Abstract
Accumulating evidence suggest that certain annexins can play a role in abiotic stress responses in plants. We found that for one member of the Arabidopsis thaliana annexin gene family, annexin 1 (AnnAt1), loss-of-function mutants are more sensitive to drought stress and gain-of-function mutants are more tolerant.1 We also found that AnnAt1 is able to regulate accumulation of H2O2 in vivo in Arabidopsis cells based on the observation that the level of ROS accumulation following induction by ABA correlates with the level of AnnAt1 protein in transgenic Arabidopsis plants. Here we provide more commentary on the antioxidant activity of AnnAt1, critically assess the evidence that AnnAt1 and other annexins possess peroxidase activity, emphasize a redox-induced posttranslational modification which occurs to AnnAt1 during ABA signaling, and discuss ways this annexin’s membrane associations could mediate stress signaling while addressing the potential that AnnAt1 is a multifunctional protein in plants.
Key words: plant annexins, stress response, oxidative burst, ABA signaling, S-glutathionylation, S3 cluster
Annexins are a multifunctional proteins in eukaryotic cells that bind membranes in a calcium-dependent manner. In plants and animals, annexins are a multigene family, and in Arabidopsis there are eight distinct genes coding for protein with annexins motif. Expression of these eight different Arabidopsis annexins is differentially regulated by a variety of abiotic stress treatments2 and over the past several years it has become clear that a key function for annexins in animal and plant cells is to help confer tolerance to stress responses.3,4 This addendum further expands on some of the main conclusions of our recent paper on the role of annexins in abiotic stress responses.1
Historically, the antioxidant effect of AnnAt1 has been suggested to be a consequence of an inherent ROS-neutralizing enzymatic activity. There are three studies that established Arabidopsis AnnAt1 could play a protective role during oxidative stress in heterologous systems. First, recognition that this annexin could protect cells from oxidative stress came from a study that used a mutant Escherichia coli strain that is lacking OxyR, a transcription factor that senses oxidative stress and is activated by the oxidation of reactive cysteines.5 After sensing oxidative stress OxyR induces the transcription of the regulon of target genes coding for different antioxidants, thus mutants lacking a functional oxyR gene (ΔoxyR) are more sensitive to the oxidative stress in comparison with wild-type E. coli. This study found that AnnAt1, which was referred to as Oxy5, could restore normal growth in the mutant in response to oxidative stress treatment (350 µM H2O2).5 It was suggested that this ability might be due to a catalase-like motif located at the N-terminus, which has a conserved histine (His40) predicted to be required for heme-binding. They also demonstrated that both bacterially produced and immunoprecipitated AnnAt1 from Arabidopsis exhibited peroxidase activity in an in vitro assay.
A follow-up report investigated the ability of AnnAt1 to protect human tumor cells from tumor necrosis factor (TNF).6 One component of TNF-induced cell death is oxidative stress, and in this study stable transfection of AnnAt1 into TNF-sensitive HeLa D98 cells resulted in decreased ROS production and resistance to TNF-induced cell death. They also found that prior to TNF treatment the transfected HeLa cells showed increased mRNA, protein and activity levels of the ROS-scavenging enzyme, manganese superoxide dismutase (MnSOD).6 A third study demonstrated that two different mammalian cell lines transfected with AnnAt1 were protected from hydrogen peroxide-induced cell death.7 The transfected cell lines showed reduced levels of superoxide ions, protein kinase C activity and tumoringenicity.
But what about AnnAt1 function in plant cells? A number of studies have confirmed that partially purified preparations of AnnAt1 protein display in vitro peroxidase activity. For example, it was reported that an overexpressed version of AnnAt1 co-migrates with a peroxidase stain in a gel assay and purified preparations of recombinant AnnAt1 protein display peroxidase activity, although the specific activity is six orders of magnitude lower than horseradish peroxidase.8 They also found that protein obtained from overexpression in Nicotiana benthamiana had much higher activity in comparison with bacterially expressed AnnAt1. This result suggests the possibility that post-translational modification or binding to an in vivo partner makes the observed peroxidase-like activity physiologically relevant, but it could also be due to different contaminations in preparations derived from eukaryotic and prokaryotic expression system.
Recently, we again showed that partially purified recombinant AnnAt1 has low levels of in vitro peroxidase activity. In contrast to previous results,8 however, we found that the mutant AnnAt1 protein, which has the His40 substituted with an alanine (H40A), also displayed in vitro peroxidase activity.1 We also retested the ability of AnnAt1 to complement the ΔoxyR mutant during oxidative stress and found that indeed AnnAt1 as well as the H40A mutant AnnAt1 could rescue the E. coli mutant at certain H2O2 concentrations.1
All of the observations that AnnAt1 has inherent peroxidase activity are based on assays of AnnAt1 preparations purified with standard chromatography affinity methods to near homogeneity. The intrinsic limitations of such methods become clear when these partially purified samples start to be analyzed with more sensitive methods such as mass spectrometry. Thus results to date cannot be interpreted confidently because it is difficult to experimentally discriminate between intrinsic activity and activation of a co-purifying enzyme.
Recently, it was reported that a purified annexin doublet from maize had a higher level of peroxidase activity than previously reported for plant annexins, approximately one order of magnitude lower than horseradish peroxidase.9 Interestingly, they found that the maize annexin doublet does not bind heme, even though the critical histidine residue is present in these maize annexins. A similar result was also reported for cotton annexin.10 These results, together with our findings, using the mutant AnnAt1 H40A protein, indicate that the peroxidase-like motif is probably not important for the in vitro peroxidase activity of annexins, but instead is important for overall structure of the annexin protein.
Interestingly, another close homolog of AnnAt1 in Brassica rapa, AnnBr1, appears to form a complex with other proteins present in anthers during flower development in turnip mustard. Although this protein complex exhibits peroxidase activity, preliminary results indicate that this activity can be separated from AnnBr1 during electrophoresis under non-reducing conditions, suggesting that it is not likely to reside with annexin (Kleinschmidt and Clark G, unpublished result). Summarizing, even though it is tempting to link the antioxidant properties of annexins with their reported in vitro peroxidase activity, the question of whether AnnAt1 and other annexins possess inherent peroxidase activity that is physiologically relevant in vivo is a difficult one to experimentally address, and until a mechanism of annexin peroxidase activity is understood this important question remains an open one.
The close homolog of Arabidopsis AnnAt1 in Brassica juncea, AnnBj1, also displays weak in vitro peroxidase activity, and transgenic tobacco lines ectopically expressing AnnBj1 show tolerance to a variety of abiotic stress treatments.11 Although oxidative stress is a component of most abiotic stress stimuli, the fact that these transgenic tobacco lines also show tolerance to chemically applied oxidative stress (10 mM H2O2) at the seedling stage makes it clear that the oxidative protective properties of annexin 1 extends to plant cells as well as bacterial and mammalian cells discussed earlier.
One major consequence of oxidative stress is lipid peroxidation of membranes, so the finding that transgenic tobacco lines also showed reduced levels of lipid peroxidation in response to drought, salinity and heavy metal treatment was a significant one. Thus it may be that during oxidative stress this annexin protects membrane integrity or even repairs damage to the membrane caused by oxidation of lipids. Consistent with this hypothesis, certain animal annexins are required for membrane repair,12–14 so the possibility that AnnAt1 is acting as a membrane repair protein needs to be experimentally addressed in the future.
Diverse studies indicate that AnnAt1 is an important participant in membrane signaling. One study found that salt treatment induced a redistribution of AnnAt1 protein from the cytoplasmic fraction to the membrane fraction.15 Several Arabidopsis proteomic studies have confirmed the association of AnnAt1 with purified plasma membrane preparations. 16,17 In one of these studies the membrane-localized AnnAt1 protein had two different molecular masses, 34 and 39 kD, with the 34 kD isoform found mainly in the detergent phase of TX-114 partition, while the 39 kD isoform had no TX-114 phase preference and was less abundant in the membrane after washing with carbonate buffer.16 Thus, the 34 kD isoform of AnnAt1 behaved more like an integral membrane protein, similar to the reported membrane-binding properties for a wheat annexin during response to cold treatment.18
Animal annexins have been suggested to function directly as novel types of calcium channels or indirectly as regulators of ion channels. Results from the animal annexin field along with the membrane-binding properties of plant annexins led to the proposal that certain plant annexins can serve as hyperpolarization-activated cation channels (HACC).19 A recent study provided further evidence that a certain fraction of AnnAt1 associates with membranes in a Ca2+-independent manner and that AnnAt1 could serve as an ion channel during stress responses.20 More recently, a study in maize revealed that annexins are able to affect calcium influx indirectly and/or directly.9 In this study, maize annexins facilitated Ca2+ influx when added either to root epidermal protoplasts (indicating that extracellular annexins could modulate existing Ca2+ channels) or lipid bilayers.
Increases in cytosolic Ca2+ occur in response to abiotic stress and H2O2 can lead to opening of hyperpolarization-activated Ca2+ channels in plants.21,22 These results point to another way in which AnnAt1 and other annexins could function during stress responses, which is via generating and/or modulating the stress-induced calcium signals. Again, this proposed function is difficult to experimentally prove, and until genetic evidence showing that a plant loss-of-function mutant has an altered calcium signature for a specific physiological response it will have to remain only a hypothesized function for plant annexins.
It has become clear recently that extra-cellular nucleotides act as important signals to regulate plant growth and development, and one of the earliest downstream steps in extracellular nucleotide signaling is an increase in cytosolic calcium levels.23 It has recently been proposed that plant annexins may act as receptors for extracellular ATP (eATP) and may participate in Ca2+ influx.24 Root cells of an Arabidopsis AnnAt1 mutant, annAt1, show an impaired release of ATP into the extracellular matrix (ECM) as well as impaired Ca2+ influx during response to salt stress.25 It needs to be determined if the altered calcium signature of the annAt1 mutant is directly due to decreased levels of eATP or due to altered Ca2+ channel activity or both, but further studies of this mutant could yield the first genetic evidence that AnnAt1 might function directly as a Ca2+ channel or as a regulator of a Ca2+ channel in plant cells.
AnnAt1 is able to form oligomers, and the oligomerization state of AnnAt1 is affected by changes in redox state.8 The function of plant annexin oligomerization remains unclear; however it was proposed that it can constitute a structural basis of an oxidative stress response.26 The formation of oligomers by AnnAt1 in vitro can be induced by H2O2 and partially prevented by reducing agents.8 Oligomerization of mammalian annexin A5 is a sine qua non condition for its ability to carry out membrane repair.14
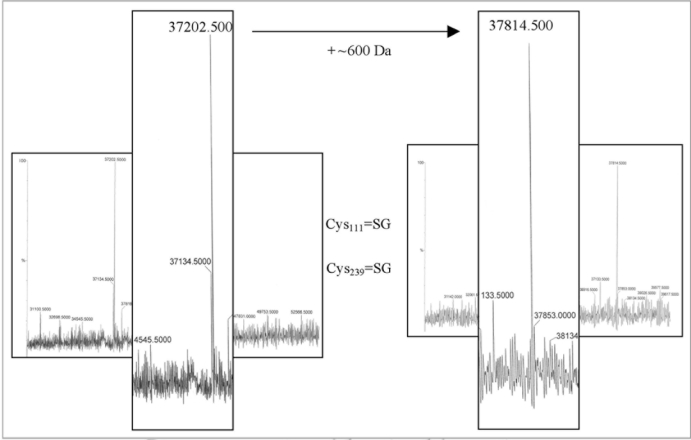
We have found that redox-driven oligomerization of AnnAt1 is achieved not via covalent S-S bonding but occurs via a different type of interaction.1 However, we found that both cysteine residues (Cys111 and Cys239) of AnnAt1 protein are target for S-glutathionylation in vitro (Fig. 1). The molecular weights of collected AnnAt1 samples determined with MALDI-TOF were 37,814 and 37,202 Da for gluthationylated and non-modified protein, respectively, which suggests the incorporation of 2 mol of GSH per mol of AnnAt1 (molecular mass change of about 600 daltons in AnnAt1 = mass change of two glutathione molecules) indicating that both Cys111 and Cys239 cross react with glutathione oxidant. This result shows that the cysteine residues are reactive and available for modification. Is S-glutathionylation of AnnAt1 physiologically relevant? We addressed this question and found that AnnAt1 is glutathionylated in vivo after ABA treatment and that S-glutathionylation decreases the calcium affinity of this annexin.1 It will be important to obtain a crystallized version of the glutathionylated form of AnnAt1 in order to further determine the structurefunction implications of this post-translational modification.
Figure 1.
Mass spec analyses of in vitro S-glutathionylated AnnAt1. Purified heterologous wild type AnnAt1-His(6) was dissolved in guanidium-HCl and treated with excess of oxidized gluthathione for 30 minutes in room temperature. After dilution protein was then purified using HPLC and was then sequenced with MS-MS to confirm modification of individual cysteine residues.
Reactive oxygen species (ROS) are produced continuously during normal cell metabolism. Cell redox homeostasis is maintained by the pool of lower molecular mass antioxidants, mainly glutathione and ascorbic acid. Glutathione is a tripeptide (Glutamate-Cysteine-Glycine) and is the most abundant non-protein thiol found in eukaryotic cells with concentrations in the millimolar range. These antioxidant molecules can undergo the reversible cycles of oxidation and reduction. Thus, on one hand they can protect intracellular components (e.g., proteins, lipids) from irreversible oxidation, or, on the other hand, they can regulate gene expression and other responses to ROS, especially during the response to many stress conditions that disrupt cellular homeostasis. In this situation the production of ROS is significantly enhanced, exceeding the rate of their breakdown, due to the increased activity of specific enzymatic systems (e.g., NADPH oxidase) that catalyze such reactions. This ROS accumulation, the so called oxidative burst, is the process that is believed to underlay the plant cross-tolerance to biotic and abiotic stresses.
One cellular response to oxidative stress is S-glutathionylation of cysteine residues on target proteins. In plants, the addition of a glutathione molecule to target proteins appears to occur by both non-enzymatic and enzymatic mechanisms.27 This modification can do more than just provide the modified protein protection from irreversible oxidation, as it can also be a reversible post-translational modification which regulates the activity of proteins that participate in redox signaling. Thus it would seem that AnnAt1 is a candidate for sensing ROS and may be able to modulate endogenous ROS-response systems or serve as a molecular switch between calcium mediated and ROS mediated signaling pathways.
ROS are also involved in signaling during plant responses to biotic stress. Tobacco transgenic lines ectopically expressing AnnBj1 showed enhanced resistance against fungal pathogen attack,11 and had increased message levels of four genes important in the defense response prior to challenge with a fungal pathogen. This result is similar to the observation that HeLa cells transfected with AnnAt1 show altered expression of MnSOD.6 Also, it was found that annexin VII knockout mice have reduced message levels for the inositol 1,4,5-trisphosphate receptor.28 Can annexins regulate gene expression, and if so do they do this as a primary effect or a secondary effect? Certainly we should at least consider that another possible way that AnnAt1 and its close relatives function during stress is via altering gene expression.
A number of studies have shown that AnnAt1 expression is regulated by abiotic stresses and abscisic acid (ABA). Other stresses, like heavy metals treatment or wounding, also regulate AnnAt1 and AnnBj1 mRNA levels, indicating that annexin 1 expression is controlled by hormonal systems other than ABA. In silico promoter studies of AnnAt1 supports the expression data for AnnAt1. We found that there are a number of conserved potential cis-binding elements for transcription factors from other families that are involved in regulating important aspects of development throughout the plant life cycle, including responses to environmental or pathogen challenge.1 Microarray and proteome studies have also well documented AnnAt1 expression in a variety of cell-types/tissues, cellular organelles and during different physiological responses.29–34 Based on its abundance and expression pattern in Arabidopsis it appears that AnnAt1 may be multifunctional and may have important functions independent of abiotic stress signaling.
In conclusion, there are numerous mechanisms by which AnnAt1 might provide protection to oxidative stress, and the mechanisms for AnnAt1-mediated protection may be different in different tissues. It is also important to consider that in Arabidopsis there are seven other annexins and the expression of each annexin in response to any single stress treatment is varied (i.e., some annexins are upregulated while others are downregulated), so the role of any individual annexin in a particular stress response may be different and needs to be tested.
Acknowledgements
This work was partially supported by grants: NAGW 1519 to S.R. and G.C. from NASA and 2P06A 007 29 to D.K.P. and PBZMNiSW-213/2006/5 to J.H. from the Polish Ministry of Education and Science. We also thank Janusz Debski, Jacek Oledzki, Agata Malinowska and Michal Dadlez from Mass Spectrometry Lab and Grazyna Goch from IBB PAS for participation in glutathionylation experiments.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10835
References
- 1.Konopka-Postupolska D, Clark G, Goch G, Debski J, Floras K, Cantero A, et al. The role of annexin 1 in drought stress in Arabidopsis. Plant Physiol. 2009;150:1394–1410. doi: 10.1104/pp.109.135228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantero A, Barthakur S, Bushart T, Morgan RO, Fernandez P, Chou S, et al. Expression profiling of the Arabidopsis annexin gene family during abiotic stress, germination and de-etiolation. Plant Physiol Biochem. 2006;44:13–24. doi: 10.1016/j.plaphy.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Mortimer JC, Laohavisit A, Macpherson N, Webb A, Brownlee C, Battey NH, et al. Annexins: multifunctional components of growth and adaptation. J Exp Bot. 2008;59:533–544. doi: 10.1093/jxb/erm344. [DOI] [PubMed] [Google Scholar]
- 4.Monastyrskaya K, Babiychuk EB, Draeger A. The annexins: spatial and temporal coordination of signaling events during cellular stress. Cell Molec Life Sci. 2009;66:2623–2642. doi: 10.1007/s00018-009-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gidrol X, Sabelli PA, Fern YS, Kush AK. Annexinlike protein from Arabidopsis thaliana rescues delta oxyR mutant of Escherichia coli from H2O2 stress. Proc Natl Acad Sci USA. 1996;93:11268–11273. doi: 10.1073/pnas.93.20.11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jänicke RU, Porter AG, Kush A. A novel Arabidopsis thaliana protein protects tumor cells from tumor necrosis factor-induced apoptosis. BBA-Mol Cell Res 1. 1998;1402:70–78. doi: 10.1016/s0167-4889(97)00147-x. [DOI] [PubMed] [Google Scholar]
- 7.Kush A, Sabapathy K. Oxy5, a novel protein from Arabidopsis thaliana, protects mammalian cells from oxidative stress. Int J Bioch Cell Biol. 2001;33:591–602. doi: 10.1016/s1357-2725(01)00040-1. [DOI] [PubMed] [Google Scholar]
- 8.Gorecka KM, Konopka-Postupolska D, Hennig J, Buchet R, Pikula S. Peroxidase activity of AnnAt1 from Arabidopsis thaliana. Biochem Biophys Res Commun. 2005;336:868–875. doi: 10.1016/j.bbrc.2005.08.181. [DOI] [PubMed] [Google Scholar]
- 9.Laohavisit A, Mortimer JC, Demidchik V, Coxon KM, Stancombe MA, Macpherson N, et al. Zea mays annexins modulate cytosolic free Ca2+ and generate a Ca2+-permeable conductance. Plant Cell. 2009;21:479–493. doi: 10.1105/tpc.108.059550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osman A, Nien-Jen H, Hofmann A. Study on calcium and heme binding to plant annexin Gh1 from cotton. 5th International Conference on Annexins. 2009 [Google Scholar]
- 11.Jami SK, Clark GB, Turlapati SA, Handley CA, Roux SJ, Kirti PB. Ectopic expression of an annexin from Brassica juncea confers tolerance to abiotic and biotic stress treatments in transgenic tobacco. Plant Physiol Biochem. 2008;46:1019–1030. doi: 10.1016/j.plaphy.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 12.McNeil AK, Rescher U, Gerke V, McNeil PL. Requirement for annexin A1 in plasma membrane repair. J Biol Chem. 2006;281:35202–35207. doi: 10.1074/jbc.M606406200. [DOI] [PubMed] [Google Scholar]
- 13.Babiychuk EB, Monastyrskaya K, Potez S, Draeger A. Intracellular Ca2+ operates a switch between repair and lysis of streptolysin O-perforated cells. Cell Death Differ. 2009;16:1126–1134. doi: 10.1038/cdd.2009.30. [DOI] [PubMed] [Google Scholar]
- 14.Brisson A, Arraud N, Berat R, Bouter A, Garnier B, Gounou C, et al. Annexin-A5: from structure to function. 5th International Conference on Annexins. 2009 [Google Scholar]
- 15.Lee S, Lee EJ, Yang EJ, Lee JE, Park AR, Song WH, et al. Proteomic identification of annexins, calciumdependent membrane binding proteins that mediate osmotic stress and abscisic acid signal transduction in Arabidopsis. Plant Cell. 2004;16:1378–1391. doi: 10.1105/tpc.021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santoni V, Rouquie D, Doumas P, Mansion M, Boutry M, Degand H, et al. Use of a proteome strategy for tagging proteins present at the plasma membrane. Plant J. 1998;16:633–641. doi: 10.1046/j.1365-313x.1998.00335.x. [DOI] [PubMed] [Google Scholar]
- 17.Alexandersson E, Saalbach G, Larsson C, Kjellbom P. Arabidopsis plasma membrane proteomics identifies components of transport, signal transduction and membrane trafficking. Plant Cell Physiol. 2004;45:1543–1556. doi: 10.1093/pcp/pch209. [DOI] [PubMed] [Google Scholar]
- 18.Breton G, Vasquez-Tello A, Danyluk J, Sarhan F. Two novel intrinsic annexins accumulate in wheat membranes in response to low temperature. Plant Cell Physiol. 2002;41:177–184. doi: 10.1093/pcp/41.2.177. [DOI] [PubMed] [Google Scholar]
- 19.White PJ, Bowen HC, Demidchik V, Nichols C, Davies JA. Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochim Biophys Acta-Biomem. 2002;1564:299–309. doi: 10.1016/s0005-2736(02)00509-6. [DOI] [PubMed] [Google Scholar]
- 20.Gorecka KM, Thouverey C, Buchet R, Pikula S. Potential role of annexin AnnAt1 from Arabidopsis thaliana in pH-mediated cellular response to environmental stimuli. Plant Cell Physiol. 2007;48:792–803. doi: 10.1093/pcp/pcm046. [DOI] [PubMed] [Google Scholar]
- 21.Mahajan S, Tuteja N. Cold, salinity and drought stresses: An overview. Arch Biochem Biophys. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Kadota Y, Furuichi T, Sano T, Kaya H, Gunji W, Murakami Y, et al. Cell cycle-dependent regulation of oxidative stress responses and Ca2+ permeable channels NtTPC1A/B in tobacco BY-2 cells. Biochem Biophys Res Comm. 2005;336:1259–1267. doi: 10.1016/j.bbrc.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Roux SJ, Steinebrunner I. Extracellular ATP: an unexpected role as a signaler in plants. Trends Plant Sci. 2007;12:522–527. doi: 10.1016/j.tplants.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Shang Z, Laohavisit A, Davies JM. Extracellular ATP activates an Arabidopsis plasma membrane Ca2+-permeable conductance. Plant Signal Behav. 2009;4:1–3. doi: 10.4161/psb.4.10.9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubio L, Laohavisit A, Mortimer JC, Dark A, Davies JM. Salt stress signalling involves ATP release and Arabidopsis annexin 1. Comp Biochem Physiol Part A: Mol Integr Physiol. 2009;153:193–194. [Google Scholar]
- 26.Hofmann A, Delmer DP, Wlodawer A. The crystal structure of annexin Gh1 from Gossypium hirsutum reveals an unusual S-3 cluster—Implications for cellulose synthase complex formation and oxidative stress response. Eur J Biochem. 2003;270:2557–2564. doi: 10.1046/j.1432-1033.2003.03612.x. [DOI] [PubMed] [Google Scholar]
- 27.Dixon DP, Skipsey M, Grundy NM, Edwards R. Stress-induced protein S-glutathionylation in Arabidopsis. Plant Physiol. 2005;138:2233–2244. doi: 10.1104/pp.104.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srivastava M, Atwater I, Glasman M, Leighton X, Goping G, Caohuy H, et al. Defects in inositol 1,4,5-trisphosphate receptor expression, Ca2+ signaling and insulin secretion in the anx7(+/−) knockout mouse. Proc Natl Acad Sci USA. 1999;96:13783–13788. doi: 10.1073/pnas.96.24.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bianchi MW, Damerval C, Vartanian N. Identification of proteins regulated by cross-talk between drought and hormone pathways in Arabidopsis wild-type and auxin-insensitive mutants, axr1 and axr2. Funct Plant Biol. 2002;29:55–61. doi: 10.1071/PP01113. [DOI] [PubMed] [Google Scholar]
- 30.Carter C, Pan SQ, Jan ZH, Avila EL, Girke T, Raikhel NV. The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell. 2004;16:3285–3303. doi: 10.1105/tpc.104.027078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honys D, Twell D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biology. 2004;5:85. doi: 10.1186/gb-2004-5-11-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleffmann T, Russenberger D, von Zychlinski A, Christopher W, Sjolander K, Gruissem W, Baginsky S. The Arabidopsis thaliana chloroplast proteome reveals pathway abundance and novel protein functions. Curr Biol. 2004;14:354–362. doi: 10.1016/j.cub.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 33.Ito J, Heazlewood JL, Millar AH. Analysis of the soluble ATP-binding proteome of plant mitochondria identifies new proteins and nucleotide triphosphate interactions within the matrix. J Proteome Res. 2006;5:3459–3469. doi: 10.1021/pr060403j. [DOI] [PubMed] [Google Scholar]
- 34.Lee KH, Kim YS, Park CM, Kim HJ. Proteomic identification of differentially expressed proteins in Arabidopsis mutant ntm1-D with disturbed cell division. Mol Cells. 2008;25:70–77. [PubMed] [Google Scholar]