Abstract
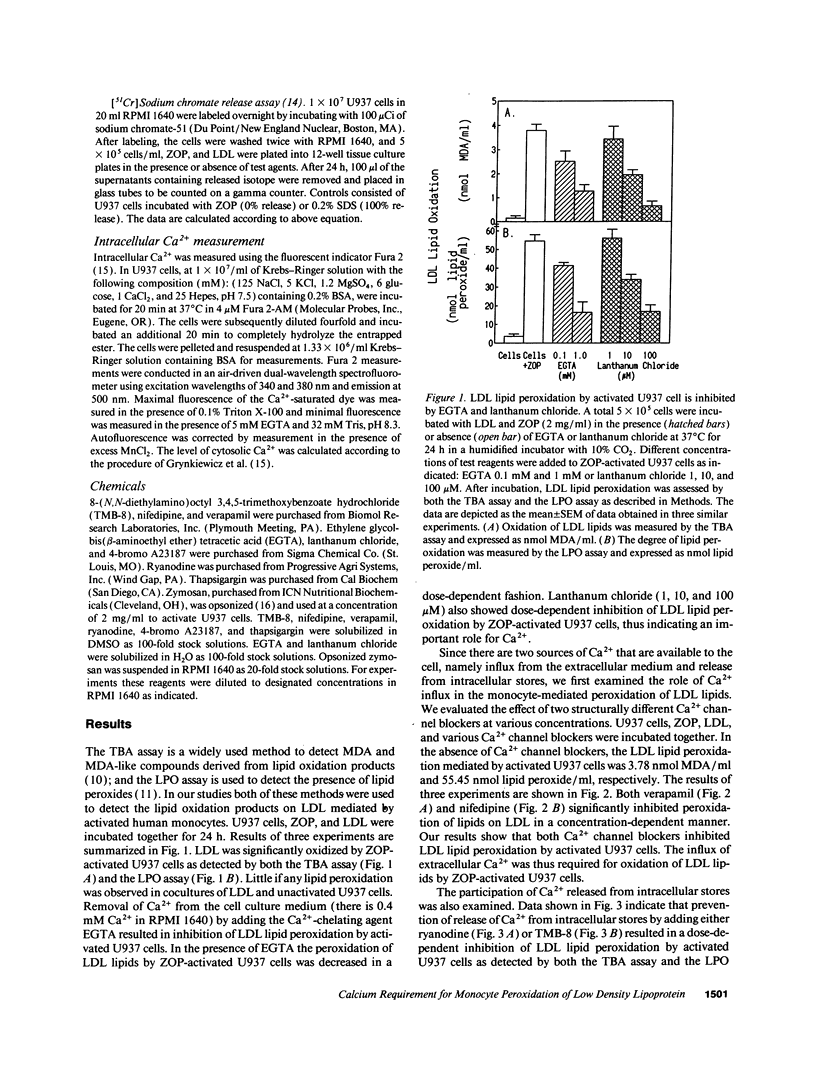
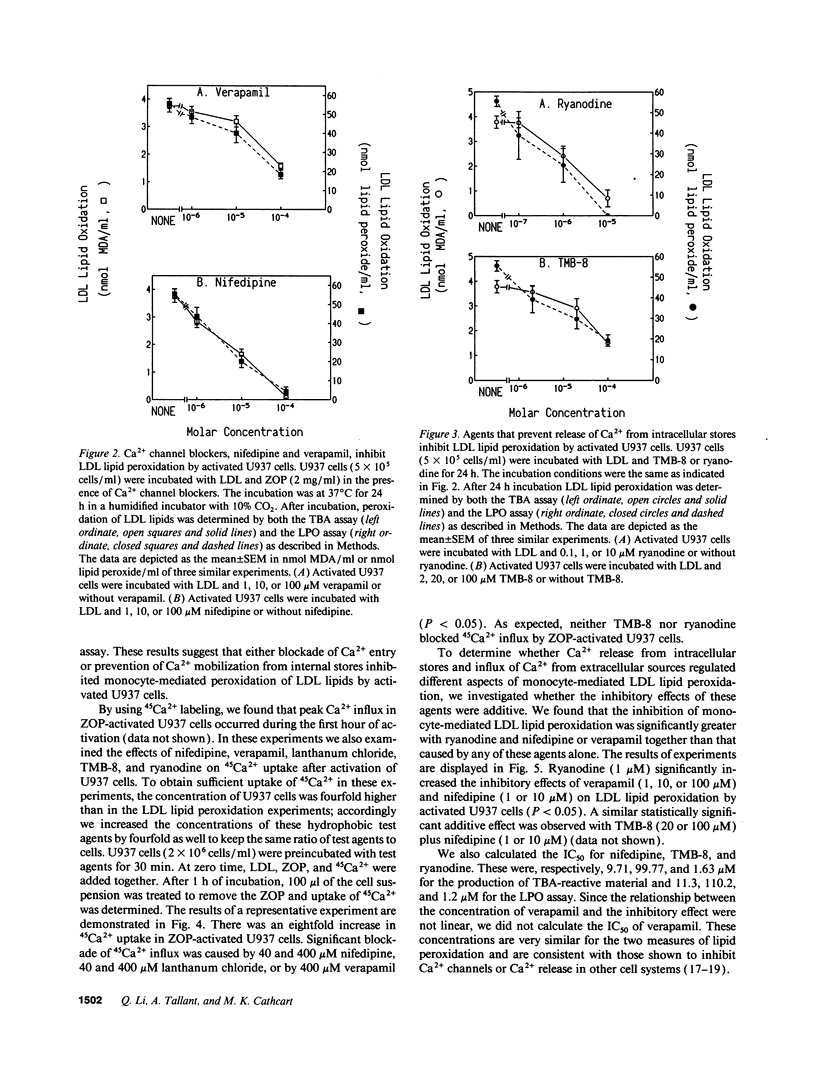
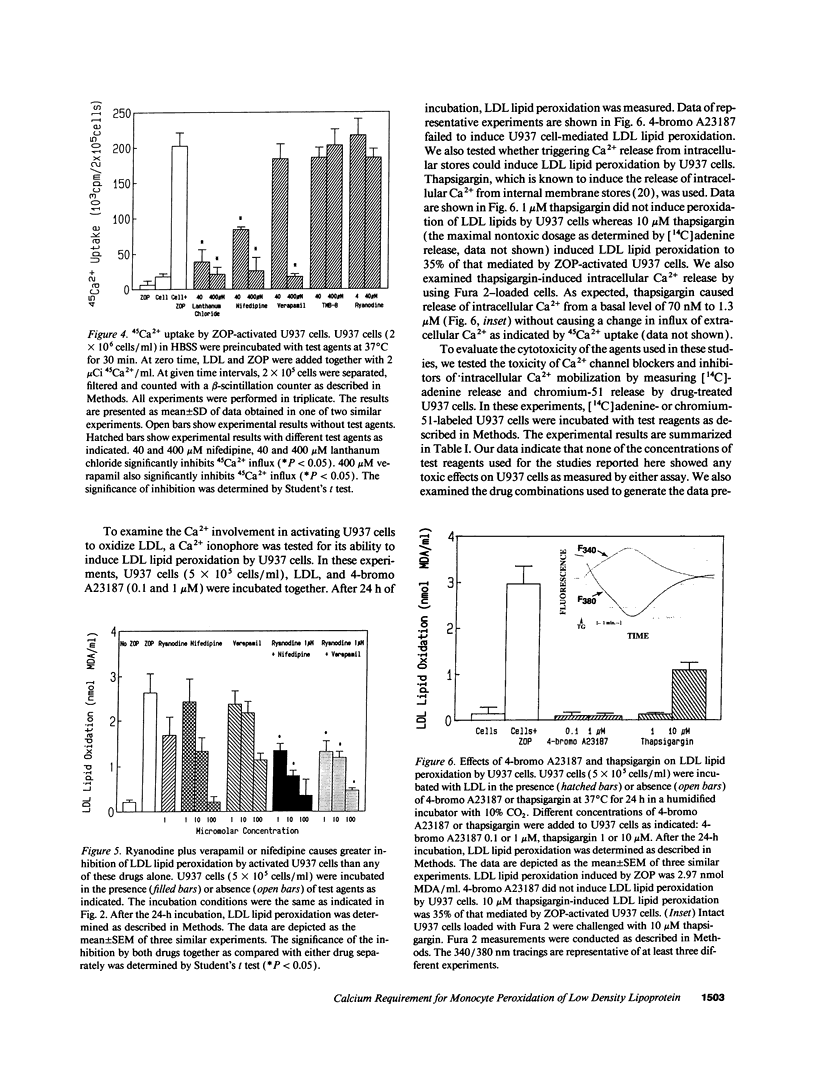
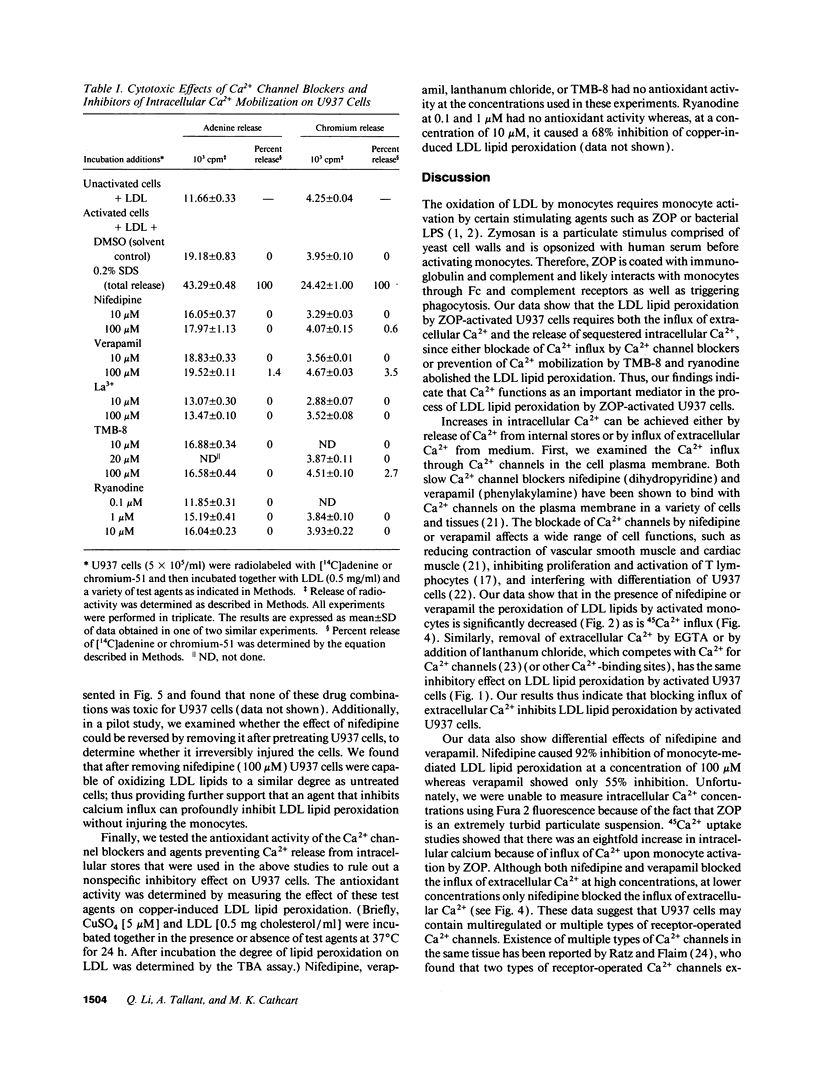
The oxidative modification of LDL seems a key event in atherogenesis and may participate in inflammatory tissue injury. Our previous studies suggested that the process of LDL oxidation by activated human monocytes/macrophages required O2- and activity of intracellular lipoxygenase. Herein, we studied the mechanisms involved in this oxidative modification of LDL. In this study, we used the human monocytoid cell line U937 to examine the role of Ca2+ in U937 cell-mediated lipid peroxidation of LDL. U937 cells were activated by opsonized zymosan. Removal of Ca2+ from cell culture medium by EGTA inhibited U937 cell-mediated peroxidation of LDL lipids. Therefore, Ca2+ influx and mobilization were examined for their influence on U937 cell-mediated LDL lipid peroxidation. Ca2+ channel blockers nifedipine and verapamil blocked both Ca2+ influx and LDL lipid peroxidation by activated U937 cells. The inhibitory effects of nifedipine and verapamil were dose dependent. TMB-8 and ryanodine, agents known to prevent Ca2+ release from intracellular stores, also caused a dose-dependent inhibition of LDL lipid peroxidation by activated U937 cells while exhibiting no effect on Ca2+ influx. Thus, both Ca2+ influx through functional calcium channels and Ca2+ mobilization from intracellular stores participate in the oxidative modification of LDL by activated U937 cells. 45Ca2+ uptake experiments revealed profound Ca2+ influx during the early stages of U937 cell activation, however, the Ca2+ ionophore 4-bromo A23187 was unable to induce activation of U937 cells and peroxidation of LDL lipids. Release of intracellular Ca2+ by thapsigargin only caused a suboptimal peroxidation of LDL lipids. Our results indicate that although increases in intracellular Ca2+ levels provided by both influx and intracellular Ca2+ mobilization are required, other intracellular signals may be involved for optimal peroxidation of LDL lipids by activated human monocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birx D. L., Berger M., Fleisher T. A. The interference of T cell activation by calcium channel blocking agents. J Immunol. 1984 Dec;133(6):2904–2909. [PubMed] [Google Scholar]
- Breugnot C., Mazière C., Auclair M., Mora L., Ronveaux M. F., Salmon S., Santus R., Morlière P., Lenaers A., Mazière J. C. Calcium antagonists prevent monocyte and endothelial cell-induced modification of low density lipoproteins. Free Radic Res Commun. 1991;15(2):91–100. doi: 10.3109/10715769109049129. [DOI] [PubMed] [Google Scholar]
- Cathcart M. K., Chisolm G. M., 3rd, McNally A. K., Morel D. W. Oxidative modification of low density lipoprotein (LDL) by activated human monocytes and the cell lines U937 and HL60. In Vitro Cell Dev Biol. 1988 Oct;24(10):1001–1008. doi: 10.1007/BF02620873. [DOI] [PubMed] [Google Scholar]
- Cathcart M. K., McNally A. K., Chisolm G. M. Lipoxygenase-mediated transformation of human low density lipoprotein to an oxidized and cytotoxic complex. J Lipid Res. 1991 Jan;32(1):63–70. [PubMed] [Google Scholar]
- Cathcart M. K., McNally A. K., Morel D. W., Chisolm G. M., 3rd Superoxide anion participation in human monocyte-mediated oxidation of low-density lipoprotein and conversion of low-density lipoprotein to a cytotoxin. J Immunol. 1989 Mar 15;142(6):1963–1969. [PubMed] [Google Scholar]
- Cathcart M. K., Morel D. W., Chisolm G. M., 3rd Monocytes and neutrophils oxidize low density lipoprotein making it cytotoxic. J Leukoc Biol. 1985 Aug;38(2):341–350. doi: 10.1002/jlb.38.2.341. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-sensitive ion channels. Science. 1988 Oct 7;242(4875):50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Chang J., Musser J. H., McGregor H. Phospholipase A2: function and pharmacological regulation. Biochem Pharmacol. 1987 Aug 1;36(15):2429–2436. doi: 10.1016/0006-2952(87)90512-0. [DOI] [PubMed] [Google Scholar]
- Chiou C. Y., Malagodi M. H. Studies on the mechanism of action of a new Ca-2+ antagonist, 8-(N,N-diethylamino)octyl 3,4,5-trimethoxybenzoate hydrochloride in smooth and skeletal muscles. Br J Pharmacol. 1975 Feb;53(2):279–285. doi: 10.1111/j.1476-5381.1975.tb07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czop J. K., Austen K. F. Functional discrimination by human monocytes between their C3b receptors and their recognition units for particulate activators of the alternative complement pathway. J Immunol. 1980 Jul;125(1):124–128. [PubMed] [Google Scholar]
- Daugherty A., Rateri D. L., Schonfeld G., Sobel B. E. Inhibition of cholesteryl ester deposition in macrophages by calcium entry blockers: an effect dissociable from calcium entry blockade. Br J Pharmacol. 1987 May;91(1):113–118. doi: 10.1111/j.1476-5381.1987.tb08989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Cusolito S., Sharp G. W. Effects of calcium antagonist TMB-8 on active Na and Cl transport in rabbit ileum. Am J Physiol. 1986 May;250(5 Pt 1):G691–G697. doi: 10.1152/ajpgi.1986.250.5.G691. [DOI] [PubMed] [Google Scholar]
- Fill M., Coronado R. Ryanodine receptor channel of sarcoplasmic reticulum. Trends Neurosci. 1988 Oct;11(10):453–457. doi: 10.1016/0166-2236(88)90198-1. [DOI] [PubMed] [Google Scholar]
- Gould R. J., Murphy K. M., Snyder S. H. [3H]nitrendipine-labeled calcium channels discriminate inorganic calcium agonists and antagonists. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3656–3660. doi: 10.1073/pnas.79.11.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Haverstick D. M., Engelhard V. H., Gray L. S. Three intracellular signals for cytotoxic T lymphocyte-mediated killing. Independent roles for protein kinase C, Ca2+ influx, and Ca2+ release from internal stores. J Immunol. 1991 May 15;146(10):3306–3313. [PubMed] [Google Scholar]
- Hessler J. R., Morel D. W., Lewis L. J., Chisolm G. M. Lipoprotein oxidation and lipoprotein-induced cytotoxicity. Arteriosclerosis. 1983 May-Jun;3(3):215–222. doi: 10.1161/01.atv.3.3.215. [DOI] [PubMed] [Google Scholar]
- Hoffman T., Brando C., Lizzio E. F., Lee Y. L., Hansen M., Tripathi A. K., Taplits M., Puri J., Bonvini E., Abrahamsen T. G. Calcium-dependent eicosanoid metabolism by concanavalin A-stimulated human monocytes in vitro. Synergism with phorbol ester indicates separate regulation of leukotriene B4 synthesis and release. J Immunol. 1991 Jan 15;146(2):692–700. [PubMed] [Google Scholar]
- Hosey M. M., Lazdunski M. Calcium channels: molecular pharmacology, structure and regulation. J Membr Biol. 1988 Sep;104(2):81–105. doi: 10.1007/BF01870922. [DOI] [PubMed] [Google Scholar]
- Jürgens G., Hoff H. F., Chisolm G. M., 3rd, Esterbauer H. Modification of human serum low density lipoprotein by oxidation--characterization and pathophysiological implications. Chem Phys Lipids. 1987 Nov-Dec;45(2-4):315–336. doi: 10.1016/0009-3084(87)90070-3. [DOI] [PubMed] [Google Scholar]
- Leslie C. C., Detty D. M. Arachidonic acid turnover in response to lipopolysaccharide and opsonized zymosan in human monocyte-derived macrophages. Biochem J. 1986 May 15;236(1):251–259. doi: 10.1042/bj2360251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mix L. L., Dinerstein R. J., Villereal M. L. Mitogens and melittin stimulate an increase in intracellular free calcium concentration in human fibroblasts. Biochem Biophys Res Commun. 1984 Feb 29;119(1):69–75. doi: 10.1016/0006-291x(84)91619-x. [DOI] [PubMed] [Google Scholar]
- Pedrinaci S., Ruiz-Cabello F., Gomez O., Collado A., Garrido F. Protein kinase C-mediated regulation of the expression of CD14 and CD11/CD18 in U937 cells. Int J Cancer. 1990 Feb 15;45(2):294–298. doi: 10.1002/ijc.2910450215. [DOI] [PubMed] [Google Scholar]
- Ratz P. H., Flaim S. F. Acetylcholine- and 5-hydroxytryptamine-stimulated contraction and calcium uptake in bovine coronary arteries: evidence for two populations of receptor-operated calcium channels. J Pharmacol Exp Ther. 1985 Sep;234(3):641–647. [PubMed] [Google Scholar]
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987 Feb 19;325(6106):717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Rosales C., Brown E. J. Calcium channel blockers nifedipine and diltiazem inhibit Ca2+ release from intracellular stores in neutrophils. J Biol Chem. 1992 Jan 25;267(3):1443–1448. [PubMed] [Google Scholar]
- Schmitz G., Hankowitz J., Kovacs E. M. Cellular processes in atherogenesis: potential targets of Ca2+ channel blockers. Atherosclerosis. 1991 Jun;88(2-3):109–132. doi: 10.1016/0021-9150(91)90074-d. [DOI] [PubMed] [Google Scholar]
- Schuh J., Fairclough G. F., Jr, Haschemeyer R. H. Oxygen-mediated heterogeneity of apo-low-density lipoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3173–3177. doi: 10.1073/pnas.75.7.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirhatti V., Krishna G. A simple and sensitive method for monitoring drug-induced cell injury in cultured cells. Anal Biochem. 1985 Jun;147(2):410–418. doi: 10.1016/0003-2697(85)90290-8. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J., Phan S. H., Gibbs D. F., Ryan U. S., Ward P. A. H2O2-mediated cytotoxicity of rat pulmonary endothelial cells. Changes in adenosine triphosphate and purine products and effects of protective interventions. Lab Invest. 1990 Nov;63(5):683–689. [PubMed] [Google Scholar]
- Warren J. B., Ryan U. S. Endothelial injury assessed by isotope release: 3H-adenine compared with 51Cr. In Vitro Cell Dev Biol. 1989 Apr;25(4):334–335. doi: 10.1007/BF02624595. [DOI] [PubMed] [Google Scholar]
- Zimányi I., Pessah I. N. Comparison of [3H]ryanodine receptors and Ca++ release from rat cardiac and rabbit skeletal muscle sarcoplasmic reticulum. J Pharmacol Exp Ther. 1991 Mar;256(3):938–946. [PubMed] [Google Scholar]
- Zobrist R. H., Giacomini K. M., Nelson W. L., Giacomini J. C. The interaction of phenylalkylamine calcium channel blockers with the 1,4-dihydropyridine binding site. J Mol Cell Cardiol. 1986 Sep;18(9):963–974. doi: 10.1016/s0022-2828(86)80010-4. [DOI] [PubMed] [Google Scholar]
- el-Saadani M., Esterbauer H., el-Sayed M., Goher M., Nassar A. Y., Jürgens G. A spectrophotometric assay for lipid peroxides in serum lipoproteins using a commercially available reagent. J Lipid Res. 1989 Apr;30(4):627–630. [PubMed] [Google Scholar]
- van de Winkel J. G., Tax W. J., Jacobs C. W., Huizinga T. W., Willems P. H. Cross-linking of both types of IgG Fc receptors, Fc gamma RI and Fc gamma RII, enhances intracellular free Ca2+ in the monocytic cell line U937. Scand J Immunol. 1990 Mar;31(3):315–325. doi: 10.1111/j.1365-3083.1990.tb02774.x. [DOI] [PubMed] [Google Scholar]