclei [28]. Nuclei from patients with HGPS are larger, have altered nuclear morphology - nuclear blebs - and a thicker nuclear lamina (see figure 2 and the first report [44]). Also, the expression of progerin has been shown to impact nuclear import due to nuclear pore mislocalization [1]. Mechanically, the over-accumulation of progerin at the nuclear envelope decreases the lamina network’s ability to deform and increases nuclear stiffness in patients with HGPS [24]. Nuclei under extreme stress can experience cracks and fissures, whereas healthy lamina networks deform relatively uniformly [24]. HGPS cells also show altered heterochromatin organization which leads to changes in cell senescence [104], telomere length [29], viability [115] and differentiation [101].
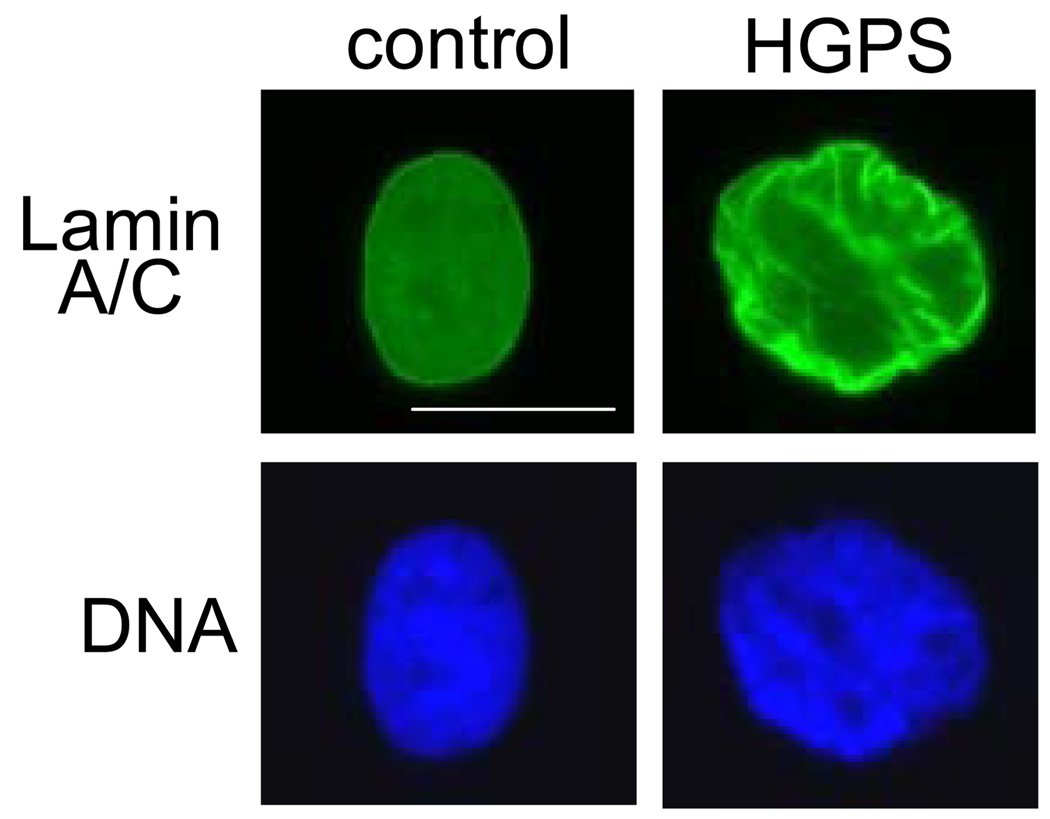
Figure 2. Lamin distribution and DNA in fibroblasts from HGPS patients.
Control primary fibroblasts, when fixed and labeled with an antibody for lamin A/C, show homogeneous distribution of lamins at the nuclear envelope with some lamins at the nuclear interior. The DNA, labeled with DAPI, is relatively evenly dispersed throughout the nucleus except for exclusion from the nucleoli. Cells from patients with HGPS (from the Progeria Research Foundation) at passage 12 begin to show redistribution of lamins to the nuclear membrane as well as an altered nuclear morphology. DNA is also redistributed heterogeneously throughout the nucleus. The scale bar is 20 µm.
6.2.3 Cellular
Although fibroblasts from HGPS patients have no apparent change in actin organization, the cells have decreased proliferation, motility and wound healing ability [115]. Fibroblasts from HGPS patients do not deform as readily as control fibroblasts due to the stiffened nucleus [115]. Cells expressing exogenous progerin are less able to adapt to stress by reorganizing proteins within the nucleus and reorganizing whole nuclear structures [96]. Also, nuclei of cells that are juxtaposed to progerin expressing cells do not show the normal response to shear stress. This suggests that only 30% expression of mutant protein [96] is sufficient to affect all cells in a flow field, possibly by altering the flow pattern and thereby shear stress of the microlayer.
7. Engineering tools in the microcirculation
7.1 Mechanical measurements of cellular and nuclear stiffness
There are a host of studies used to measure mechanical properties of cells and nuclei using hyper physiological strain and in situ methods. Many of these methods have been reviewed in detail [59]. Primarily they include (a) exerting large forces and watching the deformation over long time or (b) in situ particle tracking. Both have advantages and limitations, but combining mechanical measurements with visualization allows a better understanding of subcellular interactions. This may have important implications on tracking gene movements and reorganization in nuclei, allowing correlation of mechanics and gene expression [24].
For high force measurements, typically with compression (such as with AFM) or extension (such as with micropipette aspiration) large scale deformations can be observed and reorganizations can be mapped [23]. This technique has been used successfully in many nuclear [24] and cellular systems [53], and in some cases to determine mechanisms that may not have been visible under physiological levels of strain [24]. However, these large forces and strains may not necessarily represent the forces experienced by the cell or nucleus. In situ particle tracking allows for direct observation of mechanical properties in the native environment with the possibility of cellular manipulation and observation, and this technique has also been used successfully in nuclei [88] and cells [21]. However, drawbacks include the complications from measuring both the mechanical properties of a material and the ‘purposeful’ motion associated with molecular motors in a cell [68].
As described earlier, these techniques are/can be used before and after flow to determine changes in cellular [6] and nuclear [30] structure and mechanics related to blood flow on the vessel side.
7.2 Tracking the fluid flow and microcirculation
There is certainly more than one unique mechanical dynamic in the microcirculation. Blood flow provides a major mechanical-biological coupling since the blood provides shear stress which stimulates the endothelial layer (according to their mechanical properties), and blood perfusion is required for transport of nutrients, oxygen and waste removal [9]. Blood is a concentrated suspension of formed elements that includes erythrocytes (red blood cells, RBCs), leukocytes (white blood cells), and platelets. RBCs constitute around 45% of blood by volume. These cellular components are suspended in plasma, an aqueous solution that generally follows Newtonian fluid dynamics. In large vessels with internal diameter greater than 500 µm, blood may be modeled as a Newtonian fluid with a constant viscosity. However, in the microcirculation where vessel dimensions become comparable to cell diameters, blood behaves as a non-Newtonian fluid. In such vessels, the viscosity of blood depends on the vessel diameter [81]. Blood flow exhibits interesting and unique dynamics in microcirculation. The Fahraeus effect indicates that the hematocrit of blood in a narrow tube of less than 200 µm is lower than the discharge hematocrit exiting from the tube. This is due to the fact that RBCs move faster than the suspending medium in narrow tubes. The velocity difference originates from RBC migration away from the wall to the center of the tube, where velocities are higher. The ratio of particle velocity to suspending medium velocity depends on the ratio of particle diameter to tube diameter [128].
Topology of microvascular networks and branching patterns have been studied with several levels of idealization [8, 97], utilizing the variable morphometric and fractal approaches [107, 108] or from in vivo data [39, 99], resulting directional preference on flow induced loading. Cellular split ratios downstream of vessel braches are predicted based on pure fluid dynamics considerations [40, 41]. Physiological, disease specific adaptations and congenital malformations result complex topological arrangements either upstream or downstream of the pathological flow alteration [98, 100], such as micro arterio-arterial, veno-venous anastomoses and arterio-venous micro shunts in various tissue types [84, 110].
The migration of RBCs to the tube axis creates a cell-free plasma layer along the wall and a cell-rich central core and results in two other important blood flow anomalies; the Fahraeus–Lindqvist effect and plasma skimming. In microvessels, the cell-free layer along the wall (Fahraeus effect) is important in reducing the friction between RBCs and endothelial cells, thus reducing the flow resistance associated primarily with cell compliance and shape [58, 126, 127]. The Fahraeus–Lindqvist effect describes the decrease in apparent viscosity with decreasing micro-vessel diameter, up to a minimum diameter of ~29µm, attributed to RBC size [5]. Further decreasing vessel diameter increases apparent viscosity because the tube becomes smaller than the blood cells [33, 128]. Consequently, at individual branch points in the network, the cell-free plasma layer along the wall in the mother vessel leads to an uneven distribution of cell and plasma flow into the two daughter branches. In general, larger daughter vessels with higher volume flow exhibit increased hematocrit. For very uneven flow distributions, small vessels may even be perfused by plasma without any red cells (plasma skimming) [128].
One of the most important properties of blood is the ease with which it flows through the microvasculature. The theoretical value of the wall shear stress (WSS) distribution may be calculated by simulating the flow in the vessels (see below). If the vessel is large enough, the blood may be considered as Newtonian fluid (with constant viscosity) and the procedure is straightforward. On the other hand, in the microcirculation, flow resistance is often described in terms of ”apparent viscosity” and ”relative viscosity”, which relate blood flow to the Newtonian fluid (i.e., the plasma). However, the apparent and relative viscosities are not intrinsic properties of the blood; both vary with hematocrit, RBC aggregation state, and vessel geometry.
Allowing that it is tolerable to “average” the properties of blood for a continuum description of the flow profile in the microcirculation, there are still significant biological interactions to be considered between blood cells and endothelial cells in vessels of diameter 10–500 µm [81]. In this case, the size of the RBCs is comparable to the size of the vessels, and hence each cell must be taken into consideration in the modeling. This is a more sophisticated problem since deformation of the RBC membrane alters the fluid viscosity. Hence, the cell-fluid interaction should be considered as well [82]. In this case, the internal fluid of the cells must be taken into account along with the external fluid, the plasma, while membrane of the cells is the moving boundaries. RBCs surface deformations directly influence the WSS [82].
Four-dimensional image processing and image augmentation technologies have been developed to examine flow in model microcirculations using the interpolation of sliced confocal data [38]. This can be approximated by microscopic imaging of cellular scale flow fields induced by high hematocrit blood flowing through 300 µm square micro channels, Figure 3. In these tubes, the solid-like components of the blood, at high hematocrit, can begin to be approximated as more than an empirical factor. Increasingly sophisticated computational fluid dynamics (CFD) models that simulate 3D interactions of crowded blood cell clusters have been proposed recently by several research groups [2, 3, 56].
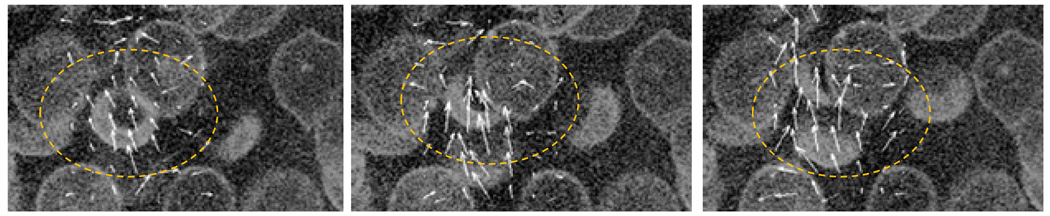
Figure 3. Imaging of single RBC trajectories in a model microcirculatory device at high hematocrit.
RBCs are labeled with a membrane stain and measured using confocal microscopy. Time-lapsed extracellular velocity fields (white vectors) are produced from particle image velocimetry analysis and show the movements induced by a single RBC as it passes through a cell cluster.
7.3 Simulating the microcirculation
Shear stress has long been studied or simulated in large arteries since Newtonian fluids and well developed fluid profiles could be used. Now, capillaries and microcirculation can be simulated to match in vitro experiments and knowledge gained from systems of larger vessels and experiments can be imaged at the microvascular level using more sophisticated technologies. Smaller regions can be studied and the inclusion of cellular components such as red blood cells can be included, such as in Figure 3.
The flow and shear stress fields can be computed both under unsteady conditions and averaged over the cardiac cycle. Flow field derived parameters include; wall shear stress (WSS), pressure, WSS gradients, WSS Angle Deviation, oscillating shear index, secondary flows, flow invariants defining coherent structures and vorticity. In addition the morphological wall shear stress sensitivity parameters [91], typically computed from two perturbed simulations, to identify the spatial regions on the cell surface that are more sensitive to local changes in mechanical loading. Comparison of 3D simulations with subject-specific biological data is not straightforward and various approaches are proposed in bio-fluids literature (Friedman, Himburg et al. 2006).
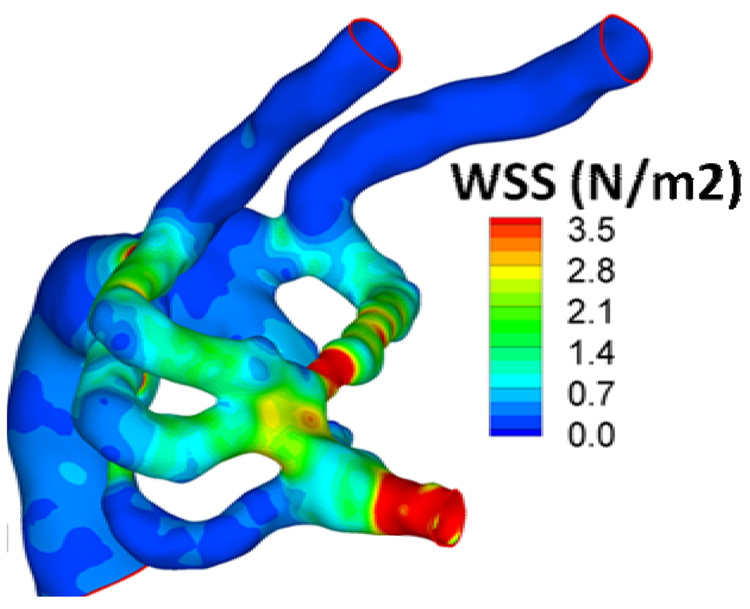
Flow-induced mechanical loading in complex cardiovascular domains at multiple geometrical scales is becoming possible with the help of high-resolved computational fluid dynamics tools developed specifically for pulsatile and unsteady hemodynamic conditions [92, 93]. Complex formulations such as the 3D pulsatile wall shear stress (WSS) distribution in early embryonic aortic arches (~40 µm diameter) are possible with the help of simulation (Figure 4 and [117]). The results allowed association of flow-induced mechanical loading (primarily WSS) at individual aortic arches to the in vivo observed prognosis of congenital aortic arch and great vessel malformations observed in vivo. Using computational fluid dynamics temporal morphological recovery from an abnormal WSS stateis possible and should be correlated with in vitro experiments.
Figure 4. Computational fluid dynamics results at the micro-scale.
Increasingly sophisticated techniques allow the simulation of complex fluid profiles and complex geometries within the microcirculation. Here, average wall shear stress (WSS; N/m2) at the mid-acceleration phase of the cardiac cycle of early embryonic aortic arch is shown for one simulation. The color map at the right shows the intensity gradients reflecting the diversity of shear stresses within one region.
8. Moving toward advancing clinical practice and patient care with regard to HGPS/aging and the microcirculation
The real questions remain, how can the study into nuclear and cellular stiffness in response to shear stress, particle tracking of blood cells combined with mathematical simulation help patients? Many traditional studies of changes in vessel stiffness during aging are related to the adventita and the collagen and elastin fibers of the extracellular matrix [105]. The microcirculation is largely ignored since the effects of cellular two-phase compliant flow make modeling more complex, in spite of the reported comparable wall shear stress values to the large artery flow [67]. Here, particularly with the HGPS pathology where the cell stiffness and mechanical response to shear stress appears to be altered, it is possible that mechanics and structure (rough endothelial layer versus smooth) could play a role in smaller vessels as well, particularly given the intimate associations with endothelium and RBCs.
If blood is excluded from regions of the microcirculation due to the stiffness or roughness of the endothelium there are huge implications for patient health. With the development of integrated clinical medicine, science and engineering tools we can begin to explore the role of endothelial response to stress in the formation of new blood vessels, for example. From this review, one hypothetical to consider would be that in effectively lowering the hematocrit of patients with HGPS or an age-related analog, one could see increased effective shear flow and possibly increased RBC access to regions of the microcirculation. This may allow regions of angiogenic blood-vessel formation to thrive [76] and may aid in tissue growth, repair and maintenance.
Acknowledgements
The authors acknowledge funding from the Progeria Research Foundation (to KND), the NIH (NRSA F30AG030905 from the National Institute On Aging to AK) and American Heart Association (BGIA 0765284U to KP). Thanks to the multiphoton confocal facility (DMR-0619424) and Michael J Patrick of MBIC/CMU for RBC labeling and assisting with confocal experiments.
References
- 1.Adelfalk C, Scherthan H, Hirsch-Kauffmann M, Schweiger M. Nuclear deformation characterizes Werner syndrome cells. Cell Biol Int. 2005;29:1032–1037. doi: 10.1016/j.cellbi.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 2.AlMomani T, Udaykumar HS, Marshall JS, Chandran KB. Micro-scale dynamic simulation of erythrocyte-platelet interaction in blood flow. Ann Biomed Eng. 2008;36:905–920. doi: 10.1007/s10439-008-9478-z. [DOI] [PubMed] [Google Scholar]
- 3.Bagchi P, Johnson PC, Popel AS. Computational fluid dynamic simulation of aggregation of deformable cells in a shear flow. J Biomech Eng. 2005;127:1070–1080. doi: 10.1115/1.2112907. [DOI] [PubMed] [Google Scholar]
- 4.Barakat AI, Leaver EV, Pappone PA, Davies PF. A flow-activated chloride-selective membrane current in vascular endothelial cells. Circ Res. 1999;85:820–828. doi: 10.1161/01.res.85.9.820. [DOI] [PubMed] [Google Scholar]
- 5.Barbee JH, Cokelet GR. Prediction of blood flow in tubes with diameters as small as 29 microns. Microvasc Res. 1971;3:17–21. doi: 10.1016/0026-2862(71)90003-3. [DOI] [PubMed] [Google Scholar]
- 6.Barbee KA. Changes in surface topography in endothelial monolayers with time at confluence: influence on subcellular shear stress distribution due to flow. Biochem Cell Biol. 1995;73:501–505. doi: 10.1139/o95-055. [DOI] [PubMed] [Google Scholar]
- 7.Barcelo F, Prades J, Encinar JA, Funari SS, Vogler O, et al. Interaction of the C-terminal region of the Ggamma protein with model membranes. Biophys. J. 2007;93:2530–2541. doi: 10.1529/biophysj.106.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassingthwaighte JB, Van Beek JH, King RB. Fractal branchings: the basis of myocardial flow heterogeneities? Ann N Y Acad Sci. 1990;591:392–401. doi: 10.1111/j.1749-6632.1990.tb15103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boisseau MR. Roles of mechanical blood forces in vascular diseases. A clinical overview. Clin Hemorheol Microcirc. 2005;33:201–207. [PubMed] [Google Scholar]
- 10.Broers JL, Machiels BM, van Eys GJ, Kuijpers HJ, Manders EM, et al. Dynamics of the nuclear lamina as monitored by GFP-tagged A-type lamins. J Cell Sci. 1999;112:3463–3475. doi: 10.1242/jcs.112.20.3463. [DOI] [PubMed] [Google Scholar]
- 11.Brooks AR, Lelkes PI, Rubanyi GM. Gene expression profiling of vascular endothelial cells exposed to fluid mechanical forces: relevance for focal susceptibility to atherosclerosis. Endothelium. 2004;11:45–57. doi: 10.1080/10623320490432470. [DOI] [PubMed] [Google Scholar]
- 12.Caille N, Thoumine O, Tardy Y, Meister JJ. Contribution of the nucleus to the mechanical properties of endothelial cells. J Biomech. 2002;35:177–187. doi: 10.1016/s0021-9290(01)00201-9. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P, Collen D. Role of vascular endothelial growth factor and vascular endothelial growth factor receptors in vascular development. Curr Top Microbiol Immunol. 1999;237:133–158. doi: 10.1007/978-3-642-59953-8_7. [DOI] [PubMed] [Google Scholar]
- 14.Casey PJ. Protein lipidation in cell signaling. Science. 1995;268:221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- 15.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 16.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–H1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 17.Chiu JJ, Chen LJ, Chang SF, Lee PL, Lee CI, et al. Shear stress inhibits smooth muscle cell-induced inflammatory gene expression in endothelial cells: role of NF-kappaB. Arterioscler Thromb Vasc Biol. 2005;25:963–969. doi: 10.1161/01.ATV.0000159703.43374.19. [DOI] [PubMed] [Google Scholar]
- 18.Chow M, Der CJ, Buss JE. Structure and biological effects of lipid modifications on proteins. Curr. Opin. Cell Biol. 1992;4:629–636. doi: 10.1016/0955-0674(92)90082-n. [DOI] [PubMed] [Google Scholar]
- 19.Corrigan DP, Kuszczak D, Rusinol AE, Thewke DP, Hrycyna CA, et al. Prelamin A endoproteolytic processing in vitro by recombinant Zmpste24. Biochem. J. 2005;387:129–138. doi: 10.1042/BJ20041359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox AD, Der CJ. Protein prenylation: more than just glue? Curr Opin Cell Biol. 1992;4:1008–1016. doi: 10.1016/0955-0674(92)90133-w. [DOI] [PubMed] [Google Scholar]
- 21.Crocker JC, Hoffman BD. Multiple-particle tracking and two-point microrheology in cells. Methods Cell Biol. 2007;83:141–178. doi: 10.1016/S0091-679X(07)83007-X. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85:9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 23.Dahl KN, Engler AJ, Pajerowski JD, Discher DE. Power-law rheology of isolated nuclei with deformation mapping of nuclear substructures. Biophys. J. 2005;89:2855–2864. doi: 10.1529/biophysj.105.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahl KN, Kahn SM, Wilson KL, Discher DE. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J Cell Sci. 2004;117:4779–4786. doi: 10.1242/jcs.01357. [DOI] [PubMed] [Google Scholar]
- 25.Dahl KN, Scaffidi P, Islam MF, Yodh AG, Wilson KL, et al. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2006;103:10271–10276. doi: 10.1073/pnas.0601058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies PF, Zilberberg J, Helmke BP. Spatial microstimuli in endothelial mechanosignaling. Circ. Res. 2003;92:359–370. doi: 10.1161/01.RES.0000060201.41923.88. [DOI] [PubMed] [Google Scholar]
- 27.De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125. [DOI] [PubMed] [Google Scholar]
- 28.Dechat T, Shimi T, Adam SA, Rusinol AE, Andres DA, et al. Alterations in mitosis and cell cycle progression caused by a mutant lamin A known to accelerate human aging. Proc. Natl. Acad. Sci. USA. 2007;104:4955–4960. doi: 10.1073/pnas.0700854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Decker ML, Chavez E, Vulto I, Lansdorp PM. Telomere length in Hutchinson-Gilford progeria syndrome. Mech Ageing Dev. 2009;130:377–383. doi: 10.1016/j.mad.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Deguchi S, Maeda K, Ohashi T, Sato M. Flow-induced hardening of endothelial nucleus as an intracellular stress-bearing organelle. J Biomech. 2005;38:1751–1759. doi: 10.1016/j.jbiomech.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 31.DePaola N, Gimbrone MA, Jr, Davies PF, Dewey CF., Jr Vascular endothelium responds to fluid shear stress gradients. Arterioscler Thromb. 1992;12:1254–1257. doi: 10.1161/01.atv.12.11.1254. [DOI] [PubMed] [Google Scholar]
- 32.Dundr M, Misteli T. Functional architecture in the cell nucleus. Biochem J. 2001;356:297–310. doi: 10.1042/0264-6021:3560297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dupin MM, Halliday I, Care CM, Alboul L, Munn LL. Modeling the flow of dense suspensions of deformable particles in three dimensions. Phys Rev E Stat Nonlin Soft Matter Phys. 2007;75 doi: 10.1103/PhysRevE.75.066707. 066707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epand RF, Xue CB, Wang SH, Naider F, Becker JM, et al. Role of prenylation in the interaction of the a-factor mating pheromone with phospholipid bilayers. Biochemistry. 1993;32:8368–8373. doi: 10.1021/bi00083a041. [DOI] [PubMed] [Google Scholar]
- 35.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escriba PV, Ozaita A, Ribas C, Miralles A, Fodor E, et al. Role of lipid polymorphism in G protein-membrane interactions: nonlamellar-prone phospholipids and peripheral protein binding to membranes. Proc Natl Acad Sci U S A. 1997;94:11375–11380. doi: 10.1073/pnas.94.21.11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 38.Frakes DH, Dasi LP, Pekkan K, Kitajima HD, Sundareswaran K, et al. A new method for registration-based medical image interpolation. IEEE Trans. Med. Imaging. 2008;27:370–377. doi: 10.1109/TMI.2007.907324. [DOI] [PubMed] [Google Scholar]
- 39.Frasher WG, Jr, Wayland H. A repeating modular organization of the microcirculation of cat mesentery. Microvasc Res. 1972;4:62–76. doi: 10.1016/0026-2862(72)90017-9. [DOI] [PubMed] [Google Scholar]
- 40.Fung YC. Stochastic flow in capillary blood vessels. Microvasc Res. 1973;5:34–48. doi: 10.1016/s0026-2862(73)80005-6. [DOI] [PubMed] [Google Scholar]
- 41.Fung YC. 1975 Eugene M. Landis Award Lecture. Microcirculation as seen by a red cell. Microvasc Res. 1975;10:246–264. doi: 10.1016/0026-2862(75)90029-1. [DOI] [PubMed] [Google Scholar]
- 42.Gant TM, Wilson KL. Nuclear assembly. Annu. Rev. Cell. Dev. Biol. 1997;13:669–695. doi: 10.1146/annurev.cellbio.13.1.669. [DOI] [PubMed] [Google Scholar]
- 43.Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP. Nuclear lamins: building blocks of nuclear architecture. Genes Dev. 2002;16:533–547. doi: 10.1101/gad.960502. [DOI] [PubMed] [Google Scholar]
- 44.Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA. 2004;101:8963–8968. doi: 10.1073/pnas.0402943101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL. The nuclear lamina comes of age. Nat. Rev. Mol. Cell Biol. 2005;6:21–31. doi: 10.1038/nrm1550. [DOI] [PubMed] [Google Scholar]
- 46.Guilak F, Tedrow JR, Burgkart R. Viscoelastic properties of the cell nucleus. Biochem. Biophys. Res. Commun. 2000;269:781–786. doi: 10.1006/bbrc.2000.2360. [DOI] [PubMed] [Google Scholar]
- 47.Haithcock E, Dayani Y, Neufeld E, Zahand AJ, Feinstein N, et al. Age-related changes of nuclear architecture in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2005:16690–16695. doi: 10.1073/pnas.0506955102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hancock R. A new look at the nuclear matrix. Chromosoma. 2000;109:219–225. doi: 10.1007/s004120000077. [DOI] [PubMed] [Google Scholar]
- 49.Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J. Cell Sci. 2001;114:4557–4565. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- 50.Hazel AL, Pedley TJ. Vascular endothelial cells minimize the total force on their nuclei. Biophys J. 2000;78:47–54. doi: 10.1016/S0006-3495(00)76571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herrmann H, Foisner R. Intermediate filaments: novel assembly models and exciting new functions for nuclear lamins. Cell Mol Life Sci. 2003;60:1607–1612. doi: 10.1007/s00018-003-3004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hierck BP, Van der Heiden K, Poelma C, Westerweel J, Poelmann RE. Fluid shear stress and inner curvature remodeling of the embryonic heart. Choosing the right lane! ScientificWorldJournal. 2008;8:212–222. doi: 10.1100/tsw.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hochmuth RM. Micropipette aspiration of living cells. J Biomech. 2000;33:15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- 54.Hofemeister H, Weber K, Stick R. Association of prenylated proteins with the plasma membrane and the inner nuclear membrane is mediated by the same membrane-targeting motifs. Mol. Biol. Cell. 2000;11:3233–3246. doi: 10.1091/mbc.11.9.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holtz D, Tanaka RA, Hartwig J, McKeon F. The CaaX motif of lamin A functions in conjunction with the nuclear localization signal to target assembly to the nuclear envelope. Cell. 1989;59:969–977. doi: 10.1016/0092-8674(89)90753-8. [DOI] [PubMed] [Google Scholar]
- 56.Jung J, Lyczkowski RW, Panchal CB, Hassanein A. Multiphase hemodynamic simulation of pulsatile flow in a coronary artery. J Biomech. 2006;39:2064–2073. doi: 10.1016/j.jbiomech.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 57.Kesner BA, Ding F, Temple BR, Dokholyan NV. N-terminal strands of filamin Ig domains act as a conformational switch under biological forces. Proteins. 2009 doi: 10.1002/prot.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kimura H, Tao Y, Roeder RG, Cook PR. Quantitation of RNA polymerase II and its transcription factors in an HeLa cell: little soluble holoenzyme but significant amounts of polymerases attached to the nuclear substructure. Mol Cell Biol. 1999;19:5383–5392. doi: 10.1128/mcb.19.8.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lammerding J, Dahl KN, Discher DE, Kamm RD. Nuclear mechanics and methods. Methods Cell Biol. 2007;83:269–294. doi: 10.1016/S0091-679X(07)83011-1. [DOI] [PubMed] [Google Scholar]
- 60.Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, et al. Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem. 2006;281:25768–25780. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- 61.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lattanzi G, Cenni V, Marmiroli S, Capanni C, Mattioli E, et al. Association of emerin with nuclear and cytoplasmic actin is regulated in differentiating myoblasts. Biochem Biophys Res Commun. 2003;303:764–770. doi: 10.1016/s0006-291x(03)00415-7. [DOI] [PubMed] [Google Scholar]
- 64.Lee JS, Hale CM, Panorchan P, Khatau SB, George JP, et al. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys. J. 2007;93:2542–2552. doi: 10.1529/biophysj.106.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med. 2006;259:381–392. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- 66.Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem. 1993;268:16321–16326. [PubMed] [Google Scholar]
- 67.Lipowsky HH. Microvascular rheology and hemodynamics. Microcirculation. 2005;12:5–15. doi: 10.1080/10739680590894966. [DOI] [PubMed] [Google Scholar]
- 68.Liu J, Gardel ML, Kroy K, Frey E, Hoffman BD, et al. Microrheology probes length scale dependent rheology. Phys Rev Lett. 2006;96:118104. doi: 10.1103/PhysRevLett.96.118104. [DOI] [PubMed] [Google Scholar]
- 69.Lutz RJ, Trujillo MA, Denham KS, Wenger L, Sinensky M. Nucleoplasmic localization of prelamin A: implications for prenylation-dependent lamin A assembly into the nuclear lamina. Proc. Natl. Acad. Sci. USA. 1992;89:3000–3004. doi: 10.1073/pnas.89.7.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maniotis AJ, Bojanowski K, Ingber DE. Mechanical continuity and reversible chromosome disassembly within intact genomes removed from living cells. J Cell Biochem. 1997;65:114–130. [PubMed] [Google Scholar]
- 71.Marshall CJ. Protein prenylation: a mediator of protein-protein interactions. Science. 1993;259:1865–1866. doi: 10.1126/science.8456312. [DOI] [PubMed] [Google Scholar]
- 72.Maske CP, Hollinshead MS, Higbee NC, Bergo MO, Young SG, et al. A carboxyl-terminal interaction of lamin B1 is dependent on the CAAX endoprotease Rce1 and carboxymethylation. J. Cell Biol. 2003;162:1223–1232. doi: 10.1083/jcb.200303113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McClintock D, Ratner D, Lokuge M, Owens DM, Gordon LB, et al. The Mutant Form of Lamin A that Causes Hutchinson-Gilford Progeria Is a Biomarker of Cellular Aging in Human Skin. PLoS ONE. 2007;2:e1269. doi: 10.1371/journal.pone.0001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCue S, Noria S, Langille BL. Shear-induced reorganization of endothelial cell cytoskeleton and adhesion complexes. Trends Cardiovasc Med. 2004;14:143–151. doi: 10.1016/j.tcm.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 75.Meaburn KJ, Misteli T. Cell biology: chromosome territories. Nature. 2007;445:379–781. doi: 10.1038/445379a. [DOI] [PubMed] [Google Scholar]
- 76.Meeson A, Palmer M, Calfon M, Lang R. A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development. 1996;122:3929–3938. doi: 10.1242/dev.122.12.3929. [DOI] [PubMed] [Google Scholar]
- 77.Moir RD, Spann TP, Goldman RD. The dynamic properties and possible functions of nuclear lamins. Int Rev Cytol. 1995;162B:141–182. doi: 10.1016/s0074-7696(08)62616-9. [DOI] [PubMed] [Google Scholar]
- 78.Morris GE, Manilal S. Heart to heart: from nuclear proteins to Emery-Dreifuss muscular dystrophy. Hum. Mol. Genet. 1999;8:1847–1851. doi: 10.1093/hmg/8.10.1847. [DOI] [PubMed] [Google Scholar]
- 79.Moss SF, Krivosheyev V, de Souza A, Chin K, Gaetz HP, et al. Decreased and aberrant nuclear lamin expression in gastrointestinal tract neoplasms. Gut. 1999;45:723–729. doi: 10.1136/gut.45.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mounkes LC, Burke B, Stewart CL. The a-type lamins. Nuclear structural proteins as a focus for muscular dystrophy and cardiovascular diseases. Trends Cardiovasc Med. 2001;11:280–285. doi: 10.1016/s1050-1738(01)00126-8. [DOI] [PubMed] [Google Scholar]
- 81.Nesheim ME, Nesheim FJ. An experimental and mathematical model for fibrinolysis. FASEB Journal. 1988;2:A1412. [Google Scholar]
- 82.Nesheim ME, Tracy RP, Mann KG. "Clotspeed," a mathematical simulation of the functional properties of prothrombinase. J Biol Chem. 1984;259:1447–1453. [PubMed] [Google Scholar]
- 83.Newport JW, Wilson KL, Dunphy WG. A lamin-independent pathway for nuclear envelope assembly. J Cell Biol. 1990;111:2247–2259. doi: 10.1083/jcb.111.6.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ninomiya H, Inomata T. Microvascular anatomy of the pig eye: scanning electron microscopy of vascular corrosion casts. J Vet Med Sci. 2006;68:1149–1154. doi: 10.1292/jvms.68.1149. [DOI] [PubMed] [Google Scholar]
- 85.Noria S, Xu F, McCue S, Jones M, Gotlieb AI, et al. Assembly and reorientation of stress fibers drives morphological changes to endothelial cells exposed to shear stress. Am J Pathol. 2004;164:1211–1223. doi: 10.1016/S0002-9440(10)63209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ohura N, Yamamoto K, Ichioka S, Sokabe T, Nakatsuka H, et al. Global analysis of shear stress-responsive genes in vascular endothelial cells. J Atheroscler Thromb. 2003;10:304–313. doi: 10.5551/jat.10.304. [DOI] [PubMed] [Google Scholar]
- 87.Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- 88.Panorchan P, Schafer BW, Wirtz D, Tseng Y. Nuclear envelope breakdown requires overcoming the mechanical integrity of the nuclear lamina. J. Biol. Chem. 2004;279:43462–43467. doi: 10.1074/jbc.M402474200. [DOI] [PubMed] [Google Scholar]
- 89.Papadaki M, Eskin SG. Effects of fluid shear stress on gene regulation of vascular cells. Biotechnol. Prog. 1997;13:209–221. doi: 10.1021/bp970029f. [DOI] [PubMed] [Google Scholar]
- 90.Parnaik VK, Manju K. Laminopathies: multiple disorders arising from defects in nuclear architecture. J Biosci. 2006;31:405–421. doi: 10.1007/BF02704113. [DOI] [PubMed] [Google Scholar]
- 91.Pekkan K, Dasi L, Dur O, Keller B, Fogel M, et al. In vitro hemodynamics of embryonic heart with Tetralogy of Fallot at late gestation; Fifth International Caltech 27 Bio-Fluid Symposium and Workshop; Pasadena, California: California Institute of Technology; 2008. [Google Scholar]
- 92.Pekkan K, de Zelicourt D, Ge L, Sotiropoulos F, Frakes D, et al. Physics-driven CFD modeling of complex anatomical cardiovascular flows-a TCPC case study. Ann Biomed Eng. 2005;33:284–300. doi: 10.1007/s10439-005-1731-0. [DOI] [PubMed] [Google Scholar]
- 93.Pekkan K, Dur O, Sundareswaran K, Kanter K, Fogel M, et al. Neonatal aortic arch hemodynamics and perfusion during cardiopulmonary bypass. J. Biomech. Eng. 2008;130:061012. doi: 10.1115/1.2978988. [DOI] [PubMed] [Google Scholar]
- 94.Peter M, Heitlinger E, Haner M, Aebi U, Nigg EA, et al. Disassembly of in vitro formed lamin head-to-tail polymers by CDC2 kinase. Embo J. 1991;10:1535–1544. doi: 10.1002/j.1460-2075.1991.tb07673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Petzold T, Orr AW, Hahn C, Jhaveri K, Parsons JT, et al. Focal adhesion kinase modulates activation of NF-{kappa}B by flow in endothelial cells. Am J Physiol Cell Physiol. 2009 doi: 10.1152/ajpcell.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Philip JT, Dahl KN. Nuclear mechanotransduction: response of the lamina to extracellular stress with implications in aging. J. Biomech. 2008;41:3164–3170. doi: 10.1016/j.jbiomech.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 97.Pozrikidis C. Numerical simulation of blood flow through microvascular capillary networks. Bull Math Biol. 2009;71:1520–1541. doi: 10.1007/s11538-009-9412-z. [DOI] [PubMed] [Google Scholar]
- 98.Pries AR, Reglin B, Secomb TW. Structural adaptation of microvascular networks: functional roles of adaptive responses. Am J Physiol Heart Circ Physiol. 2001;281:H1015–H1025. doi: 10.1152/ajpheart.2001.281.3.H1015. [DOI] [PubMed] [Google Scholar]
- 99.Pries AR, Secomb TW, Gaehtgens P. Design principles of vascular beds. Circ Res. 1995;77:1017–1023. doi: 10.1161/01.res.77.5.1017. [DOI] [PubMed] [Google Scholar]
- 100.Quick CM, Hashimoto T, Young WL. Lack of flow regulation may explain the development of arteriovenous malformations. Neurol Res. 2001;23:641–644. doi: 10.1179/016164101101198938. [DOI] [PubMed] [Google Scholar]
- 101.Scaffidi P, Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol. 2008 doi: 10.1038/ncb1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Segura-Totten M, Kowalski AK, Craigie R, Wilson KL. Barrier-to-autointegration factor: major roles in chromatin decondensation and nuclear assembly. J Cell Biol. 2002;158:475–485. doi: 10.1083/jcb.200202019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shiu YT, Li S, Yuan S, Wang Y, Nguyen P, et al. Shear stress-induced c-fos activation is mediated by Rho in a calcium-dependent manner. Biochem Biophys Res Commun. 2003;303:548–555. doi: 10.1016/s0006-291x(03)00388-7. [DOI] [PubMed] [Google Scholar]
- 104.Shumaker DK, Dechat T, Kohlmaier A, Adam SA, Bozovsky MR, et al. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc. Natl. Acad. Sci. USA. 2006;103:8703–8708. doi: 10.1073/pnas.0602569103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Silver FH, Horvath I, Foran DJ. Viscoelasticity of the vessel wall: the role of collagen and elastic fibers. Crit Rev Biomed Eng. 2001;29:279–301. doi: 10.1615/critrevbiomedeng.v29.i3.10. [DOI] [PubMed] [Google Scholar]
- 106.Sinensky M, Fantle K, Trujillo M, McLain T, Kupfer A, et al. The processing pathway of prelamin A. J Cell Sci. 1994;107(Pt 1):61–67. doi: 10.1242/jcs.107.1.61. [DOI] [PubMed] [Google Scholar]
- 107.Spilker RL, Feinstein JA, Parker DW, Reddy VM, Taylor CA. Morphometry-based impedance boundary conditions for patient-specific modeling of blood flow in pulmonary arteries. Ann Biomed Eng. 2007;35:546–559. doi: 10.1007/s10439-006-9240-3. [DOI] [PubMed] [Google Scholar]
- 108.Steele BN, Wan J, Ku JP, Hughes TJ, Taylor CA. In vivo validation of a one-dimensional finite-element method for predicting blood flow in cardiovascular bypass grafts. IEEE Trans Biomed Eng. 2003;50:649–656. doi: 10.1109/TBME.2003.812201. [DOI] [PubMed] [Google Scholar]
- 109.Stierle V, Couprie J, Ostlund C, Krimm I, Zinn-Justin S, et al. The carboxyl-terminal region common to lamins A and C contains a DNA binding domain. Biochemistry. 2003;42:4819–4828. doi: 10.1021/bi020704g. [DOI] [PubMed] [Google Scholar]
- 110.Syed Ali S. Angioarchitecture of the pancreas of the cat. Light-, scanning- and transmission electron microscopy. Cell Tissue Res. 1984;235:675–682. doi: 10.1007/BF00226968. [DOI] [PubMed] [Google Scholar]
- 111.Tarbell JM, Weinbaum S, Kamm RD. Cellular fluid mechanics and mechanotransduction. Ann Biomed Eng. 2005;33:1719–1723. doi: 10.1007/s10439-005-8775-z. [DOI] [PubMed] [Google Scholar]
- 112.Traub O, Ishida T, Ishida M, Tupper JC, Berk BC. Shear stress-mediated extracellular signal-regulated kinase activation is regulated by sodium in endothelial cells. Potential role for a voltage-dependent sodium channel. J Biol Chem. 1999;274:20144–20150. doi: 10.1074/jbc.274.29.20144. [DOI] [PubMed] [Google Scholar]
- 113.van den Berg BM, Nieuwdorp M, Stroes ES, Vink H. Glycocalyx and endothelial (dys) function: from mice to men. Pharmacol Rep. 2006;58 Suppl:75–80. [PubMed] [Google Scholar]
- 114.Varga R, Eriksson M, Erdos MR, Olive M, Harten I, et al. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA. 2006;103:3250–3255. doi: 10.1073/pnas.0600012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Verstraeten VL, Ji JY, Cummings KS, Lee RT, Lammerding J. Increased mechanosensitivity and nuclear stiffness in Hutchinson-Gilford progeria cells: effects of farnesyltransferase inhibitors. Aging Cell. 2008;7:383–393. doi: 10.1111/j.1474-9726.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Y, Chang J, Chen KD, Li S, Li JY, et al. Selective adapter recruitment and differential signaling networks by VEGF vs. shear stress. Proc Natl Acad Sci U S A. 2007;104:8875–8879. doi: 10.1073/pnas.0703088104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y, Dur O, Patrick MJ, Tinney JP, Tobita K, et al. Aortic arch morphogenesis and flow modeling in the chick embryo. Ann Biomed Eng. 2009;37:1069–1081. doi: 10.1007/s10439-009-9682-5. [DOI] [PubMed] [Google Scholar]
- 118.Wasserman SM, Topper JN. Adaptation of the endothelium to fluid flow: in vitro analyses of gene expression and in vivo implications. Vasc Med. 2004;9:35–45. doi: 10.1191/1358863x04vm521ra. [DOI] [PubMed] [Google Scholar]
- 119.Weber K, Plessmann U, Traub P. Maturation of nuclear lamin A involves a specific carboxy-terminal trimming, which removes the polyisoprenylation site from the precursor; implications for the structure of the nuclear lamina. FEBS Lett. 1989;257:411–414. doi: 10.1016/0014-5793(89)81584-4. [DOI] [PubMed] [Google Scholar]
- 120.White CR, Frangos JA. The shear stress of it all: the cell membrane and mechanochemical transduction. Philos Trans R Soc Lond B Biol Sci. 2007;362:1459–1467. doi: 10.1098/rstb.2007.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilson KL, Zastrow MS, Lee KK. Lamins and disease: insights into nuclear infrastructure. Cell. 2001;104:647–650. [PubMed] [Google Scholar]
- 122.Wu CC, Li YS, Haga JH, Kaunas R, Chiu JJ, et al. Directional shear flow and Rho activation prevent the endothelial cell apoptosis induced by micropatterned anisotropic geometry. Proc Natl Acad Sci U S A. 2007;104:1254–1259. doi: 10.1073/pnas.0609806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wydner KL, McNeil JA, Lin F, Worman HJ, Lawrence JB. Chromosomal assignment of human nuclear envelope protein genes LMNA, LMNB1, and LBR by fluorescence in situ hybridization. Genomics. 1996;32:474–478. doi: 10.1006/geno.1996.0146. [DOI] [PubMed] [Google Scholar]
- 124.Young KG, Kothary R. Spectrin repeat proteins in the nucleus. Bioessays. 2005;27:144–152. doi: 10.1002/bies.20177. [DOI] [PubMed] [Google Scholar]
- 125.Zhang C, Clarke PR. Chromatin-independent nuclear envelope assembly induced by Ran GTPase in Xenopus egg extracts. Science. 2000;288:1429–1432. doi: 10.1126/science.288.5470.1429. [DOI] [PubMed] [Google Scholar]
- 126.Zhao R, Antaki JF, Naik T, Bachman TN, Kameneva MV, et al. Microscopic investigation of erythrocyte deformation dynamics. Biorheology. 2006;43:747–765. [PubMed] [Google Scholar]
- 127.Zhao R, Kameneva MV, Antaki JF. Investigation of platelet margination phenomena at elevated shear stress. Biorheology. 2007;44:161–177. [PubMed] [Google Scholar]
- 128.Zhao R, Marhefka JN, Shu F, Hund SJ, Kameneva MV, et al. Micro-flow visualization of red blood cell-enhanced platelet concentration at sudden expansion. Ann Biomed Eng. 2008;36:1130–1141. doi: 10.1007/s10439-008-9494-z. [DOI] [PMC free article] [PubMed] [Google Scholar]