Abstract
We shed new light on the expression and function of the proteinase-activated receptor (PAR) family, associated with inflammation and hyperalgesia, in human granulocytes. Resting cells expressed constitutive levels of PAR-2 and PAR-3 mRNA but not PAR-1 or PAR-4. Based on flow cytometry, stimulation with opsonized bacteria (Bop) specifically up-regulated cell surface expression of PAR-2 in a concentration-dependent and time-dependent manner, independent of transcription or de novo protein synthesis. Primary granules were identified as a source of preformed PAR-2 that can readily be mobilized at the surface on fusion with the plasma membrane. Cellular response to PAR-2 activation, measured as changes in intracellular calcium concentration, was enhanced in PAR-2 up-regulated cells. Increase of cell-surface PAR-2 and of cell responsiveness were dependent specifically on the engagement of immunoglobulin (Ig)-binding receptors. Together, our results reveal that mobilization of intracellular granules, in response to Ig-receptor activation, up-regulates PAR-2 surface expression and makes neutrophils more responsive to proteinase activity. This enhanced response to PAR-2 activation indicates that molecular communication between pain and inflammation may be more important than previously believed.—St-Onge, M., Lagarde, S., Laflamme, C., Rollet-Labelle, E., Marois, L., Naccache, P. H., Pouliot, M. Proteinase-activated receptor-2 up-regulation by Fcγ-receptor activation in human neutrophils.
Keywords: inflammation, polymorphonuclear leukocytes, PARs, primary granules
Proteinase-activated receptors (PARs) are G-protein-coupled 7-transmembrane-domain receptors that are activated through proteolytic cleavage of their extracellular N-terminal domain, revealing a tethered ligand that binds and activates the receptor itself (1, 2). All four PAR isotypes cloned up to now have distinct cleavage sites and ligand sequences. PAR-1, PAR-3, and PAR-4 are activated by thrombin and are responsible for thrombin-induced platelet activation. In contrast, PAR-2 can be activated by trypsin, elastase (3), and mast cell tryptase (2). Synthetic peptides corresponding to hydrolytic fragments of certain proteins can also activate PARs, for example, H-Ser-Leu-Ile-Gly-Lys-Val-NH2 (SLIGKV), which specifically activates PAR-2 (4). PAR-3 differs from the other isotypes in that it is not activated by any peptide corresponding to its tethered ligand. It is considered a silent receptor that acts as a cofactor to facilitate PAR-4 activation by thrombin (5).
PARs are present in the membranes of cells of many tissues, including airways, skin, the gastrointestinal tract, the kidneys, the brain, and the peripheral nervous system, as well as vasculature and immune cells. They regulate numerous events such as contraction of vascular and tracheal smooth muscle cells, bronchi and the intestines, relaxation of the aorta, cell survival and proliferation, pain sensations, and release of lipid mediators, cytokines, and neuropeptides (6). In vivo, activated PARs can induce all of the symptoms associated with inflammation, namely edema, vasodilation, burning sensations and pain (7,8,9,10), and also mediate contraction of human endothelial cells and immune cell migration to the tissues, prostanoid generation, and enhanced expression of platelet-derived growth factor (7, 11). PAR-2 has received attention as a major proinflammatory mediator in inflammatory diseases, particularly in a mouse model of chronic arthritis induced by Freund’s complete adjuvant, in which it was found responsible for joint swelling and vasodilation (12). In experimentally induced allergic and toxic contact dermatitis, ear-swelling responses, plasma extravasation, and leukocyte adherence were attenuated significantly in PAR-2-null mutant mice (13). Moreover, PAR-2 has been implicated in inflammatory bowel diseases, based on observations that myeloperoxidase activity, macroscopic damage score, bowel thickness, leukocyte rolling, and adhesion were significantly lower in PAR-2-null mutant mice (14).
Polymorphonuclear leukocytes (granulocytes) are the first leukocytes to migrate to inflammatory sites, where they exert host defense functions, including the synthesis and release of an array of factors such as antimicrobial proteins, extracellular matrix proteins, and several cytokines and chemokines. Granulocytes, the vast majority of which are neutrophils, also generate oxygen-derived reactive agents and release proteolytic enzymes, in addition to their principal role as phagocytes, engulfing invading microorganisms and cell debris. As such, neutrophils play a major role in orchestrating the early stages of host-defense inflammatory responses. However, unchecked activation of neutrophils is associated with pathological states such as ischemia, sepsis, chronic obstructive pulmonary disease, and rheumatoid arthritis (15,16,17). Regulatory checkpoints are therefore required to ensure that neutrophil functions are tightly regulated. The presence of PAR-2 in neutrophils was reported more than a decade ago, along with its activation by SLIGKV, and the latter was modest or even absent in some donors (18). Since then, PAR-2 has been reported to elicit secretion of lactoferrin and interleukin (IL)-8 by neutrophils, to modulate expression of their adhesion molecules, and to stimulate neutrophil migration (19,20,21).
However, despite clear indication of the involvement of PAR-2 in inflammation, nociception, and neutrophil functions, data on the regulation of its expression and activity in neutrophils remain scarce. In the present work, we deciphered mRNA and cell surface expression of the PAR family in resting and activated human neutrophils. Our results indicate that neutrophils express PAR-2 and PAR-3 only and harbor a pool of preformed PAR-2 located in intracellular granules. Activation of the phagocytosis-linked, Fcγ receptors (FcγRs) causes mobilization of these granules to the cell surface and in turn up-regulates PAR-2 expression, independent of transcription or protein synthesis. This up-regulation in cell surface PAR-2 correlates with increased PAR-2-specific cell responsiveness. PAR-2 was thus identified as the main functional PAR subtype in neutrophils, while FcγR activation was found to be its principal modulator, unraveling the mystery surrounding a pivotal regulatory checkpoint in their responsiveness to the proteinase system.
MATERIALS AND METHODS
Materials
Phycoerythrin-labeled mouse anti-PAR-1 (product no. SC-13503PE), fluorescein-isothiocyanate-(FITC)-labeled mouse anti-PAR-2 (SC-13504FITC), FITC-labeled anti-PAR-3 (SC-53819FITC), phycoerythrin (PE)-labeled mouse monoclonal IgG2b (SC-53853PE, directed against mouse IgD Fc, used here as a control antibody), goat anti-PAR-2 (SC-8206), rabbit anti-PAR-2 (SC-5597), PE-labeled mouse anti-PAR-1 (SC-13503PE), and rabbit anti-PAR-4 (SC-25466) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). PE-labeled purified mouse immunoglobulin isotype IgG2a (Product No. 12–4724; also used as a control antibody) was obtained from eBioscience (San Diego, CA, USA). FITC-labeled anti-rabbit and Dy light 488 anti-goat were obtained from Jackson Immunoresearch (West Grove, PA, USA). Formylated-methionyl-leucyl-phenylalanine (fMLP), phorbol-myristate-acetetate (PMA), C5a, trypsin, and cytochalasin B (CB) were obtained from Sigma-Aldrich (St. Louis, MO, USA). C3b was from Alpha Diagnostic International (San Antonio, TX, USA). PGE2 and LTB4 were purchased from Cayman Chemical (Ann Arbor, MI, USA). Lipopolysaccharide (LPS; from Escherichia coli 0111:B4) was purchased from Calbiochem-Novalbiochem Corp. (San Diego, CA, USA). Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-1β, IL-8, and tumor necrosis factor (TNF)-α were from Peprotech Inc. (Rocky Hill, NJ, USA). The PAR-2-specific agonist H-Ser-Leu-Ile-Gly-Lys-Val-NH2 was purchased from AnaSpec (San Jose, CA, USA), Fura-2-AM was from Molecular Probes (Eugene, OR, USA), and Pelicluster, a blocking antibody for FcγRIIIb, was from Sanquin (Amsterdam, Netherlands). Anti-CD63 and anti-CD66b antibodies were obtained from Beckman Coulter (Mississauga, ON, Canada). Monoclonal antibody IV.3 was purified from ascites of mice inoculated with hybridoma HB-217 obtained from the American Type Culture Collection (Manassas, VA, USA). This antibody recognizes a native extracellular epitope of FcγRIIa and was used for blocking experiments. Anti-CD16 was purchased from Antigenix America Inc. (Huntington Station, NY, USA).
Isolation of human neutrophils and peripheral blood mononuclear cells (PBMCs)
Blood granulocytes, obtained from healthy donors, were isolated as described previously (22). Granulocytes (>95% neutrophils, <5% eosinophils) contained <0.05% monocytes, as determined by esterase staining. Viability was >98%, as determined by trypan blue dye exclusion. PBMCs were isolated from platelet-rich plasma obtained by centrifugation of whole blood at 400 g. One volume of 1 M sodium citrate was added to 10 vol of supernatant, followed by adjusting the pH to 6.4 with 1 M citric acid and centrifuging for 10 min at 900 g.
Preparation of bacteria
Salmonella typhimurium was cultured overnight in Luria-Bertani broth and transferred (1:20) to fresh broth for a second overnight culture. Cells from 100 ml of this second culture were recovered by centrifuging for 10 min at 1000 g, washed twice in HBSS, and held at 100°C for 1 h. Half was kept as nonopsonized bacteria (Bnop), while the other (Bop) was centrifuged (10 min, 1000 g), opsonized in 20 ml of sterile nondecomplemented human serum (pooled from 20 donors), incubated at 37°C for 1 h, and washed twice in HBSS.
Preparation of aggregated IgG
A 25 mg/ml solution of IgG from human serum (Sigma-Aldrich) was heated at 63°C for 60 min, then cooled on ice and stored at 4°C. Heat-aggregated IgG was used as a specific and potent activator of FcγRs (23).
Isolation of leukocyte genomic DNA
Granulocytes (30×106) were resuspended in 500 μl of extraction buffer (500 mM Tris-HCl, 0.5 M Na2EDTA, 5 M NaCl) containing 0.2 mg/ml proteinase K and 0.5% β-mercaptoethanol and incubated for 2 h at 55°C. A half-volume (250 μl) of saturated NaCl was added, and the samples were laid on ice for 15 min, followed by centrifugation at 12,000 g for 1 h at 4°C. Supernatants (250 μl) were recovered, and 3 vol of 100% ethanol was added. Samples were mixed thoroughly, centrifuged at 4000 g for 5 min at 4°C, and then washed twice in 70% ethanol. The pellets thus obtained were allowed to air-dry for 15–20 min and then dissolved in 10 μl of sterile water.
RNA isolation
Leukocyte or PBMC total RNA was isolated using Trizol (Invitrogen, Burlington, ON, Canada) according to the manufacturer’s protocol, with modifications (22). Briefly, 30 × 106 leukocytes, or platelets, from 20 ml of platelet-rich plasma were homogenized in 1 ml Trizol, and 200 μl of chloroform was added. After mixing, the sample was centrifuged at 12,000 g for 15 min (4°C). The upper aqueous phase (450 μl) was transferred to a tube containing an equal volume of isopropanol. After mixing, the samples were kept at room temperature for 10 min and then centrifuged at 12,000 g for 10 min (4°C). The supernatant was discarded, and the precipitated RNA pellet was washed twice using 500 μl of 75% ethanol and centrifuging at 12,000 g for 5 min (4°C). The final pellet was allowed to air-dry for 5–10 min and was then resuspended in RNase-free water. RNA was quantitated using a Qubit™ fluorometer (Invitrogen).
Determination of human leukocyte PAR/GAPDH mRNA by real-time PCR
First-strand cDNA synthesis was performed using 1 μg of total RNA with Superscript II® reverse transcriptase (Invitrogen) under the recommended conditions, using 500 ng of random hexamers. Amplification of granulocyte cDNA was performed in a real-time PCR Rotor-Gene 3000 analyzer operated with Rotor Gene software version 6.0.19 (Corbett Research, Mortlake, NSW, Australia). Each sample consisted of 1 μl cDNA, 1.3 mM MgCl2, 0.2 mM dNTP, 500 nM of primers, 0.3 U of TaqDNA polymerase (Roche Diagnostics, Indianapolis, IN, USA) and Sybr Green dye (Molecular Probes; 1:30,000 dilution) in a reaction volume of 20 μl. Amplification was achieved using 35 cycles of 95°C, 58°C, and 72°C for 20 s at each temperature. Reaction specificity was verified after each amplification using the Melt® procedure (58–99°C; 1°C/5 s) according to the manufacturer’s protocol. Threshold cycle (Ct) values were recorded for the genes of interest and the housekeeping gene (i.e., GAPDH). Results were analyzed essentially as described earlier (24). The following primers were used: GAPDH, sense CGAGATCCCTCCAAAATCAA and antisense TTCACACCCATGACGAACAT; PAR-1, sense CATCGTTGTGTTCATCCTGA and antisense CTTAAAGGGGAGCACAGACA; PAR-2, sense CATACATGGCAACAACTGGA and antisense TTCACGATGACCCAATACCT; PAR-3, sense TCTTGTTCAGCCAGACATCA and antisense CGCCTTAACATACCACAACC; PAR-4, sense CTGTTGGGCTGTTTCCTG and antisense CGTAGGCACCATAGAGGTTG.
Flow cytometry
Cells were stimulated with the bacterial preparations and/or purified agonists as indicated at a concentration of 5 × 106 cells/ml in HBSS with 1.6 mM Ca2+, then resuspended in a total volume of 100 μl for labeling with 10 μl of control isotype, 10 μl of specific antibody (PAR-2, PAR-3, CD63, CD66b), or both. Cells were incubated for 30 min on ice in the dark, washed with 0.5 ml HBSS, centrifuged, and resuspended in 0.5 ml HBSS for analysis by flow cytometry with a FACSCanto II apparatus (BD Biosciences, Franklin Lakes, NJ, USA). Unless stated otherwise, flow cytometry experiments with PAR-2 were performed using mouse anti-PAR-2 antibody (SC-13504FITC) raised against PAR-2 aa 37–50. For validation, goat anti-PAR-2 recognizing the N-terminal region and rabbit anti-PAR-2 recognizing aa 230–328 were also used when indicated.
Response of intracellular Ca2+ to stimulation
Cells (1×107/ml) in HBSS with 1.6 mM Ca2+ were preincubated or not with Bop (25 bacteria/cell) at 37°C for the indicated times, then incubated with 1 μM Fura-2-AM for 30 min. Cells were washed and diluted to 5 × 106 cells/ml in HBSS with 1.6 mM Ca2+ and maintained at 37°C in stirred cuvettes. PAR-2 agonist peptide SLIGKV, trypsin, thrombin, or IL-8 was injected and Fura-2-AM fluorescence emission at 510 nm was measured with excitation at 340 and 380 nm using a fluorescence spectrophotometer (Fluorolog-SPEX from Jobin Yvon Inc. Edison, NJ, USA). The change in intracellular Ca2+ (which represents cell responsiveness in this study) was calculated in arbitrary units as the area under the plot of the ratio fluorescence340/fluorescence380 for 50 s from the point of injection of the stimulating agent, minus the corresponding area under resting conditions.
Statistical analysis
Significant differences between groups were assessed using nonparametric ANOVA (Kruskal–Wallis test). Pairwise comparisons were performed using the Mann and Whitney U test. Differences were considered significant at P < 0.05.
RESULTS
Expression of PAR-2 and PAR-3 mRNA in human granulocytes
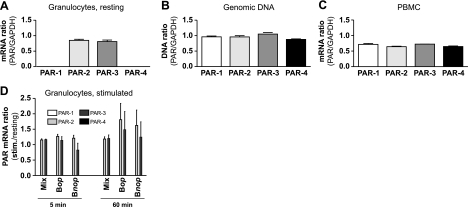
Figure 1 shows expression of mRNA corresponding to the 4 PAR subtypes in human granulocytes as measured using real-time PCR. PAR-2 and PAR-3 mRNA were readily detected in resting cells, while PAR-1 and PAR-4 mRNA were absent (Fig. 1A). Granulocyte genomic DNA or PBMC mRNA was used as a template to validate the efficiency of the primers (Fig. 1B, C). Agonists well known to induce gene expression in granulocytes (25,26,27,28) and to engage cellular functions (29,30,31,32) were also assessed for effect on PAR mRNA expression. These included the chemokine interleukin CXCL8 (100 nM), lipid mediators prostaglandin E2 (1 μM) and leukotriene B4 (100 nM) as well as complement fragments C3b and anaphylatoxin C5a (10 nM, data not shown) and a mixture of proinflammatory agonists that provide comprehensive gene induction in neutrophils (Fig. 1D) (33). This mixture contained growth factor GM-CSF (1 nM), proinflammatory cytokines TNF-α (100 ng/ml) and IL-1β (30 pM) and bacterial-derived components LPS (0.1 μg/ml) and fMLP (100 nM). In stimulations of up to 60 min, none of these agonists had significant effect on PAR-2 or PAR-3 mRNA, nor did they induce any detectable expression of PAR-1 and PAR-4 mRNA. Finally, Bop or Bnop were used as a stimulus known to engage phagocytosis in granulocytes. Opsonization, or covering of the microorganism with plasma molecules such as immunoglobulins and complement fragments, helps to slow down microorganisms and make it easier for phagocytes such as neutrophils to entrap and engulf them. Incubation with Bop for 60 min marginally increased PAR-2 mRNA levels, but this did not reach statistical significance (P>0.05). These results indicate that circulating human granulocytes constitutively express mRNA for PAR-2 and PAR-3 but not for PAR-1 or PAR-4.
Figure 1.
Proteinase-activated receptor mRNA expression in human granulocytes, as determined by real-time PCR. A) PAR mRNA expression in freshly purified, resting human granulocytes. B) Efficiency of primers with genomic DNA from human granulocytes. C) Efficiency of primers with mRNA from mixed PBMCs. Results are expressed as mean ± se threshold cycle ratio (PAR/GAPDH), from n = 5 (A) or n = 3 (B, C) experiments, each performed with cells from a different donor. D) PAR subtype mRNA expression in granulocytes incubated 5 or 60 min at 37°C with a mixture (Mix) of 5 neutrophil agonists (1 nM GM-CSF, 100 ng/ml TNF-α, 0.1 μg/ml LPS, 30 pM IL-1β, and 100 nM fMLP) or with Bop or Bnop Salmonella enterica serovar Typhimurium (25 bacteria/cell). Results are mean ± se threshold cycle ratio relative to resting cells from n = 5 experiments, each performed with a different donor. Primer sequences are provided in Materials and Methods.
Surface expression of PAR-2 in granulocytes: effect of Bop
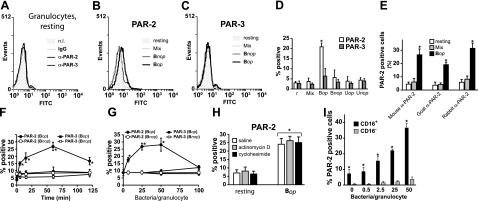
Figure 2 depicts the expression of PAR-2 on the human granulocyte cell surface, as detected by flow cytometry. In resting cells, the protein signal was negative for all four PAR subtypes, comparable to that for cells labeled with isotype-matched IgG (Fig. 2A). Stimulation with Bop significantly up-regulated PAR-2 levels, causing a shift to the right in labeling intensity for the whole cell population (Fig. 2B). In contrast, stimulation with the mixture of inflammatory agonists GM-CSF, TNF-α, IL-1β, LPS, and fMLP had no effect on PAR-2 expression (Fig. 2B–D). Monosodium urate crystals are a well-established agent in the etiology of acute gouty arthritis in which granulocytes play a pivotal role (34). However, stimulation with opsonized urate (Uop) or with nonopsonized urate (Unop) did not affect PAR-2 expression (Fig. 2D). Under all conditions, PAR-3 signals remained comparable to basal levels (Fig. 2C, D), similar to PAR-1 and PAR-4 (data not shown). In an additional set of flow cytometry experiments that yielded results similar to those obtained with the mouse anti-PAR-2 antibody (Fig. 2E), the specific stimulatory effect of Bop on PAR-2 surface expression was also observed with two additional and distinct anti-PAR-2 antibodies each recognizing different PAR-2 epitopes.
Figure 2.
Flow cytometry analysis of PAR expression on the granulocyte cell surface. A) Cell surface expression of PAR in resting granulocytes, nonlabeled (n.l.), labeled with matched-isotype IgG, or labeled with anti-(α)-PAR-2 or anti-α-PAR-3. B) Cell surface expression of PAR-2 in granulocytes stimulated for 60 min at 37°C with a mixture (Mix; 1 nM GM-CSF, 100 ng/ml TNF-α, 30 pM IL-1β, 0.1 μg/ml LPS, and 100 nM fMLP) of agonists or with Bop or Bnop (25 bacteria/cell). C) Same, PAR-3. Results for A–C are from one experiment, typical of ≥3 performed under identical conditions. D) Granulocytes from 3 different donors were stimulated for 60 min at 37°C with Mix, Bop, or Bnop bacteria as in C, or with opsonized (Uop) or nonopsonized urate crystals (Unop, 1 mg/ml). Results are means ± se. E) Granulocytes were stimulated with Mix or Bop for 60 min and assessed for PAR-2 surface expression using 3 distinct anti-PAR-2 antibodies. Mouse anti-PAR-2 antibody was raised against the sequence corresponding to aa 37–50; goat anti-PAR-2 antibody reacts with the N-terminal sequence, and rabbit anti-PAR-2 antibody recognizes aa 230–328 of PAR-2. F) Time course for PAR-2 surface expression in granulocytes stimulated with Bop or Bnop, as determined by flow cytometry. G) Concentration response. H) Cells incubated with transcription inhibitor actinomycin D (5 μg/ml) or with protein synthesis inhibitor cycloheximide (20 μg/ml) prior to stimulation with Bop (25 bacteria/cell; 60 min). Results are means ± se for ≥4 experiments. I) Total granulocytes were sorted according to CD16 surface expression; samples were stimulated with indicated Bop concentrations for 60 min at 37°C. Samples were assessed for PAR-2 surface expression by flow cytometry. Results are means ± se for 3 distinct experiments. *P < 0.05.
In time-course experiments, Bop-elicited increases of PAR-2 surface expression became significant after 5 min and peaked at around 60 min (Fig. 2F). This up-regulation was also concentration dependent and reached a plateau at a ratio of 25 to 50 bacteria/cell, above which it decreased to basal values, due possibly to interference by high numbers of bacteria with the detection system (Fig. 2G). Pretreatment of the cells with RNA transcription inhibitor actinomycin D or with the protein synthesis inhibitor cycloheximide did not hinder the increase in PAR-2 surface expression (Fig. 2H). Polymorphonuclear granulocyte preparations typically contain >90% neutrophils. Eosinophils, which play important roles in conditions such as allergies and asthma (35), make up ∼5% of granulocytes. A set of experiments was conducted to assess whether CD16-negative eosinophils (36) can significantly contribute to PAR-2 expression in Bop-stimulated granulocyte preparations. While the expression of PAR-2 did increase at the surface of purified eosinophils in response to increasing concentrations of bacteria, PAR-2 levels were modest, remaining below that observed on unstimulated, CD16-positive neutrophils (Fig. 2I), confirming the latter as the main source of granulocytes expressing PAR-2.
Contribution of intracellular primary granules to cell-surface PAR expression
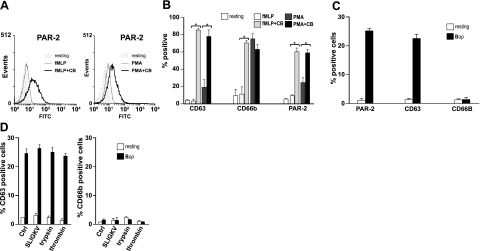
We investigated the possibility that intracellular granules constitute a pool of PAR-2 that mobilizes to produce prompt increases in cell surface PAR-2 independently of de novo synthesis. Through a classic procedure developed by Nanda et al.(37), neutrophils are stimulated with either 1 μM fMLP or 100 nM PMA in the absence or presence of CB, which causes signature profiles of mobilization for primary and secondary granules. Under these conditions, mobilization of primary granules, as revealed by the appearance of their specific marker (CD63), depends essentially on the presence of 5 μM CB in samples stimulated by fMLP and PMA. In contrast, exocytosis of secondary granules is not CB sensitive in PMA-stimulated cells, based on monitoring of their specific marker, CD66b. Under these conditions, up-regulation of PAR-2 surface expression showed clear CB dependency both in fMLP-stimulated and PMA-stimulated cells, similarly to primary granules (Fig. 3A, B). In addition, up-regulation of PAR-2 by Bop also caused the specific mobilization of primary but not secondary granules (Fig. 3C). Activation of PAR-2, whether by the PAR-2-specific peptide agonist SLIGKV or by trypsin, had no significant effect on granule mobilization, either in resting or in Bop-stimulated cells (Fig. 3D).
Figure 3.
Test for subcellular localization of a PAR-2 reservoir and effect of PAR-2 activation on the degranulation process. PAR-2, CD63, and CD66b surface expression in granulocytes stimulated with 1 μM fMLP or 100 nM PMA for 5 min at 37°C in the absence or presence of CB (5 μM), as determined by flow cytometry. A) Raw data for PAR-2 from a typical experiment among ≥3 performed under identical conditions. B) Results for PAR-2, CD63, and CD66b; means ± se; n = 3. *P < 0.05. C) PAR-2, CD63, and CD66b surface expression in granulocytes stimulated with Bop (25 bacteria/cell) for 60 min at 37°C, as determined by flow cytometry. D) CD63 and CD66b surface expression in granulocytes incubated as in C, then stimulated with 100 μM SLIGKV, trypsin (1:10 dilution) or thrombin (1U/ml), for an additional 30 min at 37°C. Results are means ± se for 3 experiments with cells from different donors.
Responsiveness of granulocytes undergoing PAR-2 up-regulation
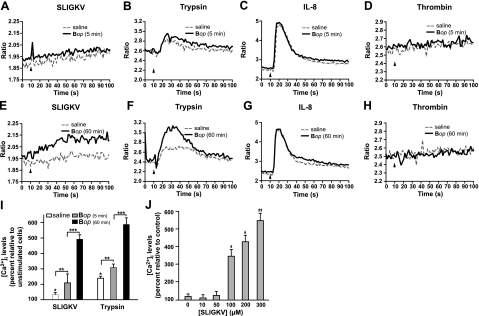
The responsiveness of human granulocytes is represented in terms of intracellular Ca2+ in Fig. 4. PAR-2 up-regulation led to stronger [Ca2+]i responses in cells preincubated with Bop and, considering donor-to-donor variability, followed the pattern of PAR-2 expression. In cells incubated for 60 min with Bop, SLIGKV-elicited [Ca2+]i responses were approximately four times stronger than in saline-treated cells (Fig. 4E, I). Stimulation with trypsin yielded a similar trend. In contrast, the chemokine CXCL8 (IL-8) elicited a classic response, which was equivalent in saline-treated and Bop-treated cells for 5 and 60 min (Fig. 4C, G). Thrombin, a PAR-1, -3, and -4 agonist, had no significant effect on [Ca2+]i, under any of the conditions tested (Fig. 4D, H). In Bop-treated cells, the [Ca2+]i response increased ∼8-fold above basal levels as SLIGKV concentration increased up to 300 μM (Fig. 4J). In these experiments, intracellular Ca2+ levels (as measured by the wavelength ratios) increased by a small amount over time because of basal cell activation, due in part to constant stirring of the samples. Together, the results obtained point to a direct association between up-regulation of surface PAR-2 originating from intracellular granules and neutrophil response to PAR-2 engagement.
Figure 4.
Effect of PAR-2 activation on intracellular Ca2+ responses. A–H) Granulocytes were incubated with or without Bop (25 bacteria/cell) for 5 min (A–D) or 60 min (E–H) at 37°C and then stimulated with the PAR-2 agonist peptide SLIGKV (100 μM; A, E), trypsin (1:10; B, F), IL-8 (100 nM; C, G), or thrombin (1U/ml; D, H) at t = 10 s. Samples were monitored for intracellular Ca2+ levels, as described in Materials and Methods. Results from one experiment are shown, typical of ≥3 distinct experiments performed in identical conditions with cells from different donors. Arrowheads indicate injection time. I) Intracellular Ca2+ responses were compiled by calculating the surface below the curves between t = 10 and 60 s. Results are mean ± se percentage change between cells treated with and without agonists; n = 3 distinct experiments. *P < 0.05 vs. no stimulation; **P < 0.05 vs. saline before stimulation; ***P < 0.05 vs. 5 min Bop before stimulation. J) Granulocytes were incubated with Bop for 60 min and stimulated with indicated concentrations of SLIGKV. Results show mean ± se intracellular Ca2+ increase vs. control (incubated 60 min with saline); n = 3 distinct experiments with cells from different donors. *P < 0.05 vs. no stimulation; **P < 0.05 vs. 100 μM SLIGKV stimulation.
FcγRs mediate the Bop-elicited up-regulation of PAR-2 and subsequent cell responsiveness
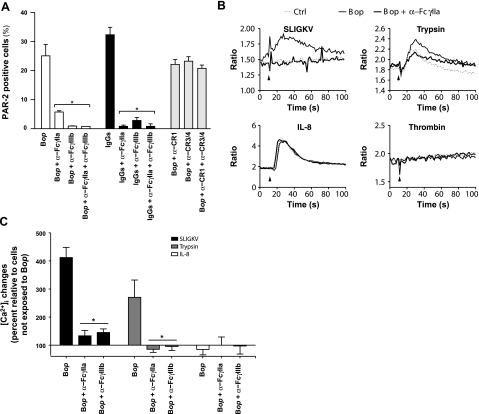
To determine the involvement of FcγRs and complement receptors (CRs) in PAR-2 up-regulation, neutrophils were incubated with a specific and potent stimulator of FcγRs, namely heat-activated, aggregated IgG (23). Cells were also incubated with blocking antibodies directed against FcγRIIa (1 μg/ml) and FcγRIIIb (4 μg/ml) or against CR1, CR3, and CR4 (4 μg/ml) prior to stimulation with Bop(38, 39). FcγRIIa blockade prevented PAR-2 up-regulation by ∼70%, while FcγRIIIb blockade diminished it by 90% (Fig. 5A). Concurrent use of both blocking antibodies did not result in stronger inhibition. Antibodies against FcγRs similarly prevented PAR-2 up-regulation elicited by aggregated IgG. In contrast, blockade of CRs, either individually or in combination, had little effect on PAR-2 up-regulation. FcγR blockade also efficiently suppressed [Ca2+]i responses to PAR-2 activation by SLIGKV or trypsin (80–100% inhibition), while sparing responses to by IL-8 (Fig. 5B, C). Cells also remained unresponsive to thrombin. These results indicate that FcγR activation is necessary for Bop-induced up-regulation of PAR-2 surface expression and increased cell responsiveness.
Figure 5.
Involvement of FcγRs on PAR-2 expression. A) Granulocytes were preincubated 5 min at 37°C with blocking antibodies for IgG-binding receptors or CRs (4 μg/ml), then stimulated for 60 min with either Bop (25 bacteria/cell) or aggregated IgGs (1 mg/ml). Samples were assessed for PAR-2 surface expression by flow cytometry. Results are mean ± se percentages of PAR-2 positive cells; n = 3 distinct experiments with cells from different donors. B) Granulocytes were preincubated 5 min at 37°C with 1 μg/ml blocking antibodies for IgG-binding receptors before incubation with Bop (25 bacteria/cell) for 60 min, and stimulation with indicated agonists. Samples were monitored for intracellular Ca2+ levels. Results from one experiment are shown, typical of ≥3 distinct experiments performed in identical conditions with cells from different donors. Arrowheads indicate injection time. C) Intracellular Ca2+ changes were compiled by calculating surface below curves between t = 10 and 60 s, above resting levels. Results are mean ± se percentage change between cells treated with and without Bop; n = 3 distinct experiments. *P < 0.05 vs. absence of blocking antibodies.
DISCUSSION
In the present study, we observed in human granulocytes the presence of constitutive mRNA for PAR-2 and PAR-3 but not for PAR-1 or PAR-4. Stimulations with an array of inflammatory agonists well recognized for inducing gene activation (33) did not significantly affect mRNA levels for any of the PAR subtypes. While it remains possible that different or longer stimulations would eventually affect PAR mRNA, no published data support a pivotal role for transcriptional activation in the regulation of PAR protein levels in neutrophils.
Resting granulocytes expressed low levels of surface PAR protein, while PAR-2 was the only up-regulated subtype following cell stimulation specifically with Bop. This enhanced cell surface expression became apparent within minutes of stimulation and was independent of mRNA regulation or de novo protein synthesis, suggesting the mobilization of a preexisting intracellular pool of PAR-2. Neutrophils contain a plethora of discrete granule subsets and vesicles, each with its characteristic protein profile. The membranes of these structures harbor receptors that provide means of communication with the environment (40, 41). Granules are traditionally subdivided into two main classes: primary granules (also called myeloperoxidase-positive or azurophil granules) and secondary granules (also termed peroxidase-negative or specific granules) (41). In the present study, mobilization of neutrophil granules coincided with up-regulation of cell surface PAR-2. Experiments aimed at determining which granule types harbor PAR-2 yielded mobilization profiles matching those of primary granules (37). These experiments thus show that PAR-2 is present in a preformed state in the granules of neutrophils and that PAR-2 can be mobilized readily in response to cell activation, thereby up-regulating its cell surface expression.
The up-regulation of PAR-2 observed in this study, specifically in response to Bop, points either to the involvement of receptors binding the Fc portion of IgG (FcγRs) or to CRs. Neutrophils express FcγRIIa (CD32A) and FcγRIIIb (CD16B) and CRs CR1 (CD35), CR3 (CD11b/CD18), CR4 (CD11c/CD18) and C5aR (CD88). CR1, CR3, and CR4 recognize the C3b fragment, while C5aR binds to C5a(42,43,44,45). In this study, aggregated IgGs (specific activator of FcγRs) closely mimicked the stimulatory effect obtained with Bop on PAR-2 surface expression, while blocking FcγRs effectively prevented PAR-2 up-regulation. Blocking either FcγRIIa or FcγRIIIb was almost as effective at preventing PAR-2 up-regulation as blocking both simultaneously. The reason for this remains unclear but may indicate a requirement for bridging between the receptors in order to obtain proper signal transduction. Experiments are underway specifically to address this point. However, complement fragments C3b and C5a have no significant effect on PAR-2 expression, and CR blockade had no effect on Bop-induced PAR-2 up-regulation. Taken together, these observations clearly identify FcγRs and not CRs as being pivotal mediators of PAR-2 up-regulation in response to Bop. Monosodium urate (MSU) crystals, the etiological agent of gouty arthritis, are particles that preferably bind complement fragments rather than IgGs when opsonized (46). In our experiments, MSU crystals failed to up-regulate PAR-2 cell surface expression in neutrophils, which is consistent with CRs not being involved.
The mediation of phagocytosis of opsonized particles by neutrophils has been attributed essentially to two distinct classes of receptors, namely FcγRs and CRs (47, 48). In the present study, engagement of FcγRs was necessary for increased cell responsiveness following specific activation of PAR-2. Indeed, neutrophils became more sensitive to PAR-2 activation as levels of surface PAR-2 increased. In contrast, cells remained unresponsive to activation of PAR-1, 3, and 4 by thrombin and their response to IL-8 (a well-characterized chemokine that signals through its own 7-membrane-domain G-coupled receptors CXCR1 and CXCR2) persisted under all tested conditions. These results clearly identify FcγRs as key controllers for adjusting neutrophil sensitivity toward the proteinase system. In the course of inflammation, neutrophils can come into contact with a wide range of proteinases secreted by resident cells or by pathogens (49, 50). Moreover, neutrophils contain many proteinases in different types of granules, which could act on surrounding cells or on their own PARs in an autocrine manner (3, 51,52,53). Under conditions in which neutrophils express high levels of PAR-2 as a result of granule fusion with the cell membrane, they can be exposed to PAR-2-activating proteinases such as elastase (54). The effect of such proteinases on the activation status of PAR-2 in our system remains unclear and is under investigation at this time. Nonetheless, as our experiments suggest, PAR-2 certainly retains some capacity for activation, either by trypsin or by its specific agonist SLIGKV. In previous studies, PAR-2 activation by SLIGKV or trypsin has been linked to an increase in intracellular calcium levels (18, 55). The apparent requirement for engaging FcγRs suggests that neutrophils must come into direct contact with Ig-coated particles (e.g., Bop) in order to become optimally responsive to the proteinase system.
In conclusion, our observations indicate that PAR-2 is the only functional PAR subtype in human neutrophils and that its surface expression and effect on cell responsiveness can be increased rapidly through stimulation of the Fcγ class of receptors. Taken together, these results unravel the mystery surrounding a pivotal regulatory checkpoint in responsiveness toward the proteinase system. Consequences of this sensitization on neutrophil functions are currently under investigation in our laboratory.
Acknowledgments
This work is supported by grants from the Canadian Institutes of Health Research (CIHR, MOP-64315; M.P.) and the U.S. National Institutes of Health (R01 AR052614). M.P. is a Senior Investigator Scholar of the Fonds de la Recherche en Santé du Québec (FRSQ). M.S.O. is the recipient of a studentship from the FRSQ. The authors declare no conflicting financial interests.
References
- Coughlin S. R. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- Ossovskaya V. S., Bunnett N. W. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- Uehara A., Sugawara Y., Sasano T., Takada H., Sugawara S. Proinflammatory cytokines induce proteinase 3 as membrane-bound and secretory forms in human oral epithelial cells and antibodies to proteinase 3 activate the cells through protease-activated receptor-2. J Immunol. 2004;173:4179–4189. doi: 10.4049/jimmunol.173.6.4179. [DOI] [PubMed] [Google Scholar]
- Sekiguchi F. Development of agonists/antagonists for protease-activated receptors (PARs) and the possible therapeutic application to gastrointestinal diseases. Yakugaku Zasshi. 2005;125:491–498. doi: 10.1248/yakushi.125.491. [DOI] [PubMed] [Google Scholar]
- Nakanishi-Matsui M., Zheng Y. W., Sulciner D. J., Weiss E. J., Ludeman M. J., Coughlin S. R. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- Steinhoff M., Buddenkotte J., Shpacovitch V., Rattenholl A., Moormann C., Vergnolle N., Luger T. A., Hollenberg M. D. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005;26:1–43. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Camerer E. PARticipation in inflammation. J Clin Invest. 2003;111:25–27. doi: 10.1172/JCI17564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N., Wallace J. L., Bunnett N. W., Hollenberg M. D. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci. 2001;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- Houle S., Papez M. D., Ferazzini M., Hollenberg M. D., Vergnolle N. Neutrophils and the kallikrein-kinin system in proteinase-activated receptor 4-mediated inflammation in rodents. Brit J Pharmacol. 2005;146:670–678. doi: 10.1038/sj.bjp.0706371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N. Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J Immunol. 1999;163:5064–5069. [PubMed] [Google Scholar]
- Garcia J. G., Patterson C., Bahler C., Aschner J., Hart C. M., English D. Thrombin receptor activating peptides induce Ca2+ mobilization, barrier dysfunction, prostaglandin synthesis, and platelet-derived growth factor mRNA expression in cultured endothelium. J Cell Physiol. 1993;156:541–549. doi: 10.1002/jcp.1041560313. [DOI] [PubMed] [Google Scholar]
- Ferrell W. R., Lockhart J. C., Kelso E. B., Dunning L., Plevin R., Meek S. E., Smith A. J., Hunter G. D., McLean J. S., McGarry F., Ramage R., Jiang L., Kanke T., Kawagoe J. Essential role for proteinase-activated receptor-2 in arthritis. J Clin Invest. 2003;111:35–41. doi: 10.1172/JCI16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeliger S., Derian C. K., Vergnolle N., Bunnett N. W., Nawroth R., Schmelz M., Von Der Weid P. Y., Buddenkotte J., Sunderkotter C., Metze D., Andrade-Gordon P., Harms E., Vestweber D., Luger T. A., Steinhoff M. Proinflammatory role of proteinase-activated receptor-2 in humans and mice during cutaneous inflammation in vivo. FASEB J. 2003;17:1871–1885. doi: 10.1096/fj.02-1112com. [DOI] [PubMed] [Google Scholar]
- Hyun E., Andrade-Gordon P., Steinhoff M., Vergnolle N. Protease-activated receptor-2 activation: a major actor in intestinal inflammation. Gut. 2008;57:1222–1229. doi: 10.1136/gut.2008.150722. [DOI] [PubMed] [Google Scholar]
- De Boer W. I., Yao H., Rahman I. Future therapeutic treatment of COPD: struggle between oxidants and cytokines. Int J Chron Obstruct Pulmon Dis. 2007;2:205–228. [PMC free article] [PubMed] [Google Scholar]
- Lynch J. P., 3rd, Flint A., Miller D. M., Fantone J. C. Noncaseating pulmonary granulomas associated with small cell carcinoma of the lung. Amer J Med. 1985;78:691–696. doi: 10.1016/0002-9343(85)90416-4. [DOI] [PubMed] [Google Scholar]
- Sawyer D. W., Donowitz G. R., Mandell G. L. Polymorphonuclear neutrophils: an effective antimicrobial force. Rev Infect Dis. 1989;11(Suppl. 7):S1532–S1544. doi: 10.1093/clinids/11.supplement_7.s1532. [DOI] [PubMed] [Google Scholar]
- Howells G. L., Macey M. G., Chinni C., Hou L., Fox M. T., Harriott P., Stone S. R. Proteinase-activated receptor-2: expression by human neutrophils. J Cell Sci. 1997;110(Pt. 7):881–887. doi: 10.1242/jcs.110.7.881. [DOI] [PubMed] [Google Scholar]
- Wang H., He S. Induction of lactoferrin and IL-8 release from human neutrophils by tryptic enzymes via proteinase activated receptor-2. Cell Biol Int. 2006;30:688–697. doi: 10.1016/j.cellbi.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Shpacovitch V. M., Varga G., Strey A., Gunzer M., Mooren F., Buddenkotte J., Vergnolle N., Sommerhoff C. P., Grabbe S., Gerke V., Homey B., Hollenberg M., Luger T. A., Steinhoff M. Agonists of proteinase-activated receptor-2 modulate human neutrophil cytokine secretion, expression of cell adhesion molecules, and migration within 3-D collagen lattices. J Leuk Biol. 2004;76:388–398. doi: 10.1189/jlb.0503221. [DOI] [PubMed] [Google Scholar]
- Shpacovitch V. M., Seeliger S., Huber-Lang M., Balkow S., Feld M., Hollenberg M. D., Sarma V. J., Ward P. A., Strey A., Gerke V., Sommerhoff C. P., Vergnolle N., Steinhoff M. Agonists of proteinase-activated receptor-2 affect transendothelial migration and apoptosis of human neutrophils. Exp Dermatol. 2007;16:799–806. doi: 10.1111/j.1600-0625.2007.00605.x. [DOI] [PubMed] [Google Scholar]
- Pouliot M., Fiset M. E., Masse M., Naccache P. H., Borgeat P. Adenosine up-regulates cyclooxygenase-2 in human granulocytes: impact on the balance of eicosanoid generation. J Immunol. 2002;169:5279–5286. doi: 10.4049/jimmunol.169.9.5279. [DOI] [PubMed] [Google Scholar]
- Rosales C., Brown E. J. Two mechanisms for IgG Fc-receptor-mediated phagocytosis by human neutrophils. J Immunol. 1991;146:3937–3944. [PubMed] [Google Scholar]
- Dussault A. A., Pouliot M. Rapid and simple comparison of messenger RNA levels using real-time PCR. Biol Proced Online. 2006;8:1–10. doi: 10.1251/bpo114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu A. D., Paquin R., Rathanaswami P., McColl S. R. Nuclear signaling in human neutrophils Stimulation of RNA synthesis is a response to a limited number of proinflammatory agonists. J Biol Chem. 1992;267:426–432. [PubMed] [Google Scholar]
- Pouliot M., McDonald P. P., Borgeat P., McColl S. R. Granulocyte/macrophage colony-stimulating factor stimulates the expression of the 5-lipoxygenase-activating protein (FLAP) in human neutrophils. J Exp Med. 1994;179:1225–1232. doi: 10.1084/jem.179.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishima S., Hoffman A. R., Vu T., Kim K. J., Zheng H., Daniel D., Kim Y., Wallace E. F., Larrick J. W., Raffin T. A. Regulation of neutrophil interleukin 8 gene expression and protein secretion by LPS, TNF-alpha, and IL-1 beta. J Cell Physiol. 1993;154:478–485. doi: 10.1002/jcp.1041540305. [DOI] [PubMed] [Google Scholar]
- Williams C., Mehrian Shai R., Wu Y., Hsu Y. H., Sitzer T., Spann B., McCleary C., Mo Y., Miller C. A. Transcriptome analysis of synaptoneurosomes identifies neuroplasticity genes overexpressed in incipient Alzheimer’s disease. PloS One. 2009;4:e4936. doi: 10.1371/journal.pone.0004936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movat H. Z., Cybulsky M. I., Colditz I. G., Chan M. K., Dinarello C. A. Acute inflammation in gram-negative infection: endotoxin, interleukin 1, tumor necrosis factor, and neutrophils. Fed Proc. 1987;46:97–104. [PubMed] [Google Scholar]
- Shanley T. P., Warner R. L., Ward P. A. The role of cytokines and adhesion molecules in the development of inflammatory injury. Mol Med Today. 1995;1:40–45. doi: 10.1016/1357-4310(95)80019-0. [DOI] [PubMed] [Google Scholar]
- Selvatici R., Falzarano S., Mollica A., Spisani S. Signal transduction pathways triggered by selective formylpeptide analogues in human neutrophils. Eur J Pharmacol. 2006;534:1–11. doi: 10.1016/j.ejphar.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Kato T., Kitagawa S. Regulation of neutrophil functions by proinflammatory cytokines. Int J Hematol. 2006;84:205–209. doi: 10.1532/IJH97.06141. [DOI] [PubMed] [Google Scholar]
- St-Onge M., Dumas A., Michaud A., Laflamme C., Dussault A. A., Pouliot M. Impact of anti-inflammatory agents on the gene expression profile of stimulated human neutrophils: unraveling endogenous resolution pathways. PloS One. 2009;4:e4902. doi: 10.1371/journal.pone.0004902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C., Poubelle P. E., Borgeat P., Pouliot M., Naccache P. H. Crystal-induced neutrophil activation: VIII Immediate production of prostaglandin E2 mediated by constitutive cyclooxygenase 2 in human neutrophils stimulated by urate crystals. Arthritis Rheum. 2003;48:1137–1148. doi: 10.1002/art.10851. [DOI] [PubMed] [Google Scholar]
- Foster P. S., Rosenberg H. F., Asquith K. L., Kumar R. K. Targeting eosinophils in asthma. Curr Mol Med. 2008;8:585–590. doi: 10.2174/156652408785748013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath R., Nutman T. B. Identification of eosinophils in lysed whole blood using side scatter and CD16 negativity. Cytometry. 1997;30:313–316. [PubMed] [Google Scholar]
- Nanda A., Brumell J. H., Nordstrom T., Kjeldsen L., Sengelov H., Borregaard N., Rotstein O. D., Grinstein S. Activation of proton pumping in human neutrophils occurs by exocytosis of vesicles bearing vacuolar-type H+-ATPases. J Biol Chem. 1996;271:15963–15970. doi: 10.1074/jbc.271.27.15963. [DOI] [PubMed] [Google Scholar]
- Barabe F., Rollet-Labelle E., Gilbert C., Fernandes M. J., Naccache S. N., Naccache P. H. Early events in the activation of Fc gamma RIIA in human neutrophils: stimulated insolubilization, translocation to detergent-resistant domains, and degradation of Fc gamma RIIA. J Immunol. 2002;168:4042–4049. doi: 10.4049/jimmunol.168.8.4042. [DOI] [PubMed] [Google Scholar]
- Fernandes M. J., Lachance G., Pare G., Rollet-Labelle E., Naccache P. H. Signaling through CD16b in human neutrophils involves the Tec family of tyrosine kinases. J Leuk Biol. 2005;78:524–532. doi: 10.1189/jlb.0804479. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Kjeldsen L., Lollike K., Sengelov H. Granules and secretory vesicles of the human neutrophil. Clin Exper Immunol. 1995;101(Suppl. 1):6–9. doi: 10.1111/j.1365-2249.1995.tb06152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Sorensen O. E., Theilgaard-Monch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28:340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Yanai M., Kubo H., Kanda A., Sasaki H., Butler J. P. Interaction of non-adherent suspended neutrophils to complement opsonized pathogens: a new assay using optical traps. Cell Res. 2006;16:887–894. doi: 10.1038/sj.cr.7310103. [DOI] [PubMed] [Google Scholar]
- O'Shea J. J., Brown E. J., Seligmann B. E., Metcalf J. A., Frank M. M., Gallin J. I. Evidence for distinct intracellular pools of receptors for C3b and C3bi in human neutrophils. J Immunol. 1985;134:2580–2587. [PubMed] [Google Scholar]
- Berger M., Sorensen R. U., Tosi M. F., Dearborn D. G., Doring G. Complement receptor expression on neutrophils at an inflammatory site, the Pseudomonas-infected lung in cystic fibrosis. J Clin Invest. 1989;84:1302–1313. doi: 10.1172/JCI114298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk P. N., Scola A. M., Madala P., Fairlie D. P. Function, structure and therapeutic potential of complement C5a receptors. Brit J Pharmacol. 2007;152:429–448. doi: 10.1038/sj.bjp.0707332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkeltaub R., Tenner A. J., Kozin F., Ginsberg M. H. Plasma protein binding by monosodium urate crystals Analysis by two-dimensional gel electrophoresis. Arthritis Rheum. 1983;26:775–783. doi: 10.1002/art.1780260612. [DOI] [PubMed] [Google Scholar]
- Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- Indik Z. K., Park J. G., Hunter S., Schreiber A. D. The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood. 1995;86:4389–4399. [PubMed] [Google Scholar]
- Oikonomopoulou K., Hansen K. K., Saifeddine M., Tea I., Blaber M., Blaber S. I., Scarisbrick I., Andrade-Gordon P., Cottrell G. S., Bunnett N. W., Diamandis E. P., Hollenberg M. D. Proteinase-activated receptors, targets for kallikrein signaling. J Biol Chem. 2006;281:32095–32112. doi: 10.1074/jbc.M513138200. [DOI] [PubMed] [Google Scholar]
- Dulon S., Leduc D., Cottrell G. S., D'Alayer J., Hansen K. K., Bunnett N. W., Hollenberg M. D., Pidard D., Chignard M. Pseudomonas aeruginosa elastase disables proteinase-activated receptor 2 in respiratory epithelial cells. Amer J Resp Cell Mol Biol. 2005;32:411–419. doi: 10.1165/rcmb.2004-0274OC. [DOI] [PubMed] [Google Scholar]
- Roche N., Stirling R. G., Lim S., Oliver B. G., Chung K. F. Regulation of protease-activated receptor-1 in mononuclear cells by neutrophil proteases. Resp Med. 2003;97:228–233. doi: 10.1053/rmed.2003.1437. [DOI] [PubMed] [Google Scholar]
- Chin A. C., Lee W. Y., Nusrat A., Vergnolle N., Parkos C. A. Neutrophil-mediated activation of epithelial protease-activated receptors-1 and -2 regulates barrier function and transepithelial migration. J Immunol. 2008;181:5702–5710. doi: 10.4049/jimmunol.181.8.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulon S., Cande C., Bunnett N. W., Hollenberg M. D., Chignard M., Pidard D. Proteinase-activated receptor-2 and human lung epithelial cells: disarming by neutrophil serine proteinases. Amer J Respir Cell Mol Biol. 2003;28:339–346. doi: 10.1165/rcmb.4908. [DOI] [PubMed] [Google Scholar]
- Pham C. T. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- Knecht W., Cottrell G. S., Amadesi S., Mohlin J., Skaregarde A., Gedda K., Peterson A., Chapman K., Hollenberg M. D., Vergnolle N., Bunnett N. W. Trypsin IV or mesotrypsin and p23 cleave protease-activated receptors 1 and 2 to induce inflammation and hyperalgesia. J Biol Chem. 2007;282:26089–26100. doi: 10.1074/jbc.M703840200. [DOI] [PubMed] [Google Scholar]