Abstract
Lever pressing during tests for resistance to extinction and cue-induced reinstatement of cocaine seeking in rats progressively increases over the first 2 months of withdrawal. In the present report, we investigated the generality of these findings in rats trained to self-administer sucrose, a non-drug reinforcer. We also examined whether the time-dependent changes in cocaine seeking correlate with the levels of the dopamine transporter (DAT) and tyrosine hydroxylase (TH) proteins in the amygdala, nucleus accumbens, prefrontal cortex and orbitofrontal cortex. Rats were trained to self-administer cocaine (0.5 mg/kg/i.v. infusion) or 10% sucrose (0.2 ml/infusion into a liquid drop receptacle) for 10 days (6 h/day); each reward delivery was paired with a tone+light cue. Tests for cocaine seeking were conducted following 1 or 15 reward-free days. On the test day, rats were initially tested for resistance to extinction during 6–7 60-min extinction sessions in the absence of the tone–light cue, until they reached the extinction criterion of less than 15 responses/60 min. Subsequently, rats were tested for cue-induced reinstatement during a 60-min session in which each lever press led to a contingent presentation of the tone–light cue. Lever pressing during the tests for reward seeking was significantly greater on day 15 than on day 1 following withdrawal from both cocaine and sucrose self-administration training. The levels of DAT, but not TH, were greater in the prefrontal cortex of cocaine-trained rats than in sucrose-trained rats on both days 1 and 15 of withdrawal. The levels of DAT and TH in other brain areas were not altered following withdrawal from cocaine or sucrose self-administration. These data suggest that the withdrawal period can modulate reward seeking of both drug and non-drug reinforcers, and that alterations in DAT and TH levels in the brain regions examined do not mediate this effect.
Keywords: cocaine withdrawal, craving, dopamine transporter, reinstatement, relapse, sucrose, tyrosine hydroxylase, rat
INTRODUCTION
Cocaine addiction is characterized by high rates of relapse after prolonged drug withdrawal periods (Mendelson and Mello, 1996). In an attempt to explain this long-term increase in relapse, Gawin and Kleber (1986) hypothesized that during the first weeks of withdrawal human addicts become progressively more sensitive to drug-associated cues that provoke cocaine craving. Cues previously paired with cocaine taking are known to induce drug craving in abstinent individuals (Childress et al., 1992). However, several studies failed to find increases in self-reports of craving during the first month of cocaine withdrawal (Weddington et al., 1990; Satel et al., 1991). In these latter studies, however, craving induced by re-exposure to cocaine cues was not measured following drug withdrawal.
The role of conditioned drug cues in relapse to cocaine seeking can be studied in a reinstatement model in laboratory animals (Davis and Smith, 1976; Meil and See, 1996). In this model, lever presses for cocaine infusions during drug self-administration training are paired with discrete conditioned stimuli (CSs). Subsequently, lever-pressing behavior is extinguished in the absence of cocaine and the drug-paired CSs over several days. Rats are then tested for cue-induced reinstatement of drug seeking in a single session in which each lever press leads to delivery of the CSs, which serve as conditioned reinforcers during testing.
We recently used a modified version of the above reinstatement procedure to study time-dependent changes in cue-induced reinstatement following withdrawal from cocaine self-administration. In this modified procedure, rats are trained to self-administer cocaine during 10 daily sessions, in which each cocaine infusion is paired with a discrete tone–light cue. Following different withdrawal periods, lever-pressing behavior during tests for resistance to extinction (in the absence of the cue) and cue-induced reinstatement are determined on the same test day. We found that cocaine seeking during these tests progressively increases from 1 to 60 days following self-administration training. Based on these data, we speculated that craving (a motivational state elicited by drug or drug-associated stimuli) incubates following withdrawal from cocaine self-administration (Grimm et al., 2001).
In the present study, we addressed two questions regarding this `incubation' effect. The first was whether the incubation effect generalizes to a non-drug reinforcer such as sucrose. Previous studies have found that following the last exposure to non-drug rewards, conditioned responses to appetitive stimuli are either relatively constant (Catania, 1992; Riccio et al., 1992) or decay (Trowill et al., 1969) over time (but see Shalev et al., 2001). Other studies, however, have shown time-dependent increases in responsiveness to cues previously associated with aversive events in the conditioned fear (Houston et al., 1999) and the conditioned taste aversion (Richardson et al., 1984) procedures. Thus, to examine reward seeking following withdrawal from a non-drug reward, we assessed lever-pressing behavior during tests for resistance to extinction and cue-induced reinstatement following 1 and 15 days of withdrawal from sucrose self-administration.
The second question was whether the regulation of selected molecular neuroadaptations within the mesocorticolimbic dopamine (DA) reward circuit correlates with the time-dependent changes in responsiveness to cocaine cues following withdrawal from cocaine. DA is known to be involved in the conditioned rewarding effects of drug and non-drug reinforcers (Everitt et al., 1999; Jentsch and Taylor, 1999). We focused on the regulation of tyrosine hydroxylase (TH), the rate-limiting enzyme for DA synthesis, and the DA transporter (DAT), the primary mechanism for reuptake of DA from the extracellular space (Wightman and Zimmerman, 1990), because of their important roles in regulating extracellular DA levels. Previous studies have shown that repeated exposure to cocaine results in time-dependent changes in the levels of DAT in the nucleus accumbens (NAc) following drug withdrawal (Kuhar and Pilotte, 1996). There is also one report on time-dependent regulation of TH in the NAc following withdrawal from cocaine self-administration (Schmidt et al., 2001). Based on these reports, in the present study we determined the levels of DAT and TH proteins in the amygdala, nucleus accumbens, prefrontal cortex (PFC), and orbitofrontal cortex (OFC) following 1 and 15 days of withdrawal from sucrose or cocaine self-administration. The above-mentioned terminal regions of the mesocorticolimbic DA system (Swanson, 1982) were chosen because of their role in the conditioned rewarding effects of drugs and non-drug reinforcers (Everitt et al., 1999), cue-induced reinstatement (See, 2002), and extinction behavior (Neisewander et al., 2000).
In the present experiment, rats were trained to self-administer sucrose or cocaine for 10 days. Different groups of rats were then tested for resistance to extinction and cue-induced reinstatement following 1 or 15 days of reward withdrawal. The behavioral data from two of the cocaine groups were included in our previous report (Grimm et al., 2001). We found that the incubation effect of reward cues generalizes to rats with a history of sucrose self-administration, and that the time-dependent changes in cocaine seeking following withdrawal do not correlate with the levels of DAT and TH in the brain regions examined.
METHODS
Subjects and surgery
Male Long–Evans rats (Charles River, Raleigh, NC, USA; 350–400 g) were used. Buprenorphine (0.01 mg/kg) was given as an analgesic before surgery and rats were surgically implanted with intravenous catheters (Shaham and Stewart, 1995) under anesthesia (xylazine, 10 mg/kg + ketamine 100 mg/kg, i.p.). The catheter was secured to the jugular vein with a silk suture and passed subcutaneously to the top of the skull, where it was connected to a modified 22-gauge cannula (Plastics One, Roanoke, VA, USA), which was mounted to the skull with dental cement. Catheters were flushed with sterile saline every 48 h. The sucrose-trained rats also underwent the i.v. surgery. Rats were transferred to the self-administration chambers 5–7 days after surgery, where they lived for 24 h/day on a reversed 12–12 h light–dark cycle, with food and water freely available. The procedures followed the NIH guidelines for animal care. As mentioned, data from two of the cocaine-trained groups were previously presented (Grimm et al., 2001), but all of the experimental groups described below were run during the same time-period.
Apparatus
The self-administration boxes, controlled by a Med Associates (Georgia, VT, USA) system, had two levers located 9 cm above the floor, but only one lever (an active, retractable lever) activated the infusion pump. Presses on the other lever (an inactive, stationary lever) were also recorded. The modified cannula on the rat's skull was connected to a liquid swivel with PE-50 tubing, protected by a metal spring, which was connected to the syringe of the infusion pump. The sucrose solution was delivered into liquid drop receptacles for oral consumption (Med Associates).
Procedures
The experiment included three phases. During the training phase (10 days), rats were trained to lever press for cocaine or sucrose. During the withdrawal period (1 or 15 days), rats were not exposed to the drug cues or to the retractable lever. During the testing phase (over the course of 1 day), lever presses were not reinforced with cocaine or sucrose. Rats were allowed to lever press on the previously active lever for 6–7 h (`extinction of lever-pressing behavior') in the absence of the discrete tone–light cue. Rats were then tested for cue-induced reinstatement during a 1-hour session, wherein lever presses led to cue presentations.
Training phase
Rats were trained to self-administer cocaine HCl (dissolved in sterile saline; 0.5 mg/kg/infusion, i.v.; infusion volume of 0.13 ml; delivered over 1.6 s) or 10% sucrose (0.2 ml/reward delivery into liquid drop receptacle). Training was conducted during two 3-h daily sessions, separated by 3 h, for 10 days, under a continuous reinforcement schedule (each lever press is reinforced), with a 40-s time-out after each earned reward. Each session began with the insertion of the active lever and the illumination of a red houselight that remained on for the entire session. A 5-second tone (2900 Hz, 20 dB above background)–light (7.5 W white light above the active lever) discrete compound cue accompanied each reward delivery. At the end of each session, the houselight was turned off and the active lever retracted. The number of rewards earned was limited to 40 per 3 h.
Withdrawal phase
At the end of the training phase, two cocaine-trained and two sucrose-trained groups (n=9–11 per group) were assigned to one of the reward-withdrawal periods (1 or 15 days). Cocaine-trained rats were disconnected from the swivels and all rats were left undisturbed in the self-administration boxes, with the exception of their daily handling.
Testing phase: Extinction of lever-pressing behavior
On the test day, the cocaine-trained rats were reconnected to the liquid swivels and all rats were given 6–7 1-h extinction sessions that were separated by 10 min until they reached an extinction criterion of less than 15 responses/hour on the previously active lever. The tone–light discrete cue was not present during these sessions. Each 1-h session began with the introduction of the active lever and illumination of the houselight. At the end of each session, the houselight was turned off and the active lever was retracted.
Testing phase: Cue-induced reinstatement
The test for cue-induced reinstatement consisted of a 1-h session wherein responses on the previously active lever led to the presentation of the tone–light cue on a continuous reinforcement schedule with a 40-s time-out. This session started 10 min after the last 1-h extinction session. A single non-contingent (priming) presentation of the cue was given 30 s into the session because, toward the end of extinction, several rats did not approach the active lever at the start of the 1-h sessions. In a final 1-h session, rats were given another extinction session in the absence of the reward-paired tone–light cue.
Immunoblotting of DAT and TH proteins
Six rats were randomly selected from each group for the molecular assays. Two additional sucrose-trained and two further cocaine-trained groups (n=6–8 per group) were trained as described above and tested on day 1 or 15 of withdrawal, but were not exposed to the tone–light cue. These groups served as control conditions for assessing acute changes in DAT and TH levels in the sucrose- and cocaine-trained groups exposed to the discrete cue during the 1-h test for cue-induced reinstatement. Immediately after the final 1-h test session, rats were rapidly decapitated and brains were frozen by immersion into −40° isopentane. One-millimeter-thick coronal brain sections were cut on a cryostat at the level of the NAc (core and shell; +1.7 from bregma), the amygdala (central and basolateral, −2.3 from bregma), and the PFC and the OFC (+3.7 from bregma) (Paxinos and Watson, 1998). Bilateral micropunches, using 14- (NAc, PFC, OFC) or 12- (amygdala) gauge needles, were collected and stored at −70°C. The tissue punches were homogenized in 1% sodium dodecyl sulfate (SDS), and protein concentrations were assayed using the BCA assay (Pierce Chemical Company; Rockford, IL, USA). Protein concentrations were equalized by diluting with 1% SDS. Samples were subjected to SDS-polyacrylamide gel electrophoresis (10% acrylamide/0.27% N,N′-methylenebisacrylamide resolving gel) for 3 h at 150 V and transferred electrophoretically to Immobilon-P transfer membranes (Millipore Corp; Bedford, MA, USA) at 0.3 A for 2 h. Immunoblotting for the DAT and TH proteins was performed using procedures previously described (Hope et al., 1994). The antibodies used were anti-DAT (1:1000 dilution; a gift from Dr G. Uhl, NIDA/IRP, Baltimore, USA), anti-TH (1:10 000; Diasorin, Stillwater, MN, USA), and actin (1:10 000; Chemicon, Temecula, CA, USA). The enhanced chemiluminescence signals were detected and quantified using a Fluor-S MultiImager (Biorad; Hercules, CA, USA). Results were interpolated from standard curves, using increasing levels of known amounts of total protein. Values of DAT and TH were divided by actin values for each sample, to yield a ratio value.
Statistical analyses
Data from the extinction sessions and tests for reinstatement were analyzed separately for total non-reinforced responses on the previously active lever and responses on the inactive lever. These data were analyzed separately for the cocaine-and sucrose-trained rats. Data were analyzed with ANOVAs, using the appropriate within- and between-subject factors (see Results section). Data from the Western blot assays were analyzed with ANOVA. Post-hoc analyses were done with a Fisher PLSD test (two-tailed) and significant differences are reported for P<0.05.
RESULTS
Figure 1 presents the data from the training phase of the experiment for all rats that were trained to self-administer cocaine or sucrose. Rats demonstrated reliable cocaine and sucrose self-administration behavior and no significant differences were observed within the sucrose and cocaine conditions between the groups tested following 1 or 15 days of reward withdrawal (Ps>0.5). In addition, within each reward condition (sucrose or cocaine), there were no differences between the experimental rats tested for cue-induced reinstatement and the control rats that were added for the biochemical measures and were not tested for cue-induced reinstatement (P>0.5). As expected, the statistical analyses revealed significant main effects of Training Day [F(1,26)=9.6, and F(1,32)=5.2, P>0.01, for the sucrose and cocaine groups, respectively]. The number of responses on the inactive lever was low during the 10 days of training (less than 5 presses/3 h; data not shown).
FIGURE 1.
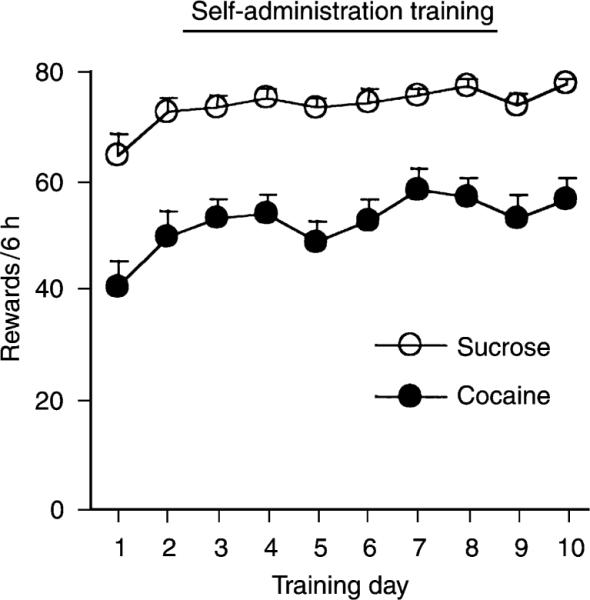
Cocaine and sucrose self-administration behavior during the training phase. Mean (± SEM) daily number of earned cocaine (0.5 mg/kg/infusion) and 10% sucrose (0.2 ml) rewards, during the 10 days of self-administration training. Rats were trained for two 3-h sessions/day, separated by 3 h (n=36 and 30 for cocaine and sucrose, respectively).
Extinction of lever-pressing behavior
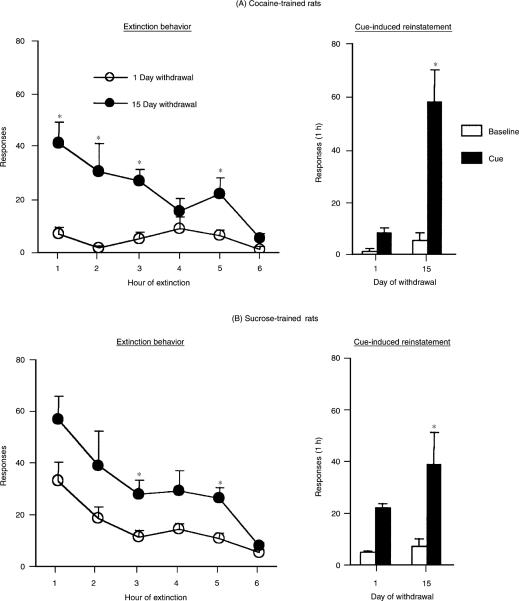
Figure 2 (left panels) shows the responses on the previously active lever in the absence of the discrete tone–light cue, during the first 60-min sessions of extinction, for the cocaine- and sucrose-trained rats following 1 or 15 days of reward withdrawal. Data were analysed with a mixed-model ANOVA, using the between-subjects factor of Withdrawal Period (1 versus 15 days) and the within-subjects factor of Test Session (the first six 60-min sessions during which all rats from all groups were exposed to the extinction conditions). The number of active lever presses during extinction was higher following 15 days than 1 day of withdrawal from cocaine or sucrose, an effect that was most pronounced during the first 60-min of extinction. As expected, regardless of the duration of the reward withdrawal period, rate of responding decreased over time.
FIGURE 2.
Time-dependent changes in resistance to extinction behavior and cue-induced reinstatement following withdrawal from cocaine (A) or sucrose (B). Mean (± SEM) number of responses on the previously active lever during the first six 60-min sessions of extinction [conducted in the absence of the discrete tone–light cue previously paired with cocaine infusions (A) or sucrose presentations (B)] and during the test for cue-induced reinstatement, which followed the extinction sessions. During the test for cue-induced reinstatement, lever presses led to presentations of the tone–light cue. Baseline: The last 60-min extinction session on which the rats reached the extinction criterion prior to the test for cue-induced reinstatement. *Significantly different from day1 withdrawal (Fisher PLSD test, P<0.05), n=9–11 per group. Data only include rats tested for both extinction responding and cue-induced reinstatement.
Cocaine-trained rats
The statistical analysis revealed significant effects of Withdrawal Period [F(1,18)=20.3, P<0.001], Test Session [F(1,18)=20.3, P<0.001] and Withdrawal Period by Test Session [F[1,18]=16.9, P<0.01]. Post-hoc group differences are indicated on Figure 2A. Inactive lever responses were low (less than 5/60-min session) and no group differences were observed (P>0.05; data not shown).
Sucrose-trained rats
The statistical analysis revealed significant effects of Withdrawal Period [F(1,16)=7.7, P<0.05] and Test Session [F(1,16)=28.9, P<0.01). These data are presented in Figure 2B. Inactive lever responses were low (less than 5/60-min session) and no group differences were observed (P>0.05; data not shown).
Cue-induced reinstatement
Figure 2 (right panels) also shows the responses on the previously active lever during the 60-min test session for cue-induced reinstatement, during which lever presses led to the presentation of the tone–light cue previously paired with earned cocaine or sucrose. The data used for the analyses were the total non-reinforced responses from the last 60-min extinction session, during which rats reached the extinction criterion (baseline condition), and the responses made during the 1-h test for cue-induced reinstatement. A mixed-model ANOVA was conducted, using the between-subjects factor of Withdrawal Period (1 versus 15 days) and the within-subjects factor of Test Session (baseline versus cue). The number of active lever presses during the test for cue-induced reinstatement was higher following 15 days than 1 day of withdrawal from cocaine or sucrose.
Cocaine-trained rats
The statistical analysis revealed significant effects of Withdrawal Period [F(1,18)=19.8, P<0.001], Test Session [F(1,18)=25.9, P<0.001] and Withdrawal Period by Test Session [F(1,18)=15.1, P<0.01]. No differences were observed for responses on the inactive lever (P values >0.05), which were very low in the baseline and cue conditions (less than 5/60 min; data not shown). Finally, lever presses during the final 60-min session in the absence of the tone–light were similar to those of the baseline session (data not shown). Post-hoc group differences are indicated in Figure 2A.
Sucrose-trained rats
The statistical analysis revealed significant effects of Withdrawal Period [F(1,16)=7.0, P<0.05], Test Session [F(1,16)=74.0, P<0.001] and Withdrawal Period by Test Session [F(1,16)=6.5, P<0.05]. No differences were observed for responses on the inactive lever (Ps>0.05), which was very low in the baseline and cue conditions (less than 5/60 min; data not shown). Finally, lever presses during the final 60-min session in the absence of the tone–light were similar to those of the baseline session (data not shown). Post-hoc group differences are indicated in Figure 2B.
DAT and TH protein levels
The data from the immunoblotting assays are presented in Table 1. Initial analysis revealed no differences in DAT or TH levels between the rats from the sucrose- and cocaine-trained groups that were exposed to the tone–light cue during the test for reinstatement and the control groups that were not exposed to the cue. Therefore, the data from these groups were pooled. Subsequent ANOVAs were conducted for each brain area and protein, using Reward Type (cocaine versus sucrose) and Withdrawal Period (day 1 versus 15) as the between-subjects factors. Within each brain area, there were no significant differences between days 1 or 15 of withdrawal from cocaine or sucrose. The statistical analyses revealed a significant increase in DAT in the PFC from rats that had self-administered cocaine versus that in rats that self-administered sucrose [Reward Type, F(1,40)=7.5, P<0.01]. No other significant effects were found.
TABLE 1.
Dopamine transporter (DAT) and tyrosine hydroxylase (TH) protein levels in the nucleus accumbens, amygdala, prefrontal cortex and orbitofrontal cortex following 1 or 15 days of withdrawal from sucrose or cocaine self-administration
| Sucrose withdrawal |
Cocaine withdrawal |
|||
|---|---|---|---|---|
| Day 1 | Day 15 | Day 1 | Day 15 | |
| DAT | ||||
| Nucleus accumbens | 5.62 ± 0.52 | 6.01 ± 1.08 | 5.79 ± 0.91 | 5.21 ± 0.86 |
| Amygdala | 3.58 ± 0.55 | 3.09 ± 0.43 | 3.89 ± 0.62 | 3.69 ± 0.52 |
| Prefrontal cortex | 3.85 ± 0.32 | 3.99 ± 0.53 | 5.49 ± 0.84* | 5.47 ± 0.52* |
| Orbitofrontal cortex | 4.57 ± 0.57 | 4.83 ± 0.35 | 5.67 ± 0.66 | 5.28 ± 0.36 |
| TH | ||||
| Nucleus accumbens | 0.95 ± 0.13 | 0.99 ± 0.19 | 0.88 ± 0.12 | 1.18 ± 0.28 |
| Amygdala | 1.01 ± 0.14 | 0.80 ± 0.12 | 1.03 ± 0.14 | 1.13 ± 0.16 |
| Prefrontal cortex | 0.77 ± 0.06 | 0.67 ± 0.09 | 0.92 ± 0.09 | 0.81 ± 0.04 |
| Orbitofrontal cortex | 4.14 ± 0.58 | 4.10 ± 0.32 | 4.11 ± 0.47 | 4.41 ± 0.50 |
Data are presented as the ratio of values for each protein measurement divided by the actin value for that sample (means ± SEMs).
Significantly different from the sucrose-trained groups (P <0.01).
DISCUSSION
We recently found that resistance to extinction and cue-induced reinstatement of cocaine seeking in rats progressively increase over the first 2 months of withdrawal from cocaine (Grimm et al., 2001). Based on these data we speculated that cocaine craving incubates following withdrawal (Grimm et al., 2001). Here we studied the generality of these findings to rats trained to self-administer sucrose, a non-drug reward. We also studied whether the time-dependent changes in cocaine seeking following withdrawal correlate with the levels of DAT and TH proteins in the amygdala, NAc, PFC and OFC. We found that the putative incubation effect generalizes to a non-drug reinforcer, sucrose, and that the time-dependent changes in reward seeking are not associated with alterations in DAT and TH protein levels in the brain areas examined. These findings are discussed below.
Time-dependent changes in sucrose and cocaine seeking following withdrawal
Lever presses during extinction and cue-induced reinstatement tests on day 15 of withdrawal from sucrose and cocaine were higher than on day 1. In addition, lever-pressing behavior during tests for extinction (in the absence of the discrete tone–light cue) and cue-induced reinstatement follow a similar time course and were highly correlated for both sucrose- and cocaine-trained rats. On day 15 the Pearson product–moment correlation coefficients for responding during extinction and cue-induced reinstatement were r=0.84 and 0.66 in the cocaine-and sucrose-trained rats, respectively (Ps<0.01). This high correlation is not surprising because, while the discrete tone–light cue was not presented during the initial extinction sessions, other conditioned cues – predictive of cocaine availability (houselight, lever insertion) – were present, and thus could have provoked differential cocaine seeking at the different withdrawal periods (McFarland and Ettenberg, 1997; Crombag and Shaham, 2002).
Based on data from previous studies from the learning literature, the time-dependent changes in reward seeking following withdrawal from sucrose self-administration training were unexpected. There are many reports that after the last exposure to non-drug rewards, conditioned responses to appetitive stimuli are relatively constant or decay over time (Trowill et al., 1969; Riccio et al., 1992). However, the present data on increases in sucrose seeking following withdrawal are in agreement with our initial observation that following 6 days of withdrawal from sucrose, responding during extinction (in the presence of the discrete CSs) was higher than that following 1 day (Shalev et al., 2001). Other studies also have shown time-dependent `incubation' of the response to cues associated with aversive events (Richardson et al., 1984; Houston et al., 1999).
The difference in responsiveness to the reward-associated cues on day 1 versus day 15 of withdrawal was more pronounced in cocaine-trained than in sucrose-trained rats. The reasons for the larger `incubation' effect with cocaine-trained rats are not known, and they may be related to the differences in response rates for the rewards during training (see Figure 1). The difference in the magnitude of incubation between the reward conditions was largely due to the very low responding on day 1 of withdrawal from cocaine (Figure 2A). It is possible that on day 1 of cocaine withdrawal rats were experiencing `anhedonia' (Markou and Koob, 1991), and thus were less responsive to cocaine-associated cues (Arroyo et al., 1998). Another possibility is that early in withdrawal the memory for the `anxiogenic' effects of cocaine (see Ettenberg and Geist, 1991) is greatest, and consequently the rats are avoiding the lever when cocaine itself is not available.
Extinction responding of the cocaine-trained rats on day 1 of withdrawal was lower than that typically observed in cocaine self-administration studies. This unusually low level of extinction responding (Figure 2A) is due in part to extinction of lever pressing being performed in the absence of the cocaine-paired tone–light cue, which influences cocaine self-administration behavior under simple (Schenk and Partridge, 2001) and complex (Goldberg, 1976) reinforcement schedules. However, this explanation cannot explain why response rates on day 1 of cocaine withdrawal (Figure 2A) were lower than those in previous studies of See and colleagues, in which extinction of lever pressing was also conducted in the absence of the tone–light cue (See, 2002). A likely possibility for this difference is that our rats self-administered higher amounts of cocaine in the 6 h/day sessions than in the 3 h/day self-administration sessions in the previous studies, and therefore were more likely to experience `anhedonia' (Markou and Koob, 1991) and `anxiety' (Sarnyai et al., 2001) during early withdrawal, both of which may decrease cocaine seeking (see above).
The time-dependent changes in cocaine seeking following withdrawal have temporal characteristics that, to some degree, resemble the time-dependent changes in the expression of locomotor sensitization to psychostimulants in response to a drug challenge following withdrawal (see Paulson et al., 1991), a phenomenon dependent on DA neurotransmission (Robinson and Berridge, 1993). Robinson and Berridge (1993) hypothesized that enhanced DA neurotransmission following repeated drug exposure and withdrawal sensitizes putative incentive motivational processes, which in turn lead to increased responsiveness to drug and drug-associated cues. However, while the enhanced responsiveness to cocaine cues following withdrawal is in agreement with predictions of the `incentive sensitization' model, it is not clear whether the present data support this theory. Specifically, sucrose self-administration, which has not been shown to enhance DA neuro-transmission, also resulted in time-dependent changes in reward seeking following withdrawal.
DAT and TH levels in terminal DAergic regions following withdrawal from cocaine and sucrose
In this initial molecular characterization of the incubation phenomenon, we did not find significant time-dependent alterations in DAT protein levels in the NAc, amygdala, PFC or OFC following 1 or 15 days of reward withdrawal. Although not regulated in a time-dependent manner, DAT levels in the PFC of cocaine-trained rats were increased following 1 and 15 days of withdrawal. This increase in DAT protein levels may be related to the attenuated acute cocaine-induced increase in extracellular DA levels in the PFC, as well as increased DA clearance in this brain area following repeated cocaine exposures (Meiergerd et al., 1997; Sorg et al., 1997). Such an effect may be relevant to the expression of locomotor sensitization to cocaine following withdrawal (Sorg et al., 1997), but it is clearly not associated with the time-dependent changes in drug-seeking described here. In addition, it should be noted that, in contrast to the present data on upregulation of the DAT protein in the PFC, previous studies have found that DAT binding levels in the PFC are either decreased (Hitri et al., 1996) or unaltered (Farfel et al., 1992) following withdrawal from non-contingent cocaine exposure. The different methods used to measure the DAT, the cocaine dose and route of exposure, as well as the use of contingent drug administration in our study, may have been responsible for the differences in DAT regulation in the PFC following withdrawal.
Significant alterations in DAT levels were not observed in any other brain region. Our data on the lack of regulation of DAT protein levels in the NAc are in agreement with other studies, where DAT levels were also not altered following cocaine withdrawal (Kula and Baldessarini, 1991; Claye et al., 1995; Hitri et al., 1996; Burchett and Bannon, 1997; Letchworth et al., 1999; Arroyo et al., 2000). However, there are previous reports that DAT binding is initially increased in the NAc (1–2 days of withdrawal), and then decreased following 7–60 days of withdrawal (Wilson et al., 1994; Kuhar and Pilotte, 1996).
TH protein levels were not altered in any brain region following 1 or 15 days of reward withdrawal. This finding agrees with previous studies showing no regulation of TH levels in the NAc following short or prolonged withdrawal from cocaine (Beitner-Johnson and Nestler, 1991; Sorg et al., 1993; Vrana et al., 1993). In contrast, Todtenkopf et al. (2000) found an increase in TH immunoreactivity in the shell of the NAc following 14, but not 2, days of withdrawal, while Trulson et al. (1987) reported a decrease in TH immunoreactivity in the NAc and frontal cortex following 2 months of withdrawal. In addition, in the one report that examined TH regulation following cocaine self-administration, Schmidt et al. (2001) found a decrease in TH levels in the NAc shell following 7 days of withdrawal. Once again, differences in cocaine doses, duration of exposure and treatment regimens (e.g. contingent versus non-contingent drug administration), as well as the method of assaying TH, may account for the different outcomes for TH levels following withdrawal.
Finally, the lack of time-dependent changes in DAT and TH levels following withdrawal in the present study does not rule out a role for DA in the time-dependent changes in cocaine (and possibly sucrose) seeking. Enhanced responsiveness to reward cues following cocaine withdrawal may still be associated with time-dependent changes in DA release in terminal regions (Rossetti et al., 1992), DA receptor supersensitivity (White and Kalivas, 1998), or DAT regulation of dendritic DA release (Falkenburger et al., 2001).
Concluding remarks
It has been suggested that relapse to cocaine taking in humans becomes increasingly under the influence of conditioned drug cues over the course of cocaine abstinence (Gawin and Kleber, 1986). In the present report and in our previous study (Grimm et al., 2001), we describe a similar phenomenon in an animal model of relapse to drugs (Stewart, 2000; Shalev et al., 2002). In our initial molecular characterization of the time-dependent changes in cocaine seeking, we found that alterations in DAT and TH protein levels in several terminal regions of the mesocorticolimbic DA system are not associated with the profound changes in behavior observed following cocaine withdrawal. Finally, the time-dependent changes in reward seeking were also observed following withdrawal from sucrose self-administration. This observation strongly suggests that changes in memory and/or motivational processes, associated with exposure to rewards in general (Balleine and Dickinson, 1998; Schultz, 2000; Hyman and Malenka, 2001), are likely to contribute to the time-dependent changes in cocaine as well as heroin (see Shalev et al., 2001) seeking following withdrawal periods.
Acknowledgements
Supported by the National Institute on Drug Abuse, Intramural Research Program.
REFERENCES
- Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacology. 1998;140:331–344. doi: 10.1007/s002130050774. [DOI] [PubMed] [Google Scholar]
- Arroyo M, Baker WA, Everitt BJ. Cocaine self-administration in rats differentially alters mRNA levels of the monoamine transporters and striatal neuropeptides. Brain Res Mol Brain Res. 2000;83:107–120. doi: 10.1016/s0169-328x(00)00205-9. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Nestler EJ. Morphine and cocaine exert common chronic actions on tyrosine hydroxylase in dopaminergic brain reward regions. J Neurochem. 1991;57:344–347. doi: 10.1111/j.1471-4159.1991.tb02133.x. [DOI] [PubMed] [Google Scholar]
- Burchett SA, Bannon MJ. Serotonin, dopamine and norepinephrine transporter mRNAs: heterogeneity of distribution and response to `binge' cocaine administration. Brain Res Mol Brain Res. 1997;49:95–102. doi: 10.1016/s0169-328x(97)00131-9. [DOI] [PubMed] [Google Scholar]
- Catania CA. Learning. Prentice-Hall; Englewood Cliffs: 1992. [Google Scholar]
- Childress AR, Ehrman R, Rohsenow DJ, Robbins SJ, O'Brien CP. Classically conditioned factors in drug dependence. In: Lowinson JW, Luiz P, Millman RB, Langard G, editors. Substance Abuse: a comprehensive textbook. Williams and Wilkins; Baltimore: 1992. pp. 56–69. [Google Scholar]
- Claye LH, Akunne HC, Davis MD, DeMattos S, Soliman KF. Behavioral and neurochemical changes in the dopaminergic system after repeated cocaine administration. Mol Neurobiol. 1995;11:55–66. doi: 10.1007/BF02740684. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Davis WM, Smith SG. Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. Pavlovian J Biol Sci. 1976;11:222–236. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Qualitative and quantitative differences in the operant runway behavior of cocaine and heroin reinforced rats. Pharmacol Biochem Behav. 1991;57:145–150. doi: 10.1016/0091-3057(93)90298-8. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala–ventral striatal subsystems. Ann NY Acad Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Falkenburger BH, Barstow KL, Mintz IM. Dendrodendritic inhibition through reversal of dopamine transport. Science. 2001;293:2465–2470. doi: 10.1126/science.1060645. [DOI] [PubMed] [Google Scholar]
- Farfel GM, Kleven MS, Woolverton WL, Seiden LS, Perry BD. Effects of repeated injections of cocaine on catecholamine receptor binding sites, dopamine transporter binding sites and behavior in rhesus monkey. Brain Res. 1992;578:235–243. doi: 10.1016/0006-8993(92)90252-5. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Goldberg SR. Stimuli associated with drug injections as events that control behavior. Pharmacol Rev. 1976;27:325–340. [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitri A, Little KY, Ellinwood EH., Jr Effect of cocaine on dopamine transporter receptors depends on routes of chronic cocaine administration. Neuropsychopharmacology. 1996;14:205–210. doi: 10.1016/0893-133X(95)00090-Z. [DOI] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, et al. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Houston FP, Stevenson GD, McNaughton BL, Barnes CA. Effects of age on the generalization and incubation of memory in the F344 rat. Learn Mem. 1999;6:111–119. [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharamacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Pilotte NS. Neurochemical changes in cocaine withdrawal. Trends Pharmacol Sci. 1996;17:260–264. doi: 10.1016/0165-6147(96)10024-9. [DOI] [PubMed] [Google Scholar]
- Kula NS, Baldessarini RJ. Lack of increase in dopamine transporter binding or function in rat brain tissue after treatment with blockers of neuronal uptake of dopamine. Neuropharmacology. 1991;30:89–92. doi: 10.1016/0028-3908(91)90047-f. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Sexton T, Childers SR, Vrana KE, Vaughan RA, Davies HM, Porrino LJ. Regulation of rat dopamine transporter mRNA and protein by chronic cocaine administration. J Neurochem. 1999;73:1982–1989. [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia: an animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- McFarland K, Ettenberg A. Reinstatement of drug-seeking behavior produced by heroin-predictive environmental stimuli. Psychopharmacology. 1997;131:86–92. doi: 10.1007/s002130050269. [DOI] [PubMed] [Google Scholar]
- Meiergerd SM, Schenk JO, Sorg BA. Repeated cocaine and stress increase dopamine clearance in the rat medial prefrontal cortex. Brain Res. 1997;773:203–237. doi: 10.1016/s0006-8993(97)00926-8. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Management of cocaine abuse and dependence. N Engl J Med. 1996;334:965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology. 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Riccio DC, Ackil J, Burch-Vernon A. Forgetting of stimulus attributes: methodological implications for assessing associative phenomena. Psychol Bull. 1992;112:433–445. doi: 10.1037/0033-2909.112.3.433. [DOI] [PubMed] [Google Scholar]
- Richardson R, Williams C, Riccio DC. Stimulus generalization of conditioned taste aversion in rats. Behav Neural Biol. 1984;41:41–53. doi: 10.1016/s0163-1047(84)90706-4. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–244. [PubMed] [Google Scholar]
- Satel SL, Price LH, Palumbo JM, McDougle CJ, Krystal JH, Gawin F, et al. Clinical phenomenology and neurobiology of cocaine abstinence: a prospective inpatient study. Am J Psychiatry. 1991;148:1712–1716. doi: 10.1176/ajp.148.12.1712. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Influence of a conditioned light stimulus on cocaine self-administration in rats. Psychopharmacology. 2001;154:390–396. doi: 10.1007/s002130000608. [DOI] [PubMed] [Google Scholar]
- Schmidt EF, Sutton MA, Schad CA, Karanian DA, Brodkin ES, Self DW. Extinction training regulates tyrosine hydroxylase during withdrawal from cocaine self-administration. J Neurosci. 2001;21:RC137. doi: 10.1523/JNEUROSCI.21-07-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin self-administration behavior in drug-free animals: An effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope BT, Yap J, Shaham Y. Time dependent changes in extinction behavior and stress-induced reinstatement of drug seeking during heroin withdrawal. Psychopharamacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Chen SY, Kalivas PW. Time course of tyrosine hydroxylase expression after behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;266:424–430. [PubMed] [Google Scholar]
- Sorg BA, Davidson DL, Kalivas PW, Prasad BM. Repeated daily cocaine alters subsequent cocaine-induced increase of extracellular dopamine in the medial prefrontal cortex. J Pharmacol Exp Ther. 1997;281:54–61. [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug-and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, De Leon KR, Stellar JR. Repeated cocaine treatment alters tyrosine hydroxylase in the rat nucleus accumbens. Brain Res Bull. 2000;52:407–411. doi: 10.1016/s0361-9230(00)00277-x. [DOI] [PubMed] [Google Scholar]
- Trowill JA, Panksepp J, Gandelman R. An incentive model of rewarding brain stimulation. Psychol Rev. 1969;76:264–281. doi: 10.1037/h0027295. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Joe JC, Babb S, Raese JD. Chronic cocaine administration depletes tyrosine hydroxylase immunoreactivity in the meso-limbic dopamine system in rat brain: quantitative light microscopic studies. Brain Res Bull. 1987;19:39–45. doi: 10.1016/0361-9230(87)90163-8. [DOI] [PubMed] [Google Scholar]
- Vrana SL, Vrana KE, Koves TR, Smith JE, Dworkin SI. Chronic cocaine administration increases CNS tyrosine hydroxylase enzyme activity and mRNA levels and tryptophan hydroxylase enzyme activity levels. J Neurochem. 1993;61:2262–2268. doi: 10.1111/j.1471-4159.1993.tb07468.x. [DOI] [PubMed] [Google Scholar]
- Weddington WW, Brown BS, Haertzen CA, Cone EJ, Dax EM, Herning RI, Michaelson BS. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled, residential study. Arch Gen Psychiatry. 1990;47:861–868. doi: 10.1001/archpsyc.1990.01810210069010. [DOI] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res Rev. 1990;15:135–144. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Nobrega JN, Carroll ME, Niznik HB, Shannak K, Lac ST, et al. Heterogeneous subregional binding patterns of 3H-WIN 35,428 and 3H-GBR 12,935 are differentially regulated by chronic cocaine self-administration. J Neurosci. 1994;14:2966–2979. doi: 10.1523/JNEUROSCI.14-05-02966.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]