Abstract
Calcium is one of the essential nutrients for growth and development of plants. It is an important component of various structures in cell wall and membranes. Besides some fundamental roles under normal condition, calcium functions as a major secondary-messenger molecule in plants under different developmental cues and various stress conditions including salinity stress. Also changes in cytosolic pH, pHcyt, either individually, or in coordination with changes in cytosolic Ca2+ concentration, [Ca2+]cyt, evoke a wide range of cellular functions in plants including signal transduction in plant-defense responses against stresses. It is believed that salinity stress, like other stresses, is perceived at cell membrane, either extra cellular or intracellular, which then triggers an intracellular-signaling cascade including the generation of secondary messenger molecules like Ca2+ and protons. The variety and complexity of Ca2+ and pH signaling result from the nature of the stresses as well as the tolerance level of the plant species against that specific stress. The nature of changes in [Ca2+]cyt concentration, in terms of amplitude, frequency and duration, is likely very important for decoding the specific downstream responses for salinity stress tolerance in planta. It has been observed that the signatures of [Ca2+]cyt and pH differ in various studies reported so far depending on the techniques used to measure them, and also depending on the plant organs where they are measured, such as root, shoot tissues or cells. This review describes the recent advances about the changes in [Ca2+]cyt and pHcyt at both cellular and whole-plant levels under salinity stress condition, and in various salinity-tolerant and -sensitive plant species.
Key words: cytosolic calcium, ionic toxicity, osmotic stress, pH, salinity stress, salt tolerance, signaling
Introduction
Soil salinity increases.
Soil salinity is a worldwide problem and poses a serious threat to world agriculture, since it reduces the crop yield in the affected areas. There are two groups of plants based on their responses to salt stress: halophytes and glycophytes. Halophytes are native in saline environment and grow well under that condition, whereas glycophytes cannot tolerate salt level to the same degree as halophytes. However, halophytes constitute only 1% of the world’s flora.1 Most of the terrestrial plants are glycophytes with varying level of salt tolerance. Among them most of the crop plants are very sensitive to salt stress. Although the exact information on global extent of salinity-affected areas is varying in different studies, the general perception is that more than 20 per cent of the irrigated land in the world is affected by soil salinity.2–6 This is a big concern for attaining self-sufficiency in food production for the ever-increasing world population. Moreover, the loss of cultivable land, due to expansion of salinity areas through irrigation practices, as well as sea-level rising, is likely to increase over time and impinge on world food supplies.
Salinity stress in plants.
Salinity stress reduces crop growth and yield in different ways. However, NaCl, the dominant salt in nature, elicits two primary effects on plants: osmotic stress and ionic toxicity. Under normal condition the osmotic pressure in plant cells is higher than that in soil solution. Plant cells use this higher osmotic pressure to take up water and essential minerals in root cells from the soil solution. Under salt stress the osmotic pressure in the soil solution exceeds the osmotic pressure in plant cells due to the presence of high salt, and thus, reduces the ability of plants to take up water and minerals like K+ and Ca2+.7,8 On the other hand, Na+ and Cl− ions can enter into the cells and have direct toxic effects on cell membranes, as well as on metabolic activities in the cytosol.9–11 These primary effects of salinity stress cause some secondary effects like reduced cell expansion, assimilate production and membrane function, as well as decreased cytosolic metabolism and production of reactive oxygen intermediates (ROSs).
Growth inhibition by Na+ and/or Cl− toxicity is one of the principal adverse effects of salt stress in plants. However, for Graminaceae crop like rice, Na+ is the principal reason for causing damage (Tester and Davenport 2003).12 The sodium ion (Na+) is very harmful for most plant cells when it is present in the cytosol at concentrations higher than 10 mM. The potassium ion (K+), on the other hand, is one of the essential and most abundant monovalent cations in cells, and needs to be maintained within 100–200 mM range in the cytosol for efficient metabolic functioning. 13–15 As a co-factor in cytosol, K+ activates more than 50 enzymes, which are very susceptible to high cytosolic Na+ and high Na+/K+ ratios.8 Therefore, apart from low cytosolic Na+, maintenance of a low cytosolic Na+/K+ ratio is also critical for the function of cells.16,17
Tolerance mechanisms to salinity stress.
Under NaCl-dominated salt stress the key mechanisms against ionic stress include the reduced uptake into the cytosol of the toxic ions, such as Na+ and Cl− and also sequestration of these toxic ions either into the apoplast or into the vacuole.12,18–27 When compartmentalized into the vacuole, Na+ is no more toxic for cells,28,29 but also an advantage for growth and osmotic adjustment,24,30 particularly since the vacuole may occupy more than 95% of the volume of a mature cell. Cytosolic Na+ also can be compartmentalized in some other sub-cellular organelles like the ER and Golgi bodies.31
To combat with osmotic stress imposed by high salinity, plants need to synthesize compatible organic solutes, such as proline, glycine betaine, trehalose, sorbitol, mannitol, pinitol and sucrose in the cytosol.15,26,32–36 Alternatively, K+ and Na+, if compartmentalized into the vacuole, could be the major compatible inorganic solutes used by the plant under salinity stress. For all these defence-response mechanisms to be active during osmotic stress and ionic toxicity, plants firstly need to perceive the stress and then activate the whole signaling cascade, starting by an elevation of [Ca2+]cyt, either in coordination with changes in cytosolic pH, pHcyt, or individually.
The measurement of changes in [Ca2+]cyt under high salinity stress has been performed by using different techniques, such as fluorescence microscopy measurements in root hairs,37 in individual mesophyll protoplast38,39 and in intact whole plant by using aequorin luminescence.40–43 A number of studies also reported changes in pHcyt in different plant species under salinity stress, which seem to vary between salinity sensitive and tolerant species. An understanding of the nature of changes in [Ca2+]cyt and pHcyt in salinity-sensitive and -tolerant plant species is very important for practical implication to identify potential future strategy to develop salinity-tolerant crop species. This article reviews the recent advances concerning the [Ca2+]cyt and pHcyt changes in both salinity-tolerant and -sensitive plants under salinity stress and their possible role in the signal transduction to activate stress-response mechanisms.
The role of calcium in plants.
Calcium is an essential nutrient for growth and development of plants.44,45 It plays important structural role in producing plant tissues and enables them to grow better. Calcium increases the plant tissues’ resistance under various stress conditions including both biotic and abiotic stresses. Besides these fundamental roles, calcium has been recognized since long time as an important secondary messenger molecule in plants under various developmental cues, as well as under different stresses, including salinity stress. In plant cells the resting cytosolic concentration of calcium, [Ca2+]cyt, under normal condition is maintained at nanomolar level, mostly in the range of 10–200 nM, whereas the concentration of Ca2+ in cell wall, vacuole, endoplasmic reticulum and mitochondria is 1–10 mM.46–48 However, specific signals, such as stress can trigger a sudden increase in the [Ca2+]cyt level up to micromolar level that is toxic if it persists for longer time in the cytosol. Therefore, plants have evolved a system to take up excess Ca2+ and store it either into the apoplast or into the lumen of intracellular organelles, such as vacuole or endoplasmatic reticulum (ER). The latter stores, together with cell walls, can be used for elevating the [Ca2+]cyt level under stress conditions and transduce the signal to subsequent defense responses.
Sensing of salt stress.
Salinity stress, like many other abiotic or biotic stresses, has to be perceived before any changes of [Ca2+]cyt and pHcyt occur in the cells. Salinity stress in plants is sensed by both osmotic stress and ionic (Na+ and/or Cl−) toxicity and these stresses can be sensed either at the outer or inner surface of the plasma membrane by a trans-membrane protein, or within the cytosol by enzymes.24 Several osmo-sensors are suggested to be involved in sensing the osmotic stress imposed by high salt, but will not be further discussed here.49–54
Concerning Na+ toxicity in cells, a substantial progress was made in understanding the signal transduction under sodium stress through investigations on the Salt Overly Sensitive (SOS) pathway in Arabidopsis.34 As proposed by Zhu34 and also elaborately explained in a recent review by Mahajan et al.55 the increase in [Ca2+]cyt under salinity stress is read by SOS3, a Ca2+ sensor. The SOS3 protein interacts with a SOS2 protein kinase and the SOS3-SOS2 complex then activates the SOS1 protein, a plasma membrane Na+/H+ antiporter, thereby re-establishing Na+ homeostasis in cells. In the SOS pathway, it still remains to clarify how Na+ toxicity in cell is perceived. However, it has been suggested that the SOS1 protein, which has a long C-terminal tail residing in the cytosol, might sense Na+.24,26,56 Kader et al.38 showed that Na+ must enter into the cytosol to be sensed in rice protoplasts, which is consistent with the earlier suggestion that the SOS1 protein might sense Na+ inside the cytosol. On the other hand, for the halophytic plant quince it was shown that Na+ entry into the cell may not be necessary for cytosolic Ca2+ elevation. 39 Therefore, it remains to be clarified, what are the sensors for Na+ toxicity in planta, and if they are different in different species, such as salinity-sensitive and salinity-tolerant ones.
Changes of [Ca2+]cyt under salinity stress.
The calcium signature. Within seconds after sensing of salinity stress a transient, stable or oscillating change in [Ca2+]cyt concentration is elicited. This change is required for activating the downstream response mechanisms either through induction or downregulation of the responsive genes. The nature of the [Ca2+]cyt signal in terms of amplitude, frequency and duration of the peak or signal likely has specific role in encoding the particular information for plants under salinity stress. Specific Ca2+ signatures are important for plant cells to sense for the subsequent events in the signaling process. Such processes may change with the particular stress,57 the rate of stress development,39,43,58 preexposure to the stress40 and the tissue type.43,57 In some studies with root tissue, or root protoplasts, salt stress was reported to reduce [Ca2+]cyt. Within minutes of application of 100 mM NaCl to root cells of Arabidopsis,37,59 or to corn root protoplast,60 there was a decrease in [Ca2+]cyt. In contrast, many studies revealed an increase in [Ca2+]cyt after salt stress.11,38–42,57,61–64
From the results so far, it can be concluded that the change in [Ca2+]cyt is not uniform and varies with species, cell type or tissue type.38,39,43,57,59 It can also be considered that the specific Ca2+ signatures (the increase with different amplitudes and their duration), as well as the amount of [Ca2+]cyt increased, vary in different studies due to the techniques used and also due to the type of experimental materials used, such as whole plant, root or specific cell types.
Sodium toxicity and osmotic stress may induce different signals in roots and shoots.
The results are contrasting in studies whether osmotic stress increases or decreases [Ca2cyt].38,40,57,59 Moreover, the changes in [Ca2+]cyt differ both in terms of concentration and amplitude depending on the amount of stress given to the cell.39,43 Tracy et al. found that the osmotic and ionic components of salinity stress induced differential increases in [Ca2+]cyt in Arabidopsis root cells.43 They reported that the heterogenous [Ca2+]cyt changes under NaCl stress were restricted to the root. Also in experiments with rice protoplasts, as well as with quince protoplasts, different [Ca2+]cyt changes were obtained under sodium stress and under osmotic stress.38,39 Kiegle et al.57 reported significant quantitative differences in [Ca2+]cyt elevation in different cell types of Arabidopsis roots. Both under osmotic stress (440 mM mannitol) and salt stress (220 mM NaCl) the endodermis and pericycle cells displayed prolonged oscillation in [Ca2+]cyt that were distinct from the responses of other cell types.
The amplitude of [Ca2+]cyt differs.
In experiments with single cell protoplasts and fluorescence microscopy, the increase in [Ca2+]cyt under different types of stress or upon auxin addition was in nanomolar level.38,39,65–67 On the other hand, the increase in [Ca2+]cyt concentration was at μolar level in most of the studies that measured [Ca2+]cyt changes by use of calcium reporting protein, aequorin and luminescence microscopy.40,42,43,57 Different changes in [Ca2+]cyt could depend on a signal variation between root and shoot cells, as obtained by Tracy et al.43 Moreover, in experiments with intact plants, or tissues referred above, the increase in [Ca2+]cyt obtained shows a mean value of many cells. The luminescent signal from aequorin expressed in whole plant or tissues may not reflect the Ca2+ signal in individual cells as suggested by Dodd et al.68 It is also likely that cell-wall signaling can take place.41 In that case the transient spikes in μolar range found in experiments with tissue or intact plants could depend on cell wall signaling. In a recent study, Tracy et al.43 found through analysis of spatiotemporal [Ca2+]cyt dynamics, additional levels of complexity in the [Ca2+]cyt signal and suggested that the signals in a cell population might be the result of oscillatory changes of single cells. From the above discussion it is evident that the data on changes of [Ca2+]cyt under salinity stress vary depending on the techniques of measuring the data, duration and intensity of salinity stress imposed, osmotic and ionic components, plant species and type of cells or tissues used.
Sources for [Ca2+]cyt changes.
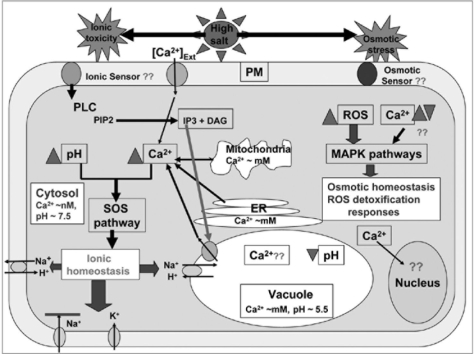
The changes in [Ca2+]cyt appear to be supplied from the the apoplast (influx of Ca2+ across the plasma membrane) or from the internal stores like ER, Golgi bodies, mitochondria or vacuole.69 Calcium-permeable channels in the plasma membrane, which are activated by membrane depolarization, are thought to lead to elevation of [Ca2+]cyt in many species after the perception of a range of stimuli.38–40,67,69–74 The generation of Ca2+ increase in the cytosol further modulates other messengers like inositol phosphate, which induces a further Ca2+ elevation in the cytosol through the opening of inositol-(1,4,5)-triphosphate (IP3)-regulated Ca2+ channels.43,69 There are some studies showing that salinity stress induces a rapid increase in IP3 concentration in the cytosol.75,76 By use of the inhibitors verapamil and nifedipine for plasma membrane Ca2+-permeable channels,38,39,67,77–79 and LiCl for inhibition of Ca2+ release from internal stores, like vacuole or ER, the major sources for [Ca2+]cyt dynamics could be suggested.38,40,80–82 In Figure 1 a proposed model of salt-stress signaling and tolerance mechanisms is included.
Figure 1.
A proposed model of salt-stress tolerance in plants at cellular level. The sensing of ionic stress induces a transient elevation of cytosolic Ca2+ and pH.6,38,39,43 Cytosolic-Ca2+ elevation is attributed with the influx of Ca2+ from cell wall, as well as from the vacuole. ER and mitochondria may also contribute to cytosolic-Ca2+ elevation. The cytosolic-pH increase seems to be linked with vacuolar-pH decreases.38 Cytosolic Ca2+ elevation and pH increase, either independently or in coordination, induces the SOS pathway for ionic homeostasis through inhibition of Na+ entry, enhancing K+ uptake into the cell, and sequestration of cytosolic Na+ either into the apoplast or vacuole.24,34,55 Cell organelles, such as nucleus, mitochondria, ER etc., might also have their own Ca2+ elevation and subsequent signaling cascade for downstream responses.85,86 Osmotic stress, in contrast to ionic toxicity, may either decrease or increase cytosolic-Ca2+ level, which might be linked with the production of reactive oxygen species (ROS).52 ROS then activates the MAPK pathways for osmotic homeostasis and detoxification responses.
Changes of Ca2+ concentration in different parts of the cell.
Since long time it has been believed that transient elevation in [Ca2+]cyt concentration plays a vital role in the signaling cascade for the downstream adaptive responses to stress conditions. Different parts of the cell, such as cell wall, ER, Mitochondria, chloroplast and vacuole are thought as sources of [Ca2+]cyt elevation, as mentioned earlier. In a few studies calcium-dependent signaling processes have been proposed to proceed in apoplast and also in some other cell organelles, for example chloroplast41,83 and mitochondria.84 More recently, cell nuclei have been suggested to generate their own calcium signals under various stimuli.85,86 It is still to be clarified whether these cell organelles have their own Ca2+-signaling system under salinity stress and, if so, what are the adaptive responses they induce by the signal. It could be suggested that a Ca2+ signal from the cell organelles appears later or even earlier than the cytosolic signal, as they all may have their distinct pathway of activating downstream mechanisms, as suggested by Mazars et al.86
The role of pH in stress signaling.
It is likely that not only Ca2+, but also protons, function as second messengers in plant cells, since there are steep differences in both calcium concentration and pH within a plant cell. Under normal condition the cell cytosol pH is around 7.5, while the apoplast and vacuolar lumen have a pH around 5.5.15 Moreover, intracellular pH can be dramatically modulated for transferring the signal to downstream responses.87,88 Changes in intracellular pH are reported for many developmental issues in plants, such as root tip growth,89–91 nodulation,92,93 elicitation of benzophenanthridine alkaloids,94 response to hormone activity such as gibberellic acid95 and abscisic acid.96 Changes in pH are also found during plant defence responses against various stresses.38,97,98 An influx of H+ from the apoplast into the cytosol was reported for many plants during elicitation of the hypersensitive response,98 whereas an efflux of H+ from the cytosol into the apoplast or vacuole was obtained in case of some other plants species.38,41
Changes of pHcyt under salinity stress.
Change in intracellular pH also acts as secondary messenger in response to different stress conditions including salinity stress.87,88,98 The [Ca2+]cyt and pH homeostasis in cells are closely linked.99 Upon shifting of [Ca2+]cyt under salinity stress, cells are challenged with the excess of other monovalent ions in the cytosol like H+.41,58,100 Transient shifts in intracellular and apoplastic pH are reported as essential steps in several signal-transduction processes, and pH is involved in cell signaling, either directly, or in cross talk with plant hormones, or Ca2+.41,87,101–104 However, the nature of cytosolic pH change also differs depending on osmotic and ionic components of salinity stress and plant species.6,38,41 Gao et al.41 reported that osmotic stress imposed by mannitol did not alter pHcyt in intact Arabidopsis roots, whereas addition of NaCl to the same roots caused a decline in pHcyt. Also osmotic stress imposed by sorbitol did not change pHcyt in rice or quince.38,39 On the other hand, the ionic component induced a transient cytosolic acidification under salinity stress in salinity-sensitive rice.38 A rise in vacuolar pH, which could be attributed to cytosolic acidification, was obtained in salt-sensitive plants upon exposure to salinity stress.105,106 In contrast, Halperin et al.37 did not find any change in cytosolic pH in Arabidopsis roots upon salt stress.
In case of salt-tolerant species reported so far, it has been found that pHcyt increased under salinity stress and that the increase was attributed to the ionic component of salinity stress, not the osmotic stress.38,39 This is consistent with the study of Caracuel et al.107 that shows that salinity tolerance in Fusarium oxysporum is correlated with the activation of PacC, a transcription factor that activates Na1-ATPase, by alkaline pH in the cytosol. By contrast, cytosolic acidification in Saccharomyces cerevisiae confers salt tolerance by activating NHA, a plasma membrane Na+/H+ antiporter.108,109 To our knowledge there is no report showing cytosolic acidification in salinity-tolerant plant species. Therefore, we believe that cytosolic alkalinization under the ionic component of salinity stress could be a unique trait of salinity-tolerant plant species. Shifting in cytosolic pH is likely related to a change in pH either in apoplast (if H+ movement occurs between cytosol and apoplast) or in vacuole (if H+ movement occurs between cytosol and vacuole). Kader et al.38 showed a vacuolar acidification in combination with cytosolic alkalinization in salt-tolerant rice cultivar Pokkali. It is quite interesting that the regulatory C-terminal domain of the tonoplast Na+/H+ antiporter resides in the vacuole,110 and the antiporter is suggested to be regulated by changes of pH in the vacuole. 111 Indeed, vacuolar compartmentalization of cytosolic Na+ is the dominant tolerance mechanism in salt-tolerant rice cultivar Pokkali under NaCl-dominated salt stress.6,27,112
Conclusion
Salinity-tolerance in plants is a multigenic trait with many quantitative trait loci (QTLs) associated with ion transport and tolerance.5 Therefore, plants need to possess a wide range of adaptation mechanisms for osmotic stress as well as ionic toxicity to be tolerant under high salinity. For the development of salinity-tolerant crop species it is imperative to have very clear understanding of the tolerance mechanisms available in plants. Generation of messenger molecules like changes in cellular Ca2+ and pH with specific amplitude and duration, and also in specific organelles, seems to play very vital role for activating the defence-response mechanisms for salinity tolerance. Until recently, studies mostly concentrated on changes of cytosolic Ca2+ and pH signaling in plants under salinity stress. However, recent reports are suggesting that changes in Ca2+ concentration, as well as changes in pH in a cell, could occur in other parts as well, such as apoplast, mitochondria, nucleus, vacuole etc. It is likely that salinity stress elicits differential Ca2+ and pH signatures in different parts of the cell to activate specific tolerance mechanisms and these signatures are likely to vary between salinity tolerant and sensitive plant species. There are few reports showing difference in cellular Ca2+ and pH signatures between salinity sensitive and tolerant plant species and, therefore, more attention to this area is needed.
Acknowledgements
This work was supported by Wallenberg foundation and Sida, Sweden.
Abbreviations
- [Ca2+]
cytosolic concentration of calcium
- ER
endoplasmatic reticulum
- pHcyt
cytosolic pH
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10740
References
- 1.Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytol. 2008;79:945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- 2.Ghassemi F, Jakeman AJ, Nix HA. Salinisation of land and water resources: Human causes, extent, management and case studies. Wallingford UK,: CAB International; 1995. p. 544. [Google Scholar]
- 3.Flowers TJ, Yeo AR. Breeding for salinity resistance in crop plants: where next? Austr J Plant Physiol. 1995;22:875–884. [Google Scholar]
- 4.Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002;25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- 5.Flowers TJ. Improving crop salt tolerance. J Exp Bot. 2004;55:307–319. doi: 10.1093/jxb/erh003. [DOI] [PubMed] [Google Scholar]
- 6.Kader MA, Lindberg S. Cellular traits for sodium tolerance in rice (Oryza sativa L) Plant Biotech. 2008;25:247–255. [Google Scholar]
- 7.Glenn EP, Brown JJ, Khan MJ. Mechanisms of salt tolerance in higher plants. In: Basra AS, Basra RK, editors. Mechanisms of Environmental Stress Resistance in Plants. the Netherlands: Harwood academic publishers,; 1997. pp. 83–110. [Google Scholar]
- 8.Munns R, James RA, Läuchli A. Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot. 2006;57:1025–1043. doi: 10.1093/jxb/erj100. [DOI] [PubMed] [Google Scholar]
- 9.Greenway H, Munns R. Mechanisms of salt tolerance in non halophytes. Ann Rev Plant Physiol. 1980;31:149–190. [Google Scholar]
- 10.Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Ann Rev Plant Physiol Plant Mol Biol. 2000;51:463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- 11.Zhu JK. Plant salt tolerance. Trends Plant Sci. 2001;6:66–71. doi: 10.1016/s1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- 12.Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Ann Bot. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker NA, Sanders D, Maathuis FJM. High-affinity potassium uptake in plants. Science. 1996;273:977–978. doi: 10.1126/science.273.5277.977. [DOI] [PubMed] [Google Scholar]
- 14.Cuin TA, Miller AJ, Laurie SA, Leigh RA. Potassium activities in cell compartments of salt-grown barley leaves. J Exp Bot. 2003;54:657–661. doi: 10.1093/jxb/erg072. [DOI] [PubMed] [Google Scholar]
- 15.Taiz L, Zeiger E. In: Plant Physiology. Fourth, editor. Sunderland, Massachusetts: Sinauer Associates, Inc., Publishers,; 2006. p. 694. [Google Scholar]
- 16.Rubio F, Gassmann W, Schroeder JI. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- 17.Zhu JK, Liu J, Xiong L. Genetic analysis of salt tolerance in Arabidopsis: Evidence for a critical role to potassium nutrition. Plant Cell. 1998;10:1181–1191. doi: 10.1105/tpc.10.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuda A, Yazaki Y, Ishikawa T, Koike S, Tanaka Y. Na+/H+ antiporter in tonoplast vesicles from rice roots. Plant Cell Physiol. 1998;39:196–201. [Google Scholar]
- 19.Fukuda A, Nakamura A, Tagiri A, Tanaka H, Miyao A, Hirochika H, et al. Function, intracellular localization and the importance in salt tolerance of a vacuolar Na+/H+ antiporter from rice. Plant Cell Physiol. 2004;45:146–159. doi: 10.1093/pcp/pch014. [DOI] [PubMed] [Google Scholar]
- 20.Apse MP, Aharon GS, Snedden WA, Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science. 1999;285:1256–1258. doi: 10.1126/science.285.5431.1256. [DOI] [PubMed] [Google Scholar]
- 21.Blumwald E. Sodium transport and salt tolerance in plants. Curr Opin Cell Biol. 2000;12:431–434. doi: 10.1016/s0955-0674(00)00112-5. [DOI] [PubMed] [Google Scholar]
- 22.Chauhan S, Forsthoefel N, Ran Y, Quigley F, Nelson DE, Bohnert HJ. Na+/myo-inositol symporters and Na+/H+-antiport in Mesembryanthemum crystallium. Plant J. 2000;24:511–522. doi: 10.1046/j.1365-313x.2000.00903.x. [DOI] [PubMed] [Google Scholar]
- 23.Hamada A, Shono M, Xia T, Ohta M, Hayashi Y, Tanaka A, et al. Isolation and characterization of a Na+/H+ antiporter gene from the halophyte Atriplex gmelini. Plant Mol Biol. 2001;46:35–42. doi: 10.1023/a:1010603222673. [DOI] [PubMed] [Google Scholar]
- 24.Zhu JK. Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol. 2003;6:441–445. doi: 10.1016/s1369-5266(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 25.Shi H, Lee BH, Wu SJ, Zhu JK. Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nature Biotech. 2003;21:81–85. doi: 10.1038/nbt766. [DOI] [PubMed] [Google Scholar]
- 26.Zhang JZ, Creelman RA, Zhu JK. From laboratory to field. Using information from Arabidopsis to engineer salt, cold and drought tolerance in crops. Plant Physiol. 2004;135:615–621. doi: 10.1104/pp.104.040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kader MA, Lindberg S. Uptake of sodium in protoplasts of salt-sensitive and salt-tolerant cultivars of rice, Oryza sativa L. determined by the fluorescent dye SBFI. J Exp Bot. 2005;56:3149–3158. doi: 10.1093/jxb/eri312. [DOI] [PubMed] [Google Scholar]
- 28.Flowers TJ, Läuchli A. Sodium versus potassium: substitution and compartmentalization. In: Läuchli A, Bieleski RL, editors. Inorganic plant nutrition. Vol. 15. Berlin: Springer-Verlag; 1983. pp. 651–681. [Google Scholar]
- 29.Subbarao GV, Ito O, Berry WL, Wheeler RM. Sodium—A functional plant nutrient. Crit Rev Plant Sci. 2003;22:391–416. [Google Scholar]
- 30.Rodríguez-Navarro A, Rubio F. High-affinity potassium and sodium transport systems in plants. J Exp Bot. 2006;57:1149–1160. doi: 10.1093/jxb/erj068. [DOI] [PubMed] [Google Scholar]
- 31.Jou Y, Chiang CP, Jauh GY, Yen HE. Functional characterization of ice plant SKD1, an AAA-type ATPase associated with the endoplasmic reticulum-golgi network, and its role in adaptation to salt stress. Plant Physiol. 2006;141:135–146. doi: 10.1104/pp.106.076786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bohnert HJ, Jensen RG. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996;14:89–97. [Google Scholar]
- 33.Chen THH, Murata N. Enhancement of tolerance to abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol. 2002;5:250–257. doi: 10.1016/s1369-5266(02)00255-8. [DOI] [PubMed] [Google Scholar]
- 34.Zhu JK. Salt and drought stress signal transduction in plants. Ann Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chinnusamy V, Jagendorf A, Zhu JK. Understanding and improving salt tolerance in plants. Crop Sci. 2005;45:437–448. [Google Scholar]
- 36.Liang C, Zhang XY, Luo Y, Wang GP, Zou Q, Wang W. Over-accumulation of glycine betaine alleviates the negative effects of salt stress in wheat. Russian J Plant Physiol. 2009;56:370–376. [Google Scholar]
- 37.Halperin SJ, Gilroy S, Lynch JP. Sodium chloride reduces growth and cytosolic calcium, but does not affect cytosolic pH, in root hairs of Arabidopsis thaliana L. J Exp Bot. 2003;54:1269–1280. doi: 10.1093/jxb/erg134. [DOI] [PubMed] [Google Scholar]
- 38.Kader MA, Lindberg S, Seidel T, Golldack D, Yemelyanov V. Sodium sensing induces different changes in free cytosolic calcium concentration and pH in salt-tolerant and salt-sensitive rice (Oryza sativa L.) cultivars. Physiol Plant. 2007;130:99–111. [Google Scholar]
- 39.D’Onofrio C, Lindberg S. Sodium induces simultaneous changes in cytosolic calcium and pH in salt-tolerant quince protoplasts. J Plant Physiol. 2009;166:1755–1763. doi: 10.1016/j.jplph.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Knight H, Trewavas AJ, Knight MR. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997;12:1067–1078. doi: 10.1046/j.1365-313x.1997.12051067.x. [DOI] [PubMed] [Google Scholar]
- 41.Gao D, Knight MR, Trewavas AJ, Sattelmacher B, Plieth C. Self-reporting Arabidopsis expressing pH and [Ca2+] indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress. Plant Physiol. 2004;134:898–908. doi: 10.1104/pp.103.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henriksson E, Henriksson KN. Salt-stress signalling and the role of calcium in the regulation of the Arabidopsis ATHB7 gene. Plant Cell Environ. 2005;28:202–210. [Google Scholar]
- 43.Tracy FE, Gilliham M, Dodd AN, Webb AA, Tester M. NaCl-induced changes in cytosolic free Ca2+ in Arabidopsis thaliana are heterogeneous and modified by external ionic composition. Plant Cell Environ. 2008;31:1063–1073. doi: 10.1111/j.1365-3040.2008.01817.x. [DOI] [PubMed] [Google Scholar]
- 44.Marschner H. Mineral nutrition of higher plants. 2nd edn. London: Academic Press; 1995. [Google Scholar]
- 45.White PJ, Broadley MR. Calcium in plants. Ann Bot. 2003;92:487–511. doi: 10.1093/aob/mcg164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy ASN. Calcium: silver bullet in signaling. Plant Sci. 2001;160:381–404. doi: 10.1016/s0168-9452(00)00386-1. [DOI] [PubMed] [Google Scholar]
- 47.Rudd JJ, Franklin-Tong VE. Unravelling response-specificity in Ca2+ signalling pathways in plant cells. New Phytol. 2001;51:7–33. doi: 10.1046/j.1469-8137.2001.00173.x. [DOI] [PubMed] [Google Scholar]
- 48.Reddy VS, Reddy ASN. Proteomics of calcium-signaling components in plants. Phytochemistry. 2004;65:1745–1776. doi: 10.1016/j.phytochem.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 49.Urao T, Yakubov B, Satoh R, Yamaguchi-Shinozaki K, Seki M, Hirayama T, et al. A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell. 1999;11:1743–1754. doi: 10.1105/tpc.11.9.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reiser V, Raitt DC, Saito H. Yeast osmosensor Sln1 and plant cytokinin receptor Cre1 respond to changes in turgor pressure. J Cell Biol. 2003;161:1035–1040. doi: 10.1083/jcb.200301099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamura T, Hara K, Yamaguchi Y, Koizumi N, Sano H. Osmotic stress tolerance of transgenic tobacco expressing a gene encoding a membrane-located receptor-like protein from tobacco plants. Plant Physiol. 2003;131:454–462. doi: 10.1104/pp.102.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boudsocq M, Lauriere C. Osmotic signaling in plants. Multiple pathways mediated by emering kinase families. Plant Physiol. 2005;138:1185–1194. doi: 10.1104/pp.105.061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tran LS, Urao T, Qin F, Maruyama K, Kakimoto T, Shinozaki K, Yamaguchi-Shinozaki K. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought and salt stress in Arabidopsis. Proc Natl Acad Sci USA. 2007;104:20623–20628. doi: 10.1073/pnas.0706547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wohlbach DJ, Quirino BF, Sussmand MR. Analysis of the Arabidopsis Histidine Kinase ATHK1 Reveals a Connection between Vegetative Osmotic Stress Sensing and Seed Maturation. Plant Cell. 2008;20:1101–1117. doi: 10.1105/tpc.107.055871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahajan S, Pandey GK, Tuteja N. Calcium- and salt stress signaling in plants: Shedding light on SOS pathway. Arc Biochem Biophys. 2008;471:146–158. doi: 10.1016/j.abb.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 56.Shabala L, Cuin TA, Newman IA, Shabala S. Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta. 2005;222:1041–1050. doi: 10.1007/s00425-005-0074-2. [DOI] [PubMed] [Google Scholar]
- 57.Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR. Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J. 2000;23:267–278. doi: 10.1046/j.1365-313x.2000.00786.x. [DOI] [PubMed] [Google Scholar]
- 58.Plieth C, Hansen UP, Knight H, Knight MR. Temperature sensing by plants: the primary characteristics of signal perception and calcium response. Plant J. 1999;18:491–497. doi: 10.1046/j.1365-313x.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- 59.Cramer GR, Jones RL. Osmotic stress and abscisic acid reduce cytosolic calcium activities in roots of Arabidopsis thaliana. Plant Cell Environ. 1996;19:1291–1298. [Google Scholar]
- 60.Lynch J, Läuchli A. Salinity affects intracellular calcium in corn root protoplasts. Plant Physiol. 1988;87:351–356. doi: 10.1104/pp.87.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bittisnich D, Robinson D, Whitecross M. Membrane-associated and intracellular free calcium levels in root cells under NaCl stress. In Plant membrane transport: The current position. In: Dainty J, de Michelis MI, Marre E, Rasi-Caldogno F, editors. Proceedings of the eighth international workshop on plant membrane transport, Venice, Italy, 25–30 June 1989. New York: Elesevier Science Publishing Company, Inc.,; 1989. pp. 681–682. [Google Scholar]
- 62.Lynch J, Polito VS, Läuchli A. Salinity stress increases cytoplasmic calcium activity in maize root protoplasts. Plant Physiol. 1989;90:1271–1274. doi: 10.1104/pp.90.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Halfter U, Ishitani M, Zhu JK. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc Natl Acad Sci USA. 2000;97:3730–3734. doi: 10.1073/pnas.040577697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knight H. Calcium signaling during abiotic stress in plants. Int Rev Cytol-Survey Cell Biol. 2000;195:269–324. doi: 10.1016/s0074-7696(08)62707-2. [DOI] [PubMed] [Google Scholar]
- 65.Lindberg S, Strid H. Aluminium induces rapid changes in cytosolic pH and free calcium and potassium concentrations in root protoplasts of wheat (Triticum aestivum) Physiol Plant. 1997;99:405–414. [Google Scholar]
- 66.Sebastiani L, Lindberg S, Vitagliano C. Cytoplasmic free Ca2+ dynamics in single tomato (Lycopersicon esculentum) protoplasts subjected to chilling temperatures. Physiol Plant. 1999;105:239–245. [Google Scholar]
- 67.Shishova M, Lindberg S. Auxin induces an increase of Ca2+ concentration in the cytosol of wheat leaf protoplasts. J Plant Physiol. 2004;161:937–945. doi: 10.1016/j.jplph.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 68.Dodd AN, Jakobsen MK, Baker AJ, Telzerow A, Hou SW, Laplaze L, et al. Time of day modulates low-temperature Ca2+ signals in Arabidopsis. Plant J. 2006;48:962–973. doi: 10.1111/j.1365-313X.2006.02933.x. [DOI] [PubMed] [Google Scholar]
- 69.Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the cross-roads of signaling. Plant Cell. 2002;14:401–417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gelli A, Blumwald E. Calcium retrieval from vacuolar pools (characterisation of a vacuolar calcium channel) Plant Physiol. 1993;102:1139–1146. doi: 10.1104/pp.102.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gelli A, Higgins VJ, Blumwald E. Activation of plant plasma membrane Ca2+ permeable channels by race-specific fungal elicitors. Plant Physiol. 1997;113:269–279. doi: 10.1104/pp.113.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamilton CA, Taylor GJ, Good AG. Vacuolar H+-ATPase, but not mitochondrial F1F0-ATPase, is required for NaCl tolerance in Saccharomyces cerevisiae. FEMS microbiol lett. 2002;208:227–232. doi: 10.1111/j.1574-6968.2002.tb11086.x. [DOI] [PubMed] [Google Scholar]
- 73.Véry AA, Davies JM. Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc Natl Acad Sci, USA. 2000;97:9801–9806. doi: 10.1073/pnas.160250397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.White PJ. Calcium channels in higher plants. Biochim biophys acta. 2000;1465:171–189. doi: 10.1016/s0005-2736(00)00137-1. [DOI] [PubMed] [Google Scholar]
- 75.Takahashi S, Katagiri T, Hirayama T, Yamaguchi-Shinozaki K, Shinozaki K. Hyperosmotic stress induces a rapid and transient increase in inositol 1,4,5-triphosphate independent of abscisic acid in Arabidopsis cell culture. Plant Cell Physiol. 2001;42:214–222. doi: 10.1093/pcp/pce028. [DOI] [PubMed] [Google Scholar]
- 76.DeWald DB, Torabinejad J, Jones CA, Shope JC, Cangelosi AR, Thompson JE, et al. Rapid accumulation of phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate correlates with calcium mobilization in salt-stressed Arabidopsis. Plant Physiol. 2001;126:759–769. doi: 10.1104/pp.126.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Polevoi V, Sinyutina NF, Salamatoma TS, Inge-Vechtomova NI, Tankelyun OV, Sharova EI, Shishova MF. Mechanism of auxin action: second messengers. In: Smith AR, editor. Plant hormone signal perception and transduction. Amsterdam: Kluwer Academic Publishers, The Netherlands; 1996. pp. 223–231. ISBN 0-7923-3768-9. [Google Scholar]
- 78.Babourina O, Shabala S, Newman I. Verapamil-induced kinetics of ion flux in oat seedlings. Austr J Plant Physiol. 2000;27:1031–1040. [Google Scholar]
- 79.White PJ, Bowen HC, Demidchik V, Nichols C, Davies JM. Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochim biophys acta. 2002;1564:299–309. doi: 10.1016/s0005-2736(02)00509-6. [DOI] [PubMed] [Google Scholar]
- 80.Gillaspy GE, Keddie JS, Oda K, Gruissem W. Plant inositol monophospatase is a lithium-sensitive enzyme encoded by a multigene family. Plant Cell. 1995;7:2175–2185. doi: 10.1105/tpc.7.12.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knight H, Trewavas AJ, Knight MR. Cold calcium signalling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell. 1996;8:489–503. doi: 10.1105/tpc.8.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liang X, Shen NF, Theologis A. Li+-regulated 1-aminocyclopropane-1-carboxylate synthase gene expression in Arabidopsis thaliana. Plant J. 1996;10:1027–1036. doi: 10.1046/j.1365-313x.1996.10061027.x. [DOI] [PubMed] [Google Scholar]
- 83.Johnson CH, Knight MR, Kondo T, Masson P, Sedbrook J, Haley A, et al. Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science. 1995;269:1863–1865. doi: 10.1126/science.7569925. [DOI] [PubMed] [Google Scholar]
- 84.Logan DC, Knight MR. Mitochondrial and cytosolic calcium dynamics are differentially regulated in plants. Plant Physiology. 2003;133:21–24. doi: 10.1104/pp.103.026047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiong T-C, Bourque S, Lecourieux D, Amelot N, Grat S, Brière C, et al. Calcium signaling in plant cell organelles delimited by a double membrane. Biochim Biophys Acta, Cell Research. 2006;1763:1209–1215. doi: 10.1016/j.bbamcr.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 86.Mazars C, Bourque S, Mithöfer A, Pugin A, Ranjeva R. Calcium homeostasis in plant cell nuclei. New Phytol. 2009;181:261–274. doi: 10.1111/j.1469-8137.2008.02680.x. [DOI] [PubMed] [Google Scholar]
- 87.Roos W. Ion mapping in plant cells: methods and applications in signal transduction research. Planta. 2000;210:347–370. doi: 10.1007/PL00008144. [DOI] [PubMed] [Google Scholar]
- 88.Roos W. Confocal pH topography in plant cells—shifts of proton distribution are involved in plant signalling. In: Rengel Z, editor. Handbook of plant growth—pH as a major variable in plant growth. New York: M. Dekker; 2001. pp. 55–86. [Google Scholar]
- 89.Gibbon BC, Kropf DL. Cytosolic pH gradients associated with tip growth. Science. 1994;263:1419–1421. doi: 10.1126/science.263.5152.1419. [DOI] [PubMed] [Google Scholar]
- 90.Robson GD, Prebble E, Rickers A, Hosking S, Denning DW, Trinci AP, et al. Polarized growth of fungal hyphae is defined by an alkaline pH gradient. Fungal Gen Biol. 1996;20:289–298. doi: 10.1006/fgbi.1996.0043. [DOI] [PubMed] [Google Scholar]
- 91.Feijó JA, Sainhas J, Hackett GR, Kunkel JG, Hepler PK. Growing pollen tubes possess a constitutive alkaline band in the clear zone and a growth-dependent acidic tip. J Cell Biol. 1999;144:483–496. doi: 10.1083/jcb.144.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Allen NS, Bennett MN, Cox DN, Shipley A, Ehrhardt DW, Long SR. Effects of nod factors on alfalfa root hair Ca++ and H+ currents and on cytoskeletal behavior. In: Daniels M, editor. Advances in Molecular Genetics. Vol. 3. Dordrecht, The Netherlands: Kluwer Academics,; 1994. pp. 107–113. [Google Scholar]
- 93.Felle HH, Kondorosi E, Kondorosi A, Schultze M. Rapid alkalinization in alfalfa root hairs in response to rhizobial lipochitooligosaccharide signals. Plant J. 1996;10:295–301. [Google Scholar]
- 94.Roos W, Evers S, Hieke M, Tschöpe M, Schumann B. Shifts of intracellular pH distribution as a part of signal mechanism leading to the elicitation of benzophenanthridine alkaloids. Plant Physiol. 1998;118:349–364. doi: 10.1104/pp.118.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Swanson SJ, Jones RL. Gibberellic acid induces vacuolar acidification in barley aleurone. Plant Cell. 1996;8:2211–2221. doi: 10.1105/tpc.8.12.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beffagna N, Romai G, Meraviglia G, Pallini S. Effects of abscisic acid and cytoplasmic pH on potassium and chloride efflux in Arabidopsis thaliana seedlings. Plant Cell Physiol. 1997;38:503–510. doi: 10.1093/oxfordjournals.pcp.a029197. [DOI] [PubMed] [Google Scholar]
- 97.Guern J, Mathieu Y, Thomine S, Jouanneau JP, Beloeil JC. Plant cells counteract cytoplasmic pH changes but likely use these pH changes as secondary messages in signal perception. Curr Top Plant Biochem Physiol. 1992;11:249–269. [Google Scholar]
- 98.Roos W, Viehweger K, Dordschbal B, Schumann B, Evers S, Steighardt J, et al. Intracellular pH signals in the induction of secondary pathways—The case of Eschscholzia californica. J Plant Physiol. 2006;163:369–381. doi: 10.1016/j.jplph.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 99.Bush DR. Proton-coupled sugar and amino-acid transporters in plants. Ann Rev Plant Physiol Plant Mol Biol. 1993;44:513–542. [Google Scholar]
- 100.Plieth C, Sattelmacher B, Hansen UP. Cytoplasmic Ca2+-H+-exchange buffers in green algae. Protoplasma. 1997;198:107–124. [Google Scholar]
- 101.Gilroy S, Trewavas A. A decade of plant signals. BioEssays. 1994;16:677–682. [Google Scholar]
- 102.Ward JM, Pei ZM, Schroeder JI. Roles of ion channels in initiation of signal transduction in higher plants. Plant Cell. 1995;7:833–844. doi: 10.1105/tpc.7.7.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blatt MR, Grabov A. Signal redundancy gates and integration in the control of ion channels for stomatal movement. J Exp Bot. 1997;48:529–537. doi: 10.1093/jxb/48.Special_Issue.529. [DOI] [PubMed] [Google Scholar]
- 104.Felle HH. pH: signal and messenger in plant cells. Plant Biol. 2001;3:577–591. [Google Scholar]
- 105.Okazaki Y, Kikuyama M, Hiramoto Y, Iwasaki N. Short-term regulation of cytosolic Ca2+, cytosolic pH and vacuolar pH under NaCl stress in the charophyte alga Nitellopsis obtuse. Plant Cell Environ. 1996;19:569–576. [Google Scholar]
- 106.Gruwel MLH, Rauw VL, Loewen M, Abrams SR. Effects of sodium chloride on plant cells; a 31P and 23Na NMR system to study salt tolerance. Plant Sci. 2001;160:785–794. doi: 10.1016/s0168-9452(00)00424-6. [DOI] [PubMed] [Google Scholar]
- 107.Caracuel Z, Casanova C, Roncero MIG, Pietro AD, Ramos J. pH response transcription factor PacC controls salt stress tolerance and expression of the P-type Na+-ATPase Ena1 in Fusarium oxysporum. Eukaryotic Cell. 2003;2:1246–1252. doi: 10.1128/EC.2.6.1246-1252.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Banuelos MA, Ruiz MC, Jimenez A, Souciet JL, Potier S, Ramos J. Role of the Nha1 antiporter in regulating K+ influx in Saccharomyces cerevisiae. Yeast. 2002;19:9–15. doi: 10.1002/yea.799. [DOI] [PubMed] [Google Scholar]
- 109.Kinclova O, Ramos J, Potier S, Sychrov H. Functional study of the Saccharomyces cerevisiae Nha1p C terminus. Mol Microbiol. 2001;40:656–668. doi: 10.1046/j.1365-2958.2001.02412.x. [DOI] [PubMed] [Google Scholar]
- 110.Yamaguchi T, Apse MP, Shi H, Blumwald E. Topological analysis of a plant vacuolar Na+/H+ antiporter reveals a luminal C terminus that regulates antiporter cation selectivity. Proc Natl Acad Sci USA. 2003;100:12510–12515. doi: 10.1073/pnas.2034966100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yamaguchi T, Aharon GS, Sottosanto JB, Blumwald E. Vacuolar Na+/H+ antiporter cation selectivity is regulated by calmodulin from within the vacuole in a Ca2+- and pH-dependent manner. Proc Natl Acad Sci USA. 2005;102:16107–16112. doi: 10.1073/pnas.0504437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kader MA, Seidel T, Golldack D, Lindberg S. Expressions of OsHKT1, OsHKT2 and OsVHA are differently regulated under NaCl stress in salt-sensitive and salt-tolerant rice (Oryza sativa L.) cultivars. J Exp Bot. 2006;57:4257–4268. doi: 10.1093/jxb/erl199. [DOI] [PubMed] [Google Scholar]