Abstract
Oomycetes are a diverse group of filamentous eukaryotic microbes comprising devastating animal and plant pathogens. They share many characteristics with fungi, including polarized hyphal extension and production of spores, but phylogenetics studies have clearly placed oomycetes outside the fungal kingdom, in the kingdom Stramenopila which also includes marine organisms such as diatoms and brown algae. Oomycetes display various specific biochemical features, including sterol metabolism. Sterols are essential isoprenoid compounds involved in membrane function and hormone signaling. Oomycetes belonging to Peronosporales, such as Phytophthora sp., are unable to synthesize their own sterols and must acquire them from their plant or animal hosts. In contrast, a combination of biochemical and molecular approaches allowed us to decipher a nearly complete sterol biosynthetic pathway leading to fucosterol in the legume pathogen Aphanomyces euteiches, an oomycete belonging to Saprolegniales. Importantly, sterol demethylase, a key enzyme from this pathway, is susceptible to chemicals widely used in agriculture and medicine as antifungal drugs, suggesting that similar products could be used against plant and animal diseases caused by Saprolegniales.
Key words: azoles, fungicides, root rot, elicitin, Saprolegnia, chromoalveolates
Sterols in Oomycetes: The First Oomycete Biosynthetic Pathway Deciphered in Aphanomyces euteiches
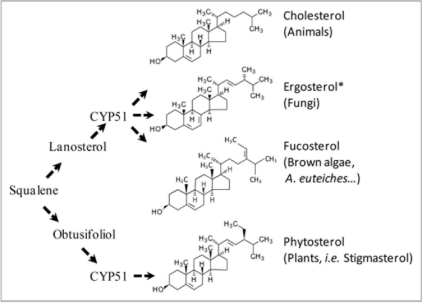
Sterols are isoprenoid compounds synthesized from a C5 isopentenyl diphosphate building block leading to the oxygenated isoprenoid oxidosqualene C30 compound. They are essential components of the membranes of all eukaryotic organisms, controlling membrane fluidity and permeability. Sterols are highly diverse in nature but only a few major sterols are found in a given biological lineage (Fig. 1). For example, cholesterol is the major sterol found in animals whereas fungi synthesize ergosterol, and plants synthesize a variety of 24-ethyl sterols such as stigmasterol. Some parasites have lost the ability to synthesize sterols and must acquire them from their host during pathogenesis. This is the case for apicomplexan parasites, such as Toxoplasma sp., and for oomycetes belonging to the Peronosporales such as Phytophthora sp. However, oomycetes belonging to the Saprolegniale group are sterol prototrophs and our study provides the first detailed analysis of a sterol biosynthesis pathway in an oomycete. Annotation of a large EST collection revealed a series of sequences unique to Aphanomyces euteiches and absent in Phytophthora genomes.1 Most of these sequences showed omology to enzymes involved in sterol metabolism. Analyses of mycelium grown in a sterolfree medium revealed that fucosterol was the major sterol produced by A. euteiches, using 3-hydroxy-3-methylglutaryl coenzyme A as a precursor.2 Lanosterol, a key intermediate found in animal and fungi, was also identified. Fucosterol was initially characterized in brown algae and the presence of fucosterol in A. euteiches strengthens the phylogenetic relationship between oomycetes and brown algae.3
Figure 1.
Sterol diversity in eukaryotes. Sterols are synthesized from the common precursor squalene. A key step involves a P450 enzyme (CYP51) which uses lanosterol in Animals, Fungi, and Stramenopiles, and obtusifoliol in green plants as substrates. The asterisk indicates that ergosterol is a sterol inducing plant immunity. Stigmasterol is shown as a representative plant sterol.
Sterols and Plant Immunity
The fungal sterol ergosterol has been shown to elicit defense responses in various plant systems.4–6 In tomato cells, alkalinization of the extracellular medium was observed in response to subnanomolar concentrations of the molecule.4 Concentrations higher than 1 nM induced an oxidative burst in tobacco cells, and a marked accumulation of capsidiol, an antimicrobial compound, was observed when more that 100 nM ergosterol was used.5 Recently, the defense-inducing activity of a high concentration of ergosterol (200 µM) was demonstrated in grape, where it triggered WRKY, VvLTP1, and stilbene synthase gene expression in plantlets. This was correlated with resveratrol accumulation and enhanced protection against Botrytis cinerea.6 In these studies, cholesterol was shown to be inactive, whereas the activity of fucosterol was not investigated. We thus recently checked the biological activity of sterols in M. truncatula by treating plantlets with 30 µM of either ergosterol, fucosterol or cholesterol. Preliminary data indicated that ergosterol induced an oxidative burst and expression of some defense genes, whereas the other two sterols did not. This suggests that plants have evolved a system for recognition of exogenous sterols that targets preferentially the fungal-specific sterol ergosterol, and not oomycete sterols (Bottin A, et al., unpublished).
Another link between plant immunity and sterols concerns elicitins, which are extracellular proteins, produced by Phytophthora sp. and other Peronosporales genera.7 These proteins have been found to bind sterols and it was proposed that they play a role as sterol carriers.8,9 Interestingly, elicitins were first discovered based on their ability to induce a hypersensitive response on tobacco plants, and it was proposed that their eliciting activity could be linked to their binding to sterols.10,11 Perception of elicitins has been suggested to play a key role in restricting the host range of Phytopthora species.12 Up to now, no elicitin gene was detected in Saprolegniales species, suggesting that expansion of the elicitin family in Peronosporales is correlated with the loss of the sterol biosynthetic pathway. Conversely, plants have evolved mechanisms which detect elicitin-sterol complexes. Thus a sterol-mediated perception of Saprolegniales seems to be absent since fucosterol does not induce a detectable plant response and elicitin genes are not present in these organisms.
Sterol Synthesis: New Target for Anti-Oomycetal Chemicals?
Sterol synthesis is a major target of fungicides which inhibit a demethylation step catalysed by a cytochrome P450 enzyme belonging to the CYP51 class. The class of triazole fungicides, which target CYP51 enzymes, represents the largest class of fungicide with more than 20% of total fungicide sales in agriculture.13 These molecules are also widely used as antifungal agents against human pathogens such Candida albicans or Aspergillus fumigatus.14 Since Phytophthora species and other Peronosporales do not possess CYP51 enzymes,15 these fungicides are inefficient against diseases caused by these pathogens. However, identification of a CYP51 enzyme in A. euteiches suggested that triazoles could be used against this pathogen. Two triazoles were found to efficiently inhibit A. euteiches mycelium growth. Sterol analyses revealed an increased amount of lanosterol, the CYP51 substrate, in triazole-treated mycelium, confirming that this enzyme is an in vivo target of triazoles. This preliminary experiment opens the way for investigating the potential use of triazoles against Aphanomyces diseases, and more generally against animal and plant disease caused by Saprolegniales.
Conclusion
The kingdom Stramenopila comprises a large number of diverse autotrophic and heterotrophic organisms with high ecological importance, such as photosynthetic algae (diatoms and brown algae) and the fungal-like oomycete parasites, for which there is a large gap in knowledge of primary and secondary metabolism. The discovery of a new sterol biosynthetic route in A. euteiches could be probably generalized to other saprophytic and parasitic Stramenopiles and this study shows that acquiring and analysing large genomic data from a small number of models can dramatically increase our knowledge on organisms which were largely ignored by the scientific community.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10551
References
- 1.Gaulin E, Madoui MA, Bottin A, Jacquet C, Mathé C, Couloux A, et al. Transcriptome of Aphanomyces euteiches: new oomycete putative pathogenicity factors and metabolic pathways. PLoS ONE. 2008;3:1723. doi: 10.1371/journal.pone.0001723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madoui MA, Bertrand-Michel J, Gaulin E, Dumas B. Sterol metabolism in the oomycete Aphanomyces euteiches, a legume root pathogen. New Phytol. 2009;183:291–300. doi: 10.1111/j.1469-8137.2009.02895.x. [DOI] [PubMed] [Google Scholar]
- 3.Baldauf SL, Roger AJ, Wenk-Siefert I, Doolittle WF. A kingdom-level phylogeny of eukaryotes based on combined protein data. Science. 2000;290:972–977. doi: 10.1126/science.290.5493.972. [DOI] [PubMed] [Google Scholar]
- 4.Granado J, Felix G, Boller T. Perception of fungal sterols in plants. Plant Physiol. 1995;107:485–490. doi: 10.1104/pp.107.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasparovsky T, Milat M-L, Humbert C, Blein J-P, Havel L, Mikes V. Elicitation of tobacco cells with ergosterol activates a signal pathway including mobilization of internal calcium. Plant Physiol Biochem. 2003;41:495–501. [Google Scholar]
- 6.Laquitaine L, Gomes E, Francois J, Marchive C, Pascal S, Hamdi S, et al. Molecular basis of ergosterol-induced protection of grape against botrytis cinerea: induction of type I LTP promoter activity, WRKY, and stilbene synthase gene expression. Mol Plant Microbe Interact. 2006;19:1103–1112. doi: 10.1094/MPMI-19-1103. [DOI] [PubMed] [Google Scholar]
- 7.Panabieres F, Ponchet M, Allasia V, Cardin L, Ricci P. Characterization of border species among Pythiaceae: several Pythium isolates produce elicitins, typical proteins from Phytophthora spp. Mycol Res. 1997;101:1459–1468. [Google Scholar]
- 8.Mikes V, Milat ML, Ponchet M, Ricci P, Blein JP. The fungal elicitor cryptogein is a sterol carrier protein. FEBS Lett. 1997;416:190–192. doi: 10.1016/s0014-5793(97)01193-9. [DOI] [PubMed] [Google Scholar]
- 9.Mikes V, Milat ML, Ponchet M, Panabieres F, Ricci P, Blein JP. Elicitins, proteinaceous elicitors of plant defense, are a new class of sterol carrier proteins. Biochem Biophys Res Commun. 1998;245:133–139. doi: 10.1006/bbrc.1998.8341. [DOI] [PubMed] [Google Scholar]
- 10.Ponchet M, Panabieres F, Milat ML, Mikes V, Montillet JL, Suty L, et al. Are elicitins cryptograms in plant-Oomycete communications? Cell Mol Life Sci. 1999;56:1020–1047. doi: 10.1007/s000180050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osman H, Vauthrin S, Mikes V, Milat ML, Panabieres F, Marais A, et al. Mediation of elicitin activity on tobacco is assumed by elicitin-sterol complexes. Mol Biol Cell. 2001;12:2825–2934. doi: 10.1091/mbc.12.9.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamoun S. Nonhost resistance to Phytophthora: novel prospects for a classical problem. Curr Opin Plant Biol. 2001;4:295–300. doi: 10.1016/s1369-5266(00)00176-x. [DOI] [PubMed] [Google Scholar]
- 13.Lamb D, Kelly D, Kelly S. Molecular aspects of azole antifungal action and resistance. Drug Resist Updat. 1999;2:390–402. doi: 10.1054/drup.1999.0112. [DOI] [PubMed] [Google Scholar]
- 14.Odds FC, Brown AJ, Gow NA. Antifungal agents: mechanisms of action. Trends Microbiol. 2003;11:272–279. doi: 10.1016/s0966-842x(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 15.Tyler BM, Tripathy S, Zhang X, Dehal P, Jiang RH, Aerts A, et al. Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science. 2006;313:1261–1266. doi: 10.1126/science.1128796. [DOI] [PubMed] [Google Scholar]