Abstract
Exogenous polyamines [cadaverine (Cad), putrescine (Put), spermidine (Spd) and spermine (Spm)] elicit the production of volatiles in Lima bean (Phaseolus lunatus). Among the tested PAs, Spm induces the production of some volatile terpenoids that are known to be induced by the spider mite Tetranychus urticae. Spm treatment elicits the biosynthesis of Jasmonic acid (JA), a phytohormone known to regulate the production of the volatile terpenoids. The treatment with JA together with Spm resulted in the increased volatile emission, and predatory mites Phytoseiulus persimilis preferred JA and Spm-treated leaves over those treated with JA alone.5 JA and Spm treatment has no effects on polyamine oxidase (PAO) and Cu-amine oxidase (CuAO) but has a significant induction of calcium influx, ROS production, enzyme activities for NADPH-oxidase complex, superoxide dismutase, catalase, ascorbate peroxidase, glutathione reductase and glutathione peroxidase, and gene expressions except for NADPH-oxidase complex.5 Here, we report that a plasma membrane potential (Vm) depolarization was observed after polyamine perfusion with an increasing trend: Spm, Cad, Put and Spd. JA perfusion did not alter Vm but the perfusion of JA and the polyamines significantly increased Cad and Put Vm depolarization. When JA was perfused with polyamines, a negative correlation was found between Vm depolarization and the number of amino group of the polyamines tested.
Key words: polyamines, lima bean, herbivore-induced volatile organic compounds, calcium and ROS signalling, jasmonic acid, quantitative gene expression, transmembrane potential
Polyamines are involved in plants’ stress responses and growth. By activating biosynthesis of nucleic acids, polyamines concern the plant growth and differentiation.1–3 Furthermore, it has been reported that polyamines are involved in the response against environmental stress and plant disease.1–4 We recently reported that exogenously applied polyamines ∼diamines [cadaverine (Cad), putrescine (Put)], triamine [spermidine (Spd)] and tetraamine ]spermine (Spm)]∽ induce volatile emission in Lima bean leaves.5 Membrane potentials (Vm) and intracellular calcium variations were also studied in Lima bean leaves after perfusion with the polyamines and with these addition of JA and here we report on these additional results.
The primary candidate for intercellular signaling in higher plants is the stimulus-induced change in Vm.6 The plasma membrane potential (Vm), which lies in the range of −50 to −200 mV in Lima bean leaves,7 may be shifted either to more negative (hyperpolarization) or to more positive values (depolarization) in response to various biotic or abiotic stresses.
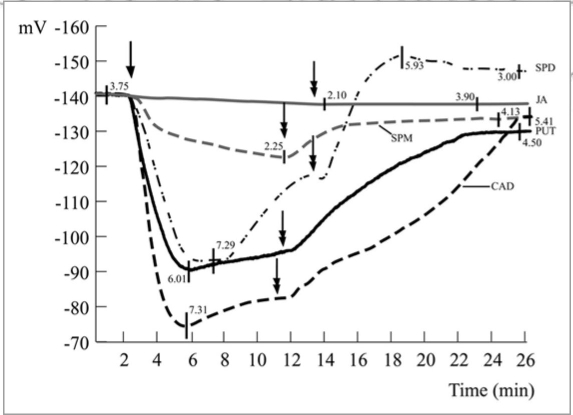
Measurement of Vm were performed and data statistically treated as previously described (ANOVA and Tukey-Kramer’s HSD test).7 Perfusion with the polyamines (Fig. 1 single arrow) shows a specific response of the leaf tissues with a different Vm depolarization, depending on the polyamine. In general, a Vm depolarization was observed after polyamine perfusion with an increasing trend: Spm, Cad, Put and Spd (Fig. 1). Spm and Spd Vm depolarization values were significantly different (p < 0.05) from all other polyamines, whereas no significant difference was found between Put and Cad Vm depolarization (p = 0.435). In all cases, Vm depolarization was reversed by washing polyamine-treated leaves with a fresh buffer solution (Fig. 1 double arrow); however, a full recovery of the Vm was observed only for Put (Fig. 1). The linearization of the data from Figure 1 allowed to calculate the rate of Vm depolarization after perfusion of the polyamines which was higher for Spd (6.0 mV min−1; R = 0.96), equal for Put and Cad (4.8 mV min−1; Put R = 0.95; Cad R = 0.97) and lower for Spm (3.0 mV min−1; R = 0.96).
Figure 1.
Effect of 1 mM polyamines (arrow) on the Vm of Lima bean palisade cells. Spermine (Spm) caused the lowest Vm depolarization, whereas spermidine (Spd) showed the highest values of Vm depolarization. intermediate values were found when putrescine (Put) and cadaverine (cad) were perfused. after washing the tissues with fresh buffer (double arrow) Vm was always hyperpolarized, however the initial potential was recovered only for Put, while for all other polyamines the Vm never reached the initial values. Metric bars indicate standard deviation.
Perfusion with JA caused a slight and not significant (p = 0.332) Vm depolarization (Fig. 2) with respect to control. The addition of JA caused a significant increase (p < 0.01) in Vm depolarization when perfused with Cad, with respect to the sole perfusion with Cad (Fig. 1). The same was observed when JA was perfused with Put, whereas not significant differences were observed when Spm (p = 0.513) and Spd (p = 0.107) were perfused with JA (Fig. 2), with respect to the sole perfusion with Spm and Spd (Fig. 1). The linearization of the data from Figure 2 allowed to calculate the rate of Vm depolarization after perfusion of the polyamines + JA, which was higher for Cad (24.40 mV min−1; R = 0.99), almost equal for Put and Spd (Put: 14.21 mV min−1, R = 0.99; Spd: 13.49 mV min−1, R = 0.99) and lower for Spm (1.34 mV min−1; R = 0.93). For JA the rate of Vm depolarization was 0.19 mV min−1 (R = 0.96). With the addition of JA, a negative correlation was found between Vm depolarization and the number of amino group of the polyamines tested.
Figure 2.
Effect of 1 mM polyamines + 0.1 mMJA (arrow) on the Vm of Lima bean palisade cells. the perfusion with Ja did not cause any variation in the Vm. addition of JA to Spm and Spd caused the same Vm depolarization observed in the absence of JA, whereas when JA was added to Put and Cad a stronger and significantly different Vm depolarization was observed. even in this case washing the tissues with fresh buffer (double arrow) caused a Vm hyperpolarized, however in this case Spd reached Vm values significantly more negative that the initial Vm. Metric bars indicate standard deviation. For abbreviations see Figure 1.
Since ion fluxes through channels directly influence Vm, it seems reasonable to assume that molecules able to act on channel activity might be considered as important factors inducing electrical signals. Among the various channels, calcium and potassium channels are predominantly involved in cell signaling.8 In the present study, rapid and reversible Vm depolarization observed upon perfusion of Lima bean mesophyll cells with polyamines was found to be significantly increased when JA was added to Cad and Put. The reversibility of the Vm may be linked to the overall physico-chemical amphiphilic properties of polyamines, probably depending on non covalent interaction with plasma membrane molecules, as polyamines occur in plants in free form, bound electrostatically to negatively charged molecules, and conjugated to small molecules and proteins.9 Liu et al.10 showed that Spm, Spd, Cad and Put strongly inhibited opening and closing of stomata in Vicia faba, suggesting that polyamines target inward potassium channels in guard cells and modulate stomatal movements, so providing a link between abiotic stress, polyamine levels and stomatal regulation. Moreover, the transport of polyamines across the plasma membrane of plant cells is energy-dependent and calcium is involved in the uptake mechanism.1,11 Both mechanisms can be correlated to the observed Vm depolarization, and the positive correlation between intracellular Ca2+ concentration5 and Vm depolarizing activity of polyamines confirms the involvement of Ca2+ during polyamine uptake.11
Acknowledgements
This work was supported by: Grant-in-Aid for Scientific Research (S) (No. 19101009 to J.T.); Global COE Program A06 of Kyoto University; CEBIOVEM (Centre of Excellence for Plant and Microbial Biosensing of the University of Turin to M.E.M.).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10848
References
- Walters DR. Polyamines in plant-microbe interactions. Physiol Mol Plant Pathol. 2000;57:137–146. [Google Scholar]
- Walters DR. Polyamines and plant disease. Phytochemistry. 2003;64:97–107. doi: 10.1016/s0031-9422(03)00329-7. [DOI] [PubMed] [Google Scholar]
- Martin-Tanguy J. Metabolism and function of polyamines in plants: recent developmentn (new approaches) Plant Growth Regul. 2001;34:135–148. [Google Scholar]
- Bouchereau A, Aziz A, Larher F, Martin-Tanguy J. Polyamines and environmental challenges: recent development. Plant Sci. 1999;140:103–125. [Google Scholar]
- Ozawa R, Bertea CM, Foti M, Narayana R, Arimura G-I, Muroi A, et al. Exogenous polyamines elicit herbivore-induced volatiles in Lima bean leaves: involvement of calcium, H2O2 and jasmonic acid. Plant Cell Physiol. 2009;50:2183–2199. doi: 10.1093/pcp/pcp153. [DOI] [PubMed] [Google Scholar]
- Maffei M, Mithöfer A, Boland W. Before gene expression: early events in plant-insect interactions. Trends Plant Sci. 2007;12:310–316. doi: 10.1016/j.tplants.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Maffei M, Bossi S, Spitfeller D, Miltho’fer A, Boland W. Effects of feeding Spodoptera littoralis on Lima beans leaves I. Membrane potentials, intracellular calcium variations, oral secretions and regurgitate components. Plant Physiol. 2004;134:1752–1762. doi: 10.1104/pp.103.034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ. Calcium channels in higher plants. Biochim Biophys Acta. 2000;1465:171–218. doi: 10.1016/s0005-2736(00)00137-1. [DOI] [PubMed] [Google Scholar]
- Martin-Tanguy J. Conjugated polyamines and reproductive development: biochemical, molecular and physiological approaches. Physiol Plantarum. 1997;100:675–688. [Google Scholar]
- Liu K, Fu H, Bei Q, Luan S. Inward potassium channel in guard cells as a target for polyamine regulation of stomatal movements. Plant Physiol. 2000;124:1315–1326. doi: 10.1104/pp.124.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antognoni F, Pistocchi R, Casali P, Bagni N. Does calcium regulate polyamine uptake in carrot protoplasts? Plant Physiol Biochem. 1995;33:701–702. [Google Scholar]