Abstract
Cardiovascular deconditioning after long duration spaceflight is especially challenging in women who have a lower orthostatic tolerance (OT) compared with men. We hypothesized that an exercise prescription, combining supine aerobic treadmill exercise in a Lower Body Negative Pressure (LBNP) chamber followed by 10 min of resting LBNP, 3 to 4 times a week, and flywheel resistive training every third day would maintain orthostatic tolerance (OT) in women during a 60-day head-down-tilt bed rest (HDBR). Sixteen women were assigned to two groups (exercise, control). Pre and post HDBR OT was assessed with a tilt/LBNP test until presyncope. OT time (mean ± SE) decreased from 17.5±1.0 min to 9.1±1.5 min (−50±6%) in control group (p<0.001) and from 19.3 ±1.3 min to 13.0 ± 1.9 min (−35±7%) in exercise group (p<0.001), with no significant difference in OT time between the two groups after HDBR (p=0.13). Nevertheless compared with controls post HDBR, exercisers had a lower heart rate (mean±SE) during supine rest (71±3 versus 85±4, p<0.01), a slower increase in heart rate and a slower decrease in stroke volume over the course of tilt/LBNP test (p<0.05). Blood volume (mean±SE) decreased in controls (−9±2%, p<0.01) but was maintained in exercisers (−4±3%, p=0.17).
Our results suggest that the combined exercise countermeasure fails to protect OT but improves cardiovascular response to subtolerance levels of orthostatic stress.
Keywords: Bed Rest, Dizziness, prevention & control, Exercise, physiology, Exercise Tolerance, physiology, Female, Head-Down Tilt, physiology, Heart Rate, physiology, Humans, Lower Body Negative Pressure, Stroke Volume, physiology, Tilt-Table Test, Time Factors, Weightlessness Simulation
Keywords: simulated microgravity, cardiovascular deconditioning, exercise countermeasure, lower body negative pressure, WISE 2005
INTRODUCTION
The transition from supine to standing posture requires complex physiological mechanisms to maintain cerebral blood flow. Exposure to actual or simulated microgravity by head-down-tilt bed rest (HDBR) alters these adaptive mechanisms and causes orthostatic intolerance as well as decreased exercise capacity (Convertino et al. 1989; Butler et al. 1991; Vernikos et al. 1993; Buckey et al. 1996; Pavy-Le Traon et al. 1999). Several factors contribute to orthostatic intolerance: moderate hypovolemia (Buckey et al. 1996; Custaud et al. 2002), decreased stroke volume (Buckey et al. 1996), myocardial atrophy (Levine et al. 1997), reduced baroreflex sensitivity (Sigaudo-Roussel et al. 2002), and increased distensibility of lower extremity blood vessels (Convertino et al. 1989; Belin de Chantemele et al. 2004b). Furthermore, females have a lower orthostatic tolerance than males (Convertino 1998; Fu et al. 2004). Lower orthostatic tolerance is observed after short-duration space flights (5–16 days) with a four times higher incidence of presyncopal symptoms during a 10 min head-up tilt test in female astronauts compared with males (28% and 7% respectively) (Harm et al. 2001). Nevertheless data concerning women in actual or simulated microgravity remain sparse.
Several countermeasures based on the microgravity-induced changes described above have proven only partly effective to counteract orthostatic intolerance. Restoration of blood volume (9 to 14 days spaceflight) (Buckey et al. 1996), moderate levels of aerobic exercise (during 9 to 16 days spaceflight or a 15-day HDBR respectively) (Lee et al. 1999; Schneider et al. 2002), or bouts of maximal exercise (90-day HDBR) (Belin de Chantemele et al. 2004a) are not able to maintain orthostatic tolerance at pre flight or pre HDBR levels. Periods of lower body negative pressure (LBNP) applied during HDBR provide beneficial effects on plasma volume, baroreflex sensitivity and lower limb vascular distensibility (Arbeille et al. 1995; Traon et al. 1995). Orthostatic tolerance is maintained by one hour, progressively increasing to two hours of daily, low-level LBNP sessions (28 mmHg) during a 30-day HDBR (Guell et al. 1991). Nevertheless, such prolonged LBNP sessions devoted only to preservation of orthostatic tolerance, are incompatible with in-flight timelines.
The combination of several countermeasures is therefore a logical approach to counteract the multiple effects of cardiovascular deconditioning. The pressure gradient generated by LBNP also produces a form of artificial gravity and allows running exercise in the supine posture with normal footward loading (Hargens et al. 1991). Furthermore, treadmill exercise within LBNP provides lower body fluid redistribution and cardiovascular responses similar to upright exercise in 1g environment (Murthy et al. 1994; Boda et al. 2000). A daily 40-min session of treadmill exercise within LBNP (LBNP/Ex) fails to protect orthostatic tolerance after a 15-day HDBR (Schneider et al. 2002). However the addition of a 5-min period of LBNP without exercise immediately after the exercise period attenuates the loss of orthostatic tolerance after a 30-day HDBR (Watenpaugh et al. 2007). Thus, increasing the resting LBNP period after exercise from 5 to 10 minutes may further preserve orthostatic tolerance during HDBR by providing additional stimulation of orthostatic reflexes.
This paper reports the results from a part of an extensive WISE study (for Women International Space Simulation for Exploration) which was conducted to evaluate whether the combination of an aerobic treadmill exercise within LBNP and resistive exercise performed on a flywheel ergometer prevents physiological deconditioning in women during 60 days of HDBR. Association of a resistive flywheel exercise to the LBNP/ex countermeasure was justified by the positive effects obtained on bone and muscle with resistive exercise after a 90-day bed rest study in men (Rittweger et al. 2005; Alkner et al. 2004). Effects on bone, muscle and other aspects of this study (e.g., effects on female endocrine responses, aerobic capacity…) will be reported elsewhere. In this paper, we tested the hypothesis that aerobic exercise in a LBNP chamber followed by 10 minutes of resting LBNP, 3 to 4 times a week, combined with flywheel resistance training of the legs, 3 times a week, maintains orthostatic tolerance in women during a 60-day HDBR.
MATERIALS AND METHODS
1. Subjects and general protocol
Sixteen healthy adult women volunteered for this study after receiving a complete description of the experimental methods, and after passing medical and psychological screening criteria. Medical tests for selection included: medical history, clinical and psychological examination, chest X-ray, electrocardiogram, abdominal echography, ultrasound imaging of lower limb veins, a 10 min head-up tilt test (intolerant subjects were excluded), peak oxygen uptake measurement (VO2pk), DEXA measurement for bone density and body composition, and laboratory tests (haematology, blood chemistry, urine analysis). The study was approved by the local Ethics Committee (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale). Each volunteer signed a consent form and was aware of her right to withdraw from the experiment without prejudice at any time. Subjects were housed in the Institute for Space Physiology and Medicine (MEDES), Toulouse, France.
The experiment protocol included 20 days of ambulatory control period, followed by 60 days of 6° HDBR and 21 days of rehabilitation. Subjects were housed in pairs and both members of the pair were in the same experimental group and performed each experiment or countermeasure on the same day. During the HDBR period, subjects were monitored by video to ensure their compliance with the head-down tilt position. During the control and rehabilitation periods, the subjects remained in the MEDES facility.
All women had a regular menstrual cycle, and oral contraception was stopped at least two months before the beginning of HDBR. Tobacco, coffee, tea, and alcohol were prohibited during the stay at the clinic. Each subject was given a caloric intake of 110% of their resting metabolic rate which was determined prior to HDBR. The maximum liquid intake of the control group was 60 ml/kg/day, and the maximum liquid intake for the exercise group was 60 ml/kg/day on the days without training and 75 ml/kg/day on days with exercise training. Caloric intake was increased for the exercise subjects to compensate for the energy costs of the exercise countermeasures.
2. Groups
Two groups of eight volunteers were selected: a control group (CON, n =8) and an exercise group (EX, n =8). Subjects were assigned to the different groups such that no between-group difference existed in mean peak oxygen uptake (VO2pk). There was no significant difference pre HDBR between CON and EX groups respectively in age (years, mean±SE): 34± versus 33±1, p=0.46, height (cm, mean±SE): 163±2 versus 165±3, p=0.49, weight (kg, mean±SE): 56.5±1.2 versus 59.5±2, p=0.34, and VO2pk (ml/min/kg, mean±SE): 38.9±6.8 versus 37.9±4.0, p=1.0.
Volunteers of the EX group performed alternately two types of exercises:
1. Aerobic exercise
Subjects ran on a vertical treadmill while supine in the LBNP chamber (Figure 1) for 40 min each session, with 10 min of resting LBNP immediately after the run. A session was scheduled for each subject approximately every three-four days/week beginning on HDBR day 1, for a total of 29 sessions during the 60-day HDBR. Unfortunately, due to constraints of other tests near the end of the HDBR period, the last LBNP countermeasure session was scheduled 62–63 hours before the end on the 60-day HDBR.
Figure 1.
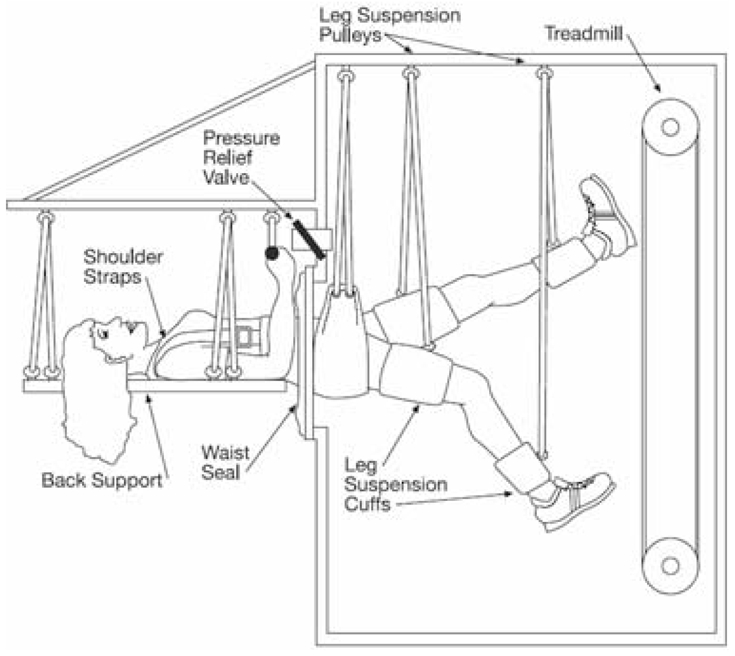
Exercise on a treadmill within lower body negative pressure (LBNP) exposure. Footward force is produced by the pressure gradient generated by LBNP. The subject is suspended at the ankles and thighs by a pulley system, and the hands are holding the suspension cables at waist level.
During supine treadmill exercise, LBNP (48 to 55 mmHg) was applied to produce footward forces equivalent to those for upright running on Earth, initially at 1.0 time body weight, increasing progressively to 1.15 times body weight according to subject tolerance. The VO2pk for each subject was determined with an upright, graded treadmill stress test before HDBR. The treadmill speeds used during exercise within the LBNP chamber were calculated from a linear regression of the oxygen consumption and exercise intensity results from the pre HDBR VO2pk test. The interval exercise protocol was similar to previous experiments (Watenpaugh et al. 2000; Schneider et al. 2002; Watenpaugh et al. 2007): 7 min to warm up at 40% VO2pk, followed by 3 min at 60%, 2 min at 40%, 3 min at 70%, 2 min at 50%, 3 min at 80%, 2 min at 60%, 3 min at 80%, 2 min at 50%, 3 min at 70%, 2 min at 40%, 3 min at 60% and 5 minutes at 40% VO2pk, with an additional 10 min of supine, stationary exposure to the same level of LBNP (resting LBNP period).
Fatigue was evaluated by the subject at the end of each stage using a visual analog scale, from 6 (very easy) to 20 (exhaustion). Any score above 18 led to immediate reduction of the treadmill speed, in order to stay at a sub-maximal effort. Heart Rate (HR) was continuously monitored by a Polar® (Polar Electro Inc, NY, USA) heart rate watch with the transmitter belt fixed around the chest.
Subjects were monitored closely during the resting LBNP period: systolic and diastolic blood pressures (SBP and DBP) were measured continuously by a non-invasive finger cuff (Finapres 2300, Ohmeda France) on the right hand placed at heart level, with a correction of the finger blood pressure every minute by comparing to an automatic arm blood pressure device (Dynamap Monitor, Critikon, Inc. Tampa, Fla, USA) on the left arm. Pain, discomfort or signs of pre-syncope were noted, and rated on a visual scale from 1 (slight symptom) to 10 (maximal intensity for symptom). If symptoms appeared, the volunteer was asked to move her legs to improve venous return, and a cold wet towel was applied to her face. The LBNP was turned off if one of the following signs occurred: rapidly increasing symptoms (nausea, clammy skin, excessive sweating, pallor, vertigo), a sudden drop in blood pressure (SBP fall > 25 mmHg/min or DBP fall > 15mmHg/min), a SBP < 70 mmHg, a sudden drop in HR (HR fall>15 beats/min). Once symptoms had disappeared, the resting LBNP period was resumed.
2. Resistive exercise
Subjects trained the back, thigh and calf muscle groups using supine squat (SS) and calf press (CP) exercises using a gravity independant inertial ergometer (Alkner and Tesch 2004). A total of 19 sessions were scheduled for each subjects approximately every third day (2–3 days/week) beginning on day 2 of HDBR. The inertial ergometer was in the 6° head-down tilt position and all resistance exercise was performed in this position. The SS exercise consisted of 4 sets of 7 maximal concentric and eccentric repetitions, while the CP exercise consisted of 4 sets of 14 maximal concentric and eccentric repetitions. This resistive exercise protocol was identical to a previous 90-day HDBR study conducted in males (Alkner and Tesch 2004).
3. Orthostatic tolerance test
Orthostatic tolerance was assessed by a tilt/LBNP test. This tilt/LBNP test was performed twice before HDBR (the first one to familiarize subjects with their presyncopal symptoms, the second one as a baseline pre HDBR test), and once immediately at the end of HDBR period (recovery day 1). Subjects were positioned and secured on a tilt table, the lower part of the body inside a LBNP chamber. LBNP was applied by enclosing the subject’s lower body up to the iliac crest in an air tight box. A footplate allowed for foot contact to maintain body position. The subject was asked to avoid leg movements during the test.
After 5 min in the supine position for collection of baseline data, the test consisted of 5 min supine rest, 10 min 80-degree head-up tilt without LBNP, followed by continued head-up tilt and progressively increasing stages of LBNP of 3 min each (10, 20, 30mmHg, etc) until presyncope. This test allows a precise and reproducible assessment of orthostatic tolerance (el-Bedawi and Hainsworth 1994). The test was ended if one of the following signs occurred: rapidly increasing symptoms (nausea, clammy skin, excessive sweating, pallor, vertigo), a sudden drop in blood pressure (SBP fall > 25 mmHg/min or DBP fall > 15mmHg/min), a SBP < 70 mmHg, a sudden drop in HR (HR fall > 15 beats/min), cardiac dysrhythmias.
During these tests, HR was obtained by standard electrocardiography and SBP and DBP were measured continuously with a non-invasive finger cuff method (Finometer®, Finapress Medical Systems, The Netherlands). A height-corrector placed at the level of the heart provided a stable measurement independent of hand movement, position or effect of tilting. Resting blood pressure values obtained with the finger cuff were calibrated to SBP and DBP values obtained with an inflatable arm cuff. Mean arterial pressure (MAP) was determined as the average value over a complete cardiac cycle. Cardiac output (Q) was estimated from the Modelflow method (Wesseling et al. 1993), which computes an aortic flow waveform from finger pressure, by simulating a non-linear three-element model of the aortic input impedance. The computed aortic flow waveform per beat provided left ventricular stroke volume (SV) and consequently Q, by multiplying SV by instantaneous HR. An estimate of total peripheral resistance (TPR) was calculated from MAP/Q. Considering the limitations of the Modelflow method in providing absolute values of SV, Q and consequently SVR (Pitt et al. 2004), these variables were presented as percent changes from supine resting values (for more details, see limitations in Discussion).
4. Blood volume
Blood volume (BV) was calculated in all subjects one day before tilt/LBNP tests pre HDBR and on day 60 of HDBR, by carbon monoxide (CO) rebreathing as described by Burge and Skinner (Burge and Skinner 1995), which was shown to provide a safe, precise and reproducible estimation of blood volume in healthy volunteers.
5. Statistical analysis
Orthostatic tolerance time (OT time), hemodynamic variables during OT tests, and BV were compared in each group and between groups before and after HDBR. Hemodynamic variables obtained during tilt/LBNP tests pre and post HDBR (HR, SV, Q, SBP, DBP, MAP, TPR) were averaged in each group over the last 30 seconds of the 4th min of test for the supine values and over the last 30 seconds of each minute during the tilt and tilt/LBNP periods. Due to onset of presyncopal symptoms, the number of subjects completing each tilt/LBNP stage decreased over time in each group: hemodynamic variables were compared every minute as long as there were data from 6 subjects per group. Normality of variables was tested by Kolmogorov-Smirnov test. Student’s paired t test was used to compare OT time and BV pre to post HDBR in EX and CON groups, and unpaired t test was used to compare OT time and BV between groups before and after HDBR. Two way analysis of variances (ANOVA) was used to determine the effects of group, HDBR, and group X HDBR interaction for hemodynamic variables during tilt/LBNP tests. An SPSS/PC+ software package was used for statistical analysis (version 15.0, SPSS, Chicago, IL). Values are expressed as means±SE. Statistical results were considered significant when p<0.05.
RESULTS
1. Compliance of exercisers to resting LBNP period
Seven out of eight of the exercisers completed at least 19 of the 29 resting LBNP sessions at the end of exercise without any break or reduction in LBNP. Conversely, the relatively low compliance of one exerciser to resting LBNP periods forced reduction of the level of LBNP, providing a very short time of training effectively achieved at a LBNP level simulating one body weight (6%). Another subject had also a low compliance to the resting LBNP period, and completed 55% of the resting LBNP time at a LBNP level simulating one body weight. The other 6 exercisers completed 88% to 100% of the prescribed resting LBNP regimen. According to the general agreement between WISE principal-investigators in Dec, 2005 at an Investigator Working Group meeting in Montreal, results are given and analyzed with all the subjects included.
2. Orthostatic tolerance time
Our combined tilt/LBNP procedure provided a clear end-point for all tests pre and post HDBR. In all cases, tilt/LBNP tests were ended because of a sudden, rapid fall in SBP, associated with a drop in HR in 5 of 7 EX and 4 of 8 CON. OT time (mean ± SE) decreased from 17.5±1.0 min to 9.1±1.5 min in CON group (−50±6%, p<0.001), and from 19.3 ±1.3 min to 13.0 ± 1.9 min (−35±7%, p<0.001) in EX group. There was no significant difference in OT time between groups before HDBR (p=0.30) and at the end of HDBR (p=0.13) (Figure 2, left).
Figure 2.
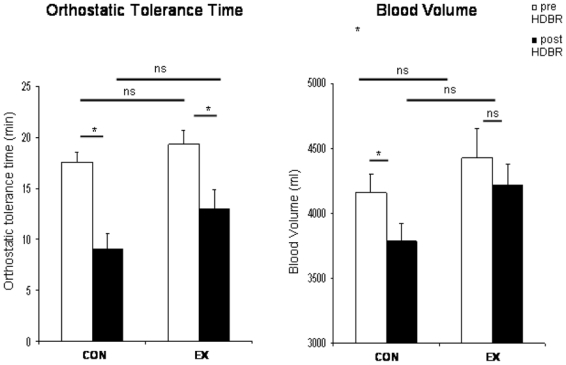
Figure 2, left. Orthostatic tolerance time (min, mean ± SE) assessed by tilt/LBNP tests pre and post HDBR in CON and EX groups. There was no significant difference between groups pre HDBR, respectively 17.5±1 min in CON and 19.3 ± 1.3 min in EX (p=0.30). Orthostatic tolerance time significantly decreased post HDBR in EX and CON groups, respectively 13.0 ± 1.9 min and 9.1±1.5 min (*: p<0.001).
Figure 2, right. Blood volume assessed by CO rebreathing method pre and post HDBR in CON and EX groups. BV decreased significantly in CON (−9±2%, p<0.01), with a non significant decrease in EX (−4±3%, p=0.17).
3. Hemodynamic variables during tilt/LBNP tests
Mean values of hemodynamic variables at presyncope were calculated and plotted on graphs to emphasize mean changes of OT time and hemodynamic variables at the presyncopal end point in CON and EX group before and after 60 days HDBR. Curves were drawn as long as there were at least 6 subjects per group: n=8 at the beginning of titl/LBNP tests in both groups, decreasing progressively related to occurrence of presyncope.
Heart rate (Figure 3)
Figure 3.
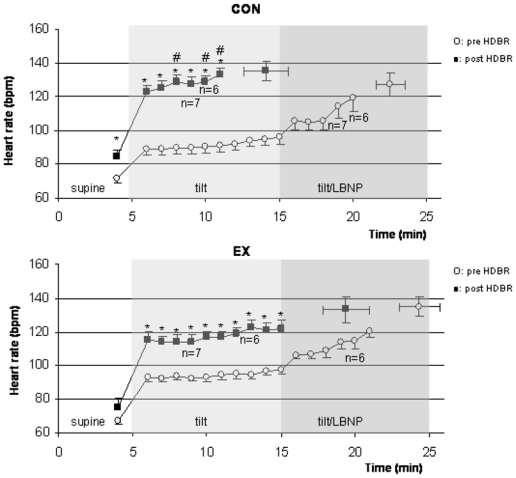
Heart rate during tilt/LBNP tests (beats per min, mean ± SE) are shown for the CON and EX conditions, before (open circles) and after (closed squares) HDBR. Isolated points are HR at presyncopal time points. * indicates significantly higher values post HDBR compared with pre HDBR values. # indicates a significantly higher HR in CON compared with EX at the same time point of the post HDBR tilt/LBNP test.
Pre HDBR, HR (bpm, mean ± SE) was similar in CON and EX groups at rest (respectively 71±3 and 68±3) and over the course of the tilt/LBNP tests, with a similar increase in the two groups after the onset of upright posture. At presyncope, HR had reached similar levels in the two groups (127±7 in CON, 135±6 in EX).
The post HDBR supine HR in CON was significantly elevated compared to pre HDBR value (85±4 versus 71±3, p<0.01), whereas it was not the case in EX (75±5 versus 68±3, NS). Over the course of the tilt/LBNP tests, a significant increase in HR compared to pre HDBR values was observed in the two groups (main effect of HDBR, p<0.01). This HR increase was more pronounced in CON compared to EX group, and the difference became significant after min 3 in upright posture with a HR of 129±4 in CON and 115±4 in EX group (significant group X HDBR interaction effect, p<0.05). At presyncope, HR had reached similar levels in the two groups (135±6 in CON, 133±8 in EX).
Blood pressure (Figure 4)
Figure 4.
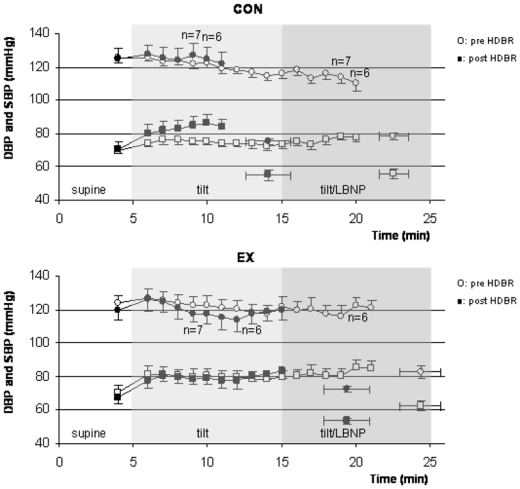
SBP (top) and DBP (bottom) during tilt/LBNP tests (mmHg, mean ±SE) are shown for the CON and EX conditions, before (SBP: open circles, DBP: open squares) and after (SBP: close circles, DBP: closed squares) HDBR period. Isolated points are SBP and DBP at presyncopal time point pre and post HDBR.
SBP and DBP (mmHg, mean ± SE) in CON and EX groups were not significantly different before HDBR at supine rest and over the course of the tilt/LBNP tests. Pre HDBR presyncopal values of SBP were 78±2 and 82±4 for CON and EX group, respectively, 56±3 and 63±3 for DBP. Supine and tilt blood pressure values were not significantly affected by HDBR, and were not different between groups, except for the earlier occurrence of presyncopal symptoms in CON group. Presyncopal blood pressure values were not different: 75±2 and 72±2 in CON and EX groups for SBP, 55±3 and 54±2 for DBP, respectively.
Stroke volume (Figure 5)
Figure 5.
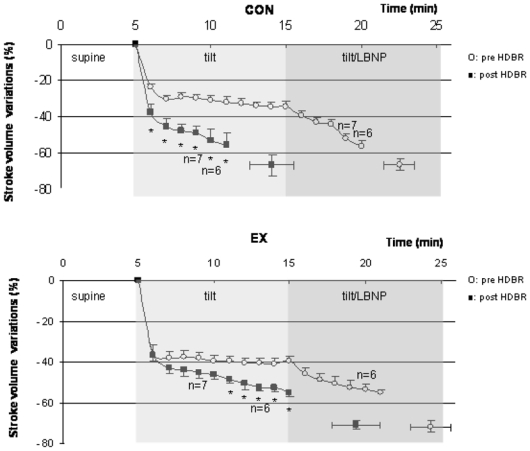
Stroke volume changes during tilt/LBNP tests (%, mean ± SE) are shown for the CON and EX conditions, before (open circles) and after (closed squares) HDBR. Isolated points are changes in SV at presyncopal time point pre and post HDBR. * indicates significant decrease in SV values post HDBR compared with pre HDBR values.
Pre HDBR, the decrease in SV (%, mean ± SE) was similar in CON and EX over the course of the tilt/LBNP tests (−34±3% versus −40±3% at min 10 in upright posture, NS) and at presyncope (− 66±4% in CON, −72±3% in EX, NS). In CON group, the main effect of HDBR was a fall in SV since the onset of upright posture (−38±4% versus −24±2% at min 1, p<0.01, −53±7 versus −31±2 at min 5, p<0.05). In EX group post HDBR, the decrease in SV over the course of the tilt/LBNP test was significantly more pronounced than pre HDBR since min 6 in upright posture (−49±2% versus −40 ±2%, p<0.05). A statistically significant group X HDBR interaction effect was observed since min 4 in upright posture, p<0.05). At presyncope, the fall in SV was similar in the two groups (−67±5% in CON group, −71±2% in EX group, NS).
Cardiac output (Figure 6)
Figure 6.
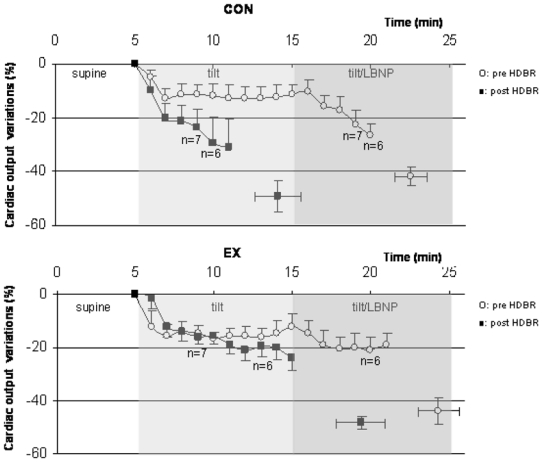
Cardiac output changes during tilt/LBNP tests are shown for the CON and EX conditions, before (open circles) and after (closed squares) HDBR. Isolated points are changes in Q at presyncopal time point pre and post HDBR. No significant change in Q was observed pre to post HDBR in CON and EX groups.
Pre HDBR, the decrease in cardiac output Q (%, mean ± SE) was similar in CON and EX groups over the course of the tilt/LBNP tests (−11±4% versus 12±5% at min 10) and at presyncope (−42±4% versus −44±5%, NS). HDBR did not affect Q in CON and EX groups, and no HDBR X group interaction was observed.
Total peripheral resistance (Figure 7)
Figure 7.
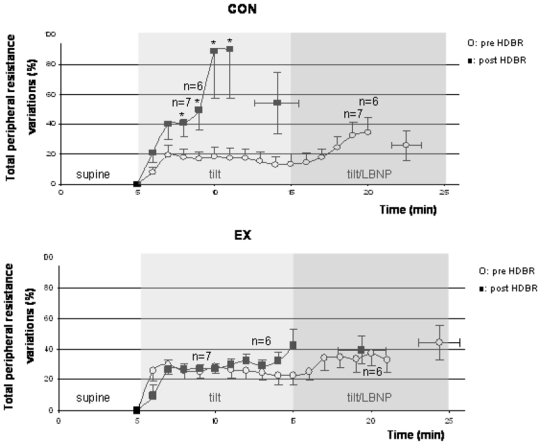
Total peripheral resistance changes (%, mean ± SE) during tilt/LBNP tests are shown for the CON and EX conditions, before (open circles) and after (closed squares) HDBR. Isolated points are TPR at presyncopal time point pre and post HDBR. * indicates significant increase in TPR values post HDBR compared with pre HDBR values.
Pre HDBR, TPR (%, mean ± SE) increased similarly in CON and EX groups over the course of the tilt/LBNP tests (+14±6% versus +23±6% at min 10, NS) and at presyncope (+26±10% versus +44±11%, NS). Significant HDBR and HDBR X group interaction effects (p<0.05) were observed after the 4th min in head-up tilt posture. In the CON group post HDBR, the increase in TPR was more pronounced than pre HDBR (+89±32% versus +18±7% at min 5, p<0.05). In the EX group, the post HDBR increase in TPR over the course of the tilt/LBNP tests was not significantly different than pre HDBR (+27±3% versus +29±5% at min 5 in upright posture, NS, +42±11% versus +23±6% at min 10, NS). At presyncope, the increase in TPR was not different in the two groups (+54±20% in CON group, +39±9% in EX group, NS).
4. Blood Volume
A technical problem (clotted blood sample) with the BV determination in one subject from the EX group at the end of HDBR led us to exclude this subject from analysis. There was no significant difference in BV between groups pre HDBR. In CON, BV (mean ± SE) decreased from 4161±142ml pre HDBR to 3783±136ml on day 60 of HDBR (−9±2%, p<0.01). In EX group, BV was maintained (4427±224ml before HDBR, 4213±168ml on day 60 of HDBR, −4±3%, p=0.17) (Figure 2, right).
DISCUSSION
The combined exercise countermeasure used in this study fails to protect orthostatic tolerance in women after a 60-day HDBR: OT time is not different after HDBR in CON and EX groups. Nevertheless some cardiovascular improvements in the EX group post HDBR are noteworthy: supine HR is maintained during HDBR, HR is lower and SV is better maintained over the course of the tilt/LBNP test. Furthermore, BV is maintained in EX and decreases significantly in CON.
Very few data exist on cardiovascular response to head-up tilt in women after long duration spaceflight or HDBR. After a 120-day HDBR in women, Maillet and co-workers (Maillet et al. 2000) observe a better maintained BP and a slower increase in HR during a 20 min head-up tilt test in a countermeasure group (60 to 80 minutes of daily supine running exercise on a treadmill with bungee cords compared to a control group (n=4 in each group). The greater HR response to an orthostatic challenge observed in their control group is also noted in our study, supporting an intact or even enhanced cardiac arm of the baroreflex. Concerning the vascular arm of the baroreflex, it is noteworthy that 1) the increase in TPR is more pronounced post HDBR in CON group than pre HDBR, 2) this maximal vascular response occurs earlier than pre HDBR, and 3) this response can not be maintained as long as pre HDBR, especially in the CON group. An early drop in SV is transiently compensated by a pronounced increase in HR (changes in Q are not significant), MAP is initially maintained by increased TPR (MAP=(SV*HR)*TPR), but these responses are rapidly overwhelmed resulting in early presyncope (Figures 4 to 8). The mechanisms involved in this altered response to orthostasis remain unclear but may include decreased blood volume, cardiac and vasculature remodelling, central nervous system dysregulation, and decreased ability to mobilise blood volume from the splanchnic area.
We did observe a maintained BV in our EX group and a significant loss after HDBR in our CON group, although less pronounced than in some previous HDBR studies of male subjects. A decrease in plasma volume of 10 to 20% over the first 30 days is a consistent finding for HDBR studies ranging from 30 to 90 days in male volunteers (Fortney et al. 1988; Fortney et al. 1994; Traon et al. 1995; Custaud et al. 2002; Belin de Chantemele et al. 2004a). There have been few previous investigations of the effects of HDBR on BV in women compared to men, and all such studies were of relatively short duration. A similar reduction of ~9% in male and female subjects was reported after a 7-day HDBR study (Custaud et al. 2002), while Fortney and collaborators reported less reduction of blood volume in women than men (−10% versus −15%) after a 13-day HDBR study (Fortney et al. 1994). A complicating factor in the present study in the comparison between groups was that BV was measured on HDBR day 60 which was approximately 36 hours after the last LBNP/Ex countermeasure session. BV is known to be reduced within the first 24-hours of HDBR (Vernikos et al. 1993). Therefore, the differences between groups might have been larger if measured on a similar schedule to that in other studies.
Concerning cardiac remodelling, it was found by Dorfman and collaborators (Dorfman et al. 2007) in the current WISE study that left and right ventricular volumes were maintained and left and right ventricular masses were increased in EX, compared to significant decreases in CON. Cardiac remodelling and atrophy (Levine et al. 1997; Dorfman et al. 2007) likely contributes to the reduced SV observed in CON in our study.
Several other factors may play a role in the better maintained cardiovascular responses in EX. Arbeille and collaborators, in this WISE study, document that the combined exercise countermeasure attenuates the bed rest effect on leg vein capacitance, with an increased pooling of blood in lower limbs veins in CON subjects compared with EX during an orthostatic stress (Arbeille et al. 2008). Edgell and co-workers observe a decreased basal leg vascular resistances in non-exercising subjects, whereas EX subjects have increased LVR post HDBR (Edgell et al. 2007). Last, Demiot and collaborators demonstrate an endothelial dysfunction in CON, with a reduced endothelium dependant vasodilation and an increased number of circulating endothelial cells, whereas those changes are not observed in EX (Demiot et al. 2007). All these factors are implicated in a better maintained venous return to the heart during an orthostatic stress in EX through a maintained vascular reactivity, resulting in the slower decrease in stroke volume.
Interestingly, the results of EX group in our study show that despite maintained cardiac mass, maintained BV, and attenuated cardiovascular changes associated with an orthostatic stress, orthostatic intolerance was not prevented. In our EX group, decreased OT while BV is maintained underlines the fact that BV is not a main factor for OT, at least after a long duration HDBR. Furthermore, in a retrospective analysis of contributory factors to orthostatic intolerance in men, Pavy-Le-Traon and collaborators observe that a reduced plasma volume is found in many tolerant subjects to orthostatism after HDBR (Pavy-Le-Traon et al. 1999).
Our OT time result is in opposition with of a previous study, where a 40 min of treadmill LBNP/Ex followed by 5 min of resting LBNP, 6 days/week, resulted in significantly less reduction in OT time in EX compared with CON (−13% versus −34%) (Watenpaugh et al. 2007). Nevertheless several differences exist between the earlier 30 day HDBR study and WISE. These factors are discussed below: 1) the HDBR period was longer (60 days); 2) WISE was conducted on female volunteers only 3) WISE combined a resistive exercise and an aerobic exercise within LBNP, resulting in a decreased number of LBNP/Ex sessions (3 to 4 per week for WISE compared with 6 per week for the 30 day HDBR study) 4) resting LBNP period after exercise was increased from 5min to 10 min in WISE 5) the OT test was scheduled 24 hours after the last LBNP/Ex session in the study of Watenpaugh and coll (Watenpaugh et al. 2007), but our OT test took place 62 –63 hours after the last LBNP/Ex session in WISE.
Increased duration of bed rest or spaceflight is associated with higher incidence of orthostatic intolerance (Meck et al. 2001), and women are more susceptible to orthostatic intolerance than men (Convertino 1998; Fu et al. 2004).
The resistive exercise performed by EX group during WISE was similar to the one performed every third day during a previous 90-day HDBR study in male subjects. This exercise showed no beneficial effect on orthostatic tolerance (Belin de Chantemele et al. 2004a) and did not prevent HDBR-induced reduction in blood volume (Belin de Chantemele et al. 2006). It is therefore unlikely that flywheel resistive exercise plays an important role in orthostatic tolerance in our female subjects.
It had been suggested by Watenpaugh and collaborators that increasing the resting LBNP period at the end of LBNP/Ex sessions might preserve OT (Watenpaugh et al. 2007). However, the poor compliance of one subject of EX group to the resting LBNP period after exercise led to decrease the level of LBNP applied (table 2 subject 8), thus compromising the countermeasure efficacy. It is noteworthy that exclusion of this non compliant subject from analysis results in significantly less reduction in OT time after HDBR in EX compared with CON (−28±5% versus −50±7%, p=0.02). Therefore the benefit for orthostatic intolerance of the resting LBNP period after exercise must be balanced with the risk of presyncope. Careful monitoring, especially during the first few training sessions, is necessary to familiarize each subject with: 1) their own presyncopal symptoms, 2) the effect of leg muscle contraction on such signs, and 3) manipulation of emergency valve to stop LBNP in case of increasing symptoms. On the other hand, the beneficial effect on orthostatic tolerance of this resting LBNP period may be related to the regular onset of a nearly-presyncopal state (Lightfoot et al. 1989). The intensity, duration and frequency of orthostatic stress should probably be adapted to each subject.
Considering the lack of impact of the flywheel countermeasures in male subjects on orthostatic tolerance (Belin de Chantemele et al. 2004a) and the slight effect of LBNP/exercise alone (Schneider et al. 2002), it is likely that the resting LBNP period after exercise plays a pivotal role for improved cardiovascular responses against an orthostatic stress in EX compared with CON in our study. Mechanisms involved are extensively described in previous paper from Watenpaugh and collaborators (Watenpaugh et al. 2007). Briefly, application of an orthostatic stimulus after exercise might stimulate an increased response of the sympathetic system to maintain blood pressure: post exercise changes could thus potentiate the cardiovascular stress imposed by the resting LBNP period. Recent work by Kimmerly and collaborators (Kimmerly et al. 2007) report a greater increase in muscle sympathetic nerve activity in subjects exposed to LBNP (−35mmHg) after a single bout of dynamic exercise compared with subjects without exercise.
Improvements in cardiovascular responses to tilt/LBNP tests in EX compared with CON group occurred even though the final exercise countermeasure session was performed 62–63 hours before the final orthostatic tolerance test. Butler and collaborators have found that only 4 hours of HDBR modifies cardiovascular responses to head-up tilt, with a marked increase in occurrence of presyncopal symptoms before ten min of head-up tilt (Butler et al. 1991). Khan and co-workers observe that after 24 hours of HDBR, the ability to augment the muscular sympathetic nerve activity at high levels of LBNP is reduced (Khan et al. 2002). Also, a recent study by Fischer and collaborators document an altered hormonal or neural control of regional vascular resistances after only 4 hours of HDBR: a reduced ability to increase splanchnic vascular resistance was observed during an orthostatic stress, that could explain the decrease in venous return and consequently in stroke volume (Fischer et al. 2007). These studies reveal the fast adaptation of the cardiovascular system to new gravitational conditions. For this present long-term HDBR study in women, numerous tests were scheduled during the last days of the HDBR period, preventing any exercise during the 2.5 days before our post HDBR tilt/LBNP orthostatic tolerance test. A better maintained orthostatic tolerance in EX may have been possible by scheduling the countermeasure closer to the end of HDBR.
From the study by Watenpaugh et al (Watenpaugh et al. 2007) and this WISE study we can formulate some conclusions: 1) 10 min of resting LBNP after exercise at a LBNP level simulating one BW can not be recommended because of a poor compliance in some subjects, 2) LBNP/Ex sessions with short post exercise resting LBNP periods should be scheduled during the very last days of HDBR or spaceflight, 3) repeated short periods of orthostatic stress during exercise may improve the efficacy of the countermeasure, especially during the last sessions (e.g., 5 min every 15 min of exercise) and 4) a short period of orthostatic stress after other modes of exercise (e.g., exercise associated with centrifugation) also may be beneficial.
Limitations of the study
An important factor that requires consideration in this study is the timing of experimentation with respect to studies of women and their menstrual phase. No subject was taking oral contraceptives for at least 2-months prior to the start of the research. In such a large study where subjects were housed in pairs and scheduling of multiple experiments was required, it is extremely difficult to balance or control the timing of an individual experiment with respect to menstrual cycle phase. As an indication of the phase of the cycle relative to experimentation, the number of subjects in the first 10 days of their cycle during the tilt/LBNP experiments was 4 CON and 5 EX during pre-HDBR and 4 CON and 4 EX at the end of HDBR. For blood volume experiments the corresponding numbers were 3 CON and 3 EX during pre HDBR testing and 2 CON and 3 EX on HDBR 60. Menstrual cycle phase does have an impact on plasma volume (Fortney et al. 1988) that might have masked the effect of HDBR and countermeasures in the current study. Differences between CON and EX groups probably did not skew the results as the number of subjects early in their cycle was similar in both groups. Nevertheless menstrual cycles were altered in many subjects, and the certain phase of their cycle could only be definitively affirmed with plasma sex hormone dosages. Rather, the period of time between the last countermeasure and the measurements of blood volume or orthostatic tolerance due to the requirement for other experimentation within this large-scale project probably reduced between-group differences.
Another limitation concerns the accuracy of the Modelflow method to assess absolute SV from finger blood pressure, and consequently Q and TPR. Controversial data exist on the reliability of absolute SV data estimated by Modelflow from non invasive finger pressure during orthostasis. Pitt and collaborators, comparing SV from Modelflow by Portapres and a rebreathing method, reveal an overestimation of Q by the Modelflow with large inter-individual variations (Pitt et al. 2004). However, Harms and collaborators observe a non-significant offset of SV by Modelflow for a 70-degree head-up tilt compared to the direct thermodilution method (Harms et al. 1999). Without an invasive calibrating method, absolute individual SV values should be viewed with caution, although relative mean changes in Modelflow-derived values seem to be reliable (Harms et al. 1999).
In conclusion, blood volume and subtolerance cardiovascular responses were better maintained after bed rest in women who performed aerobic and resistive exercise countermeasures. Interestingly, the improvements did not result in a longer orthostatic tolerance time.
Acknowledgments
We thank the outstanding 24 women who volunteered for this bed rest investigation.
We thank the nurses, staff, and entire research team at the MEDES Space Clinic (Toulouse Rangueil Hospital) for their exceptional care of the subjects during bed rest and exercise.
Special thanks to Drs M-P. Bareille and A. Beck for coordination and medical monitoring, D. Greaves for technical assistance, E. Wodey for the statistical help, and R. Tullet, S. Meuche, Alex Kowanz, Bjorn Redlich, Connie Fischer, Marius Dettmer, and Nicolas Sinanan for assistance with exercise. We also thank Dr Scott Trappe and his team for providing the flywheel resistive exercise to our subjects.
The study WISE-2005 was sponsored by the European Space Agency (ESA), the National Aeronautics and Space Administration of the USA (NASA), the Canadian Space Agency (CSA), and the French “Centre National d’Etudes Spatiales” (CNES), which has been the “Promoteur” of the study according to French law.
This work was also supported by a NASA grant NNJ04HF71G to AR Hargens, and CSA grant 9F007-046025/001/ST to RL Hughson. The study has been performed by MEDES, Institute for Space Physiology and Medicine in Toulouse, France.
BIBLIOGRAPHY
- Alkner BA, Tesch PA. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur J Appl Physiol. 2004;93:294–305. doi: 10.1007/s00421-004-1172-8. [DOI] [PubMed] [Google Scholar]
- Arbeille P, Kerbeci P, Mattar L, Shoemaker JK, Hughson RL. WISE-2005: tibial and gastrocnemius vein and calf tissue response to LBNP after a 60-day bed rest with and without countermeasures. J Appl Physiol. 2008;104:938–943. doi: 10.1152/japplphysiol.01021.2007. [DOI] [PubMed] [Google Scholar]
- Arbeille P, Pavy-le Traon A, Fomina G, Vasseur P, Guell A. Femoral flow response to lower body negative pressure: an orthostatic tolerance test. Aviat Space Environ Med. 1995;66:131–136. [PubMed] [Google Scholar]
- Belin de Chantemele E, Blanc S, Pellet N, Duvareille M, Ferretti G, Gauquelin-Koch G, Gharib C, Custaud MA. Does resistance exercise prevent body fluid changes after a 90-day bed rest? Eur J Appl Physiol. 2004a;92:555–564. doi: 10.1007/s00421-004-1121-6. [DOI] [PubMed] [Google Scholar]
- Belin de Chantemele E, Gauquelin-Koch G, Duvareille M, Pellet N, Gharib C, Custaud MA. Blood volume measurement: The comparison of pulse dye densitometry and Dill and Costill’s methods. Life Sci. 2006;78:1564–1569. doi: 10.1016/j.lfs.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Belin de Chantemele E, Pascaud L, Custaud MA, Capri A, Louisy F, Ferretti G, Gharib C, Arbeille P. Calf venous volume during stand-test after a 90-day bed-rest study with or without exercise countermeasure. J Physiol. 2004b;561:611–622. doi: 10.1113/jphysiol.2004.069468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boda WL, Watenpaugh DE, Ballard RE, Hargens AR. Supine lower body negative pressure exercise simulates metabolic and kinetic features of upright exercise. J Appl Physiol. 2000;89:649–654. doi: 10.1152/jappl.2000.89.2.649. [DOI] [PubMed] [Google Scholar]
- Buckey JC, Jr, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Moore WE, Gaffney FA, Blomqvist CG. Orthostatic intolerance after spaceflight. J Appl Physiol. 1996;81:7–18. doi: 10.1152/jappl.1996.81.1.7. [DOI] [PubMed] [Google Scholar]
- Burge CM, Skinner SL. Determination of hemoglobin mass and blood volume with CO: evaluation and application of a method. J Appl Physiol. 1995;79:623–631. doi: 10.1152/jappl.1995.79.2.623. [DOI] [PubMed] [Google Scholar]
- Butler GC, Xing HC, Northey DR, Hughson RL. Reduced orthostatic tolerance following 4 h head-down tilt. Eur J Appl Physiol Occup Physiol. 1991;62:26–30. doi: 10.1007/BF00635629. [DOI] [PubMed] [Google Scholar]
- Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol. 1998;275:R1909–1920. doi: 10.1152/ajpregu.1998.275.6.R1909. [DOI] [PubMed] [Google Scholar]
- Convertino VA, Doerr DF, Stein SL. Changes in size and compliance of the calf after 30 days of simulated microgravity. J Appl Physiol. 1989;66:1509–1512. doi: 10.1152/jappl.1989.66.3.1509. [DOI] [PubMed] [Google Scholar]
- Custaud MA, de Souza Neto EP, Abry P, Flandrin P, Millet C, Duvareille M, Fortrat JO, Gharib C. Orthostatic tolerance and spontaneous baroreflex sensitivity in men versus women after 7 days of head-down bed rest. Auton Neurosci. 2002;100:66–76. doi: 10.1016/s1566-0702(02)00132-7. [DOI] [PubMed] [Google Scholar]
- Demiot C, Dignat-George F, Fortrat JO, Sabatier F, Gharib C, Larina I, Gauquelin-Koch G, Hughson R, Custaud MA. WISE 2005: chronic bed rest impairs microcirculatory endothelium in women. Am J Physiol Heart Circ Physiol. 2007;293:H3159–3164. doi: 10.1152/ajpheart.00591.2007. [DOI] [PubMed] [Google Scholar]
- Dorfman TA, Levine BD, Tillery T, Peshock RM, Hastings JL, Schneider SM, Macias BR, Biolo G, Hargens AR. Cardiac atrophy in women following bed rest. J Appl Physiol. 2007;103:8–16. doi: 10.1152/japplphysiol.01162.2006. [DOI] [PubMed] [Google Scholar]
- Edgell H, Zuj KA, Greaves DK, Shoemaker JK, Custaud MA, Kerbeci P, Arbeille P, Hughson RL. WISE-2005: adrenergic responses of women following 56-days, 6 degrees headdown bed rest with or without exercise countermeasures. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2343–2352. doi: 10.1152/ajpregu.00187.2007. [DOI] [PubMed] [Google Scholar]
- el-Bedawi KM, Hainsworth R. Combined head-up tilt and lower body suction: a test of orthostatic tolerance. Clin Auton Res. 1994;4:41–47. doi: 10.1007/BF01828837. [DOI] [PubMed] [Google Scholar]
- Fischer D, Arbeille P, Shoemaker JK, O’Leary DD, Hughson RL. Altered hormonal regulation and blood flow distribution with cardiovascular deconditioning after short-duration head down bed rest. J Appl Physiol. 2007;103:2018–2025. doi: 10.1152/japplphysiol.00121.2007. [DOI] [PubMed] [Google Scholar]
- Fortney SM, Beckett WS, Carpenter AJ, Davis J, Drew H, LaFrance ND, Rock JA, Tankersley CG, Vroman NB. Changes in plasma volume during bed rest: effects of menstrual cycle and estrogen administration. J Appl Physiol. 1988;65:525–533. doi: 10.1152/jappl.1988.65.2.525. [DOI] [PubMed] [Google Scholar]
- Fortney SM, Turner C, Steinmann L, Driscoll T, Alfrey C. Blood volume responses of men and women to bed rest. J Clin Pharmacol. 1994;34:434–439. doi: 10.1002/j.1552-4604.1994.tb04984.x. [DOI] [PubMed] [Google Scholar]
- Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, Levine BD. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol. 2004;286:H449–457. doi: 10.1152/ajpheart.00735.2002. [DOI] [PubMed] [Google Scholar]
- Guell A, Braak L, Le Traon AP, Gharib C. Cardiovascular adaptation during simulated microgravity: lower body negative pressure to counter orthostatic hypotension. Aviat Space Environ Med. 1991;62:331–335. [PubMed] [Google Scholar]
- Hargens AR, Whalen RT, Watenpaugh DE, Schwandt DF, Krock LP. Lower body negative pressure to provide load bearing in space. Aviat Space Environ Med. 1991;62:934–937. [PubMed] [Google Scholar]
- Harm DL, Jennings RT, Meck JV, Powell MR, Putcha L, Sams CP, Schneider SM, Shackelford LC, Smith SM, Whitson PA. Invited review: gender issues related to spaceflight: a NASA perspective. J Appl Physiol. 2001;91:2374–2383. doi: 10.1152/jappl.2001.91.5.2374. [DOI] [PubMed] [Google Scholar]
- Harms MP, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, Van Lieshout JJ. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci (Lond) 1999;97:291–301. [PubMed] [Google Scholar]
- Khan MH, Kunselman AR, Leuenberger UA, Davidson WR, Jr, Ray CA, Gray KS, Hogeman CS, Sinoway LI. Attenuated sympathetic nerve responses after 24 hours of bed rest. Am J Physiol Heart Circ Physiol. 2002;282:H2210–2215. doi: 10.1152/ajpheart.00862.2001. [DOI] [PubMed] [Google Scholar]
- Kimmerly DS, Wong SW, Salzer D, Menon R, Shoemaker JK. Forebrain regions associated with postexercise differences in autonomic and cardiovascular function during baroreceptor unloading. American journal of physiology. 2007;293:H299–306. doi: 10.1152/ajpheart.00044.2007. [DOI] [PubMed] [Google Scholar]
- Lee SM, Moore AD, Jr, Fritsch-Yelle JM, Greenisen MC, Schneider SM. Inflight exercise affects stand test responses after space flight. Med Sci Sports Exerc. 1999;31:1755–1762. doi: 10.1097/00005768-199912000-00010. [DOI] [PubMed] [Google Scholar]
- Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation. 1997;96:517–525. doi: 10.1161/01.cir.96.2.517. [DOI] [PubMed] [Google Scholar]
- Lightfoot JT, Febles S, Fortney SM. Adaptation to repeated presyncopal lower body negative pressure exposures. Aviat Space Environ Med. 1989;60:17–22. [PubMed] [Google Scholar]
- Maillet A, Zaouali-Ajina M, Vorobiev D, Blanc S, Pastouchkova L, Reushkina G, Morukov B, Grigoriev AI, Gharib C, Gauquelin-Koch G. Orthostatic tolerance and hormonal changes in women during 120 days of head-down bed rest. Aviat Space Environ Med. 2000;71:706–714. [PubMed] [Google Scholar]
- Meck JV, Reyes CJ, Perez SA, Goldberger AL, Ziegler MG. Marked exacerbation of orthostatic intolerance after long- vs. short-duration spaceflight in veteran astronauts. Psychosom Med. 2001;63:865–873. doi: 10.1097/00006842-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Murthy G, Watenpaugh DE, Ballard RE, Hargens AR. Exercise against lower body negative pressure as a countermeasure for cardiovascular and musculoskeletal deconditioning. Acta Astronaut. 1994;33:89–96. doi: 10.1016/0094-5765(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Pavy-Le Traon A, Louisy F, Vasseur-Clausen P, Guell A, Gharib C. Contributory factors to orthostatic intolerance after simulated weightlessness. Clin Physiol. 1999;19:360–368. doi: 10.1046/j.1365-2281.1999.00189.x. [DOI] [PubMed] [Google Scholar]
- Pitt MS, Marshall P, Diesch JP, Hainsworth R. Cardiac output by Portapres. Clin Sci (Lond) 2004;106:407–412. doi: 10.1042/CS20030279. [DOI] [PubMed] [Google Scholar]
- Rittweger J, Frost HM, Schiessl H, Ohshima H, Alkner B, Tesch P, Felsenberg D. Muscle atrophy and bone loss after 90 days’ bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone. 2005;36:1019–29. doi: 10.1016/j.bone.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Schneider SM, Watenpaugh DE, Lee SM, Ertl AC, Williams WJ, Ballard RE, Hargens AR. Lower-body negative-pressure exercise and bed-rest-mediated orthostatic intolerance. Med Sci Sports Exerc. 2002;34:1446–1453. doi: 10.1097/00005768-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Sigaudo-Roussel D, Custaud MA, Maillet A, Guell A, Kaspranski R, Hughson RL, Gharib C, Fortrat JO. Heart rate variability after prolonged spaceflights. Eur J Appl Physiol. 2002;86:258–265. doi: 10.1007/s00421-001-0551-7. [DOI] [PubMed] [Google Scholar]
- Traon AP, Vasseur P, Arbeille P, Guell A, Bes A, Gharib C. Effects of 28-day head-down tilt with and without countermeasures on lower body negative pressure responses. Aviat Space Environ Med. 1995;66:982–991. [PubMed] [Google Scholar]
- Vernikos J, Dallman MF, Keil LC, O’Hara D, Convertino VA. Gender differences in endocrine responses to posture and 7 days of -6 degrees head-down bed rest. Am J Physiol. 1993;265:E153–161. doi: 10.1152/ajpendo.1993.265.1.E153. [DOI] [PubMed] [Google Scholar]
- Watenpaugh DE, Ballard RE, Schneider SM, Lee SM, Ertl AC, William JM, Boda WL, Hutchinson KJ, Hargens AR. Supine lower body negative pressure exercise during bed rest maintains upright exercise capacity. J Appl Physiol. 2000;89:218–227. doi: 10.1152/jappl.2000.89.1.218. [DOI] [PubMed] [Google Scholar]
- Watenpaugh DE, O’Leary DD, Schneider SM, Lee SM, Macias BR, Tanaka K, Hughson RL, Hargens AR. Lower body negative pressure exercise plus brief postexercise lower body negative pressure improve post-bed rest orthostatic tolerance. J Appl Physiol. 2007;103:1964–1972. doi: 10.1152/japplphysiol.00132.2007. [DOI] [PubMed] [Google Scholar]
- Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol. 1993;74:2566–2573. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]


