Abstract
The cytoskeleton is an important component of the plant’s defense mechanism against the attack of pathogenic organisms. Plants however, are defenseless against parasitic rootknot and cyst nematodes and respond to the invasion by the development of a special feeding site that supplies the parasite with nutrients required for the completion of its life cycle. Recent studies of nematode invasion under treatment with cytoskeletal drugs and in mutant plants where normal functions of the cytoskeleton have been affected, demonstrate the importance of the cytoskeleton in the establishment of a feeding site and successful nematode reproduction. It appears that in the case of microfilaments, nematodes hijack the intracellular machinery that regulates actin dynamics and modulate the organization and properties of the actin filament network. Intervening with this process reduces the nematode infection efficiency and inhibits its life cycle. This discovery uncovers a new pathway that can be exploited for the protection of plants against nematodes.
Key words: cytoskeleton, actin, actin depolymerizing factor, nematode, giant cells, syncytium, cytochalasin, taxol
Introduction
Sedentary plant-parasitic nematodes are major agricultural pathogens causing an estimated annual yield loss of $ 100 billion worldwide. Within this group, RKN and CN are among the most specialized phytoparasitic nematodes according to the complexity of the induced feeding sites. Both are microscopic worms (200–400 µm) that penetrate the root elongation zone of host plants as motile infective J2 and then migrate towards the stele establishing a feeding site. Afterwards these parasites become sedentary, rapidly increase in size and finally develop into fertile adults (Fig. 1A and B). The infection induces transformation of the root vascular cells into complex feeding cells that supply nutrients for the nematodes. This process is induced by parasitism proteins, which are synthesised in oesophageal glands and injected into initial feeding cells with the help of stylet.1 The complex changes in nematode feeding cell morphology are accompanied by striking increase of ploidy levels, metabolic activity and cell size (Fig. 1C and D). Feeding cells harbor a dense cytoplasm filled with small vacuoles, golgi and endoplasmic reticulum, proliferating organelles such as plastids and mitochondria2,3 and show an entirely rearranged actin and microtubular cytoskeleton.4,5
Figure 1.
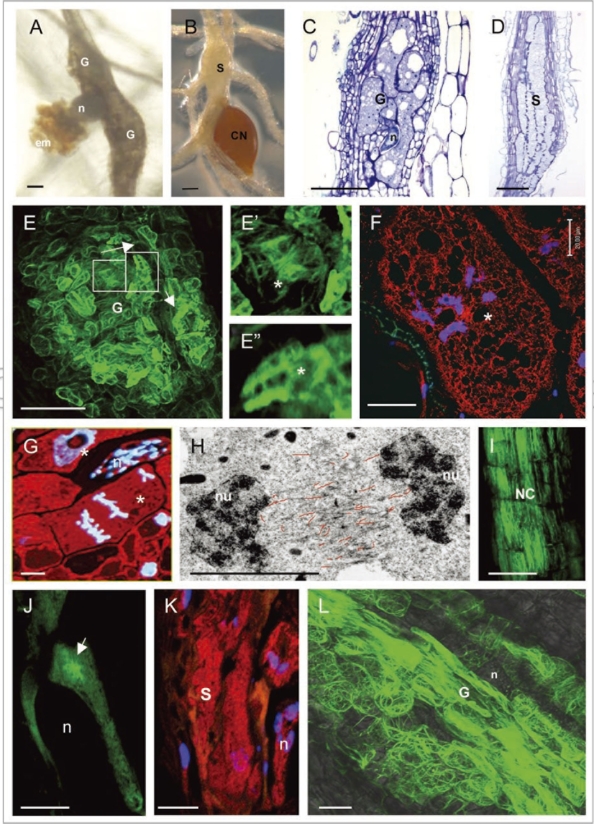
(see opposite page)Feeding site morphology and actin cytoskeleton organization in feeding cells induced by RKN and CN. (A) Root-knots or galls induced by Meloidogyne incognita in A. thaliana; (B) a syncytium induced by Heterodera schachtii in A. thaliana; (C) longitudinal section of a gall stained with toluidine blue; (D) section of a syncytium stained with toluidine blue; (E) actin organisation in a gall (E-E″ and L show labeling with Fimbrin-GFP); (E′) actin bundles in the giant cell cortex; (E″) diffuse and filamentous actin fluorescence in a giant cell; (F) actin staining with anti-actin in a giant cell of a gall (actin in red and nuclei in blue); (G) anaphase giant cell showing no structured actin label (red) between the chromosomes (blue); (H) electron micrograph showing misalignment of F-actin (traced with red lines) in a phragmoplast of a giant cell; (I) longitudinal actin bundles in neighboring cells of a syncytium (I and J show labeling with Talin-GFP); (J) young syncytium with radial actin bundles focused towards the nematode head; (K) syncytium showing diffuse and fragmented microfilaments (red) and nuclei (blue), (L) accumulation of F-actin cables in the giant cells of ADF2 knockdown line. G, gall; n, nematode; em, egg mass; CN, cyst nematode; S, syncytium; *, giant cell; nu, nucleus; NC, neighboring cells. Bars (A–E) = 100 µm; (F, G and I–L) = 50 µm; (H) = 5 µm.
Symptoms of plant infestation by root-knot nematodes include knot- or gall-like formations (Fig. 1A) consisting of six to eight giant-feeding cells surrounded by asymmetrically dividing neighboring cells within the vascular cylinder (Fig. 1C). The giant feeding cells are multinucleated as a result of multiple mitotic events with abnormal phragmoplast expansion leading consequently to incomplete cytokinesis.4,6,7 The nuclei appear to be enlarged in comparison to the surrounding cells already after the first mitotic event.8 Differently, CN induce formation of a multinucleate syncytium which expands longitudinally along the host root as a consequence of cell wall dissolution between the flanking cells (Fig. 1D).9 In this feeding site nuclei enlarge possibly due to the endoreduplication, however mitotic activity has never been observed.
The Actin Cytoskeleton Rearrangements in Nematode Feeding Sites
The plant cytoskeleton is crucial for many cellular processes including cell division, intracellular transport, maintaining cell architecture, balance between isotropic and anisotropic expansion, response to environmental stimuli and pathogen attack, and therefore is essential for plant growth and reproduction.10 Having our studies been focused on the cell biological aspects of the interaction between plants and parasitic nematodes, the interest to investigate the organization of the cytoskeleton in giant cells and syncytia induced by two types of plant parasitic nematodes was naturally pursued. Surprisingly, our observations have shown that both the actin and microtubule networks in root cells infected by nematodes are disorganized. This mini-review focuses on the actin cytoskeleton in feeding giantcells induced by RKN and to a less extent in syncytia induced by CN.
It has been shown that transcription of two members of the Arabidopis actin gene family, ACT2 and ACT7, is upregulated during gall and syncytium development suggesting that a larger pool of globular actin is required for actin filaments assembly in the cells forming feeding sites.4 In vivo analysis using actin markers such as the actin biding domain of A. thaliana fimbrin (FABD) and the actin binding domain of the mouse Talin (mTalin) fused to GFP demonstrates thick transverse and longitudinal bundles of actin filaments in the giant cell cortex through all developmental stages (Fig. 1E and E′). Numerous fine and short filaments have also been observed reflecting ongoing assembly of new actin filaments from the abundant pool of cytoplasmic free actin (Fig. 1E″), accompanied by severing.4 These observations were confirmed and complemented by immunocytochemical analyses in the model nematode host plant Arabidopsis thaliana (Fig. 1F) and in natural host Pisum sativum (pea). Mitotic giant cells reveal less endoplasmic F-actin, but instead contained diffuse actin staining (Fig. 1E″, F and G). During cell division no pre-prophase band (PPB) has been observed suggesting that organisation of cytoplasmic actin and PPB formation are either structurally linked or regulated by a common pathway altered by the parasitism proteins. The absence of actin filaments withing the cytoplasm was apparent during anaphase (Fig. 1G) in large spindles of giant cells. Normally, in mitotic cells microfilamens can only be detected in the midzone of anaphase spindle and the phragmoplast,11 but not in PPB and metaphase spindle. Actin filaments regulate positioning and fusion of cell wall forming vesicles and their depolymerization may cause slowdown of the phragmoplast expansion and phragmoplast disorientation.12 Our recent ultrastructural studies using electron microscopy show that instead of the typical two antiparallel arrays of actin filaments observed in normal phragmoplasts, actin filaments are disorganized and misaligned in phragmoplasts of giant cells (Fig. 1H; unpublished data). In agreement with these observations, giant cells are unable to accomplish cytokinesis due to the phragmoplast failure,4,7 which can be attributed to the abnormal organization of actin filaments. Cells that surround the giant cells show randomly distributed actin filaments which often form a denser network in cells flanking the giant-feeding cells (Fig. 1E). They are able to complete cytokinesis, though their phragmoplasts are often curved.
Actin filaments network in the syncytia induced by the cyst nematode Heterodera schachtii seems more disorganized and less structured than in giant cells. Analyses of the feeding site in living (Fig. 1I and J) as well as fixed (Fig. 1K) samples showed a dense actin array in cells surrounding the feeding site and a rather diffuse fluorescence in feeding cells indicating depolymerisation of actin filaments (Fig. 1J and K). Cells surrounding the syncytia displayed longitudinal actin bundles (Fig. 1I). A radial array of actin filaments focused to the nematode head can be observed in initiating syncytia (Fig. 1J). No mitotic activity seems to take place within syncytia except for neighboring cells which may undergo mitotic events prior to their incorporation into the feeding site. At early stages of syncytia development the flanking cells behave as an isolated symplastic domain without seemly functional plasmodesmata.13,14 As such, external signals that cause actin cytoskeleton disorganization within the syncytia may not affect flanking cells until the fusion.
Our findings suggest that nematode infection causes a reorganization of the actin filament network in both types of feeding cells during the entire nematodE′s life cycle, however each type of infection is characterised by the specific features. CN induce complete disassembly of actin filaments, while RKN cause only partial depolymerization and, therefore, can potentially highjack host’s actin filaments for the infection process.4,5
It remains however unclear how these cytoskeletal changes in giant cells and in syncytia take place. They may be triggered by depolymerisation of existing filamentous actin and/or by preventing G-actin monomers to polymerize. As well, these structural changes in actin organization may be caused by nematode secretions injected into the feeding cells, containing proteins that are in course of identification.
Actin Cytoskeleton Inhibitors and the Role of ABPs in Giant Cells Formation
The disorganization and relaxation of the actin filament network that accompany nematode infection can represent a pleiotropic effect of reprogramming cellular biochemistry induced by nematode signalling molecules or be an essential step in the parasite invasion. Polarization and stabilization of actin filaments is an integral part of anti-bacterial defence response. For example drug-induced disorganization of F-actin increases internalization of intracellular microbes, suppresses antimicrobial defence mechanisms and affects basal resistance.15–18 Several experiments using anti-actin (cytochalasin D, latrunculin B) and anti-microtubule (taxol, oryzalin) compounds have been applied to address the significance of cytoskeletal remodelling during nematode infection.4,8 Treatment of nematode infected roots with a low dose of cytochalasin D induces partial depolymerisation of actin filaments and, if applied during the initial stages of nematode infection, caused an arrest of gall development, whereas syncytium development was disturbed, but not arrested. When applied at later developmental stages cytochalasin D had no effect on both root-knot and cyst nematodes.4 Therefore, pharmacological treatments with inhibitors that disturb F-actin organization demonstrate that changes in actin organisation are a prerequisite for nematode infection.
Interestingly, depolymerization of microtubules has no effect on nematode infection and development, however stabilization of microtubules by taxol arrests the infection process demonstrating that nematodes dynamise/disorganise both cytoskeletal components during the infection.4,8
The actin cytoskeleton is reorganized and remodelled by a range of actin-binding proteins (ABPs).19,20 Up to date the role of few of them has been studied during nematode infection.21–23 Three formin genes, AtFH1, AtFH6 and AtFH10, are induced in giant cells. AtFH6 localises to the plasma membrane and may regulate giant cell isotropic growth by controlling the assembly of actin cables. These cables may guide vesicle trafficking required for extensive plasma membrane and cell wall biogenesis during isotropic cell expansion.21
Expression of another actin regulating protein ADF2 is required for normal Arabidopsis plant development as well as for the successful RKN infection.23 The ADF or cofilin is a key regulator of the turnover of filamentous actin.24–26 The ADF/cofilin family proteins bind G- and F-actin and facilitate F-actin turnover by severing actin filaments and by increasing the rate of dissociation of actin monomer from the pointed ends.27–31 The Arabidopsis ADF gene family consists of 11 expressed members that group phylogenetically into four ancient subclasses32,33 with different, but overlapping, expression programs.34,35 Analysis of the level of transcripts and the localization of promoter activity for seven ADF genes showed that five are highly expressed in galls, with ADF2 showing significant expression in giant cells and upregulation towards the later stages of nematode development (14 and 21 d after inoculation). Knockdown of AtADF2 promoted the net stabilization of F-actin (Fig. 1L) which still allows giant cell induction but blocks feeding cell maturation and nematode development.23 The overall effect of AtADF2 knockdown on the F-actin network of giant cells is most likely caused by a decrease in F-actin turnover leading to the less dynamic and more bundled F-actin network. Consequently, nematodes fail to mature into females and to produce eggs. These findings suggest that upregulation of ADF2 in nematode feeding cells induced by root-knot nematodes might be an essential component for successful nematode infection. As well, a loose cytoskeleton network in giant cells may facilitate nematode development, and maturation.
An extensive search to identify and characterise other ABPs possibly implicated in the reorganization of the giant cell actin cytoskeleton will be undertaken in the near future.
Conclusions and Perspectives
To date, our studies strongly favor the view that nematodes manoeuvre long-term rearrangements of the cytoskeleton during infection of roots to enable the development of giant cells and syncytia. These cytoskeleton rearrangements seem to be an essential step in nematode feeding cells initiation and expansion, as disturbed organization of microtubules and microfilaments have been observed in both types of feeding cells. Two proteins, ADF and formin, have been implicated in the reorganization of actin cytoskeleton so far, but it remains unclear how many other actin regulators are involved in this process. In particular, it would be interesting to analyse proteins capable of stabilization and bundling of actin filaments like fimbrin.36
The specific involvement of the actin depolymerizing factor 2 (ADF2) in the development of giant cells validates previously observed data obtained on feeding cell cytoskeleton upon drug treatment and provides further proof that a dynamic actin cytoskeleton is important for nematode infection and giant cell growth. The high amounts of ADF2 in giant cells might cause the destabilization of the actin cytoskeleton diminishing the density of the actin network and therefore incapacitating defence mechanisms of the host and facilitating nematode feeding. The adequate assembly and organization of the actin cytoskeleton of feeding cells depends upon the expression on an appropriate mixture of ABPs via signals mediated by the pathogen.
Developing strategies to fight against nematode parasitism becomes an important concern when considering limitations on the use of chemical pesticides which are environmentally toxic. Therefore alternative control strategies are urgently required.5 Understanding the interaction between host plants and nematodes at the cellular and molecular levels may generate novel and environmentally safe strategies to control plant-parasitic nematodes and engineer nematode-resistant plants. Per se, interfering with feeding site development via the cytoskeleton appears to be a conceivable approach to intervene with the susceptible plantnematode interactions.
Acknowledgements
We would like to acknowledge all authors who participated in our previous work and Teresa Bleve and Maria Teresa Mellilo for the electron microscopy of actin filaments. We also gratefully acknowledge Gilbert Engler for the help with confocal microscopy and critical reading.
Abbreviations
- ADF
actin depolymerizing factor
- F-actin
filamentous actin
- G-actin
globular actin
- MFs
actin microfilaments
- MT
microtubules
- RKN
root-knot nematode
- CN
cyst nematode
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10741
References
- 1.Hussey RS. Monoclonal antibodies to secretory granules in esophageal glands of meloidogyne species. J Nematol. 1989;21:392–398. [PMC free article] [PubMed] [Google Scholar]
- 2.Bird AF. The ultrastructure and histochemistry of a nematode-induced giant cell. J Biophys Biochem Cytol. 1961;11:701–715. doi: 10.1083/jcb.11.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones MGK, Dropkin VH. Scanning electron microscopy in nematode-induced giant transfer cells. Cytobios. 1976;15:149–161. [PubMed] [Google Scholar]
- 4.de Almeida Engler J, Van Poucke K, Karimi M, De Groodt R, Gheysen G, Engler G, et al. Dynamic cytoskeleton rearrangements in giant cells and syncytia of nematode infected roots. Plant J. 2004;38:12–26. doi: 10.1111/j.1365-313X.2004.02019.x. [DOI] [PubMed] [Google Scholar]
- 5.de Almeida Engler J, Favery B, Engler G, Abad P. Loss of susceptibility as an alternative for nematode resistance. Cur Opin Biotech. 2005;16:1–6. doi: 10.1016/j.copbio.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Jones MGK, Payne HL. Early stages of nematodeinduced giant-cell formation in roots of Impatiens balsamina. J Nematol. 1978;10:70–84. [PMC free article] [PubMed] [Google Scholar]
- 7.Caillaud MC, Lecomte P, Jammes F, Quentin M, Pagnotta S, Andrio E, et al. MAP65-3 microtubuleassociated protein is essential for nematode-induced giant cell ontogenesis in Arabidopsis. Plant Cell. 2008;20:423–437. doi: 10.1105/tpc.107.057422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Almeida Engler J, De Vleesschauwer V, Burssens S, Celenza JL, Jr, Inzé D, Van Montagu M, et al. Molecular markers and cell cycle inhibitors show the importance of cell cycle progression in nematodeinduced galls and syncytia. Plant Cell. 1999;11:793–808. doi: 10.1105/tpc.11.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones MGK. Host cell responses to endoparasitic nematode attack: structure and function of giant cells and syncytia. Ann Appl Biol. 1981;97:353–372. [Google Scholar]
- 10.Cyr RJ. Microtubules in plant morphogenesis: role of the cortical array. Annu Rev Cell Biol. 1994;10:153–180. doi: 10.1146/annurev.cb.10.110194.001101. [DOI] [PubMed] [Google Scholar]
- 11.Schmit AC, Lambert AM. Characterization and dynamics of cytoplasmic F-actin in higher plant endosperm cells during interphase, mitosis and cytokinesis. J Cell Biol. 1987;105:2157–2166. doi: 10.1083/jcb.105.5.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higaki T, Kutsuna N, Sano T, Hasezawa S. Quantitative analysis of changes in actin microfilament contribution to cell plate development in plant cytokinesis. BMC Plant Biol. 2008;8:80. doi: 10.1186/1471-2229-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Böckenhoff A, Prior DA, Grundler FM, Oparka KJ. Induction of phloem unloading in Arabidopsis thaliana roots by the parasitic nematode Heterodera schachtii. Plant Physiol 1. 1996;112:1421–1427. doi: 10.1104/pp.112.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golinowski W, Grundler FMW, Sobczak M. Changes in the structure of Arabodopsis thaliana during female development of the plant-parasitic nematode Heterodera schachtii. Protoplasma. 1996;194:103–116. [Google Scholar]
- 15.Kobayashi Y, Kobayashi I, Funaki Y, Fujimoto S, Takemoto T, Kunoh H. Dynamic reorganization of microfilaments and microtubules is necessary for the expression of non-host resistance in barley coleoptile cells. Plant J. 1997;11:525–537. [Google Scholar]
- 16.Kobayashi I, Kobayashi Y, Hardham AR. Inhibition of rust-induced hypersensitive response in flax cells by the microtubule inhibitor oryzalin. Aust J Plant Physiol. 1997;24:733–740. [Google Scholar]
- 17.Yun BW, Atkinson HA, Gaborit C, Greenland A, Read ND, Pallas JA, et al. Loss of actin cytoskeletal function and EDS1 activity, in combination, severely compromises non-host resistance in Arabidopsis against wheat powdery mildew. Plant J. 2003;34:768–777. doi: 10.1046/j.1365-313x.2003.01773.x. [DOI] [PubMed] [Google Scholar]
- 18.Shimada C, Lipka V, O’Connell R, Okuno T, Schulze-Lefert P, Takano Y. Non-host resistance in Arabidopsis-Colletotrichum interactions acts at the cell periphery and requires actin filament function. Mol Plant Microbe Interact. 2006;19:270–279. doi: 10.1094/MPMI-19-0270. [DOI] [PubMed] [Google Scholar]
- 19.Ayscough KR. In vivo functions of actin-binding proteins. Curr Opin Cell Biol. 1998;10:102–111. doi: 10.1016/s0955-0674(98)80092-6. [DOI] [PubMed] [Google Scholar]
- 20.Hussey PJ, Ketelaar T, Deeks MJ. Control of the actin cytoskeleton in plant cell growth. Annu Rev Plant Biol. 2006;57:109–125. doi: 10.1146/annurev.arplant.57.032905.105206. [DOI] [PubMed] [Google Scholar]
- 21.Favery B, Chelysheva LA, Lebris M, Jammes F, Marmagne A, de Almeida-Engler J, et al. Arabidopsis formin AtFH6 is a plasma membrane-associated protein upregulated in giant cells induced by parasitic nematodes. Plant Cell. 2004;16:2529–2540. doi: 10.1105/tpc.104.024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jammes F, Lecomte P, de Almeida Engler J, Bitton F, Martin-Magniette ML, Renou JP, et al. Genomewide expression profiling of the host response to root-knot nematode infection in Arabidopsis. Plant J. 2005;44:447–448. doi: 10.1111/j.1365-313X.2005.02532.x. [DOI] [PubMed] [Google Scholar]
- 23.Clement M, Ketelaar T, Rodiuc N, Banora MY, Smertenko A, Engler G, et al. Actin-depolymerizing factor2-mediated actin dynamics are essential for root-knot nematode infection of Arabidopsis. Plant Cell. 2009;21:2963–2979. doi: 10.1105/tpc.109.069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staiger CJ, Gibbon BC, Kovar DR, Zonia LE. Profilin and actin-depolymerizing factor: modulators of actin organization in plants. Trends Plant Sci. 1997;2:275–281. [Google Scholar]
- 25.Carlier MF. Control of actin dynamics. Curr Opin Cell Biol. 1998;10:45–51. doi: 10.1016/s0955-0674(98)80085-9. [DOI] [PubMed] [Google Scholar]
- 26.Maciver SK, Hussey PJ. The ADF/cofilin family: actin-remodeling proteins. Genome Biol. 2002;3:1–12. doi: 10.1186/gb-2002-3-5-reviews3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlier MF, Laurent V, Santolini J, Melki R, Didry D, Xia GX, et al. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol. 1997;136:1307–1322. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maciver SK. How ADF/cofilin depolymerises actin filaments. Curr Biol Cell Biol. 1998;10:140–144. doi: 10.1016/s0955-0674(98)80097-5. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Bernstein BW, Bamburg JR. Regulating actin filament dynamics in vivo. Trends Biochem Sci. 2000;25:19–23. doi: 10.1016/s0968-0004(99)01511-x. [DOI] [PubMed] [Google Scholar]
- 30.Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell. 2006;24:13–23. doi: 10.1016/j.molcel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Pavlov D, Muhlrad A, Cooper J, Wear M, Reisler E. Actin filament severing by cofilin. J Mol Biol. 2007;365:1350–1358. doi: 10.1016/j.jmb.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhee SY, Beavis W, Berardini TZ, Chen G, Dixon D, Doyle A, et al. The Arabidopsis Information Resource (TAIR): a model organism database providing a centralized, curated gateway to Arabidopsis biology, research materials and community. Nucl Acids Res. 2003;31:224–228. doi: 10.1093/nar/gkg076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng Y, Liu Q, Xue Q. Comparative study of rice and Arabidopsis actin-depolymerizing factors gene families. J Plant Physiol. 2006;163:69–79. doi: 10.1016/j.jplph.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Dong CH, Kost B, Xia GX, Chua NH. Molecular identification and characterization of AtADF1, AtADF5 and AtADF6 genes. Plant Mol Biol. 2001;45:517–527. doi: 10.1023/a:1010687911374. [DOI] [PubMed] [Google Scholar]
- 35.Ruzicka DR, Kandasamy MK, McKinney EC, Burgos-Rivera B, Meagher RB. The ancient subclasses of Arabidopsis actin depolymerizing factor genes exhibit novel and differential expression. Plant J. 2007;52:460–472. doi: 10.1111/j.1365-313X.2007.03257.x. [DOI] [PubMed] [Google Scholar]
- 36.Kovar DR, Staiger CJ, Weaver EA, McCurdy DW. AtFim1 is an actin filament crosslinking protein from Arabidopsis thaliana. Plant J. 2000;24:625–636. doi: 10.1046/j.1365-313x.2000.00907.x. [DOI] [PubMed] [Google Scholar]