Since the development of diagnostic criteria for Alzheimer’s disease (AD) nearly twenty-five years ago (McKhann et al. 1984), there has been an exponential growth in the number of published studies characterizing the clinical expression of the condition and its complex environmental and genetic determinants. With the advances in scientific discovery, there has been a push to refine the clinical diagnosis at earlier points in the illness with an eye towards eventual intervention. The question is, with all the progress made in clinical diagnosis, are we there yet? Are we able to take what is known about the clinical expression of AD and push back the diagnostic envelope to the early prodrome stage? Said differently, are we able to diagnose AD as the symptoms emerge and distinguish this condition from other similar brain disorders affecting cognition, behavior, and function in late life? The articles in this special issue tackle this important question, discussing the current understanding of the three known major neurodegenerative disorders: AD, Dementia with Lewy Bodies (DLB), and Frontotemporal Lobar Dementia (FTLD). Each article explores the unique clinical and neuropsychological characteristics of the disorders and addresses whether there is an identifiable prodromal stage in each condition.
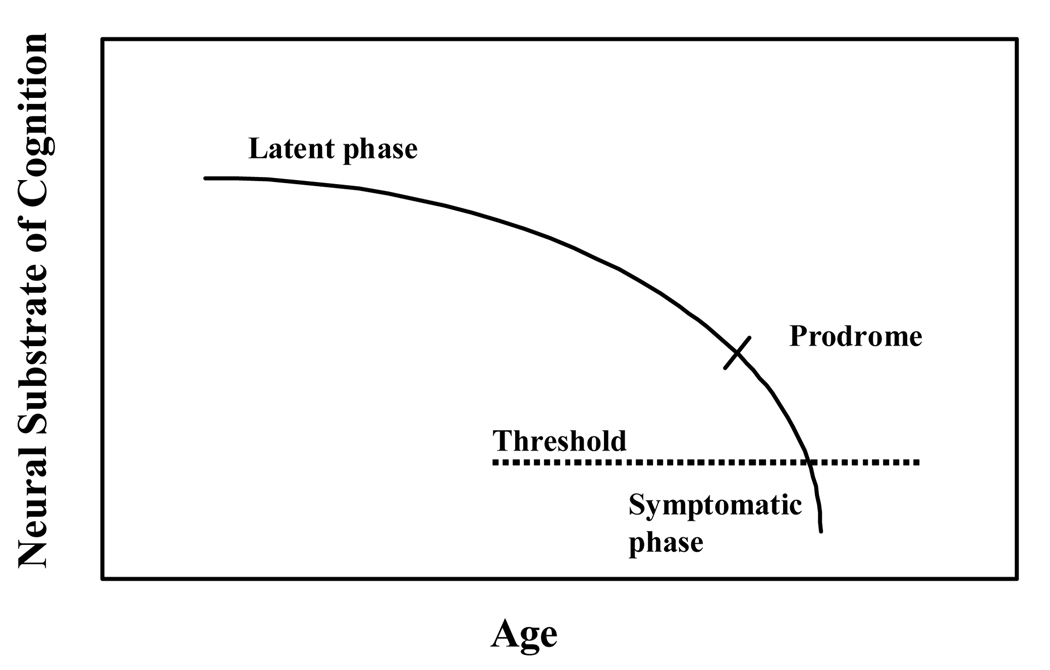
The first article by Bondi and colleagues covers the early identification of AD, illustrating how this disease process appears to follow the continuous model of disease expression, first posited by Katzman (1976). As the model suggests (Figure 1), there is a gradual decline in neurocognitive and behavioral function in AD, which is fairly predictable and appears to parallel closely the trajectory of neuropathological brain changes occurring over time in the disease (see also Welsh-Bohmer et al., 2006). In the very early stages, when the neuropathology is confined, the neuropsychological symptoms may not be apparent at all (latent phase), but as the pathology cumulates over time early symptoms emerge (prodrome stage) followed later by fully manifest clinical disease (dementia stage). The article reviews in a through and critical fashion, the burgeoning area of research on early clinical detection of disease and discusses what is now known about the prodromal neuropsychological expression of AD. Although this information alone is extremely helpful to the clinician and researcher alike, perhaps even more useful and intriguing is the careful consideration given to the effects of vascular disease and vascular risk conditions on AD symptom expression. The article considers carefully the observation that the clinical conditions of AD and VaD often co-occur (Meich et al., 2002) and that they frequently share common risk factors (Hayden et al, 2006). In considering how vascular factors may exert a role in AD symptom expression, this review poses a number of interesting hypotheses for future research, pointing the way to potential avenues for disease discovery work and for the development of clinical interventions to delay or prevent dementia onset.
Fig. 1.
Chronic Disease Model of AD
Extending the continuous model of disease to the identification of prodromes in both DLB and FTLD has conceptual appeal but is relatively untested at this point, as the authors of the next two reviews on these respective topics point out (Troster 2008; Wittenberg & colleagues, 2008). Each of these two articles considers the recent hypothesis that DLB and FTLD may have unique, dissociable clinical signatures that distinguish the early symptomatic expression of these entities from the prodrome of AD (Petersen & Morris 2006). Because the field has rapidly evolved with the introduction of disease specific diagnostic criteria for both DLB and FTLD, attention to their early disease characteristics is relatively new and reveals a whole different set of unique, diagnostic complexities. In DLB the overlap between this disorder and the closely related dementia in Parkinson’s disease (PDD) makes clinical differentiation of these two closely aligned disorders difficult. The similarity between these two dementias and AD dementia also challenges the notion that the disorders can be effectively and reliably disentangled based on neuropsychological profiles alone. Consequently, the authors suggest that while there is merit to the notion of early clinical prodromal signatures in DLB and PDD, ultimately the reliable diagnosis may rest on consideration of the neuropsychological signatures in conjunction with biomarkers specific to the underlying neuropathology.
The challenges in the identification of the prodrome in FTLD are of a different sort. Here the phenotypic expression is fairly unique and identifiable in fully expressed disease, as the prominent difficulties are not at all reminiscent of AD but rather involve impairments in behavior, personality, and language. The issue here is that these problems can often be overlooked or mistaken for other conditions, such as psychiatric problems; and currently the identification of early “prodromal” disease signatures are not as readily and reliably captured by the available neuropsychological metrics. The review of the FTLD area by Wittenberg and colleagues masterfully covers the state-of-the-art findings in this rapidly changing field and provides an encouraging perspective on how neuropsychology can contribute to the scientific direction. By applying to the study of FTLD, models of behavior developed in social psychology and constructs from personality theory, neuropsychology is in a position to advance current scientific understanding of the brain organization of complex human behaviors such as response organization, behavioral inhibition, emotional regulation, and empathy. In so doing, new tools may be developed that are better suited to the detection of the behavioral symptoms and functional disturbances that arise early in FTLD expression.
So we are back to the question originally raised at the start of this commentary: Are we there yet? Are we able to detect and diagnose patients in the early prodrome of AD, DLB, and FTLD? The answer to this question is actually neither yes nor no. In the case of AD, it is certainly true that the early clinical expression of the disease is very well understood. It is also true that a great number of early symptomatic patients may be reliably diagnosed in advance of fully manifest dementia. Hence, we might say, “yes” that we are able to diagnose the AD prodrome but we state this conclusion with some caution. It has to be noted that despite the improved resolution in early disease detection, there are many instances where the limits of diagnostic sensitivity and specificity are tested. Many patients in the AD prodrome are likely to present with atypical symptoms confounded by medical comorbidities, making early disease diagnosis ambiguous at best. Additionally, there are a number of other diagnostic scenarios where little is known regarding the variability in AD expression and information is sorely needed, such as in culturally diverse populations and in the exceptionally old (90+). Finally, it has to be said that simply because the disease is conceptualized as a continuum, does not mean that all patients with AD will inexorably pass through a discernable prodromal phase. It is entirely possible that some patients will transition very quickly from the silent stage of disease to fully expressed clinical dementia following either a major precipitant (e.g. stroke, death of spouse) or for reasons that are not yet determined. Consequently, while it is fair to say “yes” that we are able to make reliable diagnoses of early “prodromal” AD in many patients, we are not yet able to do so for all patients and that there is still much to be learned about the early disease expression in a number of understudied scenarios.
As for the newer diagnostic entities of DLB and FTLD, the identification of early prodromal states is not yet possible and naturally lags until the definition of fully expressed clinical disease becomes better defined. Given the degree of clinical heterogeneity within each disorder, it is becoming increasingly clear that the task of early clinical definition will not be an easy one, particularly if the approach relies heavily on traditional approaches and metrics. The contribution of neuropsychology in advancing the field is promising, as new hypotheses and approaches emerge building on constructs and methods from closely aligned disciplines, such as cognitive psychology, social psychology, and personality theory. By applying carefully informed approaches to the study of complex functions, such as goal directed behaviors, insight and empathy, progress is likely to be made in understanding the behaviors of DLB and FTLD that do not lend themselves readily to available metrics. This type of new information is needed and would permit refined definitions of disease expression in each condition and ultimately more precise clinical diagnoses. With this type of information and the rapid advances occurring in tandem at the neurobiological level, early “prodromal” diagnoses of AD, DLB, and FTLD are an almost certain possibility in the foreseeable future, whether these diagnoses are based on improved clinical methods alone or considered with disease specific biomarkers.
Footnotes
To this end, disease specific criteria have now been developed for DLB and FTLD, which help serve to increase the early detection of AD by reducing overall diagnostic uncertainty.
References
- Bäckman L, Jones S, Berger A, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: A meta-analysis. Neuropsychology. 2005;19:520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- for the Cache County Investigators. Hayden KM, Zandi PP, Lyketsos CG, Khachaturian A, Bastian LA, Charoonruk G, Tschanz JT, Norton MC, Pieper CF, Munger RG, Breitner JCS, Welsh-Bohmer KA. Vascular risk factors for incident Alzheimer’s disease and vascular dementia: The Cache County Study. Alzheimer’s Disease and Associated Disorders. 2006;20:93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- Katzman R. Editorial: The prevalence and malignancy of Alzheimer disease. A major killer. Archives of Neurology. 1976;33:217–218. doi: 10.1001/archneur.1976.00500040001001. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, et al. "Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease". Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- for the Cache County Study Group. Meich RA, Breitner JCS, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90’s for men, later for women: The Cache County Study. Neurology. 2002;58:209–218. doi: 10.1212/wnl.58.2.209. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Archives of Neurology. 2005;62:1160–1663. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- for the Cache County Study Group. Welsh-Bohmer KA, Breitner JCS, Hayden KM, Lyketsos C, Zandi PP, Tschanz JT, Norton MC, Munger RC. Modifying dementia risk and trajectories of cognitive decline in aging: The Cache County Memory Study. Alzheimer’s and Dementia. 2006;2:257–260. doi: 10.1016/j.jalz.2006.04.011. [DOI] [PubMed] [Google Scholar]