Abstract
Background
Peripheral arterial disease (PAD) caused by occlusive atherosclerosis of the lower extremity has 2 major clinical manifestations. Critical limb ischemia is characterized by rest pain and/or tissue loss and has a ≥40% risk of death and major amputation. Intermittent claudication causes pain on walking, has no tissue loss, and has amputation plus mortality rates of 2% to 4% per year. Progression from claudication to limb ischemia is infrequent. Risk factors in most PAD patients overlap. Thus, we hypothesized that genetic variations may be linked to presence or absence of tissue loss in PAD.
Methods and Results
Hindlimb ischemia (murine model of PAD) was induced in C57BL/6, BALB/c, C57BL/6×BALB/c (F1), F1×BALB/c (N2), A/J, and C57BL/6J-Chr7A/J/NaJ chromosome substitution strains. Mice were monitored for perfusion recovery and tissue necrosis. Genome-wide scanning with polymorphic markers across the 19 murine autosomes was performed on the N2 mice. Greater tissue loss and poorer perfusion recovery occurred in BALB/c than in the C57BL/6 strain. Analysis of 105 N2 progeny identified a single quantitative trait locus on chromosome 7 that exhibited significant linkage to both tissue necrosis and extent of perfusion recovery. Using the appropriate chromosome substitution strain, we demonstrate that C57BL/6-derived chromosome 7 is required for tissue preservation.
Conclusions
We have identified a quantitative trait locus on murine chromosome 7 (LSq-1) that is associated with the absence of tissue loss in a preclinical model of PAD and may be useful in identifying gene(s) that influence PAD in humans.
Keywords: atherosclerosis, genetics, peripheral vascular disease
Peripheral arterial disease (PAD) is caused by atherosclerosis in arterial beds other than the coronary arteries. The lower extremity is the most common site for PAD, where atherosclerotic occlusive disease impairs perfusion to the leg(s). The 2 major clinical manifestations of lower-extremity PAD are (1) intermittent claudication (IC), which is defined as leg pain during exercise that is relieved by rest and the absence of either rest pain or tissue loss, and (2) critical limb ischemia (CLI), which is defined as leg pain at rest with or without tissue loss (nonhealing leg ulcers or gangrene). Patients with IC infrequently progress to CLI and have a combined annual amputation and mortality rate of 2% to 4% per patient per year, whereas patients with CLI have a 6-month amputation risk of 25% to 40% and an annual mortality rate as high as 20%.1–3
In patients with lower-extremity PAD, the risk factors include advanced age, diabetes mellitus, hypertension, hypercholesterolemia, and smoking.2,4 Although the frequency of diabetes mellitus is higher in patients with CLI compared with patients with IC,5 a significant fraction of patients with IC also have diabetes mellitus.4,5 Similarly, whereas patients with CLI overall have a lower ankle-brachial index compared with patients with IC, and studies of CLI frequently set an ankle-brachial index cutoff of <0.5 as an entry criterion, a sizable fraction of patients with IC also have ankle-brachial index values <0.5.6–8 Thus, we hypothesized that genetic influences may be important in PAD and that these influences can be considered in at least 2 major ways. First, a polymorphism of a gene (or genes) can be associated with an increased risk for the development of lower-extremity atherosclerosis, with or without involvement of other vascular beds; examples of this include apolipoprotein E, interleukin-6, and thrombin.9–12 Second, in the setting of occlusive atherosclerosis, a polymorphism in a gene (or genes) can influence the disease severity, such as the presence or absence of tissue loss. To date, no information is available on the latter.
We took advantage of prior findings in a preclinical model of PAD in which, after surgically induced hindlimb ischemia, the C57BL/6 strain displayed good blood flow recovery with little or no tissue loss, whereas the BALB/c strain showed poor recovery and was prone to develop tissue loss or necrosis of the affected limb.13,14 This contrast suggests that sequence variation in specific gene(s) expressed in C57BL/6 and BALB/c strains may confer the observed phenotypic difference. Therefore, we sought to map the genetic loci involved in this differential recovery and test the role of the identified chromosomal region in the observed phenotypic outcome.
Methods
Mice
All mice (C57BL/6, BALB/c, A/J, C57BL/6J-Chr7A/J/NaJ) were obtained from the Jackson Laboratory (Bar Harbor, Me) either directly or bred internally (C57BL/6×BALB/c [F1] and F1×BALB/c [N2]) from a parental strain. N2 animals were derived from 1-way mating with F1 males crossed to BALB/c females. The C57BL/6J-Chr7A/J/NaJ (chromosome substitution strains [CSS]) strain is genetically identical to C57BL/6 except at chromosome 7, which is derived from the A/J strain. Mice were allowed to acclimate for a minimum of 3 days. Mice were age matched (12 to 18 weeks old for hindlimb ischemia and 8 to 12 weeks old for wound healing) and sex matched for all experiments. Studies were performed under protocols approved by the Duke University Animal Care and Use Committee.
Hindlimb Ischemia, Perfusion Recovery, Necrosis Score, and Histology
Unilateral femoral artery ligation and excision, resulting in hindlimb ischemia, was performed as described previously.15,16 Perfusion flow in the ischemic and contralateral nonischemic limbs was measured as described previously with the use of a laser Doppler perfusion imaging system (Perimed, Stockholm, Sweden).15,16 Perfusion was expressed as the ratio of the left (ischemic) to right (nonischemic) hindlimb and was performed immediately after surgery and weekly or biweekly up to 21 or 35 days postoperatively. Perfusion recovery was determined either by the absolute perfusion ratio at follow-up or as the ratio at follow-up minus the ratio immediately after surgery. In mice that developed autoamputation, the perfusion ratio obtained from the limb before autoamputation was used. The extent of necrosis was scored as follows: grade I, involving only toes; grade II, extending to dorsum pedis; grade III, extending to crus; and grade IV, extending to thigh or complete necrosis. In the ischemic and nonischemic gastrocnemius muscles, vascular density was analyzed by immunohistochemistry with a rat anti-mouse CD31 antibody by counting 3 random high-power (magnification ×200) fields for a minimum of 200 fibers and was expressed as the number of CD31+ cells per fiber, as described previously.16
Wound Healing
Full-thickness skin punch biopsies were made, as described previously by others.17 Briefly, mice were anesthetized with ketamine (50 mg/kg) and xylazine (5 mg/kg). The skin over the dorsum surface was shaved, and a 0.7- to 1.0-cm-diameter wound was generated with a disposable 0.8-cm-diameter skin punch biopsy tool (Acuderm Inc, Fort Lauderdale, Fla). Wound dressing was achieved with the use of a spray-on liquid bandage solution (Nexcare, St Paul, Minn). Wound area was measured weekly. The healing rate was defined as a percentage of original wound area or totally healed.
Genotyping
A standard high-salt procedure was used to isolate genomic DNA from mouse tails. A total of 105 N2 animals were used to identify quantitative trait locus (QTL) in the linkage scan. Single-nucleotide polymorphism (SNP) genotyping was performed with the use of the GoldenGate genotyping assay and the Illumina Bead Station (San Diego, Calif). The Illumina mouse low-density linkage panel was used, consisting of 377 SNPs covering the entire murine genome, chosen to be maximally informative in crosses between C57BL/6 and other inbred strains. In our cross between C57BL/6 and BALB/c, 239 autosomal SNP markers were informative. The average spacing per informative SNP was 6.8±1.6 cM, with the largest gap located on chromosome 2 (24.6 cM). Reported genetic map positions for the markers were retrieved from the SNP database (build 36.1) of the National Center for Biotechnology Information (NCBI).
Linkage Analysis
The MapManager QTX program (version b20) was used to localize the QTL responsible for the variability of recovery phenotypes from surgically induced hindlimb ischemia (necrosis and perfusion ratio). The source codes are available online at http://www.mapmanager.org/mmQTX.html. The significance thresholds for each phenotypic trait were determined empirically by permutation tests performed with all the informative markers and 10 000 permutation tests of data.18 This logarithmic index can be converted to a logarithm of odds (LOD) score by dividing it by 4.61. Suggestive (P=0.67), significant (P=0.05), and highly significant (P=0.001) thresholds were established on the basis of the guidelines suggested by Lander and Kruglyak.19 Single-locus association tests were performed between each marker and the phenotype to identify regions of interest in our initial genome scan. The genome-wide scans were plotted with the use of the J/QTL mapping program (version 0.8) obtained from the Jackson Laboratory.
In Silico Haplotype Analysis
To assess the possibility that QTLs can be identified only in genomic regions that differ between 3 strains of interest (C57BL/6, BALB/c, and A/J), SNP data were used to construct the haplotype map of the 31.2-Mb interval of mouse chromosome 7. SNP data were obtained from the Mouse Phenome Database (http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=snps/door). Physical map position for markers was based on the mouse genomic sequence from the NCBI build 36.1. Only SNPs polymorphic between the 3 strains were used to refine the haplotype map. SNPs missing data on 1 or 2 strains as well as SNPs that were not polymorphic between the 3 strains were omitted from the haplotype map because of lack of information.
Statistical Analysis
All continuous measures were expressed as mean±SEM. Statistical comparisons of perfusion or capillary density between multiple strains of mice were performed with the use of 1-way ANOVA; between 2 strains, the independent Student t test was used. Comparison of perfusion within each strain at different time points was analyzed by repeated-measures ANOVA. Differences in necrosis score between mice with a different genetic background were analyzed by nonparametric Kruskal-Wallis test. In all cases, P<0.05 was considered statistically significant. Correlation of perfusion recovery with necrosis was performed with the use of the Spearman correlation.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Early Identification of a QTL
C57BL/6 mice showed robust perfusion recovery after ligation, whereas far less recovery was shown by the BALB/c mice (Figure 1A). Necrosis was quite rare in the C57BL/6 strain and when present was at a low grade, whereas BALB/c mice were prone to develop necrosis (Figure 1B and the Table). The F1 mice exhibited no significant difference in perfusion recovery or necrosis from the C57BL/6 parental strain (Figure 2). This suggested that a dominant allele(s) in C57BL/6 could be responsible for the lack of tissue loss.
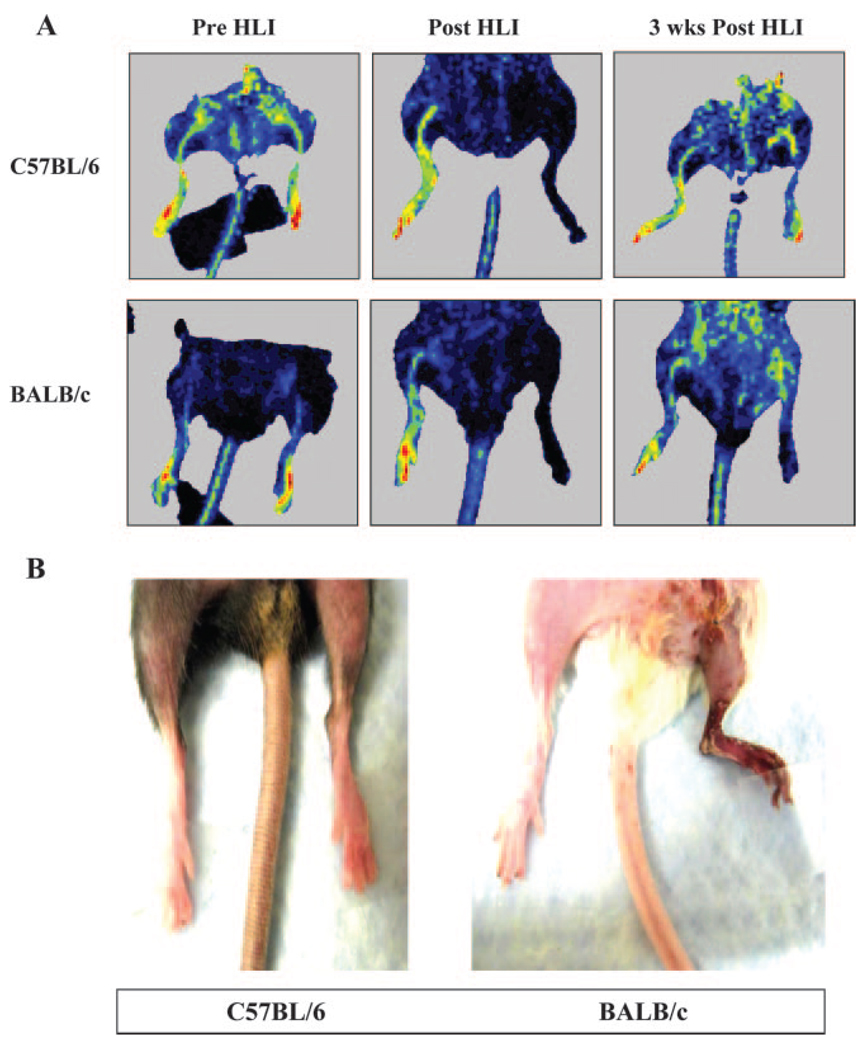
Figure 1.
C57BL/6 and BALB/c strains differ in response to hindlimb ischemia (HLI) (A). Hindlimb ischemia was induced, and perfusion was measured by laser Doppler perfusion imaging in C57BL/6 and BALB/c mice. Representative laser Doppler perfusion images of mice before surgery, immediately after surgery, and 3 weeks into recovery are shown. B, BALB/c mice are more prone to development of necrosis after induction of limb ischemia. Picture shows C57BL/6 and BALB/c mice, with BALB/c mice showing necrosis 1 week after hindlimb ischemia.
Table.
Incidence of Severity of Necrosis in Mouse Strains After Hindlimb Ischemia
| Mouse Strain | n | Mice With Any Necrosis |
Necrosis Grade | ||||
|---|---|---|---|---|---|---|---|
| Grade 0 | Grade I | Grade II | Grade III | Grade IV | |||
| BALB/c | 11 | 10/11 (90.1) | 1/11 (9) | 4/10 (40) | 3/10 (30) | 3/10 (30) | None |
| C57BL/6(B/6) | 41 | 1/41 (2.4) | 40/41 (97.6) | 1/1 (100) | None | None | None |
| B/6×BALB/cF1 | 18 | 3/18 (16.7) | 15/18 (83) | 3/3 (100) | None | None | None |
| A/J | 15 | 9/15 (60) | 6/15 (40) | 1/9 (11) | 1/9 (11) | 2/9 (22) | 5/9 (55.5) |
| CSS | 10 | 8/10 (80) | 2/10 (20) | 1/8 (12.5) | 2/8 (25) | 4/8 (50) | None |
Necrosis grade is from 0 through 4, as described in Methods. Values are expressed as number of mice affected in each category/total number of mice in the category (%). Distribution is to the right.
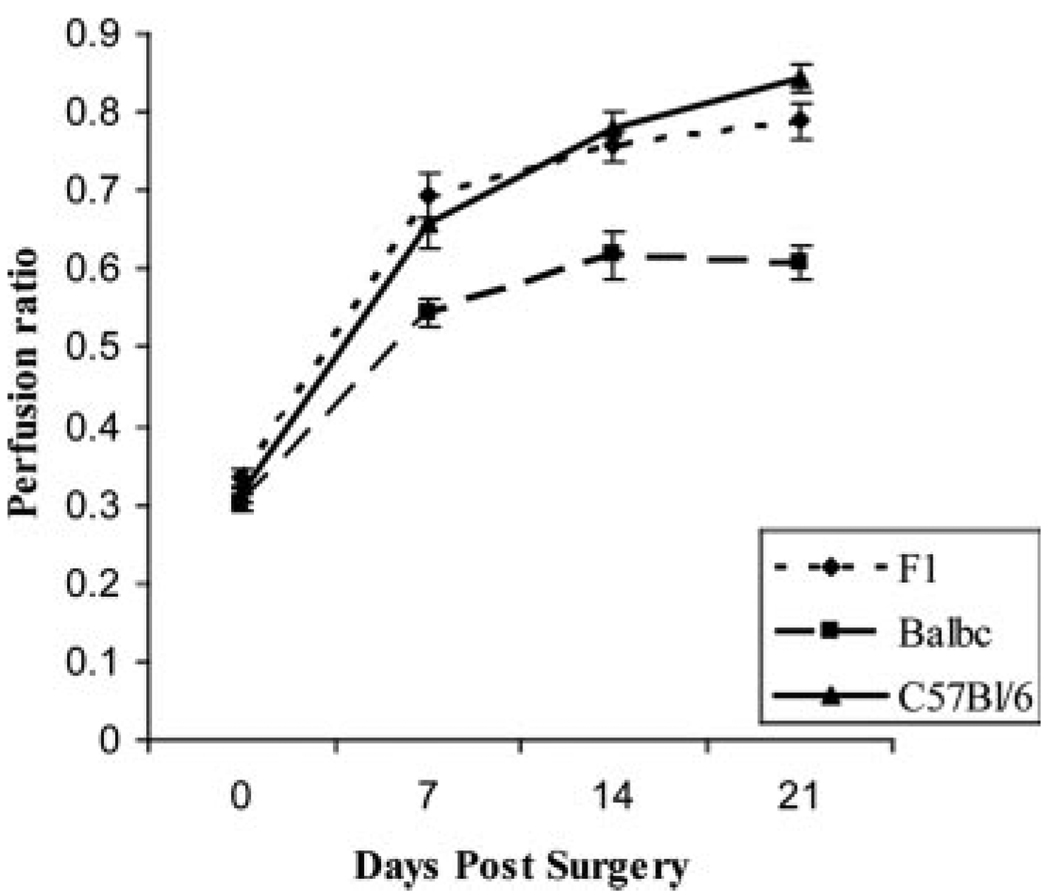
Figure 2.
C57BL/6 and C57BL/6×BALB/c F1 show similar recovery. Hindlimb ischemia was induced, and perfusion was measured by laser Doppler perfusion imaging in C57BL/6 (n=13), BALB/c (n=9), and F1 mice (n=18) strains. Immediately after surgery, no difference in perfusion was observed between C57BL/6, C57BL/6×BALB/c F1, and BALB/c (P>0.05) mice. Perfusion in C57BL/6 and C57BL/6×BALB/c F1 strains remained similar (P>0.05) but was different from BALB/c at later time points (P<0.05).
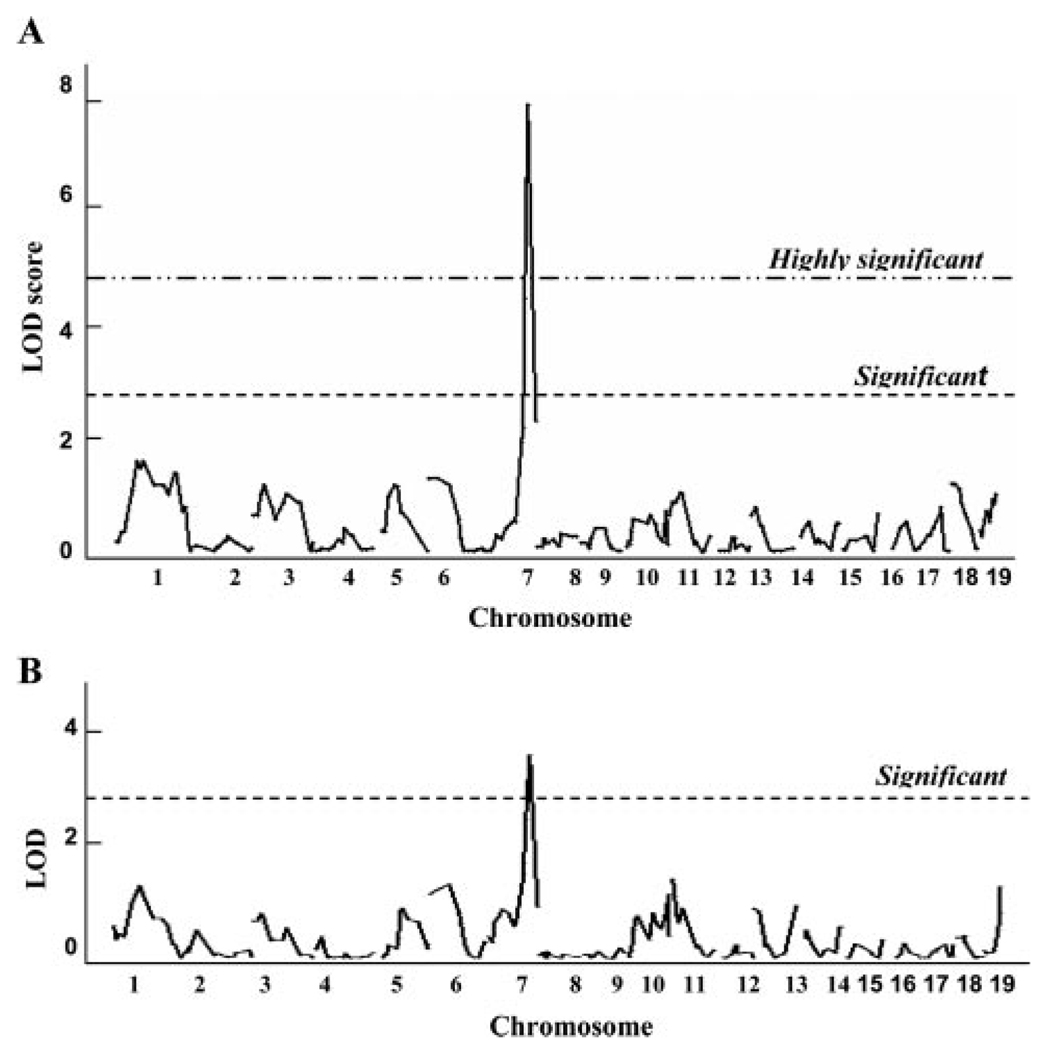
To map the allele in C57BL/6 mice, we performed an F1 backcross with BALB/c to generate 105 N2 progeny. A total of 105 N2 progeny were genotyped for the 239 SNPs that were informative in this cross. The average spacing between SNPs was 6.8±1.6 cM, affording full coverage of the mouse genome. Figure 3 illustrates the interval maps of each of the autosomes for the phenotypes of necrosis and perfusion ratio at day 21 after surgery. All but 1 of the chromosomes showed linkage profiles that were essentially flat, providing evidence for the exclusion of any strongly acting loci mapping to these chromosomes. However, we identified a single locus on chromosome 7 spanning ≈31 Mb that exhibited significant linkage to tissue necrosis and day 21 perfusion ratios, with a peak LOD score of 7.96 and 3.71, respectfully, at marker rs13479513 (Figure 4). We showed the whole genome linkage profile for reperfusion ratio at day 21 because at this time point, chromosome 7 exhibited the highest LOD score. Perfusion ratio was also measured at days 7 and 14 after surgery. For both of these time points, the chromosome 7 linkage reached the P=0.05 significance threshold (data not shown). Otherwise, across the genome, the linkage profiles for other time points are similar to that seen for the day 21 measurement. Furthermore, the linkage peak for necrosis was even more significant, far surpassing the P=0.001 threshold. The linkage peaks for perfusion ratio (Figure 3B) and necrosis (Figure 3A) were almost identical in chromosomal position, differing only in the magnitude of the strength of linkage.
Figure 3.
Genome-wide linkage scans for necrosis (A) and perfusion ratio (B) at day 21 after surgery in 105 N2 (F1×BALB/c) progeny. Chromosomes 1 through 19 are represented numerically on the ordinate. The relative width of the space allotted for each chromosome reflects the relative length of each chromosome. The abscissa represents the LOD score. The highly significant (P<0.001) and significant (P<0.05) levels of linkage were determined by 10 000 permutation tests. Only the chromosome 7 locus showed significant linkage to the 2 phenotypes.
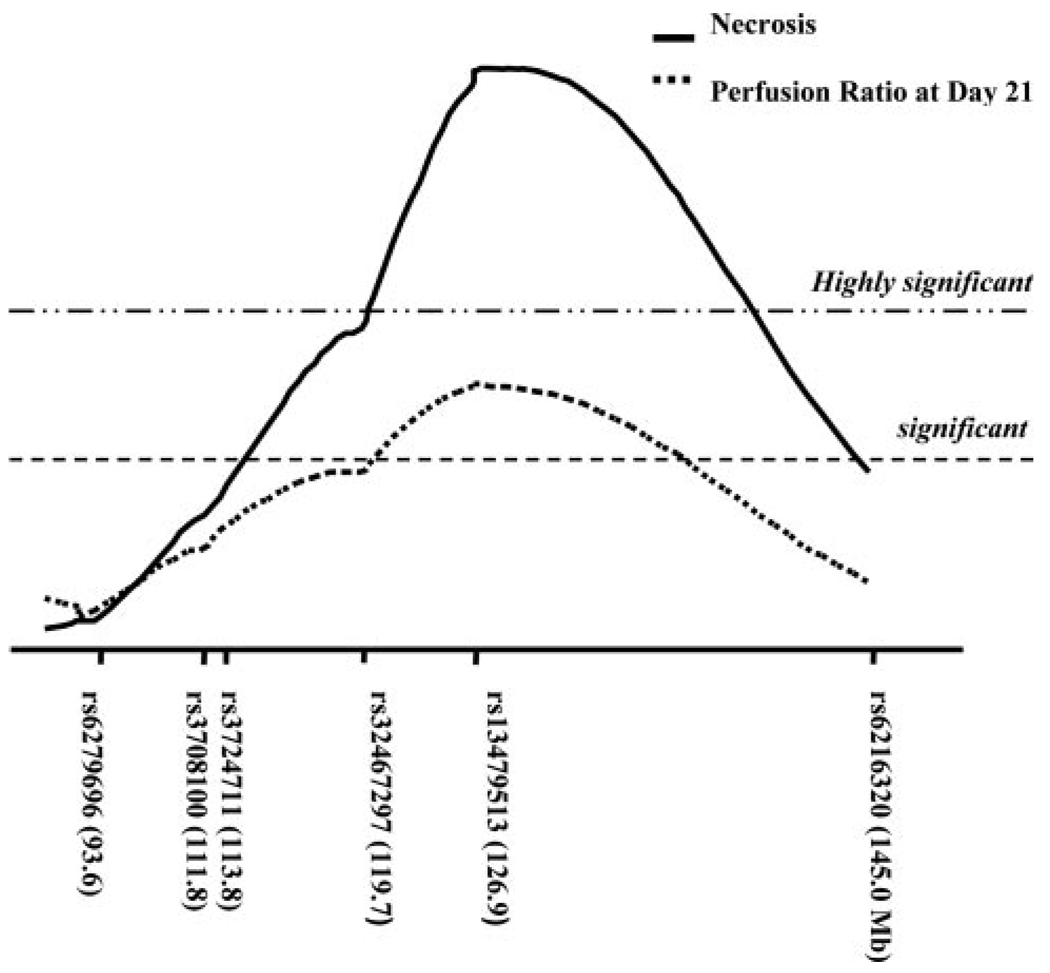
Figure 4.
Detailed interval map of distal chromosome 7. The QTL centered at SNP marker rs13479513 on chromosome 7 is significantly linked with necrosis (maximum LOD score of 8.0) and perfusion ratio at day 21 after surgery (LOD score of 3.71) in 105 N2 progeny. The chromosomal position of each SNP is indicated in parentheses (build 36). Linkage thresholds, highly significant (P<0.001) and significant (P<0.05), indicated by long dashed dot and dashed horizontal lines, were determined by permutation tests.
Because the LOD score for the necrosis trait greatly exceeded that of the perfusion ratio trait, we examined the possibility that the linkage score for the necrosis trait was artificially inflated because of a nonnormal distribution of necrosis scores between heterozygous (C57BL/6×BALB) and homozygous (BALB×BALB) animals at the chromosome 7 locus. To exclude this possibility, we recoded the necrosis scores of all the N2 mice on a scale of 0, I, or II (where all scores >II received a value of II) and again as a dichotomous trait (where any level of necrosis received a score of I). Under both recoding schemes, the linkage scores for necrosis were essentially unchanged from the original (data not shown). Because the MapManager QTX program employs means and not variance components in the analysis, the linkage scores are not unduly influenced by the nonnormal distribution of the values for the trait. Instead, it is likely that these 2 phenotypes are biologically interrelated, with necrosis being the stronger measure of the underlying pathogenesis. The coincident linkage peaks for both traits further support this possibility. No significant gender differences were present in either tissue necrosis or perfusion ratio in either parental background (data not shown) or in the progeny of the backcross.
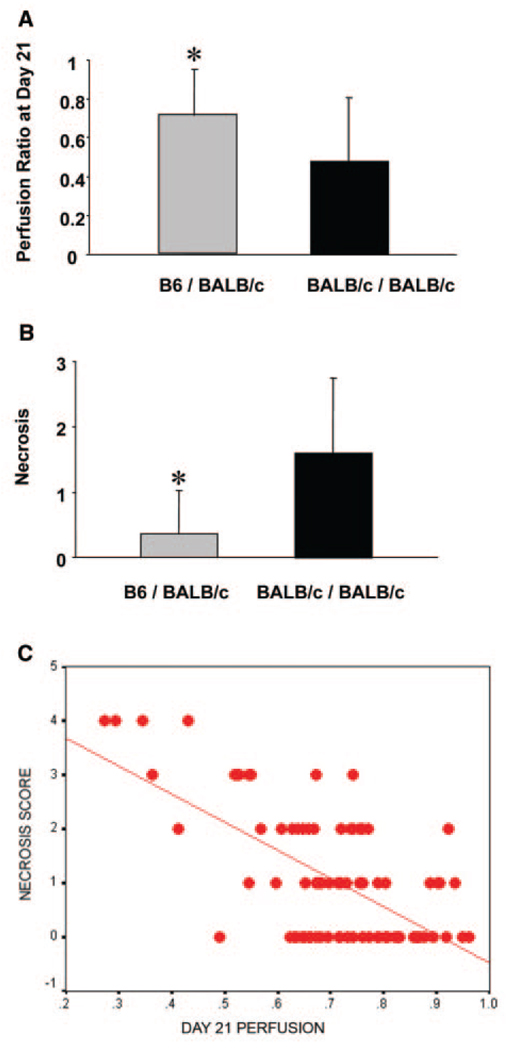
Genotype–Phenotype Correlations
To determine the allelic contribution to the effect of the locus between genotype and phenotype data, necrosis and perfusion ratio were evaluated against genotype at the chromosome 7 locus that showed the highest LOD score among all of our SNP markers. The N2 animals with a heterozygous genotype (C57BL/6×BALB) at marker rs13479513 showed minimal necrosis and higher perfusion ratio on average than their homozygous (BALB×BALB) littermates (0.37 versus 1.60 and 0.71 versus 0.47, respectively; Figure 5A and 5B). Because the necrosis score was not normally distributed, we used the nonparametric Kruskal-Wallis test, and both necrosis score and perfusion ratio were significantly different between BALB×BALB and C57BL/6×BALB animals (P<0.0001). Moreover, when we examined the correlation between necrosis and perfusion recovery in the N2 progeny, an association was present between poor final perfusion and greater necrosis in these mice (r=−0.628, P<0.001; Figure 5C). Results were similar if the perfusion recovery was measured as perfusion at follow-up minus the immediate postoperative value. Taken together, these data support the findings that region of the C57BL/6 strain is contributing a dominant protective allele (or alleles) to the recovery phenotype from hindlimb ischemia, and this allele is protective via a mechanism contributing to perfusion recovery.
Figure 5.
Allelic effects of the QTL on perfusion ratio (A) and necrosis (B) at SNP marker rs13479513. The heterozygous animals (n=50) with 1 C57BL/6 and 1 BALB/c allele have higher mean of perfusion ratio and lower mean of necrosis than homozygous BALB/c animals (n=55) (0.72 vs 0.48 and 0.37 vs 1.60, respectively), indicating that the heterozygotes show more robust recovery phenotypes after hindlimb ischemia. Each bar represents mean±SEM. *P<0.001. C, Necrosis score negatively correlates with perfusion at day 21 in the N2 progeny used for mapping. Statistics was performed by Spearman rank order correlation coefficient, resulting in r=−0.628 and P<0.001.
Requirement of QTL on C57BL/6 Chromosome 7 for Absence of Tissue Loss
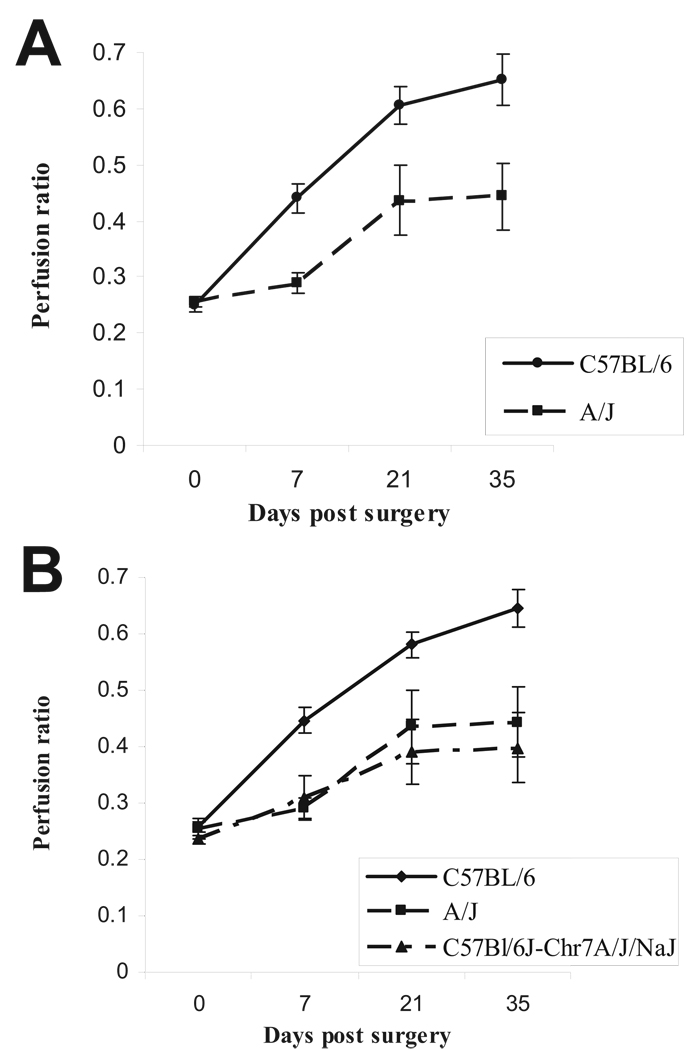
We sought to formally test whether the C57BL/6 chromosome 7 was required for the absence of tissue loss. We took advantage of the CSS strain C57BL/6J-Chr7A/J/NaJ in which the C57BL/6 chromosome 7 has been replaced by chromosome 7 from the A/J strain. To determine whether this strain will be useful in testing our hypothesis, it was important to first determine the recovery phenotype of the parental A/J strain in surgically induced hindlimb ischemia. If the A/J strain shows poor recovery, then the CSS can be used to evaluate the role of C57BL/6 chromosome 7. Hence, we performed unilateral femoral ligation and excision on A/J mice to determine its recovery phenotype; indeed, the A/J strain showed poor recovery compared with C57BL/6 (Figure 6A) and, similar to BALB/c, was prone to developing limb necrosis (Table). In light of these findings, we proceeded to test the phenotype in the CSS strain and discovered that this strain also showed poor recovery and tendency to develop limb necrosis (Table). Furthermore, although no significant difference was observed in capillary density between C57BL/6 (0.99±0.07) and A/J (0.91±0.04; P=0.38) strains in nonischemic tissues, 5 weeks after induction of limb ischemia, C57BL/6 mice showed higher capillary density (1.30±0.06) than the A/J or the CSS strain (0.97±0.10, P=0.03 or 1.01±0.11, P=0.04; n=3 per group). Therefore, these data show that the C57BL/6 chromosome 7 is required for the lack of tissue loss and greater perfusion recovery phenotype after hindlimb ischemia.
Figure 6.
A, A/J mice display poor recovery (BALB/c-like phenotype) after hindlimb ischemia. Hindlimb ischemia was induced, and perfusion was measured by laser Doppler perfusion imaging in A/J (n=15) and C57BL/6 (n=17) mice. No difference in perfusion was noted between the 2 strains immediately after induction of hindlimb ischemia (P>0.05); however, C57BL/6 showed better perfusion than A/J strain (P<0.01) at later time points. B, C57BL/6 chromosome 7 is required for its limb salvage phenotype after hindlimb ischemia. Hindlimb ischemia was induced in C57BL/6 (n=41), A/J (n=17), and CSS C57BL/6J-Chr7A/J/NaJ (n=10), which is genetically identical to C57BL/6 except at chromosome 7, which is A/J derived. Recovery was similar in CSS and A/J strains (P>0.05) but different in C57BL/6 (P<0.01).
C57BL/6 Chromosome 7 QTL Has No Effect on Wound Healing
Next, we sought to determine the role of this C57BL/6-derived chromosome 7 locus in a nonischemic tissue injury (ie, wound healing). Wounding healing rates were determined over a 2-week period. No significant difference was observed between the 2 strains in the rate of wound healing 1 week after injury (79.8±2.6% in BALB/c versus 83.9±3.2% in C57BL/6; P=0.29; n=6 per group), and all mice from both strains achieved complete wound closure by week 2 (100% in both strains). Similar results were observed in the CSS strain (90.4±4.1% at 1 week; n=4; all mice were healed at 2 weeks), suggesting that the C57BL/6-derived chromosome 7 effect may be specific for recovery after ischemic injury.
Haplotype Analysis: Toward Gene Identification
We employed ancestral haplotype sharing pattern within the inbred mouse lineage. Haplotype analysis can decrease confidence intervals by quickly identifying high-priority regions within a QTL interval that are likely to harbor the causal polymorphism.20 Approximately 97% of the genetic variation between mouse inbred strains is found in ancestral haplotype blocks that are shared between strains, with only a minority of the sequence variation being unique to any 1 strain.21 Therefore, we hypothesize that the allelic variation at the chromosome 7 recovery locus is most likely due to a gene that maps within ancestral haplotype blocks that are shared between BALB/c and A/J but differ from C57BL/6 strains. These regions are most likely to contain the causative gene for the differential recovery after ischemic tissue injury.
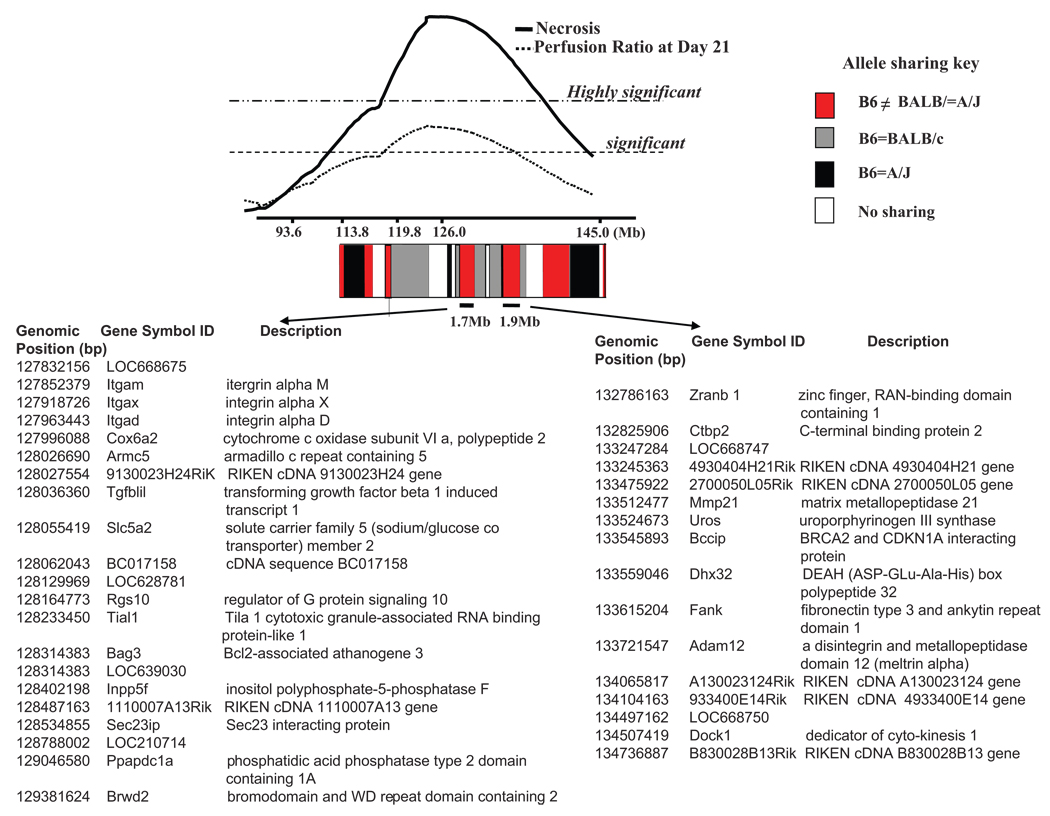
Using a dense microsatellite and SNP map of the broad chromosome 7 locus, we were able to identify the haplotype blocks that consisted of the same SNP alleles between BALB/c and A/J but were different from C57BL/6. As shown in Figure 7, 2 of the haplotype blocks in which the C57BL/6 allele differed from the shared BALB/c and A/J allele lie directly under the central region of the linkage peak (2-LOD support interval). Of primary interest are the 2 haplotype blocks of ≈1.7 and 1.9 Mb, which contain 21 and 16 known or predicted genes, respectively (Figure 7).
Figure 7.
Haplotype block patterns between C57BL/6 (B6), BALB/c, and A/J reveal 2 (1.7 and 1.9 Mb) central regions of different ancestral haplotypes residing within the region of highest linkage. The degree of allele sharing is shown by red, gray, black, and white blocks. The red blocks indicate where the C57BL/6 allele is unique compared with the same SNP allele between BALB/c and A/J strains. Arrows indicate list of genes within each block. Gene locations are based on NCBI mouse build 36.1 and represent the union of available gene annotation sources (NCBI, dbSNP, and Ensembl).
Discussion
The results of our study demonstrate, for the first time, that inherited genetic variations strongly influence the phenotypic outcome after femoral arterial occlusion, which is a preclinical model of PAD. We took advantage of known strain-specific differences in recovery after hindlimb ischemia. First, using classic genetic tools, we demonstrated that the genetic background of C57BL/6 mice was associated with greater limb perfusion and less limb loss after hindlimb ischemia compared with BALB/c. We then used 2 complementary approaches to demonstrate the pivotal role of C57BL/6-derived genetic sequence in conferring limb preservation (when present) or tissue loss (when absent). We identified an initial genetic locus covering an area of ≈19.8 Mb (Figure 4; highly significant) to 31.2 Mb (Figure 4; significant) containing 274 to 495 genes, respectively. Then, by taking advantage of ancestral haplotype blocks that are similar in strains with poor recovery but different in a strain with good recovery, we were able to quickly refine this genetic interval to 2 regions containing a total of 37 genes.
Aside from the information contained in our present report, little to no information is available on genetic influences in PAD.22 The overall approach used in our study has successfully led to the identification of genes associated with human disease.23–25 Paigen et al26 took advantage of differences in mouse strain susceptibility to the development of atherosclerosis to describe a region on chromosome 1, termed the Ath1 gene. They later refined the locus from a large region to one that contained relatively few genes.27 Subsequently, they used a combination of differential gene expression, gene targeting, and transgenic overexpression to identify Tnfsf4 as the gene responsible for the phenotype. They identified the human orthologs and performed association studies in humans to show that polymorphism of Tnfsf4 was associated with increased risk of myocardial infarction.28 The data from our study provide the first QTL associated with disease severity in PAD and suggest that we may be able to identify novel gene(s) that are linked to the clinical manifestations of PAD in humans. The results from our study can readily be used to identify specific genes with the use of methods including, but not limited to, differential expression in parental strains, allele-specific expression, transgenic expression, or targeted gene deletions.
Some prior studies have investigated the role of gene polymorphisms in the pathogenesis of PAD in humans; however, in most of these studies, the investigation centered on polymorphism of genes known to be associated with atherosclerosis or atherothrombosis.9,10,29,30 Perhaps, then, it is not surprising that associations were found between PAD and polymorphisms in genes such as apolipoprotein-B and thrombin. However, outside of genes related to atherosclerotic or thrombosis risk, genetic association studies for PAD are quite limited. We are aware of only 1 study, a family-based linkage study that identified a genetic locus conferring susceptibility to PAD.31 Gudmundsson et al31 identified a locus mapping to human chromosome 1p31 termed PAOD1. One of the strengths of this study is that it took an unbiased approach, starting with the PAD phenotype, to identify novel genetic risk factors rather than starting with a potentially biased list of candidate genes. Using a similarly unbiased genetic mapping approach, we have identified a genetic locus conferring limb preservation after murine hindlimb ischemia. Our locus (LSq-1) is not syntenic to human chromosome 1p31 but rather shows synteny to regions of human chromosomes 10, 11, and 16. It is therefore highly unlikely that PAOD1 encodes the same gene (or genes) as LSq-1, although it is plausible that genes in both species may ultimately be shown to play a role in the same physiological pathway.
Recently, Faber and colleagues32 proposed that differences in expression of vascular endothelial growth factor-A (VEGFA) may account for the differences in collateral vessel density observed between C57BL/6 and BALB/c and further suggested that this anatomic difference may underlie the different recovery phenotypes after surgically induced hindlimb ischemia. They determined that these 2 strains do not show differential expression of VEGF-A in skeletal muscle at baseline (nonischemic muscle), but expression levels differ 36 hours after ligation (ischemic muscle). Using expression QTL analysis, they also determined that the cause of this expression difference maps near the murine Vegfa gene locus. However, these data do not genetically link the VEGF-A locus to the distinct phenotypes of recovery from limb ischemia that are observed in the parental strains. VEGF-A maps to murine chromosome 17, and we did not find any evidence of a dominantly acting C57BL/6 allele on this chromosome in our cross (Figure 3A and 3B). Thus, our data support the existence of a locus distinct from the Vegfa gene that has a strong influence on the extent of recovery from ischemia.
The surgical hindlimb ischemia model is complex, and multiple mechanisms are involved in recovery, including but not limited to ischemic injury, tissue regeneration, inflammation, angiogenesis, and arteriogenesis. Although our data demonstrate that genetic variations influence perfusion recovery, the specific gene(s) and mechanism(s) by which LSq-1 contributes to strain specific recovery in this preclinical model are beyond the scope of a single study. We found that LSq-1 did not affect nonischemic wound healing, and we showed a correlation between lower perfusion recovery and greater necrosis, suggesting that the locus effect may be via a mechanism contributing to restoration of perfusion. This suggests that C57BL/6-derived genetic sequences may be important in the response to ischemia rather than for generalized tissue regeneration.
Classic studies by Schaper and colleagues showed that differences in the extent of preexisting collateral vessels and arteriogenesis play a role in perfusion recovery after hindlimb ischemia.14,33 Studies by Fukino et al13 and other groups found a differential angiogenic response in the C57BL/6 and BALB/c strains after limb ischemia, suggesting a role for angiogenesis in this phenotypic outcome.14,32 Our data showing higher vascular density in C57BL/6 tissue at late time points after ligation compared with the CSS and A/J strains are also consistent with a role for angiogenesis in strain-specific recovery. Our data do not exclude the possibility that differences in preexisting collateral vessels arteriogenesis, development of new collateral vessels via angiogenesis, adaptation to tissue ischemia, or some combination of these factors contribute to differential recovery after ischemia or that these genetic differences could work through mechanisms not yet postulated. At present, the mechanism(s) by which LSq-1 contributes to strain-dependent recovery after hindlimb ischemia is not understood completely. However, future studies that identify the specific gene(s) within the LSq-1 locus that mediate the beneficial effect, as well as their human orthologs, will likely lead to a better understanding of the pathogenesis of PAD and may also provide insight into novel therapeutic options.
CLINICAL PERSPECTIVE
When occlusions are present in the large arteries to the lower extremity, the result is the most common form of peripheral arterial disease (PAD). PAD, a major healthcare problem, has 2 major clinical presentations: intermittent claudication and critical limb ischemia. Despite the significant overlap in risk factors leading to the development of both clinical presentations, patients with critical limb ischemia frequently have ongoing or imminent tissue loss and thus have a higher rate of amputation and mortality. Patients with intermittent claudication rarely develop tissue loss. Therefore, we hypothesized that underlying genetic differences may influence the clinical outcomes in PAD. In the present study, we used a preclinical model of PAD and identified a chromosomal region in mice, termed LSq-1, that, when present, was associated with absence of tissue loss. This is the first identification of such a quantitative trait locus in PAD. This study can serve as a valuable foundation for future studies to identify specific gene(s) involved in PAD in patients. We describe how these results can be used to rapidly identify human orthologs of the mouse genes. Such information has the potential to advance our understanding of the pathophysiology of PAD, as well as new treatment options.
Acknowledgments
Sources of Funding
Dr Dokun is a Postdoctoral Fellow supported by grant T32 HL007101 from the National Institutes of Health. Dr Annex was supported in part by grant R01 HL755752 from the National Institutes of Health.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Ouriel K. Peripheral arterial disease. Lancet. 2001;358:1257–1264. doi: 10.1016/S0140-6736(01)06351-6. [DOI] [PubMed] [Google Scholar]
- 2.Da Silva A, Widmer LK, Ziegler HW, Nissen C, Schweizer W. The Basle longitudinal study: report on the relation of initial glucose level to baseline ECG abnormalities, peripheral artery disease, and subsequent mortality. J Chronic Dis. 1979;32:797–803. doi: 10.1016/0021-9681(79)90059-6. [DOI] [PubMed] [Google Scholar]
- 3.Juergens JL, Barker NW, Hines EA., Jr Arteriosclerosis obliterans: review of 520 cases with special reference to pathogenic and prognostic factors. Circulation. 1960;21:188–195. doi: 10.1161/01.cir.21.2.188. [DOI] [PubMed] [Google Scholar]
- 4.Raghunathan A, Rapp JH, Littooy F, Santilli S, Krupski WC, Ward HB, Thottapurathu L, Moritz T, McFalls EO. Postoperative outcomes for patients undergoing elective revascularization for critical limb ischemia and intermittent claudication: a subanalysis of the Coronary Artery Revascularization Prophylaxis (CARP) trial. J Vasc Surg. 2006;43:1175–1182. doi: 10.1016/j.jvs.2005.12.069. [DOI] [PubMed] [Google Scholar]
- 5.Aquino R, Johnnides C, Makaroun M, Whittle JC, Muluk VS, Kelley ME, Muluk SC. Natural history of claudication: long-term serial follow-up study of 1244 claudicants. J Vasc Surg. 2001;34:962–970. doi: 10.1067/mva.2001.119749. [DOI] [PubMed] [Google Scholar]
- 6.Rajagopalan S, Olin J, Deitcher S, Pieczek A, Laird J, Grossman PM, Goldman CK, McEllin K, Kelly R, Chronos N. Use of a constitutively active hypoxia-inducible factor-1alpha transgene as a therapeutic strategy in no-option critical limb ischemia patients: phase I dose-escalation experience. Circulation. 2007;115:1234–1243. doi: 10.1161/CIRCULATIONAHA.106.607994. [DOI] [PubMed] [Google Scholar]
- 7.Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, Hillegass WB, Rocha-Singh K, Moon TE, Whitehouse MJ, Annex BH. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359:2053–2058. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- 8.Muluk SC, Muluk VS, Kelley ME, Whittle JC, Tierney JA, Webster MW, Makaroun MS. Outcome events in patients with claudication: a 15-year study in 2777 patients. J Vasc Surg. 2001;33:251–257. doi: 10.1067/mva.2001.112210. discussion 257–258. [DOI] [PubMed] [Google Scholar]
- 9.Resnick HE, Rodriguez B, Havlik R, Ferrucci L, Foley D, Curb JD, Harris TB. Apo E genotype, diabetes, and peripheral arterial disease in older men: the Honolulu-Asia aging study. Genet Epidemiol. 2000;19:52–63. doi: 10.1002/1098-2272(200007)19:1<52::AID-GEPI4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 10.Flex A, Gaetani E, Pola R, Santoliquido A, Aloi F, Papaleo P, Dal Lago A, Pola E, Serricchio M, Tondi P, Pola P. The –174 G/C polymorphism of the interleukin-6 gene promoter is associated with peripheral artery occlusive disease. Eur J Vasc Endovasc Surg. 2002;24:264–268. doi: 10.1053/ejvs.2002.1711. [DOI] [PubMed] [Google Scholar]
- 11.Lane DA, Grant PJ. Role of hemostatic gene polymorphisms in venous and arterial thrombotic disease. Blood. 2000;95:1517–1532. [PubMed] [Google Scholar]
- 12.Monsalve MV, Robinson D, Woolcock NE, Powell JT, Greenhalgh RM, Humphries SE. Within-individual variation in serum cholesterol levels: association with DNA polymorphisms at the apolipoprotein B and AI-CIII-AIV loci in patients with peripheral arterial disease. Clin Genet. 1991;39:260–273. doi: 10.1111/j.1399-0004.1991.tb03024.x. [DOI] [PubMed] [Google Scholar]
- 13.Fukino K, Sata M, Seko Y, Hirata Y, Nagai R. Genetic background influences therapeutic effectiveness of VEGF. Biochem Biophys Res Commun. 2003;310:143–147. doi: 10.1016/j.bbrc.2003.08.134. [DOI] [PubMed] [Google Scholar]
- 14.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–787. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 15.Hazarika S, Dokun AO, Li Y, Popel AS, Kontos CD, Annex BH. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res. 2007;101:948–956. doi: 10.1161/CIRCRESAHA.107.160630. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Hazarika S, Xie D, Pippen AM, Kontos CD, Annex BH. In mice with type 2 diabetes, a vascular endothelial growth factor (VEGF)-activating transcription factor modulates VEGF signaling and induces therapeutic angiogenesis after hindlimb ischemia. Diabetes. 2007;56:656–665. doi: 10.2337/db06-0999. [DOI] [PubMed] [Google Scholar]
- 17.Jacobi J, Jang JJ, Sundram U, Dayoub H, Fajardo LF, Cooke JP. Nicotine accelerates angiogenesis and wound healing in genetically diabetic mice. Am J Pathol. 2002;161:97–104. doi: 10.1016/S0002-9440(10)64161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manly KF, Cudmore RH, Jr, Meer JM. Map Manager QTX, cross-platform software for genetic mapping. Mamm Genome. 2001;12:930–932. doi: 10.1007/s00335-001-1016-3. [DOI] [PubMed] [Google Scholar]
- 19.Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 20.Peters LL, Robledo RF, Bult CJ, Churchill GA, Paigen BJ, Svenson KL. The mouse as a model for human biology: a resource guide for complex trait analysis. Nat Rev Genet. 2007;8:58–69. doi: 10.1038/nrg2025. [DOI] [PubMed] [Google Scholar]
- 21.Wade CM, Kulbokas EJ, III, Kirby AW, Zody MC, Mullikin JC, Lander ES, Lindblad-Toh K, Daly MJ. The mosaic structure of variation in the laboratory mouse genome. Nature. 2002;420:574–578. doi: 10.1038/nature01252. [DOI] [PubMed] [Google Scholar]
- 22.Knowles JW, Assimes TL, Li J, Quertermous T, Cooke JP. Genetic susceptibility to peripheral arterial disease: a dark corner in vascular biology. Arterioscler Thromb Vasc Biol. 2007;27:2068–2078. doi: 10.1161/01.ATV.0000282199.66398.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 24.Gharani N, Benayed R, Mancuso V, Brzustowicz LM, Millonig JH. Association of the homeobox transcription factor, ENGRAILED 2, 3, with autism spectrum disorder. Mol Psychiatry. 2004;9:474–484. doi: 10.1038/sj.mp.4001498. [DOI] [PubMed] [Google Scholar]
- 25.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, Ronningen KS, Guja C, Ionescu-Tirgoviste C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SC. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 26.Paigen B, Mitchell D, Reue K, Morrow A, Lusis AJ, LeBoeuf RC. Ath-1, a gene determining atherosclerosis susceptibility and high density lipoprotein levels in mice. Proc Natl Acad Sci U S A. 1987;84:3763–3767. doi: 10.1073/pnas.84.11.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phelan SA, Beier DR, Higgins DC, Paigen B. Confirmation and high resolution mapping of an atherosclerosis susceptibility gene in mice on chromosome 1. Mamm Genome. 2002;13:548–553. doi: 10.1007/s00335-002-2196-1. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Ria M, Kelmenson PM, Eriksson P, Higgins DC, Samnegard A, Petros C, Rollins J, Bennet AM, Wiman B, de Faire U, Wennberg C, Olsson PG, Ishii N, Sugamura K, Hamsten A, Forsman-Semb K, Lagercrantz J, Paigen B. Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nat Genet. 2005;37:365–372. doi: 10.1038/ng1524. [DOI] [PubMed] [Google Scholar]
- 29.Reny JL, Alhenc-Gelas M, Fontana P, Bissery A, Julia PL, Fiessinger JN, Aiach M, Emmerich J. The factor II G20210A gene polymorphism, but not factor V Arg506Gln, is associated with peripheral arterial disease: results of a case-control study. J Thromb Haemost. 2004;2:1334–1340. doi: 10.1111/j.1538-7836.2004.00809.x. [DOI] [PubMed] [Google Scholar]
- 30.Fontana P, Gaussem P, Aiach M, Fiessinger JN, Emmerich J, Reny JL. P2Y12 H2 haplotype is associated with peripheral arterial disease: a case-control study. Circulation. 2003;108:2971–2973. doi: 10.1161/01.CIR.0000106904.80795.35. [DOI] [PubMed] [Google Scholar]
- 31.Gudmundsson G, Matthiasson SE, Arason H, Johannsson H, Runarsson F, Bjarnason H, Helgadottir K, Thorisdottir S, Ingadottir G, Lindpaintner K, Sainz J, Gudnason V, Frigge ML, Kong A, Gulcher JR, Stefansson K. Localization of a gene for peripheral arterial occlusive disease to chromosome 1p31. Am J Hum Genet. 2002;70:586–592. doi: 10.1086/339251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics. 2007;30:179–191. doi: 10.1152/physiolgenomics.00047.2007. [DOI] [PubMed] [Google Scholar]
- 33.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]