Abstract
Nociceptin is a neuropeptide sharing sequence homology with classical opioid peptides but with a distinct pharmacological profile. Through activation of its receptor, NociR, nociceptin has been linked with several physiological functions in the central nervous system including memory, locomotion, and processing of pain signals. Recently, peripheral blood neutrophils (PMNs) were demonstrated to express a functional NociR, a result suggesting that additional functions of the neuropeptide remain to be elucidated. The present study investigated the possibility that PMNs may be a source of nociceptin and whether the neuropeptide elicits PMN early responses. We observed the presence of nociceptin in the synovial fluids from arthritic patients, an inflammatory milieu typically containing high numbers of PMNs. In addition, freshly isolated PMNs were found to express and secrete nociceptin following degranulation, identifying these inflammatory cells as a novel source of the neuropeptide. Incubation of PMNs with nociceptin elicited a specific pattern of cellular protein phosphorylation on tyrosine residues in a rapid and transient fashion. Moreover, nociceptin prevented intracellular accumulation of cAMP in fMLP-stimulated PMNs, an effect mimicked by the specific NociR synthetic agonist, Ro 64-6198. Taken together, these results show that nociceptin/NociR is present and functional in human neutrophils, and the results identify a novel dialogue pathway between neural and immune tissues.
Polymorphonuclear neutrophils (PMNs)1 are the most abundant circulating leukocyte subtype and often the first to accumulate in large numbers at sites of infection. In response to chemotactic factors, PMNs migrate from the bloodstream through the vascular endothelium to the inflammatory site to perform their immune functions (1). Following appropriate stimulation, PMNs release a variety of agents including arachidonic acid-derived lipid mediators of inflammation, products of the oxidative burst, degradative enzymes, chemokines, and cytokines (2, 3). Inflammatory PMNs also act on the nociception circuitry by activating specific receptors present on sensory nerve endings through the release of factors such as leukotriene (LT)B4, prostaglandin (PG)E2, and substance P (SP) (4). LTB4 can cause hyperalgesia by activating small diameter sensory neurons and was recently identified as an endogenous agonist of the vanilloid receptor VR1 (also called a capsaicin receptor) (5). PGE2, in addition to mediating vasodilation and regulating vascular permeability, enhances pain perception elicited by bradykinin and histamine (6).
In addition to the processing of nociceptive signals, neural activity conversely bears a potential to impact on the inflammatory response. Primary afferent nerve fibers control cutaneous blood flow and vascular permeability by releasing vasoactive peptides, and neuropeptides may trigger responses from immune cells by stimulating the production of inflammatory factors, a phenomenon referred to as neurogenic inflammation (7, 8). Evidence supports the notion that neuropeptides SP and calcitonin gene-related peptide (CGRP) be major initiators of neurogenic inflammation (9); however, other factors that can potentially regulate both excitability of sensory neurons and activation of inflammatory cells, thereby mediating communication between the nervous and the immune systems, remain to be identified. The characterization of novel dialogue pathways is of particular relevance in an attempt to design better clinical treatments for diseases with inflammatory and nociceptive components such as migraine, arthritis, chronic obstructive pulmonary disease, asthma, and inflammatory bowel disease, in which pain is often a major constraint.
Nociceptin (orphanin FQ) is a 17-amino acid neuropeptide sharing high homology with dynorphin A and other classical opioids (10, 11), yet displaying a distinct pharmacological profile (12). Classical opioids constitute strong analgesics; in contrast, this novel neuropeptide caused hyperalgesia when injected via an intracerebroventricular route, and in turn, was termed nociceptin. This neuropeptide has been identified as the endogenous ligand for the opioid-like receptor-1, now called nociceptin receptor (NociR) (10, 13, 14). NociR was isolated from brain tissues as an orphan G protein-coupled receptor (GPCR) and is expressed widely in the nervous system in the cortex, amygdala, hypothalamus, and brain stem. Accumulating data indicate that it may participate in a broad range of physiological and behavioral functions such as pain processing, locomotion, learning, and anxiety (15). In particular, studies with mutant mice lacking NociR suggest that the receptor takes part in the physiological regulation of nociceptive thresholds (16).
Anatomical analysis of nociceptin precursor mRNA in the mouse central nervous system revealed that it is highly expressed in discrete neuronal sites with a pattern distinct from those of other opioid peptides (17). Aside from their presence in the central nervous system, mRNAs for NociR and its ligand precursor have been found in primary afferent nerve fibers (14), skin, visceral organs and blood vessels, and cells of the immune system. Halford et al. have observed that mouse peripheral blood lymphocytes express NociR mRNA upon mitogen activation (18). Resting human peripheral blood lymphocytes and lymphocytic cell lines express NociR mRNA, which was up-regulated by treatment with phytohemagglutinin (19). Recently, the presence of NociR in peripheral blood PMNs was documented, and its functionality was demonstrated (20). Nociceptin evoked in vitro chemotaxis at very low concentrations and elicited leukocyte recruitment in vivo, indicating an immunological role for NociR in addition to its neurological functions.
The present study investigates the possibility that PMNs may constitute a source of nociceptin. We report that nociceptin can be detected at inflammatory sites in the periphery where PMNs accumulate and that PMNs release nociceptin upon exocytosis of their granules. Nociceptin elicited early cellular responses, including protein phosphorylation on tyrosine residues and modulation of intracellular cAMP levels. Results presented herein document a functional nociceptin/NociR system in PMNs, indicating that it may constitute a novel two-way signaling pathway between immune cells and the nervous system.
MATERIALS AND METHODS
Materials
Nociceptin (orphanin FQ), cytochalasin B (CB), formyl-methionyl-leucyl-phenylalanin (fMLP), adenosine deaminase (ADA), Ro 20-1724, diisopropylfluorophosphate (DFP), and sodium orthovanadate were obtained from Sigma-Aldrich (Oakville, ON). 2-p-(2-Carboxyethyl) phenethyl-amino-5-N-ethylcarboxiamido adenosine hydrochloride (CGS 21680) was purchased from Research Biochemicals International (Natick, MA). NP-40 was obtained from Calbiochem (La Jolla, CA); aprotinin and leupeptine were from ICN Pharmaceuticals, Inc. (Costa Mesa, CA). Dextran T-500, Ficoll-Paque, and protein A sepharose were purchased from Pharmacia Biotech (Dorval, PQ). Ro 64-6198 was a generous gift from Dr. Eva-Maria Gutknecht, F. Hoffmann LaRoche Ltd. (Basel, Switzerland). The monoclonal anti-phosphotyrosine antibody (UBI 05–321, clone 4G10) was purchased from Upstate Biotechnology Inc. (Lake Placid, NY). The polyclonal anti-Hck and anti-SAM68 GST antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa-Cruz, CA). Anti-CD63 FITC-labeled and anti-CD66b PE-labeled antibodies were purchased from Beckman Coulter, Inc. (Mississauga, ON).
Synovial Exudates
Synovial fluids and autologous plasma samples from arthritic sufferers were obtained from a bank of samples constituted and maintained by Dr. Patrice E. Poubelle, Laval University, PQ.
Leukocyte Isolation
PMNs were isolated as originally described (21) with modifications (22). Briefly, venous blood collected on isocitrate anticoagulant solution from healthy volunteers was centrifuged (250g, 15 min), and the resulting platelet-rich plasma was discarded. Leucocytes were obtained following erythrocytes sedimentation in 3% Dextran T-500. PMNs were then separated from other leukocytes by centrifugation on a 10 mL Ficoll-Paque cushion. Contaminating erythrocytes were removed by a 15 s hypotonic lysis, and purified granulocytes (95% PMNs, <5% eosinophils) contained fewer than 0.2% monocytes, as determined by esterase staining. Viability was greater than 98%, as determined by trypan blue dye exclusion. The sterile whole cell isolation procedure was carried out at room temperature. PMNs were resuspended in Hank’s balanced salt solution (HBSS) containing 10 mM HEPES pH 7.4, 1.6 mM Ca2+, and no Mg2+. For total leukocytes, whole blood was water-lyzed for 25 s, and the pellet was resuspended and rinsed twice in HBSS.
Detection of Nociceptin by Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR)
First strand cDNA synthesis was performed using 1 μg of total RNA with Superscript II (Invitrogen Lifetechnology, Carlsbad, CA) in recommended conditions, using 200 ng of random hexamers. Amplification of nociceptin cDNA was performed by using 1 μg of cDNA, 1.5 mM MgCl2, 0.2 mM dNTP, 100 nM primers, and 1 unit of Taq polymerase (Amersham Biosciences, Piscataway, NJ) in a reaction volume of 50 μL and carried out in a PTC-200 thermal cycler (MJ Research Inc., Waltham, MA). Following an initial 2 min denaturation step at 94 °C, cycling was carried out using a touchdown PCR, essentially as described (23). Briefly, the annealing temperature was decreased from 60 to 50 °C during the five initial cycles, then fixed to 50 °C for an additional 35 cycles (annealing step: 45 s; denaturing step: 94 °C, 30 s; extension step: 72 °C, 1 min). A final extension step of 7 min at 72 °C was carried out after 40 cycles. PCR products were separated by electrophoresis on an agarose gel (1.0%) in 0.5X TAE, stained with ethidium bromide, and visualized under UV illumination. Primers used for detecting nociceptin in leukocyte preparations were 5′-CCT GCA CCA TGA AAG TCC TG-3′ (forward) and 5′-CCT TCC GGC TAC ACA TTA CC-3′ (reverse). Primers used to amplify the entire coding region of the PMN prepronociceptin mRNA were 5′-TAT GCT GGT GTG GCT GAG AA-3′ (forward) and 5′-TAT GGC AGT GGC AAG TCA AA-3′ (reverse).
RNA Isolation and Northern Blots
Total RNA was isolated using Trizol (Gibco, Burlington, VT) according to the manufacturer’s protocol, with modifications. Briefly, 25 × 106 PMNs was homogenized in 1 mL of Trizol and 200 μL of chloroform was added. After mixing, samples were centrifuged at 10 000g for 15 min (4 °C). The upper aqueous phase was transferred in a tube containing an equal volume of 2-propanol. Mixtures were thoroughly vortexed and centrifuged at 12 000g for 10 min (4 °C). Supernatants were discarded, and the precipitated RNA pellets were washed using 1 mL of 75% ethanol. RNA pellets were centrifuged at 12 000g for 5 min (RT). After discarding the supernatants, pellets were allowed to air-dry for 2–3 min and then were resuspended in DEPC-treated water. RNA was quantitated by UV absorbance at 260 nm. Poly-A+ RNA was purified from total RNA using a Qiagen mRNA purification kit (Qiagen Inc. Missisauga, ON) according to the manufacturer’s specifications. RNA was migrated on an agarose/formaldehyde gel and transferred onto a nylon filter (Hybond-XL), using a Vacugene transfer apparatus (Amersham Pharmacia Biotech, Baie d’Urfé, PQ). RNA was fixed using a UV-cross-linker according to the manufacturer’s specifications (Amersham Pharmacia Biotech). Filters were hybridized with a PMN-derived NociR cDNA probe labeled with [α-32P] dCTP using Neblot Kit (New England Bio labs, Beverly, MA). Bands were revealed and analyzed with a BAS-1800 bioimaging analyzer (Fuji Medical Systems USA, Stamford, CT).
Monitoring of PMN Degranulation Process by FACS
Following appropriate treatment, reactions were stopped by centrifuging samples for 10 s. Cell pellets were resuspended in 1 mL of HBSS, from which 100 μL was centrifuged and resuspended with 100 μL of a mixture containing 10 μL of anti-CD 63-PE, 10 μL of anti-CD66b-FITC, and 80 μL of HBSS (CD 63 and CD 66b being markers of azurophilic and specific granules, respectively). Cell suspensions were incubated for 30 min (RT) in the dark and then rinsed with 500 μL of HBSS + 0.01% BSA and resuspended (500 μL) for analysis by FACS.
Detection of Nociceptin
Nociceptin from synovial exudates and from PMN supernatants was quantified using a commercially available ELISA kit (Phoenix Pharmaceuticals, Belmont, CA). For synovial fluids, samples were diluted 1:1 in assay buffer prior to use in the assay.
Identification of Nociceptin by Tandem Mass Spectrometry
Following appropriate treatment, PMN suspensions were centrifuged. Supernatants were collected and loaded on a C18 SPE cartridge (Oasis HLB, Waters). The cartridge was preconditioned with 2 mL of MeOH and 2 mL of H2O. The samples were loaded, washed with 3 mL of H2O, and eluted with 1.5 mL (30% acetonitrile in water, 2% acetic acid). Samples were dried using a Speed-vac evaporator (SAVANT Instruments Inc., Holbrook NY) and then reconstituted in 300 μL of 50:50 H2O/acetonitrile + 0.17% HPLC grade acetic acid + 3.33 mM ammonium acetate. The samples were infused at a rate of 10 μL/min by an Harvard pump (South Natick, MA) and detected by an API III+ triple quadrupole mass spectrometer (P.E. Sciex, Thornhill, Canada). The mass spectrometer was operated in the Turbo Ion Spray configuration, consisting of the articulated Ion Spray inlet used in conjunction with the heated Turbo Probe desolvation unit. The Turbo Probe temperature and nitrogen gas flow rate were 400 °C and 1.0 L/min, respectively, and the nebulizer gas pressure was 60 psi (nitrogen). The analyte ions’ detection was optimized using the ion spray needle voltage set at 5 kV, the orifice voltage at 70 V, and the MS collision energy at 14 V. Argon was used as the target gas for the MS/MS experiments, at a collision gas tickness of 3 × 1015 atoms/cm2.
The authentic in-house synthesized nociceptin was scanned over the region m/z 400–1200. Ions doubly and triply charged at m/z 906 and 604, respectively, displayed stronger signals than the molecular ion itself [M + H]+ at m/z 1809. Daughter ion spectra were performed in the m/z 50–650 atomic mass units (amu) interval, and a single peak was consistently obtained at m/z 120. Thus, PMN-derived samples were analyzed for the presence of nociceptin by scanning for the reaction parent ion/daughter ion: 604:120.
Intracellular cAMP Determination
PMNs (1.5 × 107 cells/mL) were preincubated for 20 min with a phosphodiesterase IV inhibitor Ro 20-1724 (10 μM) and with adenosine deaminase (ADA; 0.1 U/mL). Cytochalasin B (CB; 10 μM) was added 5 min before incubations with fMLP (100 nM). Reactions were stopped by centrifuging for 15 s at 12 000g. Cell pellets were resuspended in 500 μL of ice-cold acidified ethanol (99% EtOH, 20 mM HCL) and kept at 4 °C. Samples were sonicated for 20 s and centrifuged at 13 000g for 45 min at 4 °C. The supernatants were collected, evaporated using a speed-vac evaporator, and stored at −20 °C until used for the cAMP measurements, which were performed using a commercially available ELISA kit (Cayman Chemicals).
Tyrosine Phosphorylation
PMNs were resuspended at a concentration of 4 × 107 cells/mL in Hank’s balanced salt solution (HBSS) containing 10 mM HEPES pH 7.4, 1.6 mM Ca2+, and no Mg2+. Before stimulation, PMNs suspensions were preincubated at room temperature with 1 mM DFP for 5 min.
Native Immunoprecipitation and Tyrosine Kinase Activity
After stimulation, the reactions were rapidly stopped and processed, as described in Gilbert et al. (24). Lysates were immunoprecipitated using 1.5 μg of anti-Hck antibodies for 60 min at 4 °C on a rotator platform with constant end-over-end mixing. Fifty μL (30% slurry) of protein A-sepharose were then added, and the samples were incubated for 60 min at 4 °C. The beads were collected and washed four times with isotonic cell lysis buffer without EDTA (ILB), as described (24). The beads were incubated at 4 °C in kinase buffer + 0.5 μg of SAM68-GST and transferred to 30 °C for the indicated times. The reactions were stopped by a quick spin, and the supernatants were precipitated with sepharose GST beads for 30 min at 4 °C before being washed with ILB buffer. Sample buffer (40 μL, 2×) was added to the beads (protein A-sepharose), which were heated for 7 min at 95 °C and stored at −20 °C until they were used for electrophoresis and immunoblotting. Aliquots (50 μL) were then subjected to 7.5–20% SDS–PAGE and transferred to Immobilon membranes (Millipore Corporation, Bedford, MA). Equal protein loading and transfer efficiency were visualized by Ponceau Red staining. The membranes were soaked for 30 min at RT in Tris-buffered saline (TBS: 25 mM Tris-HCl pH 7.6, 0.2 M NaCl, 0.15% Tween 20) containing 2% (w/v) gelatin and exposed for 30 min to an monoclonal antiphosphotyrosine antibody (1:4000) or with specific immunoprecipitation antibodies (anti-Hck (1:1000) and anti-SAM68 (1:1000)) for visualizing the amounts of precipitated protein. Membranes were then washed twice in TBS and incubated for 30 min with HRP-conjugated secondary anti-mouse or anti-rabbit antibodies (Jackson, Immune Research, Mississauga, ON) at a dilution of 1:20 000 and revealed using the Renaissance detection system ECL-Plus (NEN-Mandel, Mississauga, ON).
RESULTS
Nociceptin Can Be Found in Inflammatory Synovial Fluids
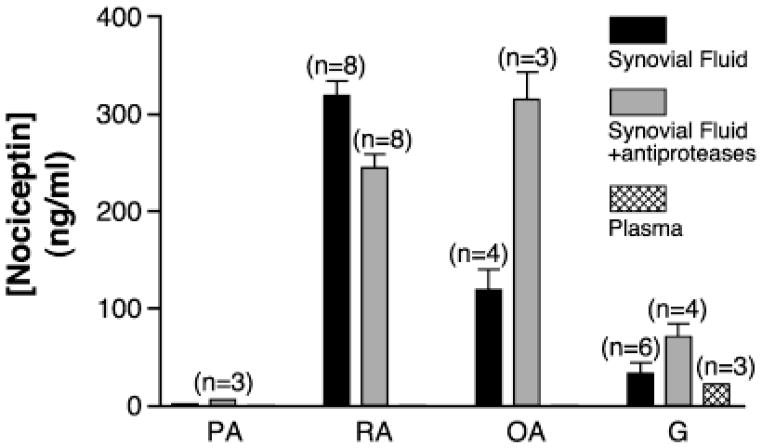
Following on the observation that nociceptin can be a chemoattractant for PMNs, both in in vitro and in vivo experimental settings (20), we sought to determine whether the neuropeptide can be found at inflammatory sites where PMNs accumulate, such as in inflamed joints. To this end, synovial exudates from joints of arthritic patients and autologous plasma samples were assayed for the presence of nociceptin. The neuropeptide was detected at various levels of the inflammatory exudates from rheumatoid-, osteo-, and gouty arthritic patients (Figure 1). These results show that the neuropeptide can be delivered in nonneural, peripheral tissues. Specificity of the assay was tested in four complementary ways. First, pretreatment of selected samples with a nociceptin antibody caused the measured concentration of nociceptin to decrease significantly. Second, when known quantities of authentic nociceptin were added to samples, resulting concentrations were accordingly increased in correlation with the amount added (data not shown). Third, the presence of an anti-protease cocktail, which sometimes may cause false positives to occur, did not consistently up-regulate the levels of nociceptin detected. Last, autologous plasma samples were consistently negative in nociceptin content. While the small number of samples, and more importantly, the health status of the patients at the moment of visiting their clinic preclude us from drawing statistically based conclusions, the detection of nociceptin in these exudates raises the possibility that inflammatory PMNs may constitute a source of nociceptin, in view of their accumulation as a typical feature of inflamed joints.
Figure 1.
Nociceptin is detected in inflammatory exudates. Nociceptin levels were determined in synovial exudates and autologous plasma samples from arthritic patients by specific ELISA as described in the Materials and Methods. Results show the mean ± SEM obtained from n separate patients. PA: psoriatic arthritis; RA: rheumatoid arthritis; OA: osteoarthritis; G: gouty arthritis.
PMNs Possess the Nociceptin/NociR System
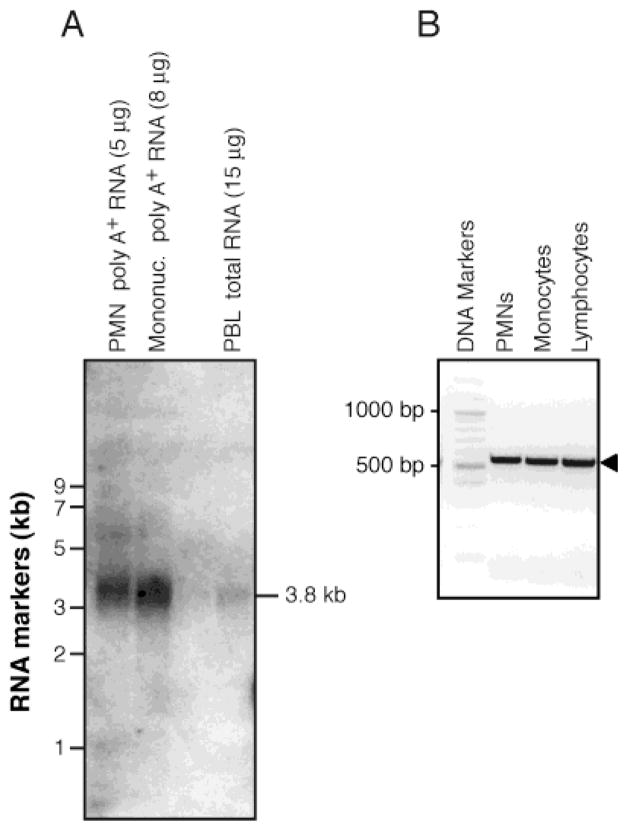
Expression of NociR mRNA was studied by Northern blot using mRNA obtained from freshly isolated, peripheral blood leukocyte preparations to further document the earlier observation based on RT-PCR results establishing the presence of NociR mRNA in PMNs (20). NociR mRNA was detected both in PMN and in mononuclear cell poly-A+ mRNA preparations (Figure 2A). In comparison, a much weaker—but detectable—signal was observed in total RNA obtained from mixed peripheral blood leukocytes.
Figure 2.
Peripheral blood leukocytes express mRNA for NociR and the ligand precursor: prepronociceptin. (A) Messenger RNA from human PMNs, mononuclear cells, or total RNA obtained from peripheral blood leukocytes (PBL) were processed for Northern blot analysis with a 32P-labeled NociR cDNA probe. (B) RT-PCRs were performed on RNA from different leukocyte subpopulations using primers specific for the ligand precursor peptide: prepronociceptin. For each panel, one experiment representative of three separate experiments with identical results is shown.
We sought to determine whether circulating leukocytes also express mRNA for prepronociceptin, the precursor of the identified endogenous ligand for NociR. First, RT-PCRs were performed with PMN, monocyte, and lymphocyte total RNA, using primers that are specific for a region common to all of the known splicing variants (25) of prepronociceptin mRNA. Positives were obtained for each leukocyte subtype assayed (Figure 2B). A pair of cDNA primers was then designed to obtain the whole coding region of prepronociceptin mRNA and was used in RT-PCR experiments on RNA from PMNs. The resulting PCR product was 1.1 kb in length, and resolution of its sequence indicated that the coding region of the PMN-derived prepronociceptin (GenBank accession no. AY335948) is identical to that observed in neural tissues (GenBank accession nos: U48263; NM_006228). The cDNA was used as a probe in Northern blots on mRNA preparations from leukocytes. In these experiments, a band of ~1 kb could be observed in PMN mRNA preparations. However, the signal was extremely weak: exposures of over 45 days with intensifying screens were necessary for detection of the band (data not shown), indicating that basal levels of nociceptin are very low in resting PMNs. Taken together, these results show that circulating PMNs express the complete nociceptin/NociR system at the mRNA level and strongly suggest that PMNs may also produce the neuropeptide at the protein level.
Nociceptin Is Released by PMNs during Granule Exocytosis
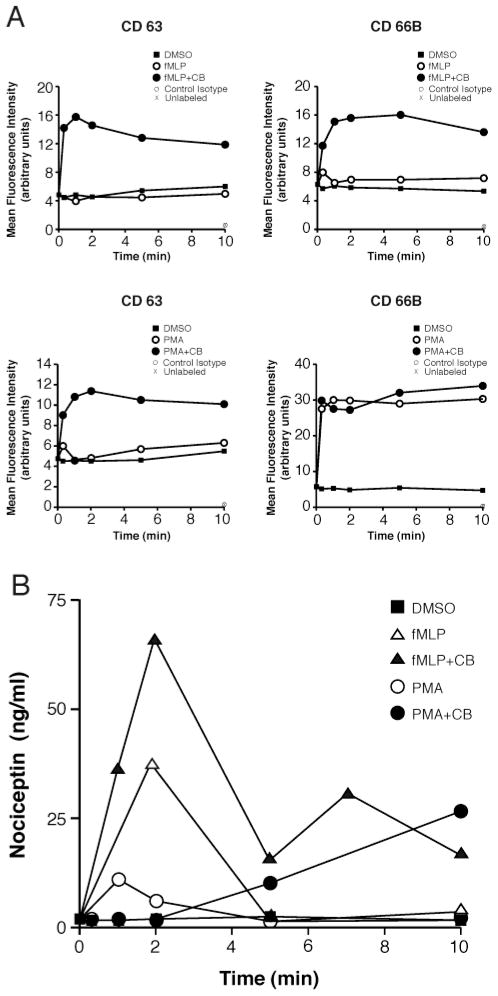
Nociceptin originating from nervous tissues can be released by exocytosis of neuropeptide-containing vesicles from activated nerve endings. Thus, we evaluated the capacity of PMNs to secrete nociceptin upon exocytosis of their granules. For these experiments, cells were stimulated either with fMLP or with PMA for increasing periods of time, in the absence or presence of cytochalasin B (CB), causing the release of PMN granules (26). Exocytosis of primary and secondary granules were respectively assessed by monitoring the appearance of the granular markers CD 63 and CD 66B at the cell surface (26). As reported (26), exocytosis of primary granules was essentially dependent on the presence of CB (Figure 3A, left panels), both in fMLP-or in PMA-stimulated samples, and this dependency was similarly observed with the release of secondary granules in fMLP-stimulated cells (top right panel). On the other hand, PMA readily caused exocytosis of secondary granules, regardless of the presence or absence of CB (bottom right panel). Supernatants of PMNs stimulated for degranulation were assayed by ELISA for the presence of nociceptin. The neuropeptide was detected mainly in the supernatants of fMLP-stimulated cells, in a CB-dependent fashion (Figure 3B), rapidly appearing in the supernatants and its concentration being highest at t = 2 min following stimulation. In comparison, PMA-stimulated cells secreted smaller amounts of nociceptin, also in a CB-dependent manner, and secretion appeared to be slower than with fMLP. While the pattern of nociceptin secretion obtained in these experiments does not allow us to determine from which granule type(s) the neuropeptide originated, these experiments nonetheless confirm that nociceptin can be secreted when PMNs are stimulated for degranulation.
Figure 3.
Human PMNs can secrete nociceptin upon degranulation. (A) PMNs [10 × 106/mL] were stimulated as indicated and then processed for CD63 and CD66B surface marker determination by FACS using PE- and FITC-labeled specific antibodies. CD 63 and CD 66B are markers of azurophilic and specific granules, respectively (fMLP: 1 μM; PMA: 100 nM; CB: 10 μM). (B) Cell-free supernatants of PMNs stimulated in panel A were assayed for the presence of nociceptin by specific ELISA. Results presented are from one experiment representative of three independent experiments performed with PMNs from different donors.
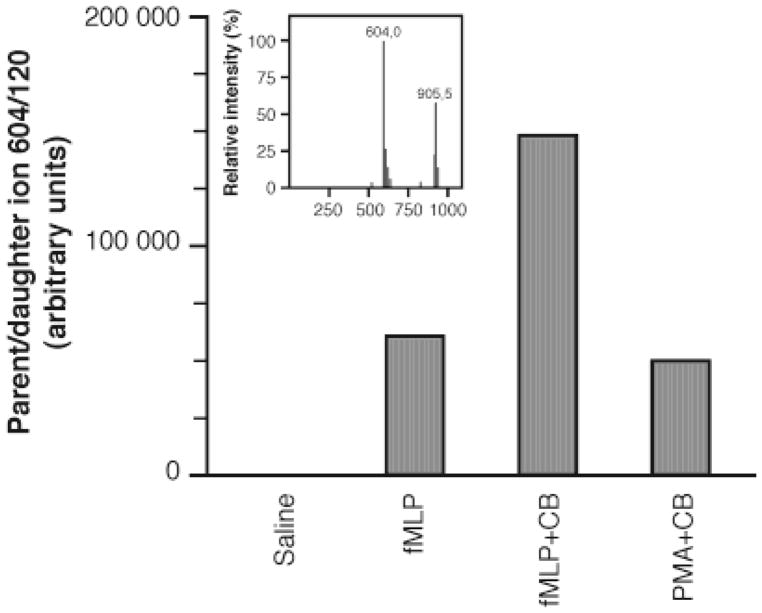
To confirm the release of nociceptin in PMNs, supernatants from cell samples stimulated as described above were analyzed by tandem mass spectrometry (MS-MS). The MS-MS signature of the neuropeptide was established using authentic nociceptin. When searching for the parent ion/daughter ion fragmentation using m/z 907 and 604 (see Materials and Methods), a single fragment was obtained at m/z 120, with the strongest signal obtained when monitoring from m/z 604 (Figure 4, inset). Therefore, monitoring of this 604:120 mother/daughter fragment was performed. As shown in Figure 4, nociceptin was detected, and the signal was strongest in CB+fMLP-stimulated PMNs, in accordance with the ELISA results. These results unequivocally establish that PMNs express and can release nociceptin upon stimulation and substantiate the existence of a novel two-way communication path between immune cells and the nervous system.
Figure 4.
Identification of nociceptin by tandem mass spectrometry (MS-MS). Supernatants from samples (t = 2 min) used in Figure 3A were analyzed for confirmation of nociceptin identity by MS-MS, as described in Materials and Methods. Inset: authentic nociceptin synthesized in-house was analyzed by MS-MS for the determination of its parent/daughter fragmentation signature.
Protein Phosphorylation on Tyrosine Residues and Stimulation of Hck by Nociceptin
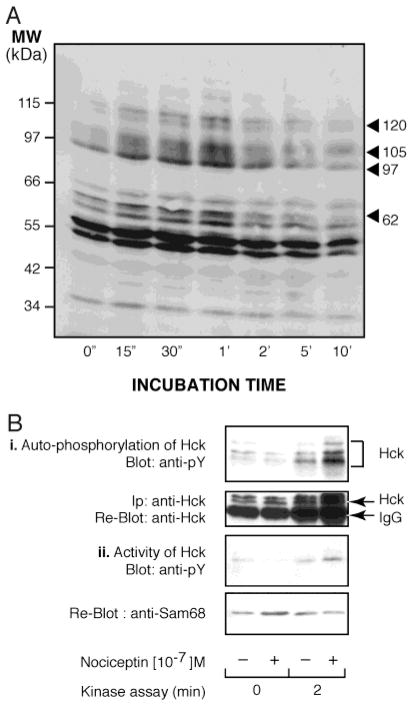
In an effort to understand the impact that the nociceptin/NociR system may have on PMN physiological functions, we endeavored to initiate identification of the signal transduction pathways utilized by this system in PMNs. Phosphorylation of target proteins on tyrosine residues represents a major means of cellular signaling in PMNs and ranks among the very first events following engagement of the receptors by their respective ligand. In turn, early tyrosine phosphorylation events were determined in PMNs incubated with exogenous nociceptin. Samples were processed for the determination of cellular proteins phosphorylated on their tyrosine residues. In these experiments, nociceptin elicited a specific, rapid, and transient tyrosine phosphorylation pattern (Figure 5A). In particular, tyrosine phosphorylation of proteins with molecular weights of approximately 120, 105, 97, and 62 kDa appeared prominent. Hck is a family of kinases present in PMNs with molecular weights ranging from 59 to 61 kDa, a region where the tyrosine phosphorylation profile elicited by nociceptin showed a great increase. Thus, samples were processed for Hck autophosphorylation and for activation assays (Figure 5B). Results show that immunoprecipitated Hck in the nociceptin stimulated cell lysate was more active than the unstimulated sample. Panel B, part i of Figure 5 shows the autophosphorylation of Hck while part ii shows the ability of Hck to phosphorylate an exogenous substrate, SAM68-GST protein. Reblots indicated that equal amounts of Hck were immunoprecipitated and loaded on gels.
Figure 5.
Nociceptin transiently induces phosphorylation of selected proteins on tyrosine residues and increases activation of Hck. (A) PMNs were incubated for the indicated times with 100 nM nociceptin and then processed for Western immunoblotting using a monoclonal anti-phosphotyrosine antibody. (B) Samples were processed as described in Materials and Methods. Panel i shows Hck autophosphorylation, and panel ii shows Hck activation assays. For each panel, autoradiograms are from one experiment representative of three separate experiments performed with different donors.
Engagement of NociR Modulates Intracellular cAMP Levels
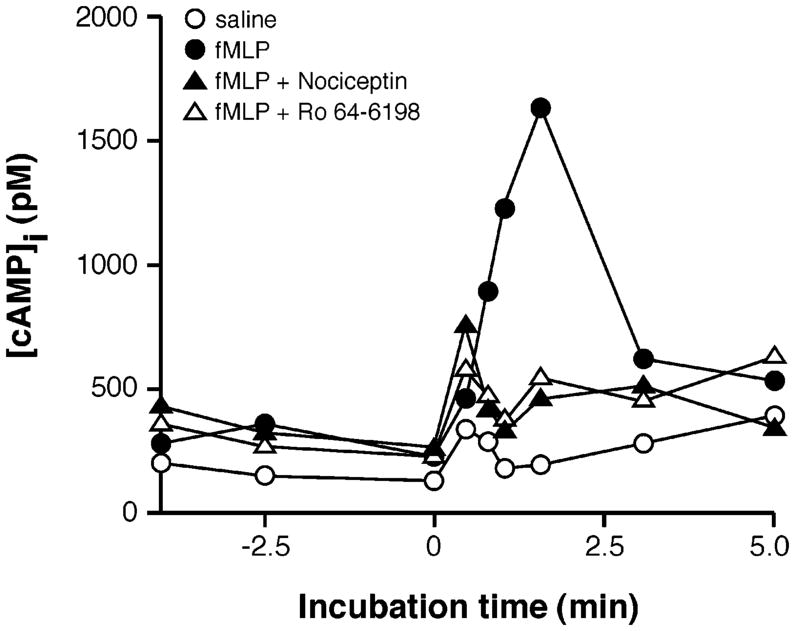
The impact of nociceptin on intracellular cAMP levels was addressed in view of the pivotal importance this second messenger pathway has in modulating a number of PMN functions (27–30). In these experiments, optimized intracellular elevation of cAMP levels was elicited by stimulating PMNs with fMLP in combination with CGS 21680, a stable agonist of the adenosine receptor A2A subtype, and with Ro 20-1724, an inhibitor of phosphodiesterase IV (31). PMNs were incubated with nociceptin or with the synthetic agonist specific for NociR, and Ro 64-6198 (32, 33) and intracellular cAMP levels were monitored in PMNs at different time points by processing samples for cAMP determination by ELISA, as described in Materials and Methods. As shown in Figure 6, the presence of nociceptin significantly reversed the elevation of cAMP induced by fMLP. Similarly, the presence of Ro 64-6198 also prevented cAMP elevation in these experiments. When used by itself, nociceptin had no significant effect on the basal levels of intracellular cAMP (data not shown). This modulation in cAMP levels by nociceptin in stimulated PMNs suggests that the neuropeptide may potentiate PMN responses.
Figure 6.
Nociceptin modulates intracellular cAMP levels in PMNs. PMNs were incubated in the presence of adenosine deaminase (0.1 U/mL) to eliminate extracellular adenosine and stimulated with fMLP (100 nM) at t = 0 min in the presence of CGS 21680, a specific agonist of the adenosine A2A receptor subtype, and with Ro 20-1724, an inhibitor of phosphodiesterase IV. When present, nociceptin or the synthetic NociR agonist, Ro 64-6198, were added to cell suspensions at t = −1 min. Reactions were stopped at the indicated time points, and intracellular cAMP levels were determined as described in the Materials and Methods. Results represent one experiment, typical of three independent sets of experiments performed with PMNs from different donors.
DISCUSSION
Nociceptin is a recently characterized neuropeptide that expression has up to now been mainly documented in neural tissues. However, detection of its mRNA in peripheral tissues has raised new questions about its potential role(s) outside the nervous system. In the present study, elevated concentrations of nociceptin were found in synovial fluids from arthritis patients. Nociceptin did not appear to be released systemically as it was consistently absent from autologous plasma samples. This is, to our knowledge, the first evidence demonstrating the presence of nociceptin at an inflammatory site. Our results contrast with one earlier report where the neuropeptide was not detected in synovial fluids (34). The reason for this discrepancy is not clear but may be related to the extensive processing of samples performed in that study that may have altered the peptide. It should not be excluded also that other cell types, including peripheral nerve endings, can release nociceptin in inflamed joints; however, the accumulation of inflammatory leukocytes at these sites support the possibility that PMNs may constitute a significant source of nociceptin.
We determined in the present study that the nociceptin/NociR system is expressed and functional in cells of the immune system, namely, in circulating leukocytes. The nociceptin precursor, prepronociceptin, was constitutively detected at the mRNA level in PMNs, monocytes, and lymphocytes. This, in conjunction with previous observations showing the presence of the NociR in these cells (20), now confirms that this ligand/receptor system is present in every circulating leukocyte subtype and suggests that nociceptin may directly participate in functions of the immune system. Of interest, PMNs were shown to secrete the neuropeptide. While the granule subtype(s) have not been identified, PMNs were shown to rapidly secrete nociceptin in the extracellular milieu upon exocytosis of their granules. Some features of nociceptin expression and secretion are noteworthy; the neuropeptide was secreted almost instantly following cell stimulation, indicating a preformed molecule stored in granules rather than de novo synthesized. Moreover, the expression of mRNA for the nociceptin was found to be very low in resting PMNs and not significantly altered upon stimulation with a number of classical agonists (data not shown). Immunocytochemistry and subcellular fractionation procedures will be necessary to specifically elucidate this point. The identity of the peptide secreted was confirmed by mass spectrometry and unequivocally demonstrated the identity of nociceptin, establishing that PMNs constitute a source of nociceptin and can release it promptly in peripheral tissues upon stimulation.
In view of the presence of nociceptin at inflammatory sites where PMNs can accumulate in large numbers, along with the earlier observation that PMNs express a functional NociR (20), it became relevant to investigate the impact of nociceptin on PMN early responses. Tyrosine phosphorylation and modulation of intracellular cAMP levels are two such responses of pivotal importance. While the former dispatches extracellular signals toward specific intracellular signal transduction pathways, the latter has a definitive impact in modulating PMN responses to extracellular agonists. In this study, nociceptin elicited phosphorylation of selected intracellular proteins. One of them has been identified as Hck, a kinase involved in degranulation, phagocytosis, and chemotaxis processes (35). Nociceptin was earlier found to stimulate PMN chemotaxis, and the present result raises the possibility that Hck may mediate this activity.
In PMNs, intracellular elevation of cAMP levels can inhibit superoxide generation, degranulation, LTB4, and cytokine generation (27–30). In our experimental settings, nociceptin modulated the increase in cAMP provoked by fMLP. Given the inhibitory consequences that elevated intracellular cAMP has in PMNs, its modulation by nociceptin could also be interpreted as a stimulatory signal. Notably, this effect was similarly observed when using Ro 64-6198, a specific NociR agonist, which confirmed the involvement of the receptor in this process. Studies are in progress in our laboratory to determine the intracellular targets of activated NociR and in particular to determine whether the adenylyl cyclase/phosphodiesterase system is directly affected in this process.
Nociceptin has been linked to a number of physiological functions, such as processing of pain signals, locomotion, and memory. However, few studies have addressed a possible role for the neuropeptide in immune responses (18, 19). It is possible that nociceptin participates in the process of neurogenic inflammation, where nerve-elicited neuropeptides induce inflammatory responses. Support for this line of thinking comes from the study demonstrating that pretreatment of mice with capsaicin to deplete sensory neuropeptides greatly reduces the subsequent influx of PMNs (36). Within this context, the most studied neuopeptide is propably SP. In addition to playing a major role in pain transmission through activation of the NK receptors (37), SP is implicated in neurogenic inflammation in a multi-pronged fashion. In mast cells, SP induces tumor necrosis factor (TNF)-α mRNA expression and stimulates IL-1β production by monocytes, IL-1β and IL-6 by bone marrow cells, IL-6 production by astrocytes, and IL-2 production by murine lymphocytes (38–42). In PMNs, SP primes the oxidative metabolism and stimulates chemotaxis, degranulation, and phagocytosis pulmonary inflammation. SP facilitates the actions of other inflammatory agents (e.g., LTB4, platelet activating factor) on PMN adhesion, migration, and biochemical reactivity (43). Conversely, our observations with nociceptin suggest that an amplification loop between pain perception and leukocyte activation may take place and be mediated, at least in part, by the nociceptin/NociR system. Nociceptin secreted by PMNs, in addition to its potential impact on leukocyte functions, may trigger nerve cells expressing NociR and cause them to release proinflammatory factors. Targeting the nociceptin pathway may thus have beneficial effects, both at the level of pain perception and at the level of leukocyte activation. Development of specific antagonists, devoid of any agonist activity, will allow us to address this hypothesis.
In summary, we showed here that PMNs possess a functional nociceptin/NociR system and that nociceptin elicits selected intracellular responses that may have stimulatory consequences on PMN pathophysiological functions. These results identify a new link between the nervous and the immune systems. Additional studies will be required to better dissect the integration of signals utilized by these two systems and in particular to delineate the role(s) that nociceptin may have in inflammatory responses.
Acknowledgments
The authors wish to thank Jean Boulanger, Serge Picard, and Lise Lemieux for excellent technical assistance and Prof. Claire Dubois (Université de Sherbrooke) for insightful discussions.
Footnotes
This work is supported by grants to M.P. from the Canadian Institutes of Health Research (CIHR) in partnership with The Arthritis Society (MOP-85251) and from the Banting Research Foundation. M.P. was a recipient of the Young Investigator Award from the Canadian Pain Society and a Scholar of the Canadian Arthritis Network. C.G. was supported by a fellowship from the K. M. Hunter Charitable Foundation in partnership with the CIHR and from the Fonds de la Recherche en Santé du Québec.
A sequence has been deposited in the GenBank database (GenBank accession no. AY335948).
Abbreviations: ADA, adenosine deaminase; CB, cytochalasin B; DEPC, diethylpyrocarbonate; DFP, diisopropylfluorophosphate; GPCR, G protein-coupled receptor; HBSS, Hank’s balanced salt solution; LT, leukotriene; NociR, nociceptin receptor; PG, prostaglandin; PMN, polymorphonuclear neutrophil; RT-PCR, reverse transcriptase-polymerase chain reaction; SP, substance P; SAM, Src-associated in mitosis.
References
- 1.Gale LM, McColl SR. Chemokines: extracellular messengers for all occasions? Bioessays. 1999;21:17–28. doi: 10.1002/(SICI)1521-1878(199901)21:1<17::AID-BIES3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Harvath L. Neutrophil chemotactic factors. EXS. 1991;59:35–52. doi: 10.1007/978-3-0348-7494-6_3. [DOI] [PubMed] [Google Scholar]
- 3.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 4.De Giorgio R, Tazzari PL, Barbara G, Stanghellini V, Corinaldesi R. Detection of substance P immunoreactivity in human peripheral leukocytes. J Neuroimmunol. 1998;82:175–181. doi: 10.1016/s0165-5728(97)00201-4. [DOI] [PubMed] [Google Scholar]
- 5.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptor byproducts of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai CC, Hong YC, Chen CC, Wu YM. Measurement of prostaglandin E2 and leukotriene B4 in the gingival crevicular fluid. J Dent. 1998;26:97–103. doi: 10.1016/s0300-5712(96)00084-x. [DOI] [PubMed] [Google Scholar]
- 7.Lotz M, Vaughan JH, Carson DA. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988;241:1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- 8.Fong TM. Molecular biology of tackykinins. In: Geppetti P, Holzer P, editors. Neurogenic Inflammation. CRC Press; New York: 1996. pp. 3–14. [Google Scholar]
- 9.Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide, and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 10.Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, Mazarguil H, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 11.Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Civelli O. Orphanin FQ—a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 12.Connor M, Christie MJ. Opioid receptor signaling mechanisms [review] Clin Exp Pharmacol Physiol. 1999;26:493–499. doi: 10.1046/j.1440-1681.1999.03049.x. [DOI] [PubMed] [Google Scholar]
- 13.Okuda-Ashitaka E, Tachibana S, Houtani T, Minami T, Masu Y, Nishi M, Takeshima H, Sugimoto T, Ito S. Identification and characterization of an endogenous ligand for opioid receptor homologue ROR-C—its involvement in allodynic response to innocuous stimulus. Mol Brain Res. 1996;43:96–104. doi: 10.1016/s0169-328x(96)00165-9. [DOI] [PubMed] [Google Scholar]
- 14.Meunier JC. Nociceptin/orphanin FQ and the opioid receptor-like ORL1 receptor [review] Eur J Pharmacol. 1997;340:1–15. doi: 10.1016/s0014-2999(97)01411-8. [DOI] [PubMed] [Google Scholar]
- 15.Harrison LM, Grandy DK. Opiate modulating properties of nociceptin/orphanin FQ [review] Peptides. 2000;21:151–172. doi: 10.1016/s0196-9781(99)00185-0. [DOI] [PubMed] [Google Scholar]
- 16.Ueda H, Yamaguchi T, Tokuyama S, Inoue M, Nishi M, Takeshima H. Partial loss of tolerance liability to morphine analgesia in mice lacking the nociceptin receptor gene. Neurosci Lett. 1997;237:136–138. doi: 10.1016/s0304-3940(97)00832-x. [DOI] [PubMed] [Google Scholar]
- 17.Houtani T, Nishi M, Takeshima H, Nukada T, Sugimoto T. Structure and regional distribution of nociceptin/orphanin FQ precursor. Biochem Biophys Res Commun. 1996;219:714–719. doi: 10.1006/bbrc.1996.0300. [DOI] [PubMed] [Google Scholar]
- 18.Halford WP, Gebhardt BM, Carr DJ. Functional role and sequence analysis of a lymphocyte orphan opioid receptor. J Neuroimmunol. 1995;59:91–101. doi: 10.1016/0165-5728(95)00030-6. [DOI] [PubMed] [Google Scholar]
- 19.Wick MJ, Minnerath SR, Roy S, Ramakrishnan S, Loh HH. Expression of alternate forms of brain opioid orphan receptor mRNA in activated human peripheral blood lymphocytes and lymphocytic cell lines. Brain Res Mol Brain Res. 1995;32:342–347. doi: 10.1016/0169-328x(95)00096-b. [DOI] [PubMed] [Google Scholar]
- 20.Serhan CN, Fierro IM, Chiang N, Pouliot M. Cutting edge: nociceptin stimulates neutrophil chemotaxis and recruitment: inhibition by aspirin-triggered 15-epi-lipoxin A4. J Immunol. 2001;166:3650–3654. doi: 10.4049/jimmunol.166.6.3650. [DOI] [PubMed] [Google Scholar]
- 21.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation and of granulocytes by combining centrifugation and sedimentation at 1g. Scand J Clin Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 22.Pouliot M, Fiset ME, Masse M, Naccache PH, Borgeat P. Adenosine up-regulates cyclooxygenase-2 in human granulocytes: impact on the balance of eicosanoid generation. J Immunol. 2002;169:5279–5286. doi: 10.4049/jimmunol.169.9.5279. [DOI] [PubMed] [Google Scholar]
- 23.Arjomand J, Evans CJ. Differential splicing of transcripts encoding the orphanin FQ/nociceptin precursor. J Neurochem. 2001;77:720–729. doi: 10.1046/j.1471-4159.2001.00219.x. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert C, Rollet-Labelle E, Caon AC, Naccache PH. Immunoblotting and sequential lysis protocols for the analysis of tyrosine phosphorylation-dependent signaling. J Immunol Methods. 2002;271:185–201. doi: 10.1016/s0022-1759(02)00347-2. [DOI] [PubMed] [Google Scholar]
- 25.Arjomand J, Cole S, Evans CJ. Novel orphanin FQ/nociceptin transcripts are expressed in human immune cells. J Neuroimmunol. 2002;130:100–108. doi: 10.1016/s0165-5728(02)00217-5. [DOI] [PubMed] [Google Scholar]
- 26.Nanda A, Brumell JH, Nordstrom T, Kjeldsen L, Sengelov H, Borregaard N, Rotstein OD, Grinstein S. Activation of proton pumping in human neutrophils occurs by exocytosis of vesicles bearing vacuolar-type H+ ATPases. J Biol Chem. 1996;271:15963–15970. doi: 10.1074/jbc.271.27.15963. [DOI] [PubMed] [Google Scholar]
- 27.Zurier RB, Weissmann G, Hoffstein S, Kammerman S, Tai HH. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974;53:297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehmeyer JE, Johnston RB., Jr Effect of antiinflammatory drugs and agents that elevate intracellular cyclic AMP on the release of toxic oxygen metabolites by phagocytes: studies in a model of tissue-bound IgG. Clin Immunol Immunopathol. 1978;9:482–490. doi: 10.1016/0090-1229(78)90144-7. [DOI] [PubMed] [Google Scholar]
- 29.Marone G, Thomas LL, Lichtenstein LM. The role of agonists that activate adenylate cyclase in the control of cAMP metabolism and enzyme release by human polymorphonuclear leukocytes. J Immunol. 1980;125:2277–2283. [PubMed] [Google Scholar]
- 30.Ham EA, Soderman DD, Zanetti ME, Dougherty HW, McCauley E, Kuehl FA. Inhibition by prostaglandins of leukotriene B4 release from activated neutrophils. Proc Natl Acad Sci USA. 1983;80:4349–4353. doi: 10.1073/pnas.80.14.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thibault N, Harbour D, Borgeat P, Naccache PH, Bourgoin SG. Adenosine receptor occupancy suppresses chemoattractant-induced phospholipase D activity by diminishing membrane recruitment of small GTPases. Blood. 2000;95:519–527. [PubMed] [Google Scholar]
- 32.Dautzenberg FM, Wichmann J, Higelin J, Py-Lang G, Kratzeisen C, Malherbe P, Kilpatrick GJ, Jenck F. Pharmacological characterization of the novel nonpeptide orphanin FQ/nociceptin receptor agonist Ro 64-6198: rapid and reversible desensitization of the ORL1 receptor in vitro and lack of tolerance in vivo. J Pharmacol Exp Ther. 2001;298:812–819. [PubMed] [Google Scholar]
- 33.Hashiba E, Lambert DG, Jenck F, Wichmann J, Smith G. Characterization of the nonpeptide nociceptin receptor agonist, Ro64-6198, in Chinese hamster ovary cells expressing recombinant human nociceptin receptors. Life Sci. 2002;70:1719–1725. doi: 10.1016/s0024-3205(02)01477-7. [DOI] [PubMed] [Google Scholar]
- 34.Kumar N, Smart D, Mason S, McKnight AT, Rowbotham DJ, Lambert DG. Neither nociceptin nor its receptor are present in human synovial fluid or tissue. Br J Anaesth. 1999;83:470–471. doi: 10.1093/bja/83.3.470. [DOI] [PubMed] [Google Scholar]
- 35.Barlic J, Andrews JD, Kelvin AA, Bosinger SE, DeVries ME, Xu L, Dobransky T, Feldman RD, Ferguson SS, Kelvin DJ. Regulation of tyrosine kinase activation and granule release through β-arrestin by CXCRI. Nat Immunol. 2000;1:227–233. doi: 10.1038/79767. [DOI] [PubMed] [Google Scholar]
- 36.Perretti M, Ahluwalia A, Flower RJ, Manzini S. Endogenous tachykinins play a role in IL-1-induced neutrophil accumulation: involvement of NK-1 receptors. Immunology. 1993;80:73–77. [PMC free article] [PubMed] [Google Scholar]
- 37.Furst S. Transmitters involved in antinociception in the spinal cord. Brain Res Bull. 1999;48:129–141. doi: 10.1016/s0361-9230(98)00159-2. [DOI] [PubMed] [Google Scholar]
- 38.Ansel JC, Brown JR, Payan DG, Brown MA. Substance P selectively activates TNF-α gene expression in murine mast cells. J Immunol. 1993;150:4478–4485. [PubMed] [Google Scholar]
- 39.Laurenzi MA, Persson MA, Dalsgaard CJ, Haeger-strand A. The neuropeptide substance P stimulates production of interleukin 1 in human blood monocytes: activated cells are preferentially influenced by the neuropeptide. Scand J Immunol. 1990;31:529–533. doi: 10.1111/j.1365-3083.1990.tb02801.x. [DOI] [PubMed] [Google Scholar]
- 40.Rameshwar P, Ganea D, Gascon P. Induction of IL-3 and granulocyte-macrophage colony-stimulating factor by substance P in bone marrow cells is partially mediated through the release of IL-1 and IL-6. J Immunol. 1994;152:4044–4054. [PubMed] [Google Scholar]
- 41.Gitter BD, Regoli D, Howbert JJ, Glasebrook AL, Waters DC. Interleukin-6 secretion from human astro-cytoma cells induced by substance P. J Neuroimmunol. 1994;51:101–108. doi: 10.1016/0165-5728(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 42.Rameshwar P, Gascon P, Ganea D. Stimulation of IL-2 production in murine lymphocytes by substance P and related tachykinins. J Immunol. 1993;151:2484–2496. [PubMed] [Google Scholar]
- 43.Mathison R, Davison JS, Befus AD. Neural regulation of neutrophil involvement in pulmonary inflammation. Comp Biochem Physiol C. 1993;106:39–48. doi: 10.1016/0742-8413(93)90252-g. [DOI] [PubMed] [Google Scholar]