Abstract
Hormones are molecules involved in virtually every step of plant development and studies in this field have been shaping plant physiology for more than a century. The model plant Arabidopsis thaliana, long used as a tool to study plant hormones, lacks significant important developmental traits, such as fleshy climacteric fruit, compound leaf and multicellular trichomes, suggesting the necessity for alternative plant models. An attractive option often used is tomato, a species also of major economic importance, being ideal to bring together basic and applied plant sciences. The tomato Micro-Tom (MT) cultivar makes it possible to combine the direct benefits of studying a crop species with the fast life cycle and small size required for a suitable biological model. However, few obscure questions are constantly addressed to MT, creating a process herein called “MT mystification”. In this work we present evidence clarifying these questions and show the potential of MT, aiming to demystify it. To corroborate our ideas we showed that, by making use of MT, our laboratory demonstrated straightforwardly new hormonal functions and also characterized a novel antagonistic hormonal interaction between jasmonates and brassinosteroids in the formation of anti-herbivory traits in tomato.
Key words: Solanum lycopersicum, Arabidopsis thaliana, hormones, plant model, jasmonates, brassinosteroids
The Need of Alternative Models to Discover Novel Hormonal Functions and Interactions
Since the classical experiments with coleoptile tips by Darwin & Darwin1 to the molecular evidences of a novel hormone controlling shoot branching and mycorrhiza formation,2 the study of plant hormones shaped plant physiology’s history. Time has shown the importance of these molecules: from root/shoot development to stress response, from light perception to trichome formation, from stomatal closure to seed germination, virtually every step of plant development is regulated by one or several hormones at the same time.3 Since one hormone class can control various different processes and, conversely, a single process can be controlled by several hormones, the discovery of novel hormonal functions and interactions is relevant to understand plant development and its interplay with the environment.
Much of what we know about plant hormones came from studies with Arabidopsis thaliana. Its small size, fast life cycle, sequenced genome (see www.arabidopsis.org/), lenience to grow in controlled conditions and, most importantly, the identification and characterization of several hormonal mutants make Arabidopsis the first and most sensible choice when conducting research on plant hormones.
On the other hand, Arabidopsis lack many traits of great economic importance and that is when tomato (Solanum lycopersicum L.) thrives as an interesting plant model. Tomato can be used to address developmental processes difficult or impossible to be studied in Arabidopsis, such as photoperiod-independent sympodial flowering;4,5 formation of fleshy climacteric fruits, compound leaves and multicellular/glandular trichomes;6,7 mycorrhizal association and agronomically relevant plant-insect and pathogen interactions.7–9
Tomato Genetics and a Remarkable Gizmo—Bringing Everything into One Cultivar
Tomato presents many characteristics of a biological model: it is an autogamous diploid species with a small genome (950 Mb) distributed in 12 chromosomes; it has a saturated genetic linkage map with numerous markers associated with traits of great economic and biological importance (solgenomics.net/). It belongs to a taxonomic group (Asterid clade) largely unexplored yet at the molecular level. Highly efficient protocols for transformation of tomato are already developed10 and rich germplasm collections are available, such as the Tomato Genetics Resource Center (tgrc.ucdavis.edu/). The tomato genome is currently being sequenced by the “International Solanaceae Genome Project” (SOL) consortium.11 Tomato is also a potentially valuable tool for research on plant hormones, due to the availability of a vast number of hormonal mutants.7 And differently from Arabidopsis, the edible crop status of tomato makes it one of the best candidates to bring basic and applied sciences together.12,13
However, two major problems are generally raised on plant hormone studies in tomato. Firstly, since many of the hormonal mutations are present in diverse cultivars, the information/comparison between them is poorly exchangeable. Secondly, due to the normal plant size of the species (>1-m tall), it requires considerable growing spaces, and the somewhat long generation time (∼4 months) makes it a not-so-easy-to-handle plant. For these reasons, the dwarf tomato cultivar Micro-Tom (MT) is now becoming increasingly used as a model system and was described as the “laboratory tomato”.14 Because of its compact plant size (∼15-cm tall) and red-ripened fruits, MT was originally described for ornamental purposes,15 but because its short life cycle ∼10 weeks) and the ability to growth at high densities, it has also become a suitable genetic model system.14
MT is now being widely used in studies of fruit development,16 hormonal interactions,7 abiotic/biotic stress responses,17,18 mycorrhizal colonization,8 small RNA gene regulation19 and even microgravity growth.20 Several genetic and physiological tools are already available in the MT cultivar, such an efficient transformation protocol, 10 comprehensive EMS and gamma-ray mutant collections,21 EST and SNP databases (www.kazusa.or.jp/jsol/microtom/index.html), and a “metabolite annotation” compilation.22 A hormonal mutant collection introgressed into the MT genotype is also publicly available,7,23 making it also an ideal model to characterize physiological and developmental functions of plant hormones and their interactions, as it has been proven recently.7
Despite its potential value, the complete acceptance of MT as a model plant has not taken off as yet because some confusing and obscure concerns still persist, creating what we called herein the “mystification of the MT cultivar”. Nonetheless, if looked closer, one will realize that once understanding the genetic concepts behind the MT and taking the necessary scientific approaches to avoid misleading conclusions, these concerns are vanished and MT becomes an ideal model system.
Demystifying Micro-Tom
Indeed, the MT cultivar harbours some distinctive mutations. The most well known mutant alleles are: dwarf (d), a brassinosteroid (BR)-related mutation24 responsible for the small plant size, and self-pruning (sp), responsible for its determinate growth habit.4,25 The miniature (mnt) allele was also suggested to contribute to the MT small plant size,14 although this has not been proven yet. Additional alleles present in MT are uniform ripening (u), Stemphylium resistance (Sm) and Immunity to Fusarium wilt (I),15 which confer the absence of green shoulders in fruits and resistance to the pathogenic fungi Stemphylium solani and Fusarium oxysporum f. lycopersici, respectively. The presence of such mutations led to the prejudgment that MT cannot be used in scientific studies because they can interfere with the results observed.
Scientists using crop species as genetic models should bear in mind that the domestication process itself was developed based on selection and recombination of mutations. Thus, although the term “wild type” (WT) is adequate for non-domesticated animal and plants, it is not entirely accurate for domesticated models, such as tomato. Moreover, every cultivar holds many mutant alleles when compared to any other genotype and this genetic assortment is exactly what defines a cultivar. To exemplify, the cultivar M82 of tomato, used to study natural genetic variations26 and induced mutagenesis,27 holds the alleles sp, obv, u, I and Ve (tgrc.ucdavis.edu/). Even for cultivars with no apparent mutations, such as Ailsa Craig and MoneyMaker, the most used “non-dwarf ” cultivars in genetic studies of tomato, allelic variations exist at least in quantitative trait loci (QTL) controlling fruit size,28 since their fruits are larger than the fruits of the wild tomato (Solanum lycopersicon var. cerasiforme). If one denies the use of MT because of its mutations, one will come to the conclusion that no cultivar in any species can be used in scientific experiments. Moreover, it is worth noting that with an appropriate control, the presence of mutations or allelic variations in a cultivar does not preclude it from being used to study the effects of specific mutations. If the event under study is influenced by that particular mutation, the alternative of generating near isogenic lines (NILs) harboring the non-mutated allele as a control fulfills the requirement of an appropriate control in the scientific method. In the case of MT, NILs with indeterminate growth habit (MT-Sp), green-shoulder fruits (MT-U) and increased BR levels (MT-D) have recently been developed (Carvalho et al. in preparation).
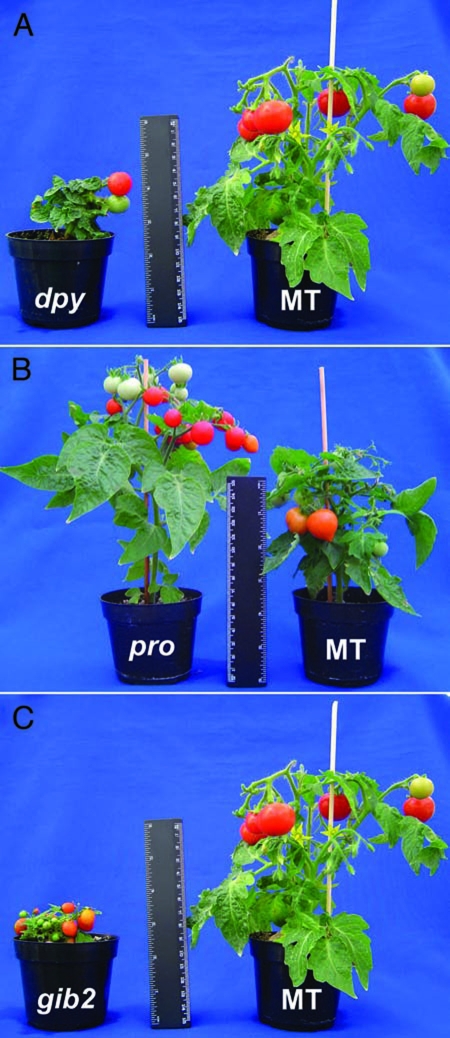
Another concern commonly addressed to MT specifically in hormonal studies is associated to its BR-related mutation and a suggested gibberellin (GA) mutation, perceiving the genotype as unsuitable for hormone studies or to analyse GA and/or BR-dependent responses. Indeed, MT contains a weak mutation (d) related to BR biosynthesis, however, two points should be mentioned: (1) Although there is no “correct” level of BR that a plant should contain, d is not a severe BR mutation like cu3 or dpy.29 Additional evidence is that the dpy mutant was introgressed (as well as cu3) into MT and the resulting NIL was completely differently than MT itself (Fig. 1A) and its original background; (2) a recent paper from our lab showed that MT can perfectly be used to study BR-dependent responses and how BR interacts with other hormones.7 The case of GA is even simpler. There is no indicative for a GA mutation in MT since: (1) MT has normal seed germination and leaf development; (2) opposite GA mutations were introgressed into MT7,23 and their phenotypes were clearly observed, such as for the mutation in the DELLA repression domain procera (Fig. 1B) and the GA deficient gibberellin deficient 2 (Fig. 1C).30,31
Figure 1.
Comparison between Micro-Tom (MT) and near isogenic lines harboring hormonal
The Micro-Tom as a Model System to Discover Novel Hormonal Functions and Interactions
An example of the usefulness of the MT cultivar in hormonal functions and interaction investigation was demonstrated in the paper that is the object of this addendum.7 Novel hormonal functions were observed when comparing several different hormonal mutants in the MT cultivar. We found out that ethylene, GAs and auxin are capable of altering glandular and non-glandular trichome density in tomato leaves, showing a possible multi-hormonal control of this trait. However, the most remarkable finding was a BR-JA (jasmonic acid) interaction, where low BR levels positively control the formation of anti-herbivory traits by means of upregulating the JA pathway. This interaction seems to be evolutionary divergent in the plant kingdom, since the opposite is observed in Arabidopsis, in which high BR is capable of upregulating OPR3, an enzyme involved in JA biosynthesis.32 Additionally to these novel results, our work also proved that although MT harbors a BR mutation, it still can be employed to study plant hormones, even on BR action and interaction.
Conclusion
The late Charles Rick once said that “If Arabidopsis is the Drosophila of plant genetics, than tomato has become the mouse”.12 If Rick was right, than MT is the best and fastest way to make tomato as widespread, as useful and perhaps as small as a mouse.
Acknowledgements
FAPESP (grant 02/00329-8 and fellowship 06/05911-8) and CNPq (grant 475494/03-2 and fellowship 308075/03-0) supported this work.
References
- 1.Darwin C, Darwin G. The power of movement in plants. New York, USA: Appleton-Century Inc; 1880. [Google Scholar]
- 2.Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- 3.Davies PJ. Plant hormones: biosynthesis, signal transduction, action! Dordrecht, Netherlands: Kluwer Academics Publisher; 2004. [Google Scholar]
- 4.Pnueli L, Carmel-Goren L, Hareven D, Gutfinger T, Alvarez J, Ganal M, et al. The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development. 1998;125:1979–1989. doi: 10.1242/dev.125.11.1979. [DOI] [PubMed] [Google Scholar]
- 5.Lifschitz E, Eviatar T, Rozman A, Goldshmidt A, Amsellem Z, Alvarez JP, et al. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. P Natl Acad Sci USA. 2006;103:6398–6403. doi: 10.1073/pnas.0601620103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannoni J. Molecular biology of fruit maturation and ripening. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- 7.Campos ML, Almeida A, Rossi ML, Martinelli AP, Junior CGL, Figueira A, et al. Brassinosteroids interact negatively with jasmonates in the formation of anti-herbivory traits in tomato. J Exp Bot. 2009;60:4347–4361. doi: 10.1093/jxb/erp270. [DOI] [PubMed] [Google Scholar]
- 8.Zsögön A, Lambais MR, Benedito VA, Figueira AVD, Peres LEP. Reduced arbuscular mycorrhizal colonization in tomato ethylene mutants. Sci Agr. 2008;65:259–267. [Google Scholar]
- 9.Arie T, Takahashi H, Kodama M, Teraoka T. Tomato as a model plant for plant-pathogen interactions. Plant Biotechnol. 2007;24:135–147. [Google Scholar]
- 10.Sun HJ, Uchii S, Watanabe S, Ezura H. A highly efficient transformation protocol for Micro-Tom, a model cultivar for tomato functional genomics. Plant Cell Physiol. 2006;47:426–431. doi: 10.1093/pcp/pci251. [DOI] [PubMed] [Google Scholar]
- 11.Mueller LA, Lankhorst RK, Tanksley SD, Giovannoni JJ, White R, Vrebalov J, et al. A snapshot of the emerging tomato genome sequence. Plant Genome. 2009;2:78–92. [Google Scholar]
- 12.Rick CM. Tomato paste: a concentrated review of genetics highlight from the beginnings to the advent of molecular genetics. Genetics. 1991;128:1–5. doi: 10.1093/genetics/128.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rick CM. Tomato mutants: freaks, anomalies and breeder’s resources. HortScience. 1986;21:917–918. [Google Scholar]
- 14.Meissner R, Jacobson Y, Melamed S, Levyatuv S, Shalev G, Ashri A, et al. A new model system for tomato genetics. Plant J. 1997;12:1465–1472. [Google Scholar]
- 15.Scott J, Harbaugh B. Micro-Tom A miniature dwarf tomato. Fla Agr Exp Sta Circ. 1989;370:1–6. [Google Scholar]
- 16.Serrani JC, Fos M, Atares A, Garcia-Martinez JL. Effect of gibberellin and auxin on parthenocarpic fruit growth induction in the cv micro-tom of tomato. J Plant Growth Regul. 2007;26:211–221. [Google Scholar]
- 17.Gratao PL, Monteiro CC, Antunes AM, Peres LEP, Azevedo RA. Acquired tolerance of tomato (Lycopersico esculentum cv. Micro-Tom) plants to cadmium-induced stress. Ann Appl Biol. 2008;153:321–333. [Google Scholar]
- 18.Hase S, Takahashi S, Takenaka S, Nakaho K, Arie T, Seo S, et al. Involvement of jasmonic acid signaling in bacterial wilt disease resistance induced by biocontrol agent Pythium oligandrum in tomato. Plant Pathol. 2008;57:870–876. [Google Scholar]
- 19.Zrachya A, Kumar PP, Ramakrishnan U, Levy Y, Loyter A, Arazi T, et al. Production of siRNA targeted against TYLCV coat protein transcripts leads to silencing of it expression and resistance to the virus. Transgenic Res. 2007;16:385–398. doi: 10.1007/s11248-006-9042-2. [DOI] [PubMed] [Google Scholar]
- 20.Colla G, Rouphael Y, Cardarelli M, Mazzucato A, Olimpieri I. Growth, yield and reproduction of dwarf tomato grown under simulated microgravity conditions. Plant Biosyst. 2007;141:75–81. [Google Scholar]
- 21.Pino-Nunes LE, Figueira AVO, Tulmann Neto A, Zsögön A, Piotto FA, Silva JA, et al. Induced mutagenesis and natural genetic variation in tomato ‘Micro-Tom’. Acta Hort. 2009;821:63–72. [Google Scholar]
- 22.Iijima Y, Nakamura Y, Ogata Y, Tanaka K, Sakurai N, Suda K, et al. Metabolite annotations based on the integration of mass spectral information. Plant J. 2008;54:949–962. doi: 10.1111/j.1365-313X.2008.03434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carvalho RF. Analysis of the interactions between phytochrome and plant hormones in plant development. Brazil: Universidade de São Paulo,; 2008. PhD Thesis, [Google Scholar]
- 24.Bishop GJ, Nomura T, Yokota T, Harrison K, Noguchi T, Fujioka S, et al. The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. P Natl Acad Sci USA. 96:1761–1766. doi: 10.1073/pnas.96.4.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martí E, Gisbert C, Bishop GJ, Dixon MS, García-Martínez JL. Genetic and physiological characterization of tomato cv. Micro-Tom. J Exp Bot. 2006;57:2037–2047. doi: 10.1093/jxb/erj154. [DOI] [PubMed] [Google Scholar]
- 26.Eshed Y, Abu-Abied M, Saranga Y, Zamir D. Lycopersicon esculentum lines containing small overlapping introgressions from L. pennellii. Theor Appl Genet. 1982;83:1027–1034. doi: 10.1007/BF00232968. [DOI] [PubMed] [Google Scholar]
- 27.Menda N, Semel Y, Peled D, Eshed Y, Zamir D. In silico screening of a saturated mutation library of tomato. Plant J. 2004;38:861–872. doi: 10.1111/j.1365-313X.2004.02088.x. [DOI] [PubMed] [Google Scholar]
- 28.Paterson H, Landerj ES, Hewiit D, Petersons S, Lincoln E, Tanksley SD. Resolution of quantitative traits into Mendelian factors by using a complete RFLP linkage map. Nature. 1988;335:721–726. doi: 10.1038/335721a0. [DOI] [PubMed] [Google Scholar]
- 29.Koka CV, Cerny RE, Gardner RG, Noguchi T, Fujioka S, Takatsuto S, et al. A putative role for the tomato genes DUMPY and CURL-3 in brassinosteroid biosynthesis and response. Plant Physiol. 2000;122:85–98. doi: 10.1104/pp.122.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassel GW, Mullen RT, Bewley JD. Procera is a putative DELLA mutant in tomato (Solanum lycopersicum): effects on the seed and vegetative plant. J Exp Bot. 2008;59:585–593. doi: 10.1093/jxb/erm354. [DOI] [PubMed] [Google Scholar]
- 31.Koornneeff M, Bosma TDG, Hanhart CJ, Van Der Veen JH, Zeevaart JAD. The isolation and characterization of gibberellin-deficient mutants in tomato. Theor Appl Genet. 1990:852–857. doi: 10.1007/BF00224204. [DOI] [PubMed] [Google Scholar]
- 32.Müssig C, Biesgen C, Lisso J, Uwer U, Weiler EW, Altmann T. The novel stress-inducible 12-oxophytodienoate reductase from Arabidopsis thaliana provides a potential link between Brassinosteroid-action and Jasmonic-acid synthesis. J Plant Physiol. 2000;157:143–152. [Google Scholar]