Abstract
The development and production of recombinant monoclonal antibodies is well established. Although most of these are IgGs, there is also great interest in producing recombinant IgAs since this isotype plays a critical role in providing immunologic protection at mucosal surfaces. the choice of expression system for production of recombinant antibodies is crucial because they are glycoproteins containing at least one N-linked carbohydrate. these glycans have been shown to contribute to the stability, pharmacokinetics and biologic function of antibodies. We have produced recombinant human IgA1 and all three allotypes of IgA2 in murine myeloma and CHo cell lines to systematically characterize and compare the N-linked glycans. Recombinant IgAs produced in murine myelomas differ significantly from IgA found in humans in that they contain the highly immunogenic Galα(1,3)Gal epitope and N-glycolylneuraminic acid residues, indicating that murine myeloma is not the optimal expression system for the production of human IgA. In contrast, IgAs produced in CHo cells contained glycans that were more similar to those found on human IgA. expression of IgA1 and IgA2 in Lec2 and Lec8 cell lines that are defective in glycan processing resulted in a less complex pool of N-glycans. In addition, the level of sialylation of rIgAs produced in murine and CHo cells was significantly lower than that previously reported for serum IgA1. these data underscore the importance of choosing the appropriate cell line for the production of glycoproteins with therapeutic potential.
Key words: recombinant antibody, IgA, glycosylation, expression system, mass spectrometry
Introduction
The development of therapeutic antibodies to combat diseases such as cancer and protect against pathogens is an important area of research. Although all of the monoclonal antibodies (mAbs) approved for clinical use to date are of the IgG isotype, there is much interest in the production of recombinant IgAs (rIgA). IgA is the most abundant class of immunoglobulin (Ig) produced in humans and is critical in conferring immunity at mucosal surfaces, which are the primary route of entry for many pathogenic organisms. Among other things, binding of IgA to bacteria and viruses neutralizes and prevents attachment of the pathogens to mucosal cells, thereby inhibiting infection and colonization. Because of this crucial role in mucosal immunity, there is much interest in engineering IgAs for clinical use. Indeed, rIgAs against pathogens such as Clostridium difficile, Streptococcus mutans and Neisseria meningitidis have been shown to confer protection.1–3 In addition to its role in immunity against microbes, IgA has also shown promise in the treatment of cancer. Studies have shown exchange that engagement of the IgA receptor FcαRI can result in tumor cell killing by neutrophils and polymorphonuclear cells,4–6 and rIgA directed against HLA class II was effective in triggering lymphoma cell lysis.7
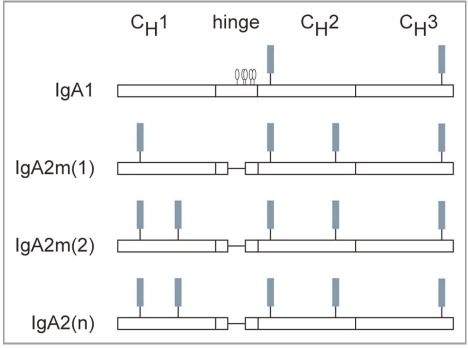
All Igs are glycoproteins, containing at least one N-linked carbohydrate. IgGs contain one N-linked glycan at Asn297 in the CH2 domain. The glycosylation pattern for human IgA is complex as it contains two to five N-linked carbohydrate addition sites, depending on the isotype and allotype of IgA. IgA1, but not IgA2, contains a hinge region with three to five O-linked carbohydrates. The IgA1 heavy (H) chain contains two N-linked carbohydrates, one in CH2 (Asn263) and the other in the tail-piece extension of CH3 (Asn459).8 In addition to these sites, IgA2 possesses an additional N-linked site in CH1 (Asn166) and in CH2 (Asn337). The IgA2m2 and IgA2n allotypes have a fifth site in CH1 (Asn211).8,9 The carbohydrate addition sites present on the IgA H chain are shown as a schematic in Figure 1. Variable region glycosylation has also been reported for some IgAs.8,10 In addition, IgA can polymerize into dimers containing J chain, which has one N-linked glycan, and can be assembled into secretory IgA (SIgA), which has an additional seven N-linked glycans on secretory component (SC).
Figure 1.
Glycosylation of human IgA. IgA1 has N-linked carbohydrate addition sites in CH2 (Asn263) and in CH3 (Asn459) and o-linked carbohydrate in its hinge region. IgA2 lacks linked carbohydrates, but has additional N-linked sites in CH1 (Asn166) and in CH2 (Asn337). the IgA2m2 and the IgA2n allotypes have a fifth N-linked site in CH1 (Asn211). ▪, N-linked glycan; O, O-linked glycan.
Several papers have described the N-glycans on IgA from normal serum and human myeloma-derived IgA. One consistent finding from these studies is that the predominant glycans found are complex biantennary structures with high levels of sialylation.10–15 These glycans vary in the presence or absence of a bisecting N-acetylglucosamine (GlcNAc) and core fucose (Fuc). Some myeloma IgAs also contain incompletely galactosylated complex glycans and some tri- and tetra-antennary and oligomannose structures.12,15 In addition, there appears to be evidence for site-specific glycan processing as fucosylation is observed in the N-glycans from the tail-piece of IgA1 but not in the CH2 glycan.15,16
As mentioned above, IgA is present in different molecular forms. It exists mostly as a monomer in serum, but is found as a dimer and as SIgA at mucosal surfaces. Interestingly, the various molecular forms of IgA differ in their carbohydrate composition. Oligomannose glycans have been found exclusively on polymeric but not monomeric serum IgA, and polymeric IgA appears to contain less disialylated glycans than monomeric IgA.14 SIgA glycans differ significantly from that of serum IgA. Serum IgA has shown to be highly sialylated with reports ranging from 65% to >90%,10,13,14 while the H chain of SIgA contains <15% sialylation.17 The H chain glycans on SIgA are less processed with 66% of the N-glycans containing a free terminal GlcNAc on one antenna and 12% constituting oligomannose structures.17 In addition, Th2 cytokines have been shown to influence the structure of IgA glycans in mice,18,19 suggesting that the microenvironment of IgA synthesis plays a role in determining which glycoforms are produced.
An important factor to consider when producing recombinant glycoproteins is that glycan processing is species- and cell type-specific. Mammalian cell lines such as murine myelomas and Chinese hamster ovary (CHO) cells have been used extensively for the production of rIgGs because they generate proteins that closely resemble human antibodies. FDA approved mAbs produced in the Sp2/0 murine myeloma cell line include cetuximab, infliximab and basiliximab, while CHO cell-produced mAbs include alemtuzumab, rituximab and trastuzumab. Although these rIgGs contain N-glycan structures that are similar to, or the same as, those found in human serum IgG, it is not clear if other antibody isotypes also undergo the same type of carbohydrate processing in these expression systems. Therefore, we produced rIgAs in the Sp2/0 murine myeloma and CHO cell lines and analyzed the structures of the N-linked carbohydrates. In the murine myeloma expression system, the glycan profiles of rIgAs were much more complex than that found in serum IgA. In addition, the glycans on rIgAs produced in murine myelomas were different from that found in rIgGs in that they contain the Galα(1,3)Gal epitope and N-glycolylneuraminic acid (NGNA) residues that are not found in humans; however, in the CHO expression system, the rIgAs generally contained carbohydrates resembling those found in human IgA.
Another advantage of using CHO cells is the availability of mutants that are defective in glycan processing. rIgA was also expressed in the Lec2 and Lec8 CHO cell lines, resulting in a less heterogeneous mixture of glycoforms. In addition, the level of sialylation for the rIgAs was found to be different from that reported for human serum IgA1. These studies underscore the importance of the choice of expression system for production of glycoproteins. In the case of human IgA, CHO cells appear to be more appropriate than murine myeloma cells for the production of rIgAs.
Results
Determination of N-glycan structures on rIgAs
The N-linked glycans on rIgGs produced in murine myelomas have been found to be similar to those found on human serum IgG; however, murine and human cells differ in carbohydrate processing. Given the number of N-glycan sites as well as the fact that the glycans are exposed on the surface of IgA, there is concern that glycans on rIgAs may differ significantly from those found on human IgA. To address this concern, we analyzed the N-glycans from rIgAs produced in Sp2/0 cells. These chimeric Igs contain the same murine variable region specific for the hapten dansyl and the human α1, α2m1, α2m2 or α2n H chain. Previous studies have shown that about 50–75% of the proteins are expressed as dimers that incorporate the endogenous mouse J chain produced by the Sp2/0 cells.20 The structures of the N-glycans were determined using MALDI-TOF. The acidic glycans were detected in the negative mode as (M-H)− ions, neutral glycans were detected in the positive mode as (M+Na)+ ions, and the corresponding mass was expressed as mass/charge (m/z). The spectral peaks were assigned as likely N-glycan structures using the Cartoonist computer software21 based on mass and published information regarding the glycans attached by the cell line. For instance, murine myelomas and CHO cells lack expression of N-acetylglucosaminyltransferase and do not add a bisecting GlcNAc.22 For some of the spectral peaks, there was ambiguity in the assignment of a glycan structure. In these cases, all possible structures were assigned to the peaks.
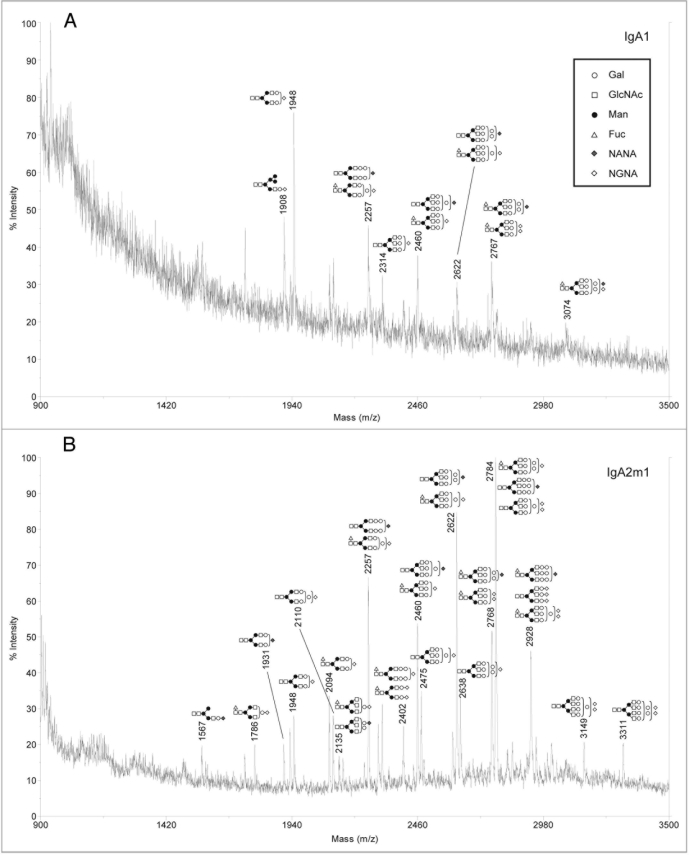
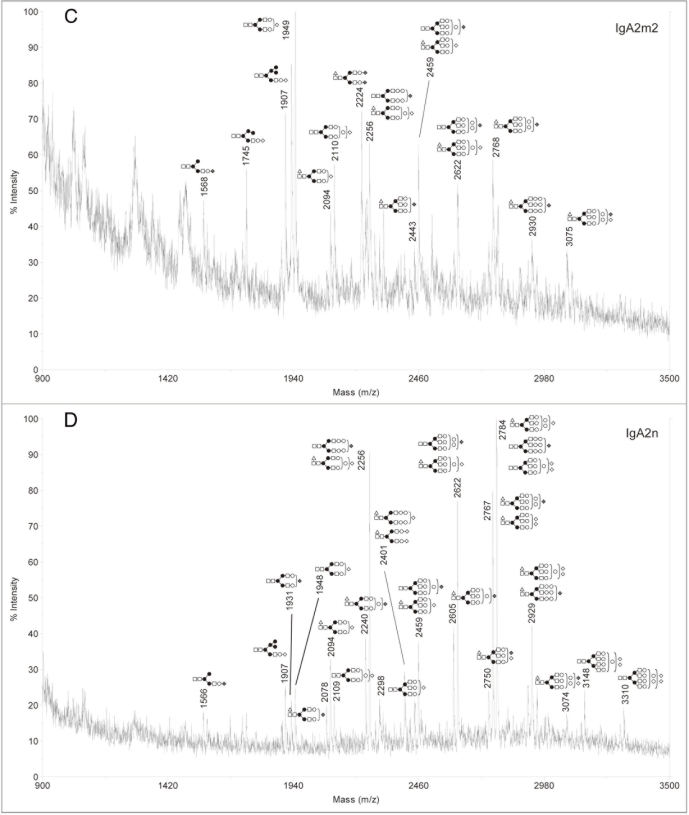
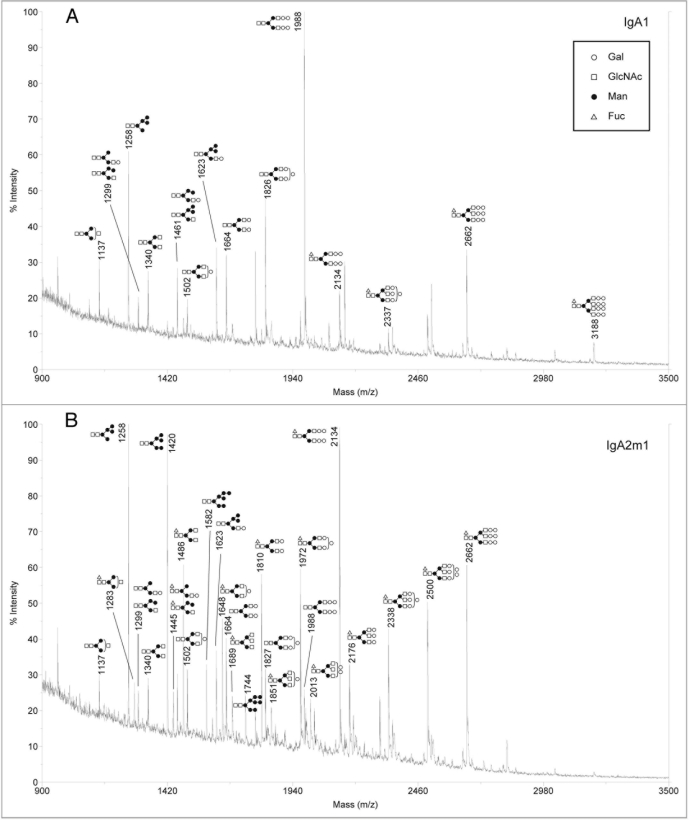
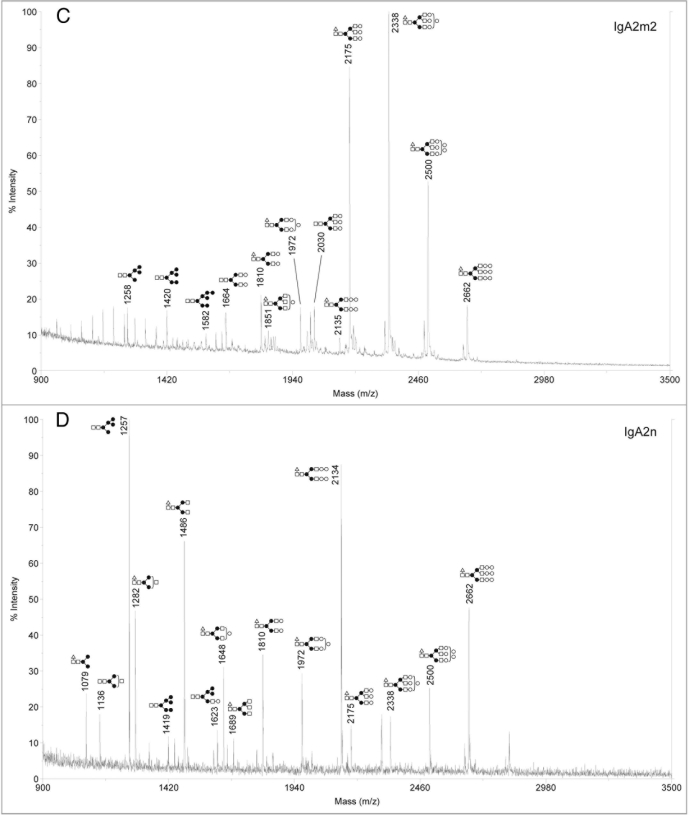
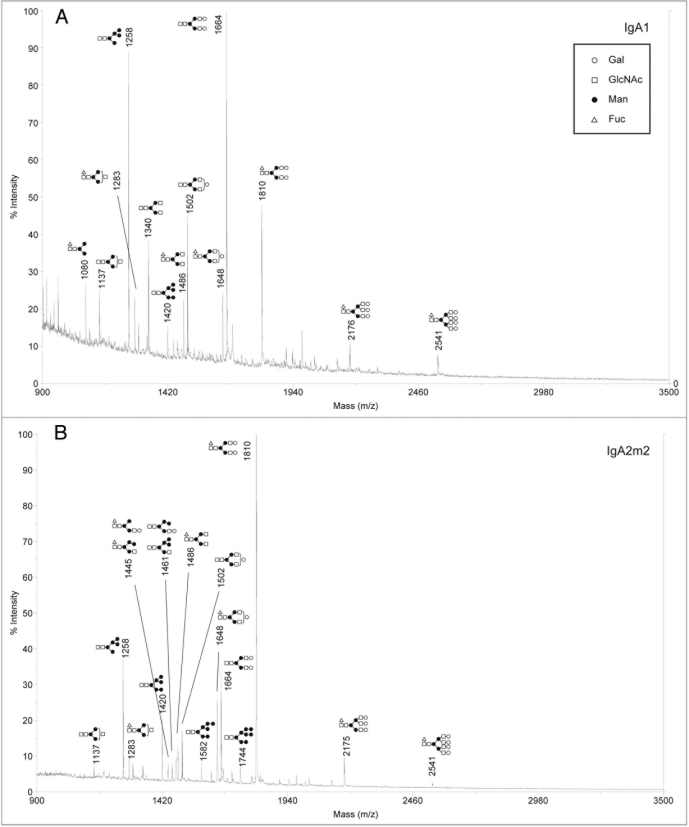
Analysis of sialylated glycans on rIgAs produced in murine myelomas showed a mixture of hybrid and complex N-linked glycans (Fig. 2). The sialylated glycan pool for the rIgA2 allotypes was more complex than that of IgA1 possibly due to the fact that IgA2 contains four or five N-linked glycans while IgA1 contains two. All the rIgAs differed dramatically from the acidic glycans found on human serum IgA1, which have been reported to be predominantly biantennary with one or two terminal sialic acid residues.10,13 In addition to the biantennary structures, rIgA contained glycans with considerably higher mass that probably represent tri- and tetra-antennary carbohydrates. Another significant difference is that rIgAs produced in murine myelomas contain both N-acetylneuraminic acid (NANA) and N-glycolylneuraminic acid (NGNA) while only NANA is incorporated into human glycoproteins. The neutral glycans on rIgAs were also analyzed and were found to contain a mixture of high mannose, hybrid and biantennary, as well as more complex higher mass structures (Fig. 3). This is significantly different from the glycans found on rIgGs produced in murine myeloma cells in which the majority of the glycans are biantennary. In addition, all isotypes and allotypes of rIgAs contained glycans with the Galα(1,3)Gal epitope. Although this highly immunogenic epitope is usually not observed for rIgGs produced in murine myelomas, it was prevalent in rIgAs. The Galα(1,3)Gal epitope was found in neutral glycans (Fig. 3) but was especially prevalent in sialylated glycans (Fig. 2). Interestingly, the high mannose structures found on rIgAs were not fucosylated.
Figure 2.
Negative ion MALDI-TOF analysis of acidic N-glycans released from rIgAs produced in murine myeloma cell line Sp2/0. the likely glycan structures are shown as a schematic and the mass of the glycans is expressed as m/z. (A) IgA1, (B) IgA2m1, (C) IgA2m2, (D) IgA2n.
Figure 3.
Positive ion MALDI-TOF analysis of neutral N-glycans released from rIgAs produced in murine myeloma cell line Sp2/0. the likely glycan structures are shown as a schematic and the mass of the glycans is expressed as m/z. (A) IgA1, (B) IgA2m1, (C) IgA2m2, (D) IgA2n.
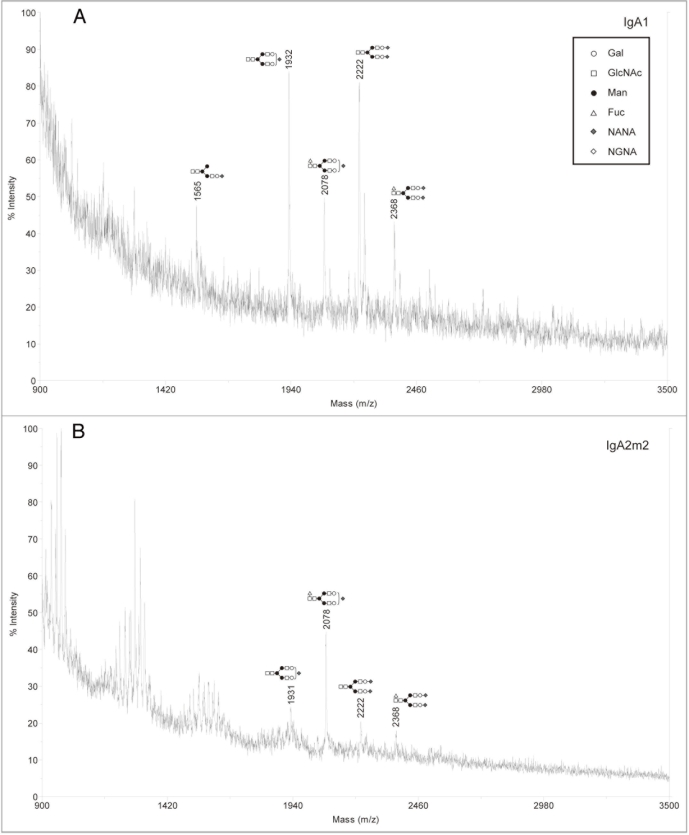
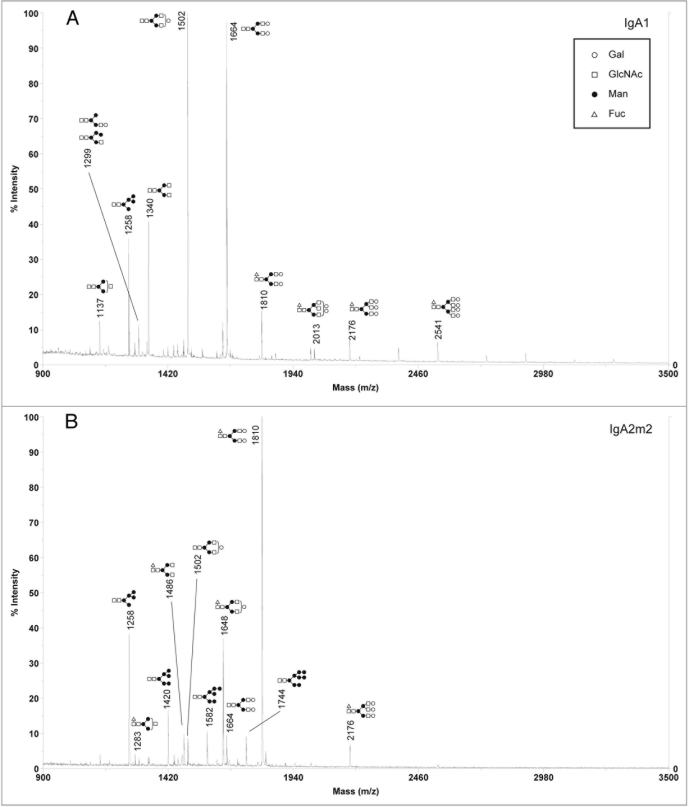
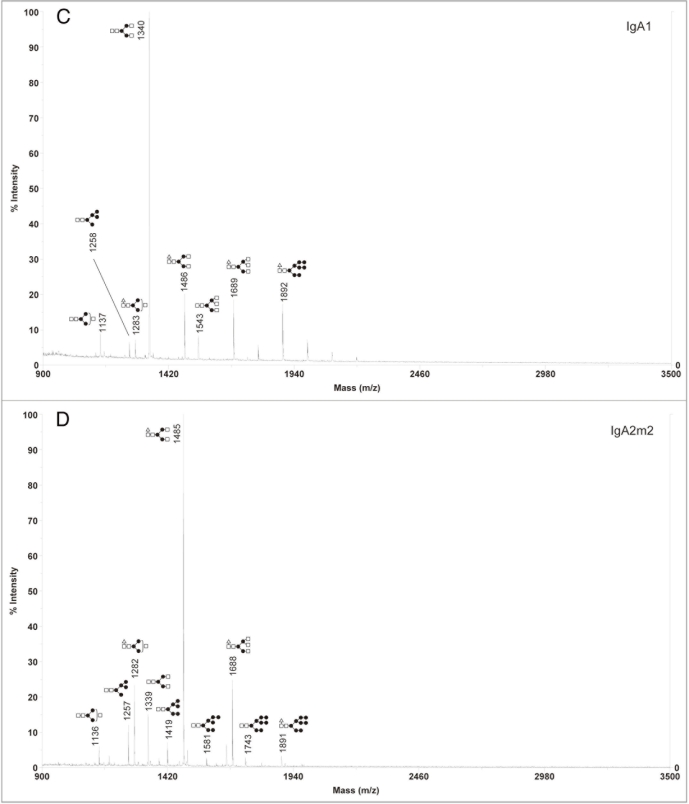
Since our analysis showed that murine myeloma is not an ideal expression system for the production of rIgAs, we next focused on the CHO cell line, which is also commonly used for the production of recombinant antibodies. rIgA1 and rIgA2m2 were produced in the wild-type Pro5 cells and the N-glycans were analyzed by MALDI-TOF. In contrast to murine myeloma derived rIgA, the sialylated glycan profiles for rIgAs produced in CHO cells were relatively simple in that only a few glycans were detected with most being biantennary with one or two NANA residues (Fig. 4). This is very similar to what is found on serum IgA1.10,13 For neutral glycans, high mannose, hybrid and complex glycans were observed (Fig. 5). Moreover, the CHO derived IgAs differed significantly from those produced in murine myelomas in that they did not contain Galα(1,3)Gal or NGNA. Similar to murine myeloma produced rIgAs, fucosylation was not observed on high mannose N-glycans.
Figure 4.
Negative ion MALDI-TOF analysis of acidic N-glycans released from rIgAs produced in CHO cells. The likely glycan structures are shown as a schematic and the mass of the glycans is expressed as m/z. (A) IgA1, (B) IgA2m2.
Figure 5.
positive ion MALDI-TOF analysis of neutral N-glycans released from rIgAs produced in CHO cells. the likely glycan structures are shown as a schematic and the mass of the glycans is expressed as m/z. (A) IgA1, (B) IgA2m2.
One of the confounding challenges in producing recombinant glycoproteins is the ability to characterize and produce homogeneous batches. The glycans on IgG and on IgA are known to contribute to antibody function. Therefore, it would be highly advantageous to be able to control the types of glycoforms that are produced. Although various approaches can be taken to address this issue, we focused on the production of rIgA in mutant CHO cell lines (Lec2 and Lec8), which have alternations in their glycosylation processing machinery. Lec2 is defective in the transport of CMP-sialic acid and therefore produces carbohydrate structures that lack sialic acid,23 while Lec8 does not attach Gal residues because is defective in the transport of UDP-Gal.24 rIgA1 and rIgA2m2 were produced in these cell lines and the N-glycans were analyzed. As expected, the Lec2 derived IgAs contained high mannose, hybrid and complex glycans with terminal Gal residues (Fig. 6A and B) while the IgAs produced in Lec8 contained only high mannose and complex glycans with terminal GlcNAc (Fig. 6C and D). Interestingly, the dominant glycans detected in the Lec2 and Lec8 cell lines differed between rIgA1 and rIgA2m2. In Lec2 cells, the dominant peak for rIgA1 was unfucosylated biantennary with one or two terminal Gal while a core fucosylated biantennary glycan with two terminal Gal predominated for rIgA2m2. In Lec8 cells, the dominant peak for rIgA1 was unfucosylated biantennary with terminal GlcNAc while a core fucosylated biantennary glycan with terminal GlcNAc predominated for rIgA2m2. In addition, Man8 containing a core Fuc was detected in rIgA1 and rIgA2m2 produced in Lec8 cells.
Figure 6.
Positive ion MALDI-TOF analysis of neutral N-glycans released from rIgAs produced in mutant CHO cell lines defective in glycan processing. The likely glycan structures are shown as a schematic and the mass of the glycans is expressed as m/z. (A) IgA1 produced in Lec2, (B) IgA2m2 produced in Lec2, (C) IgA1 produced in Lec8, (D) IgA2m2 produced in Lec8.
To determine if the IgA containing different glycoforms differed in stability, we incubated rIgA2m2 produced in myeloma, CHO, Lec2 and Lec8 cells for 1, 2 and 7 days at 37°C. The proteins were subjected to SDS-PAGE and found to be intact. Degradation of the proteins was not observed (data not shown).
Determination of levels of sialylation of rIgA1 and rIgA2
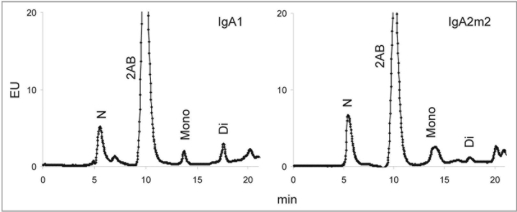
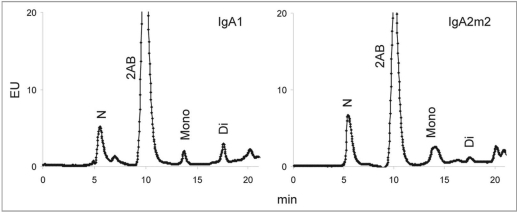
The degree of sialylation may affect various properties of glycoproteins. Terminal sialylation of N-linked glycans on IgG has been shown to impart anti-inflammatory properties,25,26 and the level of sialylation appears to be a contributing factor in the rate of serum clearance of IgA.20 WAX chromatography was performed to determine the relative levels of neutral and sialylated glycans on rIgAs. The relative levels of neutral and mono- and disialylated glycans from rIgA1, IgA2m1 and IgA2n produced in murine myelomas are shown in Figure 7. The rIgA allotypes did not differ significantly in the levels of sialylation. Similar results were obtained for rIgA1 and IgA2m2 produced in the CHO cell line (Fig. 8). The level of sialylation observed for the rIgAs was approximately 50% in both expression systems, which is lower than the >90% reported for serum IgA1.10,13
Figure 7.
WAX chromatography of N-linked glycans from IgA1, IgA2m1 and IgA2n glycans produced in murine myelomas. The peaks were assigned by comparison with glycan standards isolated from bovine fetuin (data not shown). N, neutral glycans; 2AB, free 2-AB label; Mono, mono-sialylated glycans; Di, di-sialylated glycans.
Figure 8.
WAX chromatography of N-linked glycans from IgA1, and IgA2m2 produced in CHO cells. The peaks were assigned by comparison with glycan standards isolated from bovine fetuin (data not shown). N, neutral glycans; 2AB, free 2-AB label; Mono, mono-sialylated glycans; Di, di-sialylated glycans.
Discussion
Glycosylation is an important post-translational modification that can have profound effects on the properties of proteins, and even small changes to N-glycan structure can produce significant alterations in antibody effector functions. In the case of IgG, the absence or presence of the core fucose on the CH2 glycan influenced the ability of mononuclear and polymorphonuclear cells to mediate antibody-dependent cellular cytotoxicity,27,28 and IgGs containing terminal sialic acid residues have been shown have anti-inflammatory properties.25,26 In addition, the IgG N-glycan is known to influence binding to Fcγ receptors.29 Similarly, the glycans on IgA also serve an important role. Aberrant O-linked glycosylation in the IgA1 hinge has been linked to IgA nephropathy,30–33 a disease characterized by deposition of IgA immune complexes in the glomerular mesangium, and there is some evidence that abnormalities in IgA N-glycans may also be involved.34 The N-linked glycans have been shown to contribute to the proper assembly and stability of IgA.35,36 In addition, oligosaccharides on SIgA participate in innate immunity. The O- and N-glycans of SIgA contain a variety of epitopes that can bind to bacterial adhesions, and terminal GlcNAc residues on the H chain of SIgA may be ligands for lectins such as mannose-binding lectin.17 The glycans on SC localize SIgA to the mucus lining of the epithelial surface,37 thereby linking the innate and adaptive immune responses. Various studies have addressed the role of IgA glycans in receptor binding. The crystal structure of human FcαRI with IgA1-Fc revealed that although the CH2 glycan is in close proximity to FcαRI, it does not directly contact the receptor.38 Other studies showed very little or no differences in binding to FcαRI for myeloma-derived, recombinant (produced in CHO cells) and serum monomeric IgA1 with or without the CH2 or tail-piece glycans;10,15,39 however, rIgA1 lacking the CH2 glycan produced in insect cells showed complete loss of binding to FcαRI.40 Increasing the level of sialylation resulted in a decrease in the interaction of IgA1 and IgA2 with the asialoglycoprotein receptor (ASGPR),41 while desialylation and degalactosylation of IgA1 appeared to enhance its binding to the transferrin receptor on human mesangial cells.42 N-linked glycosylation on IgA1 was not required for association with the polymeric immunoglobulin receptor,43 and the role, if any, of IgA glycans in binding to the Fcα/μ receptor which binds both IgA and IgM44 has not yet been established. These studies suggest the intriguing possibility that IgA glycoforms may bind differentially to the various IgA receptors and modulate immune responses.
To date, mammalian cell lines have been the preferred expression system for the production of therapeutic proteins because their intrinsic glycosylation machinery closely resembles that in human. Many therapeutic IgGs have been produced in murine and CHO cell lines and have demonstrated efficacy in the clinic. Analysis of IgG glycans from human serum, as well as those produced recombinantly in murine myelomas and CHO cells, has shown the glycans to be relatively homogeneous with most being complex biantennary with zero, one or two terminal galactose residues. The IgG carbohydrate is sequestered within the Fc, limiting the extent to which it can be processed. In contrast, the glycosylation of IgA is much more complex. Human IgA contains two to five glycans on each H chain that are exposed on the protein surface,38,45,46 making them accessible for processing.
Our studies showed that the N-linked carbohydrates present on IgA expressed in murine myeloma cells are much more heterogeneous than those present on IgG expressed in the same system. In addition, the glycosylation pattern on rIgAs was significantly different from that found in human serum IgA1. Our analysis revealed that one significant difference between IgG and IgA produced in murine cells is the presence of NGNA residues in rIgAs. Humans do not produce NGNA because of a mutation in the CMAH gene.47,48 NGNA is known to be immunogenic, and there is a high prevalence of anti-NGNA antibodies in normal human sera.49,50 Although not typically found in rIgGs produced in murine cells, our studies showed the prevalence of the highly immunogenic Galα(1,3)Gal epitope in rIgA produced in Sp2/0 cells. A significant amount of circulating IgG in man has been reported to specifically interact with this epitope, which is abundant on glycoconjugates of non-primate mammals, prosimians, and New World monkeys, but absent from Old World monkeys, apes and man.51 Therefore, the presence of these carbohydrate structures on therapeutic proteins would have significant consequences. The differences between IgG and IgA are probably due to the highly exposed nature of the N-glycans in IgA. This is supported by data from cetuximab, an IgG produced in Sp2/0 cells. Cetuximab is an anti-epidermal growth factor receptor mAb approved by the FDA for the treatment of various solid tumors. The Fab from cetuximab has an N-linked carbohydrate that was shown to contain NGNA residues and the Galα(1,3)Gal epitope,52 which induced anaphylaxis in some patients.53 Given these issues, murine cells are not the optimal system for the production of rIgAs.
The N-linked glycan profiles of rIgAs produced in CHO cells were much more similar to that reported for serum IgA. The immunogenic Galα(1,3)Gal epitope was absent from rIgAs produced in CHO cells. Although NGNA residues have been reported to be present in erythropoietin and fetuin produced in CHO cells,54 both rIgA1 and rIgA2m2 produced in CHO cells did not contain N-glycans bearing NGNA. Only NANA sialic acids appeared to be on the CHO derived proteins (Fig. 4). It is unclear why NGNA is not incorporated into rIgAs, but may be due to external factors such as culturing conditions. In addition, we expressed rIgA1 and rIgA2 in the Lec2 and Lec8 mutant cell lines in an effort to have N-glycans attached that are more homogeneous. Indeed, rIgAs lacking sialic acid were produced in Lec2 cells and rIgAs lacking both sialic acid and galactose were produced in Lec8 cells. Expression in these cell lines resulted in a less heterogeneous mixture of IgA glycoforms. Notably, IgA1 and IgA2 differed in the dominant structures attached when expressed in Lec2 or Lec8 cells (Fig. 6). Others have used strategies in which key enzymes in the glycosylation pathway were overexpressed or knocked down as a way to control protein glycosylation. These approaches should prove fruitful in producing therapeutics with specific effector functions and maintaining product quality attributes.
Using WAX chromatography we found that only about 50% of the glycans present on rIgAs produced by CHO and murine cells were sialylated. This contrasts with serum IgA1, in which >90% of the N-glycans contain sialic acid.10,13 It is not clear if these differences reflect the polymerization state of the different IgAs or the expression system; it may be that human plasma cells are more efficient in attaching sialic acid. This difference in the level of sialylation may have a significant impact on the functional properties of rIgA as sialylation of N-linked glycans has been shown to impart anti-inflammatory properties to IgG.25,26 In addition, the level of sialylation was shown to contribute to the serum clearance of IgA.20
Although Igs have very similar protein structure, they differ significantly in their carbohydrate composition. The carbohydrates of rIgGs produced in CHO and murine cells are very similar to those found on serum IgG; however, our data indicate that is not the case for rIgA. Therefore, careful attention should be paid in the decision to use a particular cell line for expression of recombinant proteins. It will also be imperative to consider the characteristics of the protein itself, such as whether the N-glycan sites are exposed or are buried, and what role carbohydrates play in the overall function of the protein. The availability of improved expression systems and strategies to better control the glycan processing pathways should facilitate the production of more effective therapeutic glycoproteins.
Materials and Methods
Production and purification of rIgA.
Chimeric human IgA1, IgA2m1, IgA2m2 and IgA2n specific for the hapten dansyl were produced in the Sp2/0 murine myeloma cell line55 and in the CHO cell line56 as described previously. rIgA1 and IgA2 antibodies were purified from culture supernatant by DNS-Sepharose affinity chromatography57 and the concentrations of the proteins were determined using the BCA Protein Assay (Pierce; Rockford, IL).
MALDI-TOF analysis of N-linked oligosaccharides
The N-linked carbohydrates on IgA1 and IgA2 were cleaved from the proteins using N-glycanase (Prozyme) and isolated as described by Papac et al.58 10–100 µg of IgA1 or IgA2 protein was used for the isolation of oligosaccharides. The matrices used for MALDI-TOF analysis, 2′,4′,6′-trihydroxyacetophenon monohydrate (THAP), 2,5-dihydroxybenzoic acid (DHB) and 5-methoxysalicylic acid, were purchased from Aldrich (Milwaukee, WI). The glycans were analyzed immediately using MALDI-TOF in positive reflector mode and negative linear mode. Storage of glycans for more than 5 days led to considerable loss of signal, especially in the analysis of acidic carbohydrates. Multiple clones of rIgAs were analyzed to ensure the reproducibility of the data and to rule out clonal variability.
Analysis of glycans by HPLC
N-linked glycans were released from purified IgA by cleavage using N-glycanase (Prozyme) and isolated as described above. Glycans were then fluorescently labeled with 2-aminobenzamide (AB) using the Glyko Signal 2-AB Labeling Kit (Prozyme) and the glycan samples were cleaned using GlycoClean S Cartridges (Prozyme). Weak anion exchange (WAX) chromatography was carried out using a GlycoSep-C column (size 7.5 × 75 mm; Prozyme) at 30°C using the 500 mM ammonium formate, pH 4.5 buffer system as recommended by the manufacturer. The glycan peaks were assigned by comparing with standard sugars isolated from bovine fetuin.
Acknowledgements
This work was supported by grants AI29470 and AI51415 from the NIH.
Abbreviations
- CHO
Chinese hamster ovary
- Fuc
fucose
- Gal
galactose
- GlcNAc
N-acetylglucosamine
- Ig
immunoglobulin
- mAbs
monoclonal antibodies
- NANA
N-acetylneuraminic acid
- NGNA
N-glycolylneuraminic acid
- rIg
recombinant immunoglobulin
- SC
secretory component
- SIgA
secretory IgA
- WAX
weak anion exchange
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/11802
References
- 1.Stubbe H, Berdoz J, Kraehenbuhl JP, Corthesy B. Polymeric IgA is superior to monomeric IgA and IgG carrying the same variable domain in preventing Clostridium difficile toxin A damaging of T84 monolayers. J Immunol. 2000;164:1952–1960. doi: 10.4049/jimmunol.164.4.1952. [DOI] [PubMed] [Google Scholar]
- 2.Ma JK, Hikmat BY, Wycoff K, Vine ND, Chargelegue D, Yu L, et al. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat Med. 1998;4:601–606. doi: 10.1038/nm0598-601. [DOI] [PubMed] [Google Scholar]
- 3.Vidarsson G, van Der Pol WL, van Den Elsen JM, Vile H, Jansen M, Duijs J, et al. Activity of human IgG and IgA subclasses in immune defense against Neisseria meningitidis serogroup B. J Immunol. 2001;166:6250–6256. doi: 10.4049/jimmunol.166.10.6250. [DOI] [PubMed] [Google Scholar]
- 4.Stockmeyer B, Dechant M, van Egmond M, Tutt AL, Sundarapandiyan K, Graziano RF, et al. Triggering Fcalpha-receptor I (CD89) recruits neutrophils as effector cells for CD20-directed antibody therapy. J Immunol. 2000;165:5954–5961. doi: 10.4049/jimmunol.165.10.5954. [DOI] [PubMed] [Google Scholar]
- 5.van Egmond M, van Spriel AB, Vermeulen H, Huls G, van Garderen E, van de Winkel JG. Enhancement of polymorphonuclear cell-mediated tumor cell killing on simultaneous engagement of FcgammaRI (CD64) and FcalphaRI (CD89) Cancer Res. 2001;61:4055–4060. [PubMed] [Google Scholar]
- 6.Otten MA, Rudolph E, Dechant M, Tuk CW, Reijmers RM, Beelen RH, et al. Immature neutrophils mediate tumor cell killing via IgA but not IgG Fc receptors. J Immunol. 2005;174:5472–5480. doi: 10.4049/jimmunol.174.9.5472. [DOI] [PubMed] [Google Scholar]
- 7.Dechant M, Vidarsson G, Stockmeyer B, Repp R, Glennie MJ, Gramatzki M, et al. Chimeric IgA antibodies against HLA class II effectively trigger lymphoma cell killing. Blood. 2002;100:4574–4580. doi: 10.1182/blood-2002-03-0687. [DOI] [PubMed] [Google Scholar]
- 8.Torano A, Tsuzukida Y, Liu YS, Putnam FW. Location and structural significance of the oligosaccharides in human Ig-A1 and IgA2 immunoglobulins. Proc Natl Acad Sci USA. 1977;74:2301–2305. doi: 10.1073/pnas.74.6.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chintalacharuvu KR, Raines M, Morrison SL. Divergence of human alpha-chain constant region gene sequences. A novel recombinant alpha2 gene. J Immunol. 1994;152:5299–5304. [PubMed] [Google Scholar]
- 10.Mattu TS, Pleass RJ, Willis AC, Kilian M, Wormald MR, Lellouch AC, et al. The glycosylation and structure of human serum IgA1, Fab and Fc regions and the role of N-glycosylation on Fcalpha receptor interactions. J Biol Chem. 1998;273:2260–2272. doi: 10.1074/jbc.273.4.2260. [DOI] [PubMed] [Google Scholar]
- 11.Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. J Biol Chem. 1974;249:7260–7269. [PubMed] [Google Scholar]
- 12.Endo T, Mestecky J, Kulhavy R, Kobata A. Carbohydrate heterogeneity of human myeloma proteins of the IgA1 and IgA2 subclasses. Mol Immunol. 1994;31:1415–1422. doi: 10.1016/0161-5890(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 13.Field MC, Amatayakul-Chantler S, Rademacher TW, Rudd PM, Dwek RA. Structural analysis of the N-glycans from human immunoglobulin A1: comparison of normal human serum immunoglobulin A1 with that isolated from patients with rheumatoid arthritis. Biochem J. 1994;299:261–275. doi: 10.1042/bj2990261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oortwijn BD, Roos A, Royle L, van Gijlswijk-Janssen DJ, Faber-Krol MC, Eijgenraam JW, et al. Differential glycosylation of polymeric and monomeric IgA: a possible role in glomerular inflammation in IgA nephropathy. J Am Soc Nephrol. 2006;17:3529–3539. doi: 10.1681/ASN.2006040388. [DOI] [PubMed] [Google Scholar]
- 15.Gomes MM, Wall SB, Takahashi K, Novak J, Renfrow MB, Herr AB. Analysis of IgA1 N-glycosylation and its contribution to FcalphaRI binding. Biochemistry. 2008;47:11285–11299. doi: 10.1021/bi801185b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka A, Iwase H, Hiki Y, Kokubo T, Ishii-Karakasa I, Toma K, et al. Evidence for a site-specific fucosylation of N-linked oligosaccharide of immunoglobulin A1 from normal human serum. Glycoconj J. 1998;15:995–1000. doi: 10.1023/a:1006989910120. [DOI] [PubMed] [Google Scholar]
- 17.Royle L, Roos A, Harvey DJ, Wormald MR, van Gijlswijk-Janssen D, Redwan el RM, et al. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem. 2003;278:20140–20153. doi: 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]
- 18.Chintalacharuvu SR, Emancipator SN. The glycosylation of IgA produced by murine B cells is altered by Th2 cytokines. J Immunol. 1997;159:2327–2333. [PubMed] [Google Scholar]
- 19.Chintalacharuvu SR, Nagy NU, Sigmund N, Nedrud JG, Amm ME, Emancipator SN. T cell cytokines determine the severity of experimental IgA nephropathy by regulating IgA glycosylation. Clin Exp Immunol. 2001;126:326–333. doi: 10.1046/j.1365-2249.2001.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rifai A, Fadden K, Morrison SL, Chintalacharuvu KR. The N-glycans determine the differential blood clearance and hepatic uptake of human immunoglobulin (Ig)A1 and IgA2 isotypes. J Exp Med. 2000;191:2171–2182. doi: 10.1084/jem.191.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg D, Sutton-Smith M, Paulson J, Dell A. Automatic annotation of matrix-assisted laser desorption/ionization N-glycan spectra. Proteomics. 2005;5:865–875. doi: 10.1002/pmic.200401071. [DOI] [PubMed] [Google Scholar]
- 22.Campbell C, Stanley P. A dominant mutation to ricin resistance in Chinese hamster ovary cells induces UDP-GlcNAc:glycopeptide beta-4-N-acetylglucosaminyltransferase III activity. J Biol Chem. 1984;259:13370–13378. [PubMed] [Google Scholar]
- 23.Deutscher SL, Nuwayhid N, Stanley P, Briles EIB, Hirschberg CB. Translocation across golgi vesicle membranes: A CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell. 1984;39:295–299. doi: 10.1016/0092-8674(84)90007-2. [DOI] [PubMed] [Google Scholar]
- 24.Oelmann S, Stanley P, Gerardy-Schahn R. Point mutations identified in Lec8 Chinese hamster ovary glycosylation mutants that inactivate both the UDP-galactose and CMP-sialic acid transporters. J Biol Chem. 2001;276:26291–26300. doi: 10.1074/jbc.M011124200. [DOI] [PubMed] [Google Scholar]
- 25.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 26.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 28.Peipp M, Lammerts van Bueren JJ, Schneider-Merck T, Bleeker WW, Dechant M, Beyer T, et al. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood. 2008;112:2390–2399. doi: 10.1182/blood-2008-03-144600. [DOI] [PubMed] [Google Scholar]
- 29.Jefferis R, Lund J. Interaction sites on human IgG-Fc for FcgammaR: current models. Immunol Lett. 2002;82:57–65. doi: 10.1016/s0165-2478(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 30.Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiki Y, Odani H, Takahashi M, Yasuda Y, Nishimoto A, Iwase H, et al. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001;59:1077–1085. doi: 10.1046/j.1523-1755.2001.0590031077.x. [DOI] [PubMed] [Google Scholar]
- 32.Allen AC, Bailey EM, Brenchley PE, Buck KS, Barratt J, Feehally J. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney Int. 2001;60:969–973. doi: 10.1046/j.1523-1755.2001.060003969.x. [DOI] [PubMed] [Google Scholar]
- 33.Odani H, Hiki Y, Takahashi M, Nishimoto A, Yasuda Y, Iwase H, et al. Direct evidence for decreased sialylation and galactosylation of human serum IgA1 Fc O-glycosylated hinge peptides in IgA nephropathy by mass spectrometry. Biochem Biophys Res Commun. 2000;271:268–274. doi: 10.1006/bbrc.2000.2613. [DOI] [PubMed] [Google Scholar]
- 34.Iwanami N, Iwase H, Takahashi N, Kato K, Itoh A, Takatani T, et al. Similarities between N-glycan glycoform of tonsillar IgA1 and that of aberrant IgA1 abundant in IgA nephropathy patient serum. J Nephrol. 2008;21:118–126. [PubMed] [Google Scholar]
- 35.Taylor AK, Wall R. Selective removal of alpha heavy-chain glycosylation sites causes immunoglobulin A degradation and reduced secretion. Mol Cell Biol. 1988;8:4197–4203. doi: 10.1128/mcb.8.10.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkin JD, Pleass RJ, Owens RJ, Woof JM. Mutagenesis of the human IgA1 heavy chain tailpiece that prevents dimer assembly. J Immunol. 1996;157:156–159. [PubMed] [Google Scholar]
- 37.Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthesy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 38.Herr AB, Ballister ER, Bjorkman PJ. Insights into IgA-mediated immune responses from the crystal structures of human FcalphaRI and its complex with IgA1-Fc. Nature. 2003;423:614–620. doi: 10.1038/nature01685. [DOI] [PubMed] [Google Scholar]
- 39.Oortwijn BD, Roos A, van der Boog PJ, Klar-Mohamad N, van Remoortere A, Deelder AM, et al. Monomeric and polymeric IgA show a similar association with the myeloid FcalphaRI/CD89. Mol Immunol. 2007;44:966–973. doi: 10.1016/j.molimm.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Carayannopoulos L, Max EE, Capra JD. Recombinant human IgA expressed in insect cells. Proc Natl Acad Sci USA. 1994;91:8348–8352. doi: 10.1073/pnas.91.18.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basset C, Devauchelle V, Durand V, Jamin C, Pennec YL, Youinou P, et al. Glycosylation of immunoglobulin A influences its receptor binding. Scand J Immunol. 1999;50:572–579. doi: 10.1046/j.1365-3083.1999.00628.x. [DOI] [PubMed] [Google Scholar]
- 42.Moura IC, Arcos-Fajardo M, Sadaka C, Leroy V, Benhamou M, Novak J, et al. Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. J Am Soc Nephrol. 2004;15:622–634. doi: 10.1097/01.asn.0000115401.07980.0c. [DOI] [PubMed] [Google Scholar]
- 43.Chuang PD, Morrison SL. Elimination of N-linked glycosylation sites from the human IgA1 constant region: effects on structure and function. J Immunol. 1997;158:724–732. [PubMed] [Google Scholar]
- 44.Shibuya A, Sakamoto N, Shimizu Y, Shibuya K, Osawa M, Hiroyama T, et al. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat Immunol. 2000;1:441–446. doi: 10.1038/80886. [DOI] [PubMed] [Google Scholar]
- 45.Ramsland PA, Willoughby N, Trist HM, Farrugia W, Hogarth PM, Fraser JD, et al. Structural basis for evasion of IgA immunity by Staphylococcus aureus revealed in the complex of SSL7 with Fc of human IgA1. Proc Natl Acad Sci USA. 2007;104:15051–15056. doi: 10.1073/pnas.0706028104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furtado PB, Whitty PW, Robertson A, Eaton JT, Almogren A, Kerr MA, et al. Solution structure determination of monomeric human IgA2 by X-ray and neutron scattering, analytical ultracentrifugation and constrained modelling: a comparison with monomeric human IgA1. J Mol Biol. 2004;338:921–941. doi: 10.1016/j.jmb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 1998;95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. 1998;273:15866–15871. doi: 10.1074/jbc.273.25.15866. [DOI] [PubMed] [Google Scholar]
- 49.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–381. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 50.Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galili U. Abnormal expression of alpha-galactosyl epitopes in man. A trigger for autoimmune processes Lancet. 1989;2:358–361. doi: 10.1016/s0140-6736(89)90539-4. [DOI] [PubMed] [Google Scholar]
- 52.Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem. 2007;364:8–18. doi: 10.1016/j.ab.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 53.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noguchi A, Mukuria CJ, Suzuki E, Naiki M. Immunogenicity of N-glycolylneuraminic acid-containing carbohydrate chains of recombinant human erythropoietin expressed in Chinese hamster ovary cells. J Biochem. 1995;117:59–62. doi: 10.1093/oxfordjournals.jbchem.a124721. [DOI] [PubMed] [Google Scholar]
- 55.Chintalacharuvu KR, Morrison SL. Residues critical for H-L disulfide bond formation in human IgA1 and IgA2. J Immunol. 1996;157:3443–3449. [PubMed] [Google Scholar]
- 56.Chintalacharuvu KR, Gurbaxani B, Morrison SL. Incomplete assembly of IgA2m(2) in Chinese hamster ovary cells. Mol Immunol. 2007;44:3445–3452. doi: 10.1016/j.molimm.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 57.Tao MH, Morrison SL. Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J Immunol. 1989;143:2595–2601. [PubMed] [Google Scholar]
- 58.Papac DI, Briggs JB, Chin ET, Jones AJ. A high-throughput microscale method to release N-linked oligosaccharides from glycoproteins for matrix-assisted laser desorption/ionization time-of-flight mass spectrometric analysis. Glycobiology. 1998;8:445–454. doi: 10.1093/glycob/8.5.445. [DOI] [PubMed] [Google Scholar]