Abstract
Determination of the subcellular localization of an unknown protein is a major step towards the elucidation of its function. Lately, the expression of proteins fused to fluorescent markers has been very popular and many approaches have been proposed to express these proteins. Stable transformation using Agrobacterium tumefaciens generates stable lines for downstream experiments, but is time-consuming. If only colocalization is required, transient techniques save time and effort. Several methods for transient assays have been described including protoplast transfection, biolistic bombardment, Agrobacterium tumefaciens cocultivation and infiltration. In general colocalizations are preferentially performed in intact tissues of the same species, resembling the native situation. High transformation rates were described for cotyledons of Arabidopsis, but never for roots. Here we report that it is possible to transform Arabidopsis root epidermal cells with an efficiency that is sufficient for colocalization purposes.
Key words: Arabidopsis, GFP-fusions, protein localization, root, transient transformation
Since the release of the Arabidopsis thaliana genome sequence plant biologists set the goal to elucidate the functions of all coded genes. Apart from the spatio-temporal expression patterns of genes, the subcellular localization of gene products can play an essential role in deciphering their function. Classical immunological approaches to localize proteins can be hindered by cross-reactivity, time-consuming generation of antibodies and the low temporal resolution. Expression of tagged proteins forms a suitable alternative. Lately, fusions with fluorescent proteins in combination with confocal (CLSM)1 or spinning disc microscopy2 allow real time protein localization and even subcellular trafficking at high resolution. An overview of fluorescent tagging approaches can be found elsewhere.3
Currently several techniques to introduce the coding region for a tagged protein in a plant are available. The generation of stable lines transformed by Agrobacterium tumefaciens offers a continuous source of plant material, but it is time-consuming especially when only colocalization experiments are required. Transient assays, on the other hand, offer the advantage of being fast and amenable to high throughput strategies. Each of these techniques, however, has some limitations and drawbacks. Particle bombardment (biolistics) 4–6 for example circumvents the host specificity of Agrobacterium strains, but requires expensive equipment. Moreover, it is rather disruptive and imposes a significant stress upon the plants, possibly influencing the results. Protoplasts lack a cell wall and protoplast transformation7,8 is therefore not suitable for certain experiments related to cell wall proteins or when interactions between cells on tissue level might be important.9 Moreover, protoplasts have lost their identity which might be critical for the correct functioning of certain transgenic constructs. Agrobacterium infiltration of tobacco leaves10 is regularly used and represents an efficient, fast and relatively easy transformation technique. However, tobacco leaves easily show autofluoresence due to tissue damage as a result of experimental manipulations. As it has been reported that some protein fusions expressed in an heterologous system localize to different subcellular localizations11 it is advisable not to use tobacco when localizing Arabidopsis proteins. Leaf infiltrations have been performed in Arabidopsis,12 but apparently their leaves are much more prone to mechanical damage and the leaf developmental stage is critical, complicating this technique. Cocultivation of Agrobacterium with seedlings offers a rapid and efficient approach applicable to many mono and dicot species. It was reported to work efficiently in Arabidopsis cotyledons, but not in roots.9 As an alternative method, Agrobacterium infiltration of Arabidopsis seedlings11 seems an efficient technique for transient expression. However, expression in root cells could not be obtained. Colocalizations are required in the native cells or tissue for the correct localization of an unknown protein or proteins that need interaction partners. As a consequence this technique can not be reliably used when root expressed gene products are studied. Here we show evidence that it is possible to use the described technique11 to induce transient expression in Arabidopsis roots.
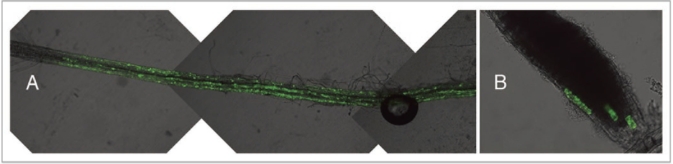
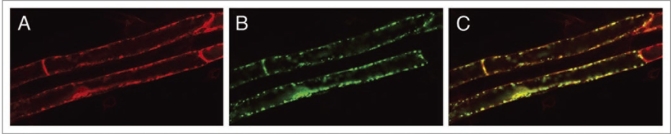
We used the Agrobacterium infiltration of Arabidopsis seedlings technique11 to colocalize several C-terminal (S65T)-sGFP fusions generated in the plant binary vector pGWB6.13 Each construct was transformed into Agrobacterium tumefaciens (C59C1RifR) containing the helper plasmid pMP90. Subsequently different stable marker lines, wild type Arabidopsis (Col-0) bearing mCherry fusion constructs,14 were transiently transformed.11 After 2 or 3 days seedlings were studied using CLSM. Besides being expressed in cotyledons fusion proteins were clearly observed in root epidermis and root cap cells (Fig. 1A and B). As reported11 the transformation efficiency in cotyledons was considerably higher than in root cells. However, in each experiment we obtained a considerable amount of transformed root epidermal cells which was more than sufficient for colocalization studies (Fig. 2). It was remarkable that transformation was repeatedly successful in groups of cells, adjacent or close to each other.
Figure 1.
Transient transformation of Arabidopsis root cells. Expression of the protein-GFP fusion product can be seen in the epidermal (A) and root cap cells (B) on fluorescence/transmission merged images. As seen in (A) high efficiencies of root transformation can be reached.
Figure 2.
Colocalization of mCherry and GFP constructs. Confocal image of the mCherry fluorescence (A), the GFP signal (B) and the merged image (C).
In contrast to what was reported earlier we show here that the Agrobacterium infiltration technique11 is perfectly capable of transiently transforming Arabidopsis root epidermal cells. It allows the transient production and study of proteins in their native environment, considerably increasing the reliability of such experiments. Additionaly the use of RFP marker constructs in colocalisation studies in the root is free of interference by the red background autofluorescence of chlorophyll.
Acknowledgements
This work was supported by a PhD grant of the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT, Vlaanderen), the Interuniversity Attraction Poles Programme—Belgian State—Belgian Science Policy [IUAP VI/33] and the Research Foundation—Flanders (FWO).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10575
References
- 1.Cox G. Biological confocal microscopy. Mater Today. 2002;5:34–41. [Google Scholar]
- 2.Gräf R, Rietdorf J, Zimmermann T. Live cell spinning disk microscopy. Adv Biochem Eng Biot. 2005;95:57–75. doi: 10.1007/b102210. [DOI] [PubMed] [Google Scholar]
- 3.Tian G-W, Mohanty A, Chary SN, Li S, Paap B, Drakakaki G, et al. High-throughput fluorescent tagging of full-length Arabidopsis gene products in planta. Plant Physiol. 2004;135:25–38. doi: 10.1104/pp.104.040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christou P. Strategies for variety-independent genetic-transformation of important cereals, legumes and woody species utilizing particle bombardment. Euphytica. 1995;85:13–27. [Google Scholar]
- 5.Klein TM, Wolf ED, Wu R, Sanford JC. Highvelocity microprojectiles for delivering nucleic-acids into living cells. Nature. 1987;327:70–73. [PubMed] [Google Scholar]
- 6.Ueki S, Lacroix B, Krichevsky A, Lazarowitz SG, Citovsky V. Functional transient genetic transformation of Arabidopsis leaves by biolistic bombardment. Nat Protoc. 2009;4:71–77. doi: 10.1038/nprot.2008.217. [DOI] [PubMed] [Google Scholar]
- 7.Pimpl P, Taylor JP, Snowden C, Hillmer S, Robinson DG, Denecke J. Golgi-mediated vacuolar sorting of the endoplasmic reticulum chaperone BiP may play an active role in quality control within the secretory pathway. Plant Cell. 2006;18:198–211. doi: 10.1105/tpc.105.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miao Y, Jiang L. Transient expression of fluorescent fusion proteins in protoplasts of suspension cultured cells. Nat Protoc. 2007;2:2348–2353. doi: 10.1038/nprot.2007.360. [DOI] [PubMed] [Google Scholar]
- 9.Li JF, Park E, von Arnim AG, Nebenfuhr A. The FAST technique: a simplified Agrobacterium-based transformation method for transient gene expression analysis in seedlings of Arabidopsis and other plant species. Plant Methods. 2009;5:6. doi: 10.1186/1746-4811-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sparkes IA, Runions J, Kearns A, Hawes C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat Protoc. 2006;1:2019–2025. doi: 10.1038/nprot.2006.286. [DOI] [PubMed] [Google Scholar]
- 11.Marion J, Bach L, Bellec Y, Meyer C, Gissot L, Faure JD. Systematic analysis of protein subcellular localization and interaction using high-throughput transient transformation of Arabidopsis seedlings. Plant J. 2008;56:169–179. doi: 10.1111/j.1365-313X.2008.03596.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim MJ, Baek K, Park C-M. Optimization of conditions for transient Agrobacterium-mediated gene expression assays in Arabidopsis. Plant Cell Rep. 2009;28:1159–1167. doi: 10.1007/s00299-009-0717-z. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, et al. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng. 2007;104:34–41. doi: 10.1263/jbb.104.34. [DOI] [PubMed] [Google Scholar]
- 14.Nelson BK, Cai X, Nebenfuhr A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 2007;51:1126–1136. doi: 10.1111/j.1365-313X.2007.03212.x. [DOI] [PubMed] [Google Scholar]