Abstract
Background
The impact of antiretroviral therapy (ART) interruption in HIV–hepatitis B virus (HBV) coinfected patients was examined in the Strategic Management of Antiretroviral Therapy (SMART) study.
Methods
Plasma HBV DNA was measured on all HBsAg positive (HBV+) participants at baseline, and at months 1, 2, 4, 6, 8, 10 and 12.
Results
Among HBV+ participants in the ART interruption (drug conservation, DC) (n=72) and ART continuation (virological suppression, VS) (n=62) arms, HBV DNA rebound of >1 log from baseline at months 1–4 was seen in 31–33% (p=0.003) and 3–4% (p=0.017), respectively. Thirteen HBV+ participants had HBV DNA rebound of >3 log, including 12 in the DC arm, of which 8 were on tenofovir (TDF)-containing regimens. Factors independently associated with a HBV DNA rebound were DC arm (p=0.0002), non-detectable HBV DNA at baseline (p=0.007), and black race (p=0.03). Time to ART reinitiation was shorter (7.5, 15.6, 17.8 months, p<0.0001) and proportion reinitiating greater (62.5, 46.5, 39.7%, p=0.0002) among HBV+ participants compared to HCV+ and non-HBV/HCV participants in the DC arm. No hepatic decompensation events occurred among HBV+ participants in either arm.
Conclusion
HBV DNA rebound following ART interruption is common and may be associated with accelerated immune deficiency in HIV-HBV coinfected patients.
Keywords: Human immunodeficiency virus, hepatitis B virus, coinfection, antiretroviral therapy, tenofovir
Introduction
Individuals with HIV-hepatitis B virus (HBV) coinfection have higher HBV DNA levels, more rapid liver disease progression and considerably higher liver disease-related mortality than those with HBV monoinfection [1–3]. Although greater immune deficiency is associated with increased HBV-related disease progression [1], high rates of HBV-related disease events continue to be seen following the introduction of highly active antiretroviral therapy (ART)[1].
Nucleos(t)ide analogues with dual HBV and HIV activity such as lamivudine, emtricitabine, and tenofovir are common agents within ART regimens. These drugs provide potent HBV DNA suppression [4–7], but drug resistance in HBV develops in around 20% per year with lamivudine (3TC) monotherapy (and presumably emtricitabine, FTC) [8]. Rates of tenofovir (TDF) HBV resistance are considerably lower [9], and early reports suggest greater beneficial impact halting HBV-related liver disease progression [10].
The Strategies for Management of Antiretroviral Therapy (SMART) was a randomized controlled trial which examined a strategy of episodic ART among HIV-infected persons with baseline CD4 counts of >350 cells/mm3, and demonstrated that CD4 guided episodic interruption was associated with higher rates of opportunistic diseases, non-AIDS clinical morbidity, and all cause mortality compared to continuous ART [11].
While recruitment of HBV co-infected patients with active hepatitis in SMART was not recommended the inclusion of some HBV coinfected participants and frequent use of anti-HBV active ART within the SMART study provided an ideal opportunity to examine aspects of HIV-HBV immunopathogenesis, in particular the impact of ART interruption on markers of HIV and HBV disease activity.
Participants and Methods
Study design
The design and data collection methods of the SMART trial have previously been reported [11]. Briefly, the SMART study was a randomized clinical trial that compared two distinct strategies of using ART in a large cohort of participants over 13 years of age with confirmed HIV-1 infection and CD4 counts >350 cells/mm3 at the time of screening. In the drug conservation (DC) strategy arm, participants interrupted or deferred ART until the CD4 count dropped below 250 cells/mm3. ART was resumed or initiated until the CD4 count reached >350 cells/mm3 and then suspended again. Cycles of ART were guided by CD4 cell count levels or the presence of HIV related symptoms or if the CD4 percentage dropped below 15%. In the viral suppression (VS) strategy arm participants continued ART with the goal of maximal viral suppression in accordance with HIV treatment guidelines. The choice of antiretroviral agents and combinations was based on clinician/patient preference and was continued without interruption. The primary outcome was the development of a new or recurrent opportunistic disease or death from any cause.
The protocol allowed for participants with chronic HBV infection to use monotherapy with specific anti-hepatitis drugs (e.g. adefovir) while not receiving ART. The protocol had no exclusion criteria based on alanine aminotransferase (ALT) level, but recommended that patients requiring continued ART for management of chronic HBV infection should not be enrolled.
Hepatitis status
During screening, patients’ medical charts were reviewed for documentation of HBV and hepatitis C virus (HCV) status. If there was no laboratory evidence of a positive hepatitis B surface antibody (HBsAb) or two positive hepatitis B surface antigen (HBsAg) results obtained at least six months apart, HBsAb and HBsAg tests were performed. If there was no evidence of a prior positive anti-HCV antibody result or a negative result from within the previous year, anti-HCV antibody tests were performed. In the absence of plasma HCV RNA results, chronic HCV infection was defined based on the presence of hepatitis C antibody and denoted as “HCV+”. Chronic HBV infection was defined as the persistence of HBsAg over a minimum of 6 months and denoted “HBV+”.
Stored baseline and follow-up plasma samples of HBV+ participants were analyzed for levels of HBV DNA using the branched DNA assay (VERSANT HBV DNA 3.0; Bayer Diagnostics, Leverkusen, Germany) with a lower level of detection of 357 IU/mL.
Data collection and follow up
Prior to randomization, the following information was collected: ART history, nadir CD4 cell count and highest HIV RNA level, prior three laboratory results for CD4 cell count and percentage, and plasma HIV RNA. Participants were seen at one month and every two months during Year 1 and every four months in Year 2 onward, with clinical assessment, CD4 cell count and HIV- RNA measurement, and storage of plasma samples. Retrospective collection of available ALT level data was undertaken following SMART study completion.
Statistical analysis
Participants were divided into three mutually exclusive groups: HBV+, HCV+ and non HBV/HCV. Participants who were both HBV+ and HCV+ (6 DC and 8 VS) were included in the HBV+ group. Baseline characteristics were compared between DC and VS HBV+, HCV+ and non HBV/HCV participants using Pearson’s χ2-test for binomial proportions for categorical variables and non-parametric rank tests for continuous variables (Wilcoxon test for treatment group comparisons and Kruskal-Wallis for comparisons across hepatitis groups). The impact of hepatitis status on time to ART reinitiation in the DC arm was evaluated by Kaplan-Meier analysis. Factors associated with ART initiation were further examined in Cox proportional hazard models adjusting for age, gender, prior AIDS, baseline and nadir CD4 count and baseline and highest plasma HIV RNA test. Median CD4+ slope from baseline to 4 months of follow-up was compared by hepatitis sub-groups in the DC arm using the Kruskal-Wallis test.
The proportion of participants with >1 log increase in plasma HBV DNA at follow-up visits was compared by Fisher’s exact test for DC and VS hepatitis sub-groups including HBV+ participants on HBV active ART (lamivudine, emtricitabine or tenofovir) at baseline. Pearson correlation coefficients were used to determine the correlation between change in plasma HBV DNA and a) change in CD4 cell count, and b) change in plasma HIV RNA.
Predictors for change in Log10 HBV DNA over time were assessed by linear regression, using an outcome of area under the curve of the Log10 HBV DNA values from baseline to Month 12. This analysis included 54 DC and 46 VS participants with HBV DNA results available at baseline and at least 1 follow-up visit. All plasma HBV DNA results available in the first 12 months and prior to 11 January 2006 were included in the analyses.
Time-to-event analyses were censored at the earliest of the date of death, the lost to follow-up date, or 11 January 2006, the date that ART-experienced DC participants were advised to reinitiate ART following the recommendations of an independent Safety Data Monitoring Board. All p-values are 2-sided. Analyses were performed using SAS (Version 9.1).
Results
Baseline characteristics
There were 5472 participants enrolled in the SMART study from January 2002 to January 2006 (2752 in the VS arm and 2720 in the DC arm). Prevalence of HBV and HCV were 2.3% (n=62) and 14.0% (n=385), respectively, in the VS arm, and 2.6% (n=72) and 15.1% (n=411), respectively, in the DC arm. The HBV prevalence includes 14 subjects (0.3%) with HBV/HCV coinfection. Baseline characteristics by hepatitis status are shown in Table 1. Demographic and clinical characteristics were similar across the hepatitis groups in both arms, apart from HIV transmission category; the proportion with IDU-acquired HIV infection was much higher among HCV+ participants. Overall, 84% of participants were on ART at entry, including 74% on HBV active ART. Among HBV+ participants in the DC arm, 67% were on HBV active ART, including 25% on TDF-containing regimens.
Table 1.
Baseline characteristics in the SMART drug conservation arm
| Virological Suppression arm | Drug Conservation arm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HBV a (n=62) |
HCV (n=385) |
Non- HBV/HCV (n=2305) |
Total (n=2752) |
HBV b (n=72) |
HCV (n=411) |
Non- HBV/HCV (n=2237) |
Total (n=2720) |
||
| Median age (IQR) (years) | 45 (38–49) | 46 (41–50) | 43 (37–50) | 44 (38–50) | 41 (38–49) | 46 (40–51) | 43 (37–50) | 43 (37–50) | |
| HIV transmission category | |||||||||
| - MSM sex contact (%) | 48 | 27 | 52 | 48 | 60 | 27 | 55 | 51 | |
| - Heterosexual sex contact(%) | 44 | 42 | 46 | 46 | 42 | 45 | 44 | 44 | |
| - IDU (%) | 6 | 57 | 2 | 10 | 8 | 53 | 2 | 10 | |
| - Other or unknown (%) | 8 | 11 | 9 | 9 | 6 | 7 | 8 | 8 | |
| Median (IQR) baseline CD4 (/mm3) | 570 (449–677) |
566 (459–726) |
605 (467–798) |
598 (465–789) |
556 430–708) |
607 (466–812) |
596 (468–792) |
597 (467–791) |
|
| Median nadir CD4 (/mm3) | 245 (120–382) |
250 (150–389) |
250 (160–354) |
250 (157–358) |
207 (93–285) |
264 (155–360) |
250 (154–359) |
250 (152–358) |
|
| Baseline HIV RNA ≤400 copies/ml (%) | 63 | 61 | 73 | 71 | 72 | 68 | 72 | 72 | |
| Prior AIDS (%) | 24 | 28 | 22 | 23 | 29 | 27 | 25 | 25 | |
| ART at entry (%) | 74 | 78 | 85 | 84 | 79 | 82 | 85 | 84 | |
| - 3TC, not TDF (%) | 40 | 56 | 58 | 57 | 42 | 55 | 57 | 56 | |
| - TDF, not 3TC (%) | 10 | 4 | 5 | 5 | 8 | 6 | 6 | 6 | |
| - 3TC/FTC and TDF | 18 | 11 | 12 | 12 | 17 | 13 | 12 | 12 | |
| HBeAg / ant-HBe (%) c | 59/36 | 50/45 | |||||||
| HBV DNA detectable at entry (%) d | 53 | 55 | |||||||
| Median (IQR) log10 HBV DNA (IU/ml) e | 7.4 (4.2– 8.4) |
8.1 (3.4– 8.5) |
|||||||
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; MSM, men who have sex with men; TDF, tenofovir; 3TC, lamivudine; FTC, emtricitabine; ART, antirretroviral therapy.
Includes 8 participants with HBV/HCV coinfection.
Includes 6 participants with HBV/HCV coinfection.
Out of 66 DC and 55 VS with stored specimens available.
Out of 66 DC and 55 VS with stored specimens available.
Out of 36 DC and 29 VS with detectable HBV DNA
Despite high rates of HBV active therapy, 54% of HBV+ participants had detectable HBV DNA at baseline, and among these participants the median log10 HBV DNA level was 7.4 and 8.1 IU/ml in the VS and DC arms, respectively. In the DC arm, baseline median log10 HBV DNA (IQR) was 2.55 (2.55 – 2.55), 3.27 (2.55 – 8.19), and 8.08 (3.77 – 8.60) in the TDF-containing, 3TC (without TDF)-containing, and no HBV active ART groups, respectively. In the DC arm, the proportion with detectable HBV DNA (>2.55 log10 IU/ml) at baseline was 23.5%, 51.9%, and 81.0% in the TDF containing, 3TC (no TDF)-containing, and no HBV active ART groups, respectively (p=0.002).
Plasma HBV DNA rebound
Among HBV+ participants in the DC (n=54) and VS (n=46) arms HBV DNA rebound of >1 log from baseline was seen in 31–33% and 3–4%, respectively, at early timepoints (months 1–4) (p=0.021, 0.004, 0.003 for months 1, 2, 4 comparisons, respectively). ART reinitiations among HBV+ participants in the DC arm reduced this proportion over subsequent timepoints, although the proportion with >1 log rebound at 12 months remained higher in the DC vs VS arms (19% vs 7%, respectively; p=0.14). Among HBV+ participants on HBV active ART at baseline in the DC arm (n=44), the proportion with >1 log rebound was higher among those receiving TDF containing regimens (with or without 3TC/FTC) (n=17) compared with those receiving 3TC only-containing regimens (n=27) over the initial 4 months (60–89% versus 0–20% p=0.044, 0.015, 0.002 for months 1, 2, 4 comparisons, respectively). The 3 HBV+ participants in the VS arm with HBV DNA rebound of >1 log at 12 months were either on no ART at baseline (n=2) or on a 3TC-containing (non-TDF) ART regimen (n=1).
Among HBV+ participants with a baseline HBV DNA <1,000 IU/ml in the DC (n=24) and VS (n=22) arms HBV DNA rebound of >1 log from baseline was seen in 42–60% and 0–8%, respectively, at early timepoints (months 1–4) (p=0.04, 0.002, 0.007 for months 1, 2, 4 comparisons, respectively).
HBV DNA rebounds of >3 log from baseline were documented in 13 HBV+ participants, 12 in the DC arm and one in the VS arm. In the DC arm, a >3 log HBV DNA rebound was more common among HBV+ participants on baseline TDF-containing regimens (7/17), compared to 3TC only-containing regimens (3/27), and no HBV active ART (2/21) (p=0.013). The one participant in the VS arm was on a 3TC only-containing regimen. Among HBV+ participants on HBV active ART in the DC arm, time to reach a >3 log HBV DNA increase varied from 1 to 10 months.
Following reinitiation of TDF-containing regimens in 7 DC participants with a >3 log HBV DNA increase, there were generally rapid reductions in plasma HBV DNA. Only 2 HBV+ participants in the DC arm received non-HIV active/HBV active agents (adefovir in both cases) during ART interruption.
Univariate and multivariate factors associated with HBV DNA changes from baseline through month 12 are shown in Table 2. In the multivariate model, factors associated with a HBV DNA rebound were DC arm (p=0.0002), non-detectable HBV DNA at baseline (p=0.007), and black race (p=0.03). Being on a TDF-containing ART regimen at baseline was marginally associated with a HBV DNA rebound (p=0.06).
Table 2.
Univariate and multivariate models for outcome of area under the curve − Log10 HBV-DNA over time for 54 DC and 46 VS patients with HBV-DNA data
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Est. (SE) | P value | Est. (SE) | P value | |
| DC group | 1.07 (0.30) | 0.0006 | 1.16 (0.29) | 0.0002 |
| Detectable baseline HBV DNA | −0.97 (0.30) | 0.002 | −0.87 (0.32) | 0.007 |
| Age (/10 years) | 0.02 (0.20) | 0.91 | 0.00 (0.18) | 0.99 |
| Female | −0.48 (0.42) | 0.25 | −0.54 (0.40) | 0.18 |
| Black race | 0.13 (0.32) | 0.69 | 0.70 (0.31) | 0.03 |
| Prior AIDS | 0.38 (0.35) | 0.27 | 0.05 (0.35) | 0.88 |
| Baseline HIV RNA ≤400 copies/ml | 0.66 (0.32) | 0.04 | 0.03 (0.37) | 0.93 |
| Baseline CD4 count (/100 cells lower) | −0.15 (0.09) | 0.09 | −0.01 (0.09) | 0.95 |
| Nadir CD4 count (/100 cells lower) | 0.10 (0.09) | 0.29 | 0.05 (0.10) | 0.61 |
| On TDF (vs. no HBV-active ART) | 1.21 (0.38) | 0.002 | 0.88 (0.47) | 0.06 |
| On 3TC (vs. no HBV- active ART) | 0.62 (0.36) | 0.09 | 0.39 (0.39) | 0.32 |
Abbreviations: DC, drug conservation; TDF, tenofovir; 3TC, lamivudine.
Retrospective ALT data including baseline and at least one follow-up level at month 4, 8, or 12 was available in 32/72 (44%) HBV+ participants in the DC arm. A hepatic flare (increase in ALT level from baseline to above 200 U/ml) was uncommon during follow-up, with only 2 subjects developing flares at month 12. The rate of hepatic flare was low in HBV+ participants in the DC arm with and without an HBV DNA rebound of >1 log (1/10 and 1/22, respectively).
During the SMART study follow-up, there were no episodes of hepatic decompensation or liver disease mortality events recorded among HBV+ participants in either the DC or VS arm.
Reinitiation of antiretroviral therapy in the DC group
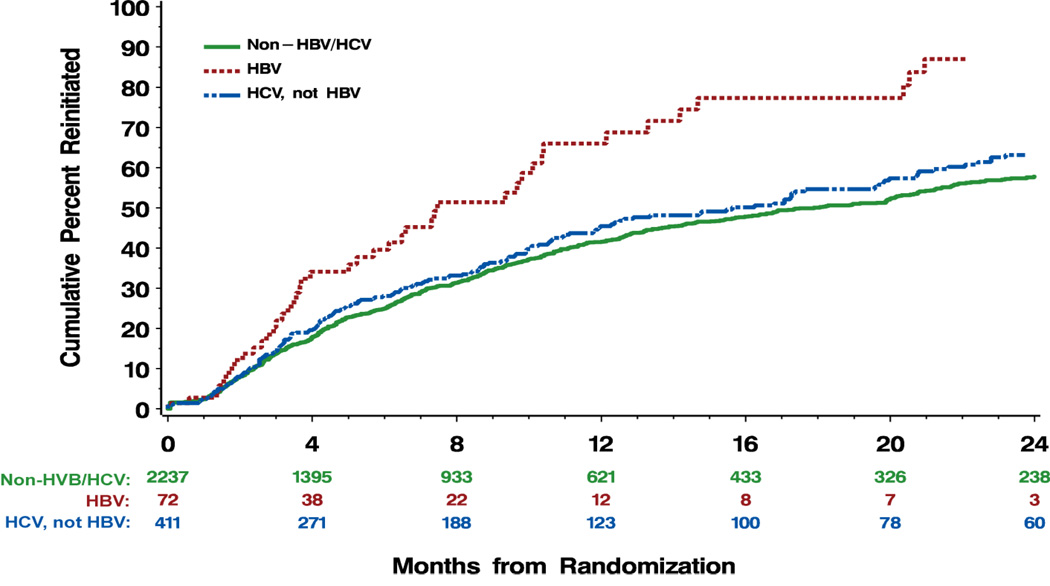
Median time to ART reinitiation was considerably shorter (7.5, 15.6, 17.8 months, p<0.0001) and the proportion of participants reinitiating greater (62.5, 46.5, 39.7%, p=0.0002) among HBV+ participants in the DC arm compared to HCV+ and non-HBV/HCV participants (Figure 1, Table 3). The median CD4 count at time of reinitiation was similar across the three groups (ranging from 233 to 241/mm3). As the CD4 count was comparable among the groups when ART was interrupted, these findings suggest that depletion of CD4+ T cells occurred more rapidly among HBV+ participants.
Figure 1.
Percentage of HBV+ participants in the DC (n=54) and VS (n=46) arms with HBV DNA rebound of >1 log from baseline through month 12. DC, drug conservation; VS, virological suppression.
Table 3.
Antiretroviral therapy reinitiation in the SMART drug conservation arm
| HBV a (n=72) |
HCV (n=411) |
Non-HBV/HCV (n=2237) |
|
|---|---|---|---|
| Reinitiated ART (%) | 62.5 | 46.5 | 39.7 |
| Estimated median (95% CI) time to ART reinitiation (months) |
7.5 (5.2–10.3) | 15.6 (11.8–19.8) | 17.8 (15.5–20.1) |
| Median (IQR) CD4 count at reinitiation (/mm3) |
240 (197–286) | 241 (205–341) | 233 (193–314) |
| - <150 (%) | 4.4 | 7.4 | 9.9 |
| - 150 – 249 (%) | 55.6 | 47.9 | 49.4 |
| - 250 – 349 (%) | 28.9 | 20.0 | 19.8 |
| - 350 – 449 (%) | 8.9 | 10.5 | 8.3 |
| - 450+ (%) | 2.2 | 14.2 | 12.5 |
| Reason for ART reinitiation | (n=45) | (n=191) | (n=888) |
| CD4 count < 250/mm3 (%) | 60.0 | 55.0 | 59.1 |
| Low CD4% (%) | 33.3 | 31.4 | 27.3 |
| Rapid CD4 decline (%) | 26.7 | 16.8 | 21.3 |
| HIV-related symptoms (%) | 8.9 | 10.5 | 10.8 |
| Progression of HIV disease (%) | 2.2 | 5.2 | 2.7 |
| High HIV RNA level (%) | 15.6 | 23.6 | 21.2 |
| Patient wish (%) | 22.2 | 29.8 | 22.4 |
| Other (%) | 17.8 | 17.3 | 15.5 |
Includes 6 participants with HBV/HCV coinfection.
Abbreviations: ART, antiretroviral therapy; HBV, hepatitis B virus; HCV, hepatitis C virus.
Over the initial four months follow-up off ART in the DC arm the median CD4 decline (slope) per month was 70.9 cells/mm3, 50.6 cells/mm3, and 53.0 cells/mm3, for HBV+, HCV+ and non-HBV/HCV participant groups (p=0.14). The reasons for ART reinitiation were similar across the groups, with CD4 count <250/mm3 and low CD4 percentage being the most common reasons in each group (Table 3).
Factors associated with ART reinitiation in multivariate analysis were HBV infection (compared to no HBV/HCV infection) (hazard ratio (HR)=1.71, 95% confidence interval (CI) (1.27–2.31), baseline CD4 count (per 100 cells/mm3 lower): 1.14 (1.11–1.18), nadir CD4 count (per 100 cells/mm3 lower): 1.50 (1.42–1.58), age (per 10 years older): 1.13 (1.06–1.20), prior AIDS: 1.41 (1.24–1.61), baseline plasma HIV RNA ≤400 copies/mL: 1.19 (1.04–1.37) and highest recorded log10 HIV RNA (per 1 log higher): 1.19 (1.11–1.28) (Table 4).
Table 4.
Multivariate model of predictors of antiretroviral therapy reinitiation in the SMART drug conservation arm
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Hazard ratio | P-value | Hazard ratio | P-value | |
| Non-HBV/HCV | 1.00 | 1.00 | ||
| HBV | 1.95 (1.45–2.63) | <0.0001 | 1.71 (1.27 – 2.31) | 0.0005 |
| HCV | 1.01 (0.87–1.18) | 0.87 | 1.04 (0.88 – 1.22) | 0.66 |
| Prior AIDS | 2.17 (1.91–2.45) | <0.0001 | 1.41 (1.24 – 1.61) | <0.0001 |
| Nadir CD4 count (/100 cells lower) |
1.67 (1.60–1.75) | <0.0001 | 1.50 (1.42 – 1.58) | <0.0001 |
| Baseline CD4 count (/100 cells lower) |
1.20 (1.16–1.23) | <0.0001 | 1.14 (1.11 – 1.18) | <0.0001 |
| Baseline HIV RNA ≤400 copies/ml |
1.18 (1.04–1.34) | 0.011 | 1.19 (1.04 – 1.37) | 0.012 |
| Highest HIV RNA (Log10) | 1.34 (1.25–1.44) | <0.0001 | 1.19 (1.11 – 1.28) | <0.0001 |
| Female | 0.97 (0.84–1.11) | 0.61 | 1.01 (0.88 – 1.16) | 0.89 |
| Age (/10 years) | 1.15 (1.08–1.22) | <0.0001 | 1.13 (1.06 – 1.20) | 0.0003 |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; AIDS, acquired immune deficiency syndrome.
The extent of HBV DNA rebound moderately correlated with the degree of CD4 count decline at several follow-up timepoints: correlation coefficients were −0.19 (p=0.40) for month 1, −0.31 (p=0.06) for month 2, −0.40 (p=0.02) for month 4, −0.49 (p=0.003) for month 6, −0.28 (p=0.11) for month 8, −0.54 (p=0.001) for month 10, and −0.26 (p=0.06) for month 12. HBV DNA rebounds also correlated significantly with plasma HIV RNA increases at several follow-up timepoints, with coefficients varying from 0.38 to 0.51.
Discussion
Structured ART interruptions have been associated with increased rates of AIDS and non-AIDS related morbidity and mortality [11]. This post-hoc substudy conducted in SMART demonstrates that ART interruption may be particularly problematic among HIV-HBV coinfected individuals. Such patients in the DC arm of SMART experienced considerable HBV DNA rebound and had to reinitiate ART more often and more rapidly than the rest. Similar reasons for reinitiation and CD4 distribution at reinitiation among HBV+ and non-HBV+ participants in SMART indicate that HBV-specific management issues did not drive more rapid reinitiation of ART. HBV+ participants with baseline HBV DNA suppression on TDF-containing regimens were at particularly high risk of HBV DNA rebound following ART interruption. HBV DNA rebound following ART interruption was associated with CD4 decline and HIV RNA increase, although the explanation for this relationship is unclear.
Plasma HBV DNA rebound following cessation of HBV antiviral therapy has been documented in both HBV monoinfected and HIV-HBV coinfected individuals [12–15]. Such rebounds have been associated with flares in liver enzymes and occasionally with hepatic decompensation. HBV DNA rebounds following development of lamivudine resistance have also led to hepatic inflammation and worsening of liver function [16, 17].
A smaller study of ART interruption (STACCATO) recently documented HBV DNA rebounds and ALT flares following interruption of HBV-active containing ART regimens [18]. However, this SMART substudy is the first to indicate that such rebounds among HIV-HBV coinfected individuals may lead to accelerated immune deterioration in terms of drop in CD4 counts. The mechanisms underlying such an interaction between HBV viraemia and immune status are unclear. HIV is associated with higher HBV DNA and faster liver disease progression [1, 3, 19], but so far there is no evidence that HBV influences HIV disease progression [20, 21]. CD4 count recovery following ART initiation may be marginally impaired in the initial few months of therapy among HBV coinfected individuals, but by 12 months responses are similar to non-HBV coinfected individuals [22]. Furthermore, HIV suppression following ART initiation is not affected by HBV coinfection [22, 23]. In the EuroSIDA Cohort study, around 500 HIV-HBV coinfected participants (8.7% of the total cohort) had a higher rate of liver-disease related and overall mortality, but no increased HIV disease progression or AIDS-related mortality [24].
In contrast to stable chronic HBV infection, a rapid increase in HBV replication following treatment cessation as observed in this study may potentially alter HIV replication and CD4+ T-cell turnover by several mechanisms. First, a rapid increase in HBV replication may have led to an increased number of activated HBV-specific T-cells as observed following acute HBV infection and hepatic flare [25, 26]. An increase in activated CD4+ T-cells could provide a larger pool of target cells for HIV replication leading to accelerated CD4+ T-cell decline. Second, an acute increase in HBV replication may have potentially led to altered T-cell trafficking with recruitment of both HBV-specific and non-HBV specific T-cells to the liver from the periphery [27], however in the absence of hepatic flare, this explanation seems less likely. Finally, although primarily a hepatotropic virus, HBV can infect lymphocytes at low levels and therefore could potentially interact directly with HIV [28, 29]. The HBV genome encodes a 17-kDa protein, termed HBx, that acts synergistically with the HIV protein, Tat, to induce HIV replication and cellular activation in Jurkat cells, an immortalised T-cell line [30]. It is possible that this direct interaction between HBV x-protein and HIV may occur in primary CD4+ T cells in vivo.
Potential consequences of HBV DNA rebound and accelerated immune deterioration include both enhanced HBV and HIV disease morbidity. HBV DNA rebound could lead to increased hepatic inflammation and liver disease progression as well as enhanced susceptibility to HBV drug resistance. Although ALT data collection was only undertaken retrospectively and available in a minority of subjects, the low rate of hepatic flare even in those with significant HBV DNA rebound is somewhat reassuring. The subsequent HBV DNA declines following reinitiation of TDF-containing regimens and a lack of reported major liver disease events among HBV+ participants provides further reassurance that adverse HBV clinical outcomes will be limited if HBV therapy is subsequently resumed. In a separate post-hoc SMART analysis, both HBV+ and HCV+ participants had increased AIDS and non-AIDS morbidity and mortality in the DC arm compared to non-hepatitis coinfected participants, indicating that ART interruption is particularly hazardous for hepatitis coinfected individuals [31]. Despite this association, liver disease related events were limited and not the explanation for the increased non-AIDS related morbidity.
The major clinical implication of our study findings, along with previously reported adverse outcomes following cessation of HBV antiviral therapy, would appear to be that ART interruption, particularly of regimens containing HBV active therapy, should be avoided in HIV-HBV coinfected individuals. A higher rate of HBV DNA rebound following interruption of TDF-containing regimens is consistent with greater baseline HBV DNA suppression as a result of the high potency and genetic barrier for resistance of this agent [5, 6, 9]. If ART interruption is required for any reason, a non-HIV active HBV drug should be commenced, particularly if there is previously documented evidence of HBV viraemia or markers of disease activity such as presence of HBeAg, elevated liver enzymes and/or significant liver fibrosis. The recent demonstration of HIV suppression and associated M184V mutation development in HIV-HBV coinfected individuals receiving entecavir but no ART has reduced the choices available [32]. The nucleotide analogue adefovir produces sustained HBV DNA suppression in a large proportion of HIV-HBV coinfected individuals [33, 34], has no significant HIV activity at the 10 mg daily HBV therapeutic dose, and does not seem to select for HIV mutations (including K65R) [35]. Education of HIV clinicians on the importance of maintaining HBV viral control is required, as only 2 of 72 SMART HBV+ participants who interrupted ART were commenced on HBV active therapy during interruptions.
Several limitations of the study must be recognized. First, is the post-hoc nature of the analyses, albeit within a well characterized population from a randomized controlled trial population. Second, the lack of prospective and systematically collected ALT data limited evaluation of the impact of HBV DNA rebound on hepatic disease parameters. Third, the exclusion of HBV coinfected individuals from the SMART study if they were assessed as requiring ongoing ART for management of chronic HBV infection meant the HBV prevalence was relatively low and therefore impaired the generalization of the study findings.
In conclusion, ART interruption among HIV-HBV coinfected participants in the SMART study was associated with frequent plasma HBV DNA rebound, and more rapid and higher rates of ART reinitiation. Such outcomes indicate that ART interruption may be particularly hazardous for this subpopulation of HIV-infected individuals.
Acknowledgements
We would like to acknowledge the SMART participants, the SMART study team (see N Engl J Med, 2006:355:2294–2295 for list of investigators), and the INSIGHT Executive Committee. The National Centre in HIV Epidemiology and Clinical Research is funded by the Australian Government Department of Health and Ageing and is affiliated with the Faculty of Medicine, University of New South Wales. GD is supported by a NHMRC Practitioner Fellowship.
Financial support:
Support provided by: NIAID, NIH grants U01AI042170 and U01AI46362.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
No authors have a potential conflict of interest in relation to the content of the manuscript.
Clinical Trials.gov identifier: NCT00027352.
Conference presentation:
Preliminary findings were presented at the 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention, Sydney, Australia, July 2007.
Contributor Information
Gregory J. Dore, Email: gdore@nchecr.unsw.edu.au.
Vicente Soriano, Email: vsoriano@dragonet.es.
Jürgen Rockstroh, Email: Juergen.Rockstroh@ukb.uni-bonn.de.
Bernd Kupfer, Email: bernd.kupfer@yahoo.de.
Ellen Tedaldi, Email: Ellen.Tedaldi@tuhs.temple.edu.
Lars Peters, Email: lpe@cphiv.dk.
Jacqueline Neuhaus, Email: jacquie@ccbr.umn.edu.
Massimo Puoti, Email: m.puoti@iol.it.
Marina B. Klein, Email: marina.klein@muhc.mcgill.ca.
Amanda Mocroft, Email: a.mocroft@pcps.ucl.ac.uk.
Bonaventura Clotet, Email: BClotet@irsicaixa.es.
Jens D. Lundgren, Email: jdl@cphiv.dk.
References
- 1.Thio CL, Seaberg EC, Skolasky R, Jr, Phair J, Visscher B, Munoz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 2.Dore GJ, Cooper DA. The impact of HIV therapy on co-infection with hepatitis B and hepatitis C viruses. Current Opinion in Infectious Diseases. 2001;14:749–755. doi: 10.1097/00001432-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Gilson RJ, Hawkins AE, Beecham MR, Ross E, Waite J, Briggs M, et al. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS. 1997;11:597–606. doi: 10.1097/00002030-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Dore GJ, Cooper DA, Barrett C, Goh LE, Thakrar B, Atkins M. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. J Infect Dis. 1999;180:607–613. doi: 10.1086/314942. [DOI] [PubMed] [Google Scholar]
- 5.Dore GJ, Cooper DA, Pozniak AL, DeJesus E, Zhong L, Miller MD, et al. Efficacy of tenofovir disoproxil fumarate in antiretroviral therapy-naive and -experienced patients coinfected with HIV-1 and hepatitis B virus. J Infect Dis. 2004;189:1185–1192. doi: 10.1086/380398. [DOI] [PubMed] [Google Scholar]
- 6.Benhamou Y, Fleury H, Trimoulet P, Pellegrin I, Urbinelli R, Katlama C, et al. Anti-hepatitis B virus efficacy of tenofovir disoproxil fumarate in HIV-infected patients. Hepatology. 2006;43:548–555. doi: 10.1002/hep.21055. [DOI] [PubMed] [Google Scholar]
- 7.Nunez M, Perez-Olmeda M, Diaz B, Rios P, Gonzalez-Lahoz J, Soriano V. Activity of tenofovir on hepatitis B virus replication in HIV-co-infected patients failing or partially responding to lamivudine. AIDS. 2002;16:2352–2354. doi: 10.1097/00002030-200211220-00023. [DOI] [PubMed] [Google Scholar]
- 8.Matthews GV, Bartholomeusz A, Locarnini S, Ayres A, Sasaduesz J, Seaberg E, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS. 2006;20:863–870. doi: 10.1097/01.aids.0000218550.85081.59. [DOI] [PubMed] [Google Scholar]
- 9.Sheldon J, Camino N, Rodes B, Bartholomeusz A, Kuiper M, Tacke F, et al. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antivir Ther. 2005;10:727–734. [PubMed] [Google Scholar]
- 10.Matthews GV, Cooper DA, Dore GJ, et al. Improvements in parameters of end-stage liver disease in patients with HIV/HBV-related cirrhosis treated with tenofovir. Antivir Ther. 2007;12:119–122. [PubMed] [Google Scholar]
- 11.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 12.Honkoop P, de Man RA, Niesters HG, Zondervan PE, Schalm SW. Acute exacerbation of chronic hepatitis B virus infection after withdrawal of lamivudine therapy. Hepatology. 2000;32:635–639. doi: 10.1053/jhep.2000.16333. [DOI] [PubMed] [Google Scholar]
- 13.Lim SG, Wai CT, Rajnakova A, Kajiji T, Guan R. Fatal hepatitis B reactivation following discontinuation of nucleoside analogues for chronic hepatitis B. Gut. 2002;51:597–599. doi: 10.1136/gut.51.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altfeld M, Rockstroh JK, Addo M, Kupfer B, Pult I, Will H, Spengler U. Reactivation of hepatitis B in a long-term anti-HBs-positive patient with AIDS following lamivudine withdrawal. J Hepatol. 1998;29:306–309. doi: 10.1016/s0168-8278(98)80017-2. [DOI] [PubMed] [Google Scholar]
- 15.Neau D, Schvoerer E, Robert D, Dubois F, Dutronc H, Fleury HJ, Ragnaud JM. Hepatitis B exacerbation with a precore mutant virus following withdrawal of lamivudine in a human immunodeficiency virus-infected patient. J Infect. 2000;41:192–194. doi: 10.1053/jinf.2000.0724. [DOI] [PubMed] [Google Scholar]
- 16.Thabut D, Thibault V, Benhamou Y, Bernard B, Aubron-Olivier C, Poynard T, Di Martino V. Successful control of subfulminant hepatitis related to lamivudine-resistant hepatitis B virus in an HIV-infected patient. AIDS. 2001;15:2463–2464. doi: 10.1097/00002030-200112070-00020. [DOI] [PubMed] [Google Scholar]
- 17.Liaw YF, Chien RN, Yeh CT, Tsai SL, Chu CM. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology. 1999;30:567–572. doi: 10.1002/hep.510300221. [DOI] [PubMed] [Google Scholar]
- 18.Nuesch R, Ananworanich J, Srasuebkul P, Chetchotisakd P, Prasithsirikul W, Klinbuayam W, et al. Interruptions of tenofovir/emtricitabine-based antiretroviral therapy in patients with HIV/hepatitis B virus co-infection. AIDS. 2008;22:152–154. doi: 10.1097/QAD.0b013e3282f303bf. [DOI] [PubMed] [Google Scholar]
- 19.Thomas DL. Growing importance of liver disease in HIV-infected persons. Hepatology. 2006;43:S221–S229. doi: 10.1002/hep.21033. [DOI] [PubMed] [Google Scholar]
- 20.Lincoln D, Petoumenos K, Dore GJ. HIV/HBV and HIV/HCV coinfection, and outcomes following highly active antiretroviral therapy. HIV Med. 2003;4:241–249. doi: 10.1046/j.1468-1293.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- 21.Bonacini M, Louie S, Bzowej N, Wohl AR. Survival in patients with HIV infection and viral hepatitis B or C: a cohort study. AIDS. 2004;18:2039–2045. doi: 10.1097/00002030-200410210-00008. [DOI] [PubMed] [Google Scholar]
- 22.Law WP, Duncombe CJ, Mahanontharit A, Boyd MA, Ruxrungtham K, Lange JM, et al. Impact of viral hepatitis co-infection on response to antiretroviral therapy and HIV disease progression in the HIV-NAT cohort. AIDS. 2004;18:1169–1177. doi: 10.1097/00002030-200405210-00010. [DOI] [PubMed] [Google Scholar]
- 23.De Luca A, Bugarini R, Lepri AC, Puoti M, Girardi E, Antinori A, et al. Coinfection with hepatitis viruses and outcome of initial antiretroviral regimens in previously naive HIV-infected subjects. Archives of Internal Medicine. 2002;162:2125–2132. doi: 10.1001/archinte.162.18.2125. [DOI] [PubMed] [Google Scholar]
- 24.Konopnicki D, Mocroft A, de Wit S, Antunes F, Ledergerber B, Katlama C, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19:593–601. doi: 10.1097/01.aids.0000163936.99401.fe. [DOI] [PubMed] [Google Scholar]
- 25.Penna A, Artini M, Cavalli A, Levrero M, Bertoletti A, Pilli M, et al. Long-lasting memory T cell responses following self-limited acute hepatitis B. J Clin Invest. 1996;98:1185–1194. doi: 10.1172/JCI118902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marinos G, Torre F, Chokshi S, Hussain M, Clarke BE, Rowlands DJ, et al. Induction of T-helper cell response to hepatitis B core antigen in chronic hepatitis B: a major factor in activation of the host immune response to the hepatitis B virus. Hepatology. 1995;22:1040–1049. doi: 10.1016/0270-9139(95)90607-x. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Zhao JH, Wang PP, Xiang GJ. Expression of CXC chemokine IP-10 in patients with chronic hepatitis B. Hepatobiliary Pancreat Dis Int. 2008;7:45–50. [PubMed] [Google Scholar]
- 28.Laure F, Zagury D, Saimot AG, Gallo RC, Hahn BH, Brechot C. Hepatitis B virus DNA sequences in lymphoid cells from patients with AIDS and AIDS-related complex. Science. 1985;229:561–563. doi: 10.1126/science.2410981. [DOI] [PubMed] [Google Scholar]
- 29.Zeldis JB, Mugishima H, Steinberg HN, Nir E, Gale RP. In vitro hepatitis B virus infection of human bone marrow cells. J Clin Invest. 1986;78:411–417. doi: 10.1172/JCI112591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez-Gonzalo M, Carretero M, Rullas J, Lara-Pezzi E, Aramburu J, Berkhout B, et al. The hepatitis B virus X protein induces HIV-1 replication and transcription in synergy with T-cell activation signals: functional roles of NF-kappaB/NF-AT and SP1-binding sites in the HIV-1 long terminal repeat promoter. J Biol Chem. 2001;276:35435–35443. doi: 10.1074/jbc.M103020200. [DOI] [PubMed] [Google Scholar]
- 31.Tedaldi EM, Peters L, Neuhaus J, Puoti M, Rockstroh J, Klein MB, et al. Opportunistic disease and mortality in patients coinfected with hepatitis B or C virus in the Strategic Management of Antiretroviral Therapy (SMART) study. Clin Infect Dis. 2008;47:1468–1475. doi: 10.1086/593102. [DOI] [PubMed] [Google Scholar]
- 32.McMahon MA, Jilek BL, Brennan TP, Shen L, Zhou Y, Wind-Rotolo M, et al. The HBV drug entecavir - effects on HIV-1 replication and resistance. N Engl J Med. 2007;356:2614–2621. doi: 10.1056/NEJMoa067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benhamou Y, Bochet M, Thibault V, Calvez V, Fievet MH, Vig P, et al. Safety and efficacy of adefovir dipivoxil in patients co-infected with HIV-1 and lamivudine-resistant hepatitis B virus: an open-label pilot study. Lancet. 2001;358:718–723. doi: 10.1016/s0140-6736(01)05840-8. [DOI] [PubMed] [Google Scholar]
- 34.Benhamou Y, Thibault V, Vig P, Calvez V, Marcelin AG, Fievet MH, et al. Safety and efficacy of adefovir dipivoxil in patients infected with lamivudine-resistant hepatitis B and HIV-1. J Hepatol. 2006;44:62–67. doi: 10.1016/j.jhep.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Sheldon JA, Corral A, Rodes B, Mauss S, Rockstroh J, Berger F, et al. Risk of selecting K65R in antiretroviral-naive HIV-infected individuals with chronic hepatitis B treated with adefovir. AIDS. 2005;19:2036–2038. doi: 10.1097/01.aids.0000189563.79976.05. [DOI] [PubMed] [Google Scholar]