Abstract
Protein phosphatase type 2A (PP2A) activity is required for the sucrose induction of fructan metabolism in wheat leaves, as shown in experiments with the addition of the specific inhibitor okadaic acid (OA) together with sucrose. However, a decrease in total PP2A activity has been found along sucrose treatment. Here we analyze the effect of sucrose feeding to wheat leaves on PP2A activity profiles after Deae-Sephacel and Superose separation, in comparison with those of control leaves. The results show no evidence of changes in PP2A activity profiles as a consequence of sucrose feeding. In all, our data suggest that constitutive levels of PP2A activity may be sufficient for the sucrose-mediated induction of fructan metabolism and that general decrease of PP2A activity produced by long-term treatment with sucrose may be due to a negative feedback regulation.
Key words: fructan; protein phosphatases 2A; sucrose:fructan 6-fructosyltransferase, sugar sensing; Triticum aestivum
Protein phosphorylation and dephosphorylation are necessary for the sugar-mediated induction of fructan synthesizing enzymes (FSS, 6-sucrose:fructan fructosyltransferase and 1-sucrose:sucrose fructosyltransferase);1,2 specifically, CDPK and PP2A activities are required for this process. Recently, we also showed that sucrose decreases general PP2A activity in parallel with a decreasing sugar uptake and that PP2A is involved in sugar uptake in wheat leaves.3 Very little is known about PP2A isoforms in wheat, as well as about the number of their encoding sequences.4–6 Here we discuss further the role of PP2A in sugar sensing and possible causes of the effect of sucrose on PP2A activity in wheat.
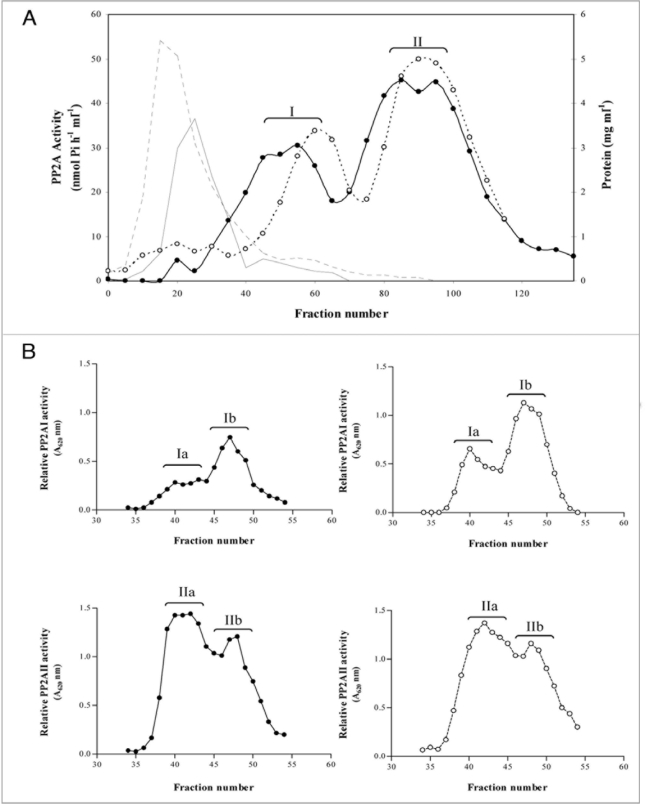
The fact that PP2A activity is necessary for fructan induction by sucrose while a decrease in total PP2A activity occurs along sucrose treatment,3 led us to investigate whether this could be the result of a modification of a specific PP2A activity. Then, we partially purified PP2A present in either sucrose- or water-treated leaves. Total protein leaf extracts from 6 h sugar treated or control (water treated) leaves were loaded onto Deae-Sephacel columns. Two major peaks of PP activity were obtained (named PP2AI and PP2AII). Sugar treatment modified the elution position of both PP activities: in the case of sucrose-treated leaf extracts, PP2AI and PP2AII eluted at 260 mM and 375 mM NaCl, respectively, and when control leaf extracts were chromatographed, PP2AI and PP2AII eluted at 345 mM and 430 mM NaCl, respectively. PP2AII activity was higher than that of PP2AI for wheat leaves treated either with sucrose or water. The specific activity for the concentrated proteins were c.a. 30 nmol/min/mg protein and 110 nmol/min/mg protein for PP2AI and PP2AII, respectively for the sucrose treatment (Fig. 1A). Incubating leaves for 24 h with sucrose or water rendered essentially the same activity profile as for 6 h. The fractions under each Deae-Sephacel peak were pooled, concentrated and loaded into Superose-12 size-exclusion columns. The elution pattern of these chromatographies showed two peaks from each peak eluted from the Deae-Sephacel column, designated as PP2AIa, PP2AIb, PP2AIIa and PP2AIIb for both treatments (Fig. 1B). They corresponded to an approximately 120–130 kDa and 30–35 kDa proteins, which are in accordance to the molecular weight of the PP2A core and the PP2A catalytic subunit, respectively.7,8 PP2AIb activity was higher than that of PP2AIa and PP2AIIa activity was higher than that of PP2AIIb for both treatments. In comparison with PP2A isolated from other tissues, wheat leaf PP2A specific activities were similar to PP2A purified from wheat embryo and from maize seedlings.4,9
Figure 1.
Elution profiles of wheat leaves PP2A activity. Crude extracts from leaves treated for 6 h with water (control) or 0.5 M sucrose were loaded onto Deae-Sephacel columns. Elutions were done with a linear gradient of NaCl 0-0.5 M (A). PP-containing fractions from water-(—◯—) or sucrose-(—•—) fed leaves were concentrated (PP2AI and PP2AII peaks) and loaded onto Superose 12 columns (B). PP2A activity was assayed with the non-radioactive method. Lines without markers at the left of the graph indicate total protein for water (——) and sucrose (—) treatments.
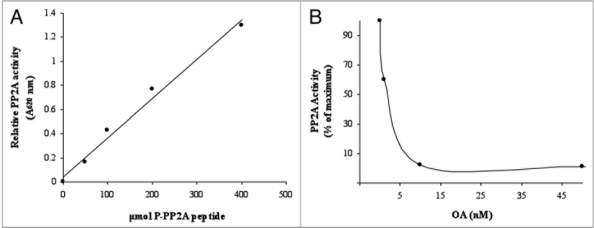
To characterize the partially purified PPs we tested their activity on phosphopeptide RR(pT)VA, which is substrate for Ser/Thr PP2A but is a poor substrate of PP1 (Fig. 2A). Also, to ensure that we measured specific PP2A activities we included imidazole and EDTA in the reaction buffer, to inhibit alkaline phosphatases and PP2B and PP2C activity.10 Moreover, purified enzymes were active with the general substrate p-NPP. In contrast, these PPs did not catalyze the dephosphorylation of nonprotein phosphomonoesters such as Glc-6P or PEP at substrate concentration (100 µM). Finally, partially purified PP activities were assayed with different reported effectors of animal and plant PP2A. OA, a potent inhibitor of PP2A activity, completely inhibited the purified PPs at 10 nM. The OA IC50 value was 1 nM (Fig. 2B), which is in the normal range of values described for PP2As.10 They were also inhibited by the general phosphatase inhibitor NaF but not by inhibitor 2 (I-2), which inhibits PP1 specifically.10 Thus, the PP activities that we partially purified belong to the PP2A family.
Figures 2.
Phosphopeptide concentration-dependent and okadaic acid sensitivity of the sucrose-partially purified wheat PP2As. The pooled fractions of Superose 12 chromatographies with PP2A activity were incubated with different concentration of the phosphopeptide (A) or with different concentrations of OA (B). PP2A activity was assayed with the non-radioactive method.
These results are in accordance with the proposed model,3 where PP activity may be required for sucrose uptake into leaf tissues,11 and possibly also for maintaining this transporter in a dephosphorylatedactive form.12 Within this scheme, PP2A activity required to initiate sucrose signaling leading to fructan synthesis induction is present before the signal. On the long term, sucrose may decrease general PP2A activity in a negative feedback.3 Moreover, further steps in the sucrose signaling pathway may require PP2A activity, since adding 1 µM OA 6 h after the beginning of sucrose feeding (when most sucrose uptake had already taken place, and both 6-SFT mRNA level and FSS activity had significantly increased), blocks any further increase in FSS activity (Martínez-Noël et al. unpublished). Further research is needed to elucidate whether the different PP2A isoforms here described are associated with different steps in this signaling process.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10924
References
- 1.Martínez-Noël G, Tognetti JA, Pontis HG. Protein kinase and phosphatase activities are involved in fructan synthesis initiation mediated by sugars. Planta. 2001;213:640–646. doi: 10.1007/s004250100550. [DOI] [PubMed] [Google Scholar]
- 2.Martínez-Noël G, Nagaraj VJ, Caló G, Wiemken A, Pontis HG. Sucrose regulated expression of a Ca2+-dependent protein kinase (TaCDPK1) gene in excised leaves of wheat. Plant Physiol Biochem. 2007;45:410–419. doi: 10.1016/j.plaphy.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Martínez-Noël G, Tognetti J, Nagaraj V, Wiemken A, Pontis H. Protein phosphatase activity and sucrose-mediated induction of fructan synthesis in wheat. Planta. 2009;230:1071–1079. doi: 10.1007/s00425-009-1002-7. [DOI] [PubMed] [Google Scholar]
- 4.Polya G, Haritou M. Purification and characterization of two wheat-embryo protein phosphatases. Biochem J. 1988;251:357–363. doi: 10.1042/bj2510357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacKintosh C, Cohen P. Identification of high levels of type 1 and type 2A protein phosphatases in higher plants. Biochem J. 1989;262:335–339. doi: 10.1042/bj2620335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu C, Jing R, Mao X, Jia X, Chang X. A wheat (Triticum aestivum) protein phosphatase 2A catalytic subunit gene provides enhanced drought tolerance in tobacco. Ann Bot. 2007;99:439–450. doi: 10.1093/aob/mcl285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechward K, Awotunde O, Swiatek W, Muszynska G. Protein phosphatase 2A: Variety of forms and diversity of functions. Acta Biochim Pol. 2001;48:921–933. [PubMed] [Google Scholar]
- 9.Awotunde O, Sugajka E, Zolnierowicz S, Muszynska Characterization of two protein phosphatase 2A holoenzymes from maize seedlings. Biochem Biophys Acta. 2000;1480:65–76. doi: 10.1016/s0167-4838(00)00097-2. [DOI] [PubMed] [Google Scholar]
- 10.MacKintosh C, MacKintosh RW. Inhibitors of protein kinases and phosphatases. Trends Biochem Sci. 1994;19:444–448. doi: 10.1016/0968-0004(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 11.Ransom-Hodgkins WD, Vaughn MW, Bush DR. Protein phosphorylation plays a key role in sucrosemediated transcriptional regulation of a phloemspecific proton-sucrose symporter. Planta. 2003;217:483–489. doi: 10.1007/s00425-003-1011-x. [DOI] [PubMed] [Google Scholar]
- 12.Roblin G, Sakr S, Bonmort J, Delrot S. Regulation of a plant plasma membrane sucrose transporter by phosphorylation. FEBS Letts. 1998;424:165–168. doi: 10.1016/s0014-5793(98)00165-3. [DOI] [PubMed] [Google Scholar]