Abstract
Protein degradation is the cell’s mechanism of eliminating misfolded or unwanted proteins. The pathway by which proteins are degraded occurs through the ubiquitin-proteasome system. Ubiquitin is a small 9-kilodalton (kDa) protein that is attached to proteins. A minimum of four ubiquitins is required for proteins to be recognized by the degradation machinery, known as the 26S proteasome. Defects in ubiquitination have been identified in a number of diseases, including cancer, neurodegenerative diseases, and metabolic disorders. We sought to exploit the delicate balance between protein synthesis and degradation to treat cancer by designing a chimeric molecule, known as Protac (Proteolysis Targeting Chimeric molecule). Protacs are heterobifunctional nanomolecules that are approximately 10 nanometers (nm) in size and can recruit proteins that cause cancer to the ubiquitin-proteasome machinery for degradation. In this review, we discuss the development of this novel technology for the treatment of cancer.
Ubiquitin-Proteasome System
The ubiquitin-proteasome system (UPS) is a major pathway that regulates the levels of intracellular proteins and provides a fine balance between protein synthesis and degradation. In addition to protein synthesis, the UPS regulates a number of critical functions within cells, including cell cycle progression, apoptosis, protein localization, growth signaling pathways, autophagy, and deoxyribonucleotide (DNA) repair (1–3). Multi-ubiquitination of proteins with a minimum of 4 ubiquitins attached to proteins results in degradation by the 26S proteasome (4). Monoubiquitination regulates other cellular functions, e.g. transcription, localization, signal transduction, DNA repair (1,5). Recently a class of proteases known as deubiquitinating enzymes (DUBs) have been identified, which act to remove or remodel ubiquitin moieties from target proteins (6). Since the UPS plays an important role in so many functions, they are likely targets for cancer therapy.
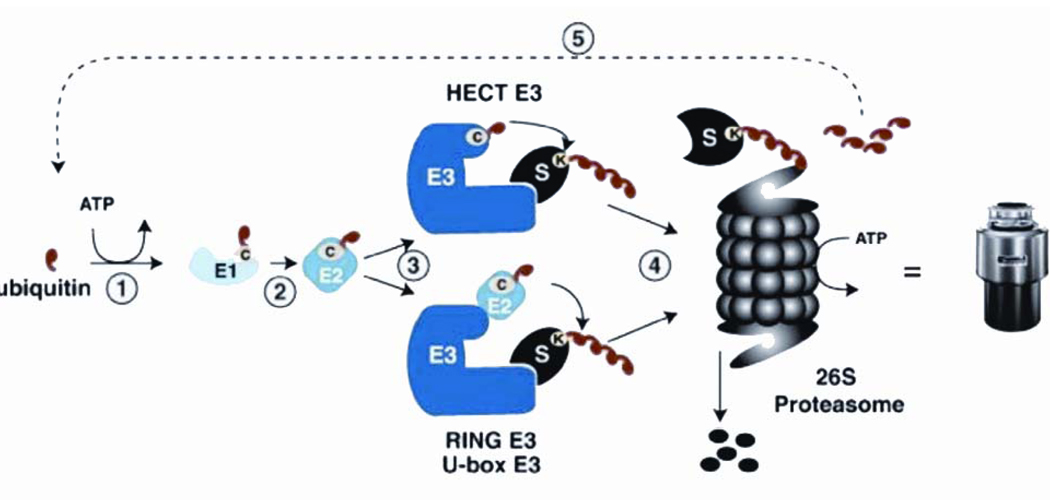
Ubiquitin is a small 9-kDa protein that consists of 76 amino acids and is conserved throughout evolution from yeast to humans (7). The UPS consists of ubiquitin, a three-enzyme complex known as the ubiquitin ligase, and the 26S proteasome (8). The components of the UPS exist in the cytoplasm and the nucleus (5,9,10) and act in a sequential manner to ubiquitinate target proteins. First, the ubiquitin activating enzyme or E1 attaches the ubiquitin to a substrate in an adenosine triphosphate (ATP)-dependent reaction. Second, the ubiquitin is transferred to the ubiquitin-conjugating enzyme or E2, which transfers the ubiquitin together with an E3 ubiquitin ligase, to the protein (Figure 1). The E3 ubiquitin ligase confers specificity to the protein target. In the human genome, there is one E1 enzyme, approximately 50 E2 enzymes, and more than 600 E3 enzymes (4,5,11,12).
Figure 1.
Ubiquitin Proteasome System. Adapted from Heuze ML et al. 2008 Blood Cells Mol Dis 40:200–210. Copyright © 2007 Elsevier Inc, with permission.
Mutations or abnormal regulation of ubiquitin ligases have been identified in cancer. In addition, several targets of E3 ubiquitin ligases are mutated or are overexpressed in tumors. Among these proteins are the tumor suppressors p53, (breast cancer1, early onset) BRCA1, cyclin inhibitor kinase inhibitor 1B (p27Kip1), and oncogenes epidermal growth factor receptor, Cyclin E, Von Hippel-Lindau, human papilloma virus protein E6-associated protein (AP), and most recently the E3 ligase Casitas B-lineage Lymphoma (c-Cbl) in myeloid leukemia (5,13). Therefore, E3 ligases are potential targets for cancer therapy.
Protacs to target cancer-causing proteins for destruction
We developed an approach to treat human disease that recruits a cancer-causing protein to an E3 ligase for subsequent ubiquitination and degradation. This technology is known as Protac or Proteolysis Targeting Chimeric Molecule. The concept was first described by Zhou and Howley in which they used a gene therapy approach to target proteins for ubiquitination and degradation (14). Zhou et al. made a fusion protein between a subunit of the ubiquitin ligase known as SCF (Skp1-Cullin-F-box protein) in yeast, cell division cycle protein 4 (Cdc4), with the binding partner of the phosphorylated Retinoblastoma (Rb) protein, known as E7N (N-terminus of the human papilloma virus E7 protein). The fusion protein recruited the pRb to the SCF ubiquitin ligase resulting in the ubiquitination and degradation of Rb (14). Unfortunately, this technology was limited by low efficiency of transduction and use of potentially harmful lentiviruses, thereby making it difficult to translate this to clinical application (14).
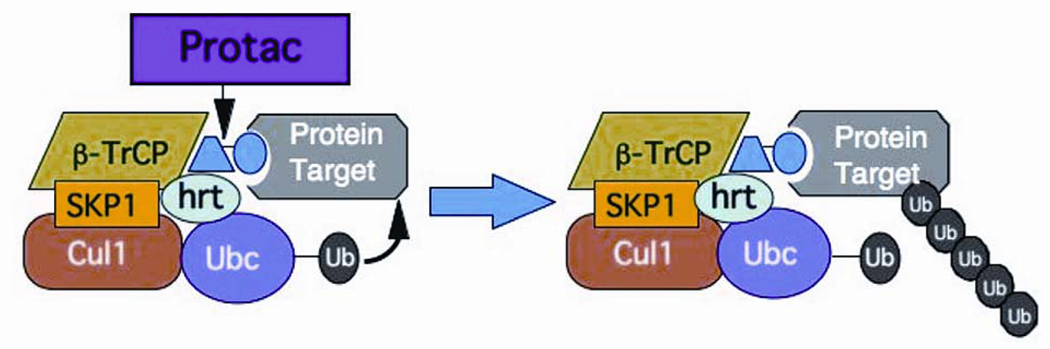
The goal of Protac technology is to create a chimeric molecule that bridges any cancer-causing protein to an E3 ligase. In terms of its structure, Protacs consist of one moiety, e.g. a peptide, which is recognized by the E3 ligase. This moiety is then chemically and covalently linked to a small molecule or a protein that recognizes the target protein. In this manner, the chimeric molecule would bring the target protein to the E3 ligase in close enough proximity for the ligase to attach ubiquitins onto the protein, thereby resulting in degradation (Figure 2). Other advantages of Protacs include the fact that it is catalytic and can be used to recruit any protein that exists in a multisubunit complex (15).
Figure 2.
Protac can theoretically target any protein for ubiquitination and degradation. In this case, the protein is recruited to the SCFβ-TRCP E3 ubiquitin ligase, resulting in destruction of the protein target.
In addition to the use of Protacs for the treatment of human disease, these molecules provide a chemical genetic approach to “knock down” proteins to study their function (16). Furthermore, Protacs are specific and do not require transfections or transduction. Protacs can be directly applied to cells or injected into animals without the use of vectors. Given the increased number of E3 ligases in the human genome, the possibilities for different combinations of Protacs that target specific disease-causing proteins to different ligases are unlimited. This review describes general strategies of testing the efficacy of Protacs using two E3 ligases as examples: SCFβ-TRCP and Von Hippel Lindau (VHL) (17,18). Three different targets will be described: methionine aminopeptidase-2 (MetAP-2), estrogen receptor (ER), and androgen receptor (AR).
Protacs target proteins that bind covalently for ubiquitination and degradation
As proof of principle, we first generated a Protac molecule that binds to the protein MetAP-2 for ubiquitination and degradation. MetAP-2 cleaves the N-terminal methionine from nascent polypeptides and is one of the targets of angiogenesis inhibitors fumagillin and ovalicin (19–21). Ovalicin binds covalently to MetAP-2 at the His-231 active site. Inhibition of MetAP-2 is thought to block endothelial cell proliferation by causing G1 arrest (22). MetAP-2 is a stable protein that is not known to be ubiquitinated or an endogenous substrate of the E3 ligase SCFβ-TRCP. For these reasons, Met-AP2 was chosen to be the initial protein to test Protacs.
The multisubunit ubiquitin ligase, SCFβ-TRCP (Skp1-Cullin-Fbox-Hrt1), was selected because the F-box protein β-TRCP/E3RS was previously shown to bind to IκBα (inhibitor of NFκBα) through a minimal phosphopeptide sequence, DRHDSGLDSM (23,24). This 10 amino acid phosphopeptide was chemically linked to ovalicin to form the Protac as previously described (25). Our results demonstrated that in vitro not only was Protac able to bind to MetAP-2, but it also induced its ubiquitination. Furthermore, proteasomes in Xenopus extracts could degrade this MetAP-2-Protac complex(25).
Protacs target proteins for ubiquitination and degradation through noncovalent interactions
The next challenge was to demonstrate that Protacs could associate cancer-causing proteins through noncovalent interactions. Steroid hormone receptors and their ligands associate through high affinity interactions. Both the estrogen receptor (ER) and androgen receptor (AR) are members of the steroid hormone receptor superfamily whose ligands (estradiol and testosterone, respectively) have been well defined and implicated in tumorigenesis. The ER has been implicated in the progression of breast cancer (26–28). Similarly, hormone-dependent prostate cancer cells grow in response to androgens (29,30). Therefore, both ER and AR are excellent targets for Protac technology. To target ER for ubiquitination and degradation, we synthesized a version of Protac containing the IκBα phosphopeptide linked to estradiol (the ligand for ER) (31). Not only was Protac able to ubiquitinate purified ER efficiently in vitro, but the ubiquitinated ER was effectively degraded by a purified preparation of the yeast proteasome (31).
Development of Protacs to target proteins in cells
Clinical application of Protacs is dependent on successful ubiquitination and degradation of the protein target by endogenous ubiquitin ligases and proteasomes within cells. Because the first generation of Protacs contained a phosphopeptide from IκBα, the molecule was found to be very polar and did not penetrate into cells. Microinjection of this version of Protac was successful (31); however, this approach would not be viable in clinical practice.
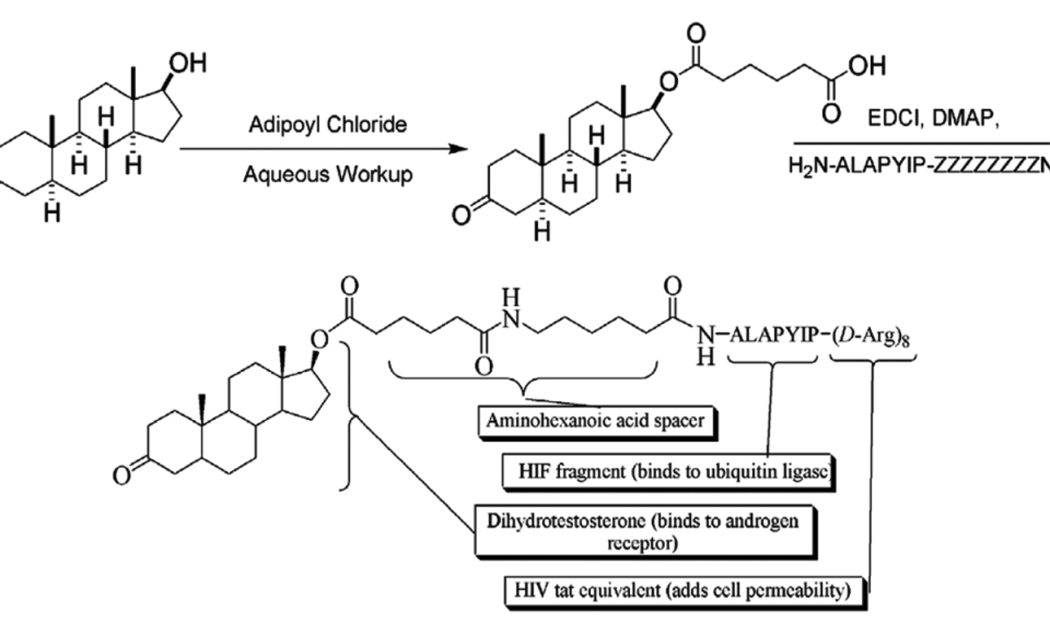
To this end, a HIF1α-DHT Protac was synthesized to increase permeability. Given the lack of small molecules that bind to E3 ligase, the seven amino acid sequence ALAPYIP of HIF1, a natural substrate of VHL, was chosen for the E3 recognition domain of this HIF1-Protac (16). The HIF1 sequence has been demonstrated to be the minimum recognition domain for the von Hippel-Lindau tumor suppressor protein (VHL) (17,18). VHL is part of the VBC-Cul2 E3 ubiquitin ligase complex. Under normoxic conditions, a proline hydroxylase catalyzes the hydroxylation of hypoxia inducible factor 1α (HIF1α) at P564 (32). P564 is the central proline in the ALAPYIP sequence, resulting in recognition and polyubiquitination by VHL. HIF1α is constitutively ubiquitinated and degraded under normoxic conditions (17,18). In addition, a poly-D-arginine tag derived from human immunodeficiency virus (HIV) tat was added to the carboxyl terminus of the peptide sequence to confer cell permeability and prevent nonspecific proteolysis (Figure 3) (33,34). This Protac should then enter the cell, be recognized and hydroxylated by a prolyl hydroxylase, and subsequently be bound by both the VHL E3 ligase and the target, AR.
Figure 3.
Synthesis and chemical structure of a cell permeable Protac with HIF1 peptide and testosterone.
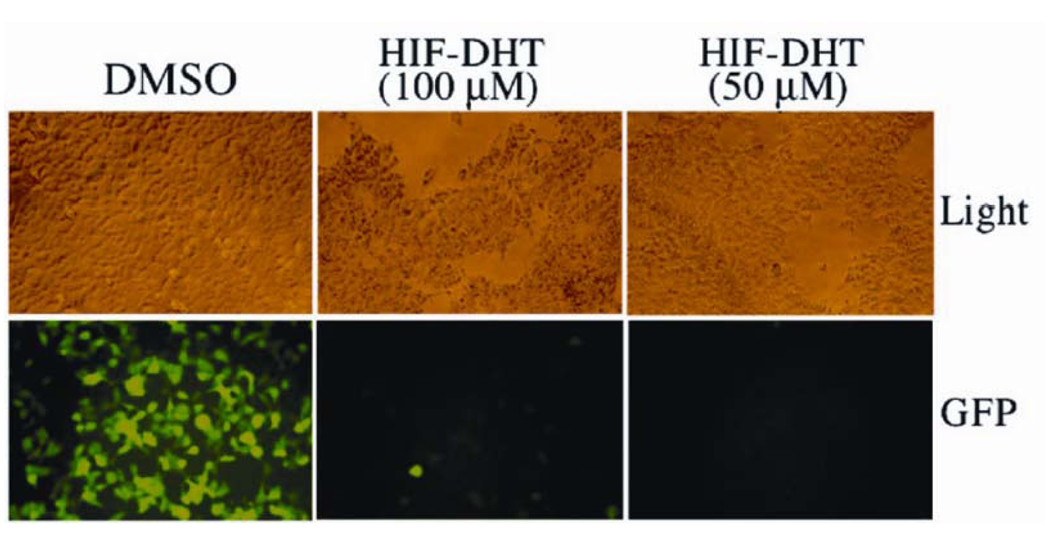
To test this version of HIF1-Protac, we bath-applied 293 cells stably expressing the androgen receptor-green fluorescent protein (AR-GFP) fusion protein with Protac, resulting in complete disappearance of AR-GFP signal within hours after treatment (Figure 4) (31). This effect was completely inhibited by pretreatment of cells with the proteasome inhibitor epoxomicin (31). Our results suggested that Protacs could in fact degrade specific cancer-promoting targets within cells, provided that the chimeric molecules could permeate into cells.
Figure 4.
Treatment of 293 human embryonic kidney cells expressing GFP-AR with HIF1-testosterone Protac resulted in degradation of AR.
Protacs to target endogenous proteins in cancer cells
Despite the encouraging results of the HIF1-testosterone Protacs in GFP-AR expressing 293 human embryonic kidney cells, this does not represent the endogenous AR expressed in prostate cancer cells. Therefore, we next tested whether Protacs were effective in AR positive prostate cancer cells and ER positive breast cancer cells. When androgen-dependent LnCaP prostate cancer cells were treated with HIF1-testosterone Protac, we observed a significant decrease in the levels of endogenous AR and inhibition of proliferation (35). In androgen-independent cells, there was no effect of Protac on AR levels or cell proliferation. Similarly, when estrogen-dependent MCF7 cells were treated with HIF1-estrogen Protac, cells stopped growing and ER levels decreased (35). These results demonstrated that when hormone-dependent cells are treated with Protac, not only are the receptors downregulated, but they are also functionally inhibited. Interestingly, the immediate effects of Protacs on prostate and breast cancer cells appear to be growth arrest in G1. With prolonged exposure, the cells entered apoptosis, similar to what is observed under conditions of androgen or estrogen-deprivation. This further supports the efficacy of Protacs through its ability to “starve” cells of their hormone dependency, resulting in cell death.
Despite these encouraging results, the IC50 for Protacs remained high for a possible drug for clinical application. The lowest IC50 with derivatization was 3.8µM for prostate and breast cancer cells, which is still quite high. Other groups have since confirmed the effectiveness of Protacs in prostate cancer cells using a testosterone-based Protac (36). Clearly, further development of Protacs is clearly needed to bring this technology to clinical trials in humans.
Other applications of Protacs have been recently reported (20,37). Protacs were used to block the aryl hydrocarbon receptor (AHR) pathway. Activation of AHR by agonists and environmental pollutants, such as dioxin, leads to carcinogenesis. To downregulate this pathway, Protacs were design with the ligand for AHR covalently linked to HIF1 peptide to target AHR to the VHL E3 ubiquitin ligase. This chimeric molecule was shown to induce degradation of AHR in epithelial cells and inhibit its function at a concentration of 10µM (20,37). Therefore, as seen with our own data, although Protacs are effective, the concentrations necessary for their effects are quite high.
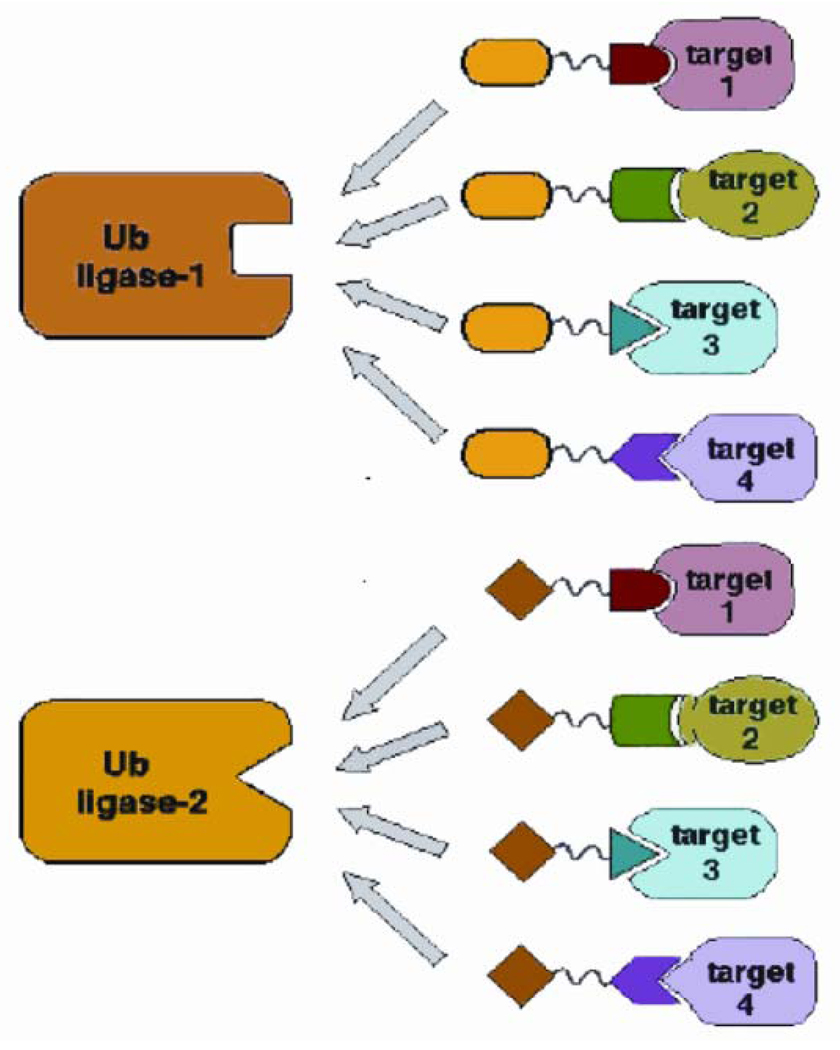
To overcome the problem with permeability and penetration into tumor cells, one approach is to replace the peptide with a small molecule that binds either the protein target or the E3 ligase. This may result in more efficient Protacs and provide enough reagent to test these molecules in vivo using mouse models of prostate and breast cancer. Another hurdle is the expense and labor of producing peptides as drugs for cancer therapy. The chemistry involved is time consuming and expensive, therefore not practical. To this end, Schneekloth et al. designed a Protac that consisted of a non-steroidal androgen receptor ligand and the mouse double minute (MDM2) ligand known as nutlin (38,39). In this version of Protac, a polyethylene glycol (PEG)-based linker was used to promote stability. The selective androgen receptor modulator (SARM)-nutlin Protac was able to recruit the androgen receptor to the E3 ubiquitin ligase MDM2, resulting in ubiquitination and degradation of the androgen receptor at a concentration of 10 µM. The model system used was a HeLa cell line expressing the AR. The degradation was inhibited by the proteasome inhibitor epoxomicin, suggesting that the Protac induced AR degradation through the UPS (38). The benefit of Protac technology is that it is versatile and can theoretically recruit any cancer or disease-promoting protein to an E3 ubiquitin ligase for degradation (Figure 5). Despite the advances in Protac technology, the molecule will require further derivation and chemical modifications prior to use in animal models and humans.
Figure 5.
Potential versatility of Protacs. Any number of ligands or binding partners could be chemically linked to any peptide or small molecule that associates with any E3 ubiquitin ligase to promote the ubiquitination and degradation of a cancer-causing protein. Reprinted from Sakamoto KM et al. 2001 Proc Natl Acad Sci USA 98:8554–8559. Copyright © 2001, The National Academy of Sciences, with permission.
Conclusions and summary
Protacs provide an entirely new nanotechnology-based approach to target cancer-causing proteins for ubiquitination and degradation. Experiments over the past decade have demonstrated that Protacs are specific, versatile, and biologically active to induce degradation of proteins that promote tumorigenesis and inhibit the growth of cancer cells. Future directions will focus on further development of Protacs to convert them into more practical drugs for clinical application.
Acknowledgments
Financial Support: This work was supported by the NIH (R21 CA108545) and Department of Defense (USA) Prostate Cancer Research Program (W81XWH-06-1-0192).
Abbreviations
- AHR
aryl hydrocarbon receptor
- AR
androgen receptor
- ER
estrogen receptor
- GFP
green fluorescent protein
- HIF1
hypoxia inducible factor
- MetAP-2
Methionine aminopeptidase-2
- Protac
Proteolysis Targeting Chimeric Molecule
- SCF
Skp1-Cullin-F-box protein
- UPS
ubiquitin proteasome system
- VHL
Von Hippel Lindau
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pray TR, Parlati F, Huang J, Wong BR, Payan DG, Bennett MK, Issakani SD, Molineaux S, Demo SD. Cell cycle regulatory E3 ubiquitin ligases as anticancer targets. Drug Resist Updat. 2002;5:249–258. doi: 10.1016/s1368-7646(02)00121-8. [DOI] [PubMed] [Google Scholar]
- 2.Vlachostergios PJ, Patrikidou A, Daliani DD, Papandreou CN. The Ubiquitin-Proteasome System in cancer, a major player in DNA Repair. Part 2: Transcriptional regulation. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00824.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol. 2009;10:398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]
- 5.Sakamoto KM. Ubiquitin-dependent proteolysis: its role in human diseases and the design of therapeutic strategies. Mol Genet Metab. 2002;77:44–56. doi: 10.1016/s1096-7192(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 6.Reyes-Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem. 2009;78:363–397. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mani A, Gelmann EP. The ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol. 2005;23:4776–4789. doi: 10.1200/JCO.2005.05.081. [DOI] [PubMed] [Google Scholar]
- 8.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 9.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 10.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 11.Handley PM, Mueckler M, Siegel NR, Ciechanover A, Schwartz AL. Molecular cloning, sequence, and tissue distribution of the human ubiquitin-activating enzyme E1. Proc Natl Acad Sci USA. 1991;88:258–262. doi: 10.1073/pnas.88.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nalepa G, Rolfe M, Harper JW. Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov. 2006;5:596–613. doi: 10.1038/nrd2056. [DOI] [PubMed] [Google Scholar]
- 13.Sanada M, Suzuki T, Shih LY, Otsu M, Kato M, Yamazaki S, Tamura A, Honda H, Sakata-Yanagimoto M, Kumano K, Oda H, Yamagata T, Takita J, Gotoh N, Nakazaki K, Kawamata N, Onodera M, Nobuyoshi M, Hayashi Y, Harada H, Kurokawa M, Chiba S, Mori H, Ozawa K, Omine M, Hirai H, Nakauchi H, Koeffler HP, Ogawa S. Gain-of-function of mutated C-CBL tumour suppressor in myeloid neoplasms. Nature. 2009;460:904–908. doi: 10.1038/nature08240. [DOI] [PubMed] [Google Scholar]
- 14.Zhou P, Bogacki R, McReynolds L, Howley PM. Harnessing the ubiquitination machinery to target the degradation of specific cellular proteins. Mol Cell. 2000;6:751–756. doi: 10.1016/s1097-2765(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto KM. Chimeric molecules to target proteins for ubiquitination and degradation. Methods Enzymol. 2005;399:833–847. doi: 10.1016/S0076-6879(05)99054-X. [DOI] [PubMed] [Google Scholar]
- 16.Schneekloth JS, Jr, Fonseca FN, Koldobskiy M, Mandal A, Deshaies R, Sakamoto K, Crews CM. Chemical genetic control of protein levels: selective in vivo targeted degradation. J Am Chem Soc. 2004;126:3748–3754. doi: 10.1021/ja039025z. [DOI] [PubMed] [Google Scholar]
- 17.Kaelin WG., Jr The von hippel-lindau tumor suppressor protein: an update. Methods Enzymol. 2007;435:371–383. doi: 10.1016/S0076-6879(07)35019-2. [DOI] [PubMed] [Google Scholar]
- 18.Kaelin WG., Jr The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8:865–873. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- 19.Griffith EC, Su Z, Turk BE, Chen S, Chang YH, Wu Z, Biemann K, Liu JO. Methionine aminopeptidase (type 2) is the common target for angiogenesis inhibitors AGM-1470 and ovalicin. Chem Biol. 1997;4:461–471. doi: 10.1016/s1074-5521(97)90198-8. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Chang YH. Amino-terminal protein processing in Saccharomyces cerevisiae is an essential function that requires two distinct methionine aminopeptidases. Proc Natl Acad Sci USA. 1995;92:12357–12361. doi: 10.1073/pnas.92.26.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sin N, Meng L, Wang MQ, Wen JJ, Bornmann WG, Crews CM. The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc Natl Acad Sci USA. 1997;94:6099–6103. doi: 10.1073/pnas.94.12.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeh ET. SUMOylation and De-SUMOylation: wrestling with life's processes. J Biol Chem. 2008;284:8223–8227. doi: 10.1074/jbc.R800050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Neriah Y. Regulatory functions of ubiquitination in the immune system. Nat Immunol. 2002;3:20–26. doi: 10.1038/ni0102-20. [DOI] [PubMed] [Google Scholar]
- 24.Chu WM, Ostertag D, Li ZW, Chang L, Chen Y, Hu Y, Williams B, Perrault J, Karin M. JNK2 and IKKbeta are required for activating the innate response to viral infection. Immunity. 1999;11:721–731. doi: 10.1016/s1074-7613(00)80146-6. [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci USA. 2001;98:8554–8559. doi: 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howell A. Selective oestrogen receptor downregulator. Eur J Cancer. 2002;38:S61–S62. doi: 10.1016/s0959-8049(02)00289-7. [DOI] [PubMed] [Google Scholar]
- 27.Howell A, Howell SJ, Evans DG. New approaches to the endocrine prevention and treatment of breast cancer. Cancer Chemother Pharmacol. 2003;52:S39–S44. doi: 10.1007/s00280-003-0645-5. [DOI] [PubMed] [Google Scholar]
- 28.Howell SJ, Johnston SR, Howell A. The use of selective estrogen receptor modulators and selective estrogen receptor down-regulators in breast cancer. Best Pract Res Clin Endocrinol Metab. 2004;18:47–66. doi: 10.1016/j.beem.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Debes JD, Schmidt LJ, Huang H, Tindall DJ. p300 mediates androgen-independent transactivation of the androgen receptor by interleukin 6. Cancer Res. 2002;62:5632–5636. [PubMed] [Google Scholar]
- 30.Debes JD, Tindall DJ. The role of androgens and the androgen receptor in prostate cancer. Cancer Lett. 2002;187:1–7. doi: 10.1016/s0304-3835(02)00413-5. [DOI] [PubMed] [Google Scholar]
- 31.Sakamoto KM, Kim KB, Verma R, Ransick A, Stein B, Crews CM, Deshaies RJ. Development of Protacs to target cancer-promoting proteins for ubiquitination and degradation. Mol Cell Proteomics. 2003;2:1350–1358. doi: 10.1074/mcp.T300009-MCP200. [DOI] [PubMed] [Google Scholar]
- 32.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 33.Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc Natl Acad Sci USA. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirschberg TA, VanDeusen CL, Rothbard JB, Yang M, Wender PA. Arginine-based molecular transporters: the synthesis and chemical evaluation of releasable taxol-transporter conjugates. Org Lett. 2003;5:3459–3462. doi: 10.1021/ol035234c. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Gonzalez A, Cyrus K, Salcius M, Kim K, Crews CM, Deshaies RJ, Sakamoto KM. Targeting steroid hormone receptors for ubiquitination and degradation in breast and prostate cancer. Oncogene. 2008;27:7201–7211. doi: 10.1038/onc.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang YQ, Han BM, Yao XQ, Hong Y, Wang Y, Zhao FJ, Yu SQ, Sun XW, Xia SJ. Chimeric molecules facilitate the degradation of androgen receptors and repress the growth of LNCaP cells. Asian J Androl. 2009;11:119–126. doi: 10.1038/aja.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puppala D, Lee H, Kim KB, Swanson HI. Development of an aryl hydrocarbon receptor antagonist using the proteolysis-targeting chimeric molecules approach: a potential tool for chemoprevention. Mol Pharmacol. 2008;73:1064–1071. doi: 10.1124/mol.107.040840. [DOI] [PubMed] [Google Scholar]
- 38.Schneekloth AR, Pucheault M, Tae HS, Crews CM. Targeted intracellular protein degradation induced by a small molecule: En route to chemical proteomics. Bioorg Med Chem Lett. 2008;18:5904–5908. doi: 10.1016/j.bmcl.2008.07.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 40.Heuze ML, Lamsoul I, Moog-Lutz C, Lutz PG. Ubiquitin-mediated proteasomal degradation in normal and malignant hematopoiesis. Blood Cells Mol Dis. 2008;40:200–210. doi: 10.1016/j.bcmd.2007.07.011. [DOI] [PubMed] [Google Scholar]