Abstract
Background/Objectives. Rapid risk stratification of the patient with acute chest pain is essential to select the best management. We investigated the value of the ECG at first medical contact to determine size of the ischaemic myocardial area and thereby severity of risk.
Methods. In 386 patients with acute chest pain, ECG findings were correlated with the coronary angiogram. Using ST-segment deviation patterns the location of the coronary culprit lesion was predicted and thereby size of the area at risk. Four groups of patients were present. Those with a narrow QRS and a total 12-lead ST-segment deviation score of ≥5 mm (group 1) or ≤4 mm (group 2); a QRS width of ≥120 ms (group 3), and patients with previous coronary bypass grafting (CABG) or percutaneous coronary intervention (PCI) (group 4).
Results. Correct coronary culprit lesion localisation was possible in 84% of the 185 patients in group 1, 40% of the total cohort. Accurate prediction was not possible in most patients in groups 2, 3 and 4, in spite of extensive coronary artery disease in group 3 and 4.
Conclusions. Using the 12-lead ECG the size of the myocardial area at risk can be accurately predicted when the total ST-segment deviation score is ≥5 mm, allowing identification of those in need of a PCI. In most patients with bundle branch block, previous CABG or PCI, the ECG can not localise the culprit lesion. This approach simplifies and accelerates decision-making at first medical contact. (Neth Heart J 2010;18:301–6.)
Keywords: Chest Pain, Coronary Disease, Electrocardiography, Risk Assessment, Time Factors
In acute cardiac ischaemia the size of the cardiac area at risk is related to the site of occlusion of the culprit coronary artery. The closer to the origin of the coronary vessel the larger the jeopardised area. Therefore the purpose of our study was to find out how good the ECG is at the time of first medical contact to give information about the size of the area at risk, helping decision-making about the best and quickest way to prevent myocardial damage.
The initial ECG changes in acute cardiac ischaemia take place in the ST segment with ST-segment elevation and depression in the different leads. Specific patterns of ST-segment deviations in the 12-lead ECG allow a more accurate localisation of the occlusion site in the culprit coronary artery, giving a better estimate of the size of the area at risk.1-16
Methods
From January 2006 to September 2008, 400 patients with acute chest pain were taken by ambulance to the Marienhospital of the Med Klinik II of the Ruhr University in Herne, Germany. A 12-lead ECG was recorded outside the hospital and a coronary angiogram performed within one hour. To predict the location of the coronary culprit lesion, an algorithm was developed based on earlier described ECG characteristics16 and results from a retrospective study during 2004 and 2005 in 499 patients with acute chest pain admitted to the Isala Clinics in Zwolle, the Netherlands.
The algorithm uses computer-assisted ST-deviation measurements in the 12 ECG leads at 60 ms after the J point with the TP segment as the reference line, measuring both ST elevation and ST depression. The direction of ST-segment deviation indicates the occlusion site in the coronary artery (figure 1).16-19 To differentiate lesions in the proximal and distal right coronary artery and the circumflex artery11,12 lead V4R was recorded instead of lead V4, after we found that ST deviation in lead V4 is the mean of ST deviations in leads V3 and V5. Apart from ST-segment deviation measurement (elevated, isoelectric or depressed) in the individual lead, the sum of deviations in all leads was determined and measured in mm’s, resulting in the total ST-segment deviation score. Therefore, in each patient both the total ST-segment deviation score and the ST-segment deviation direction were determined.
Figure 1.
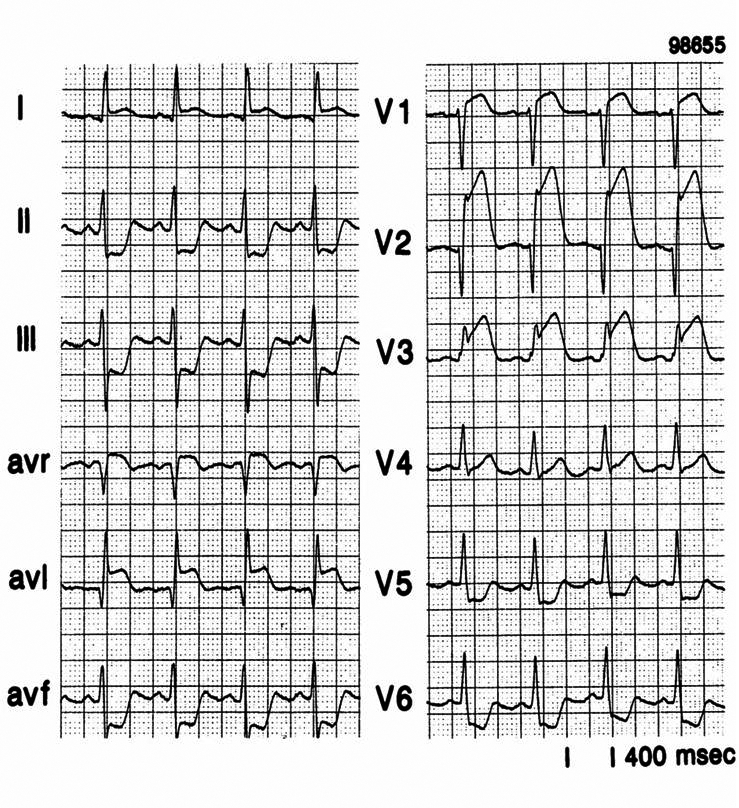
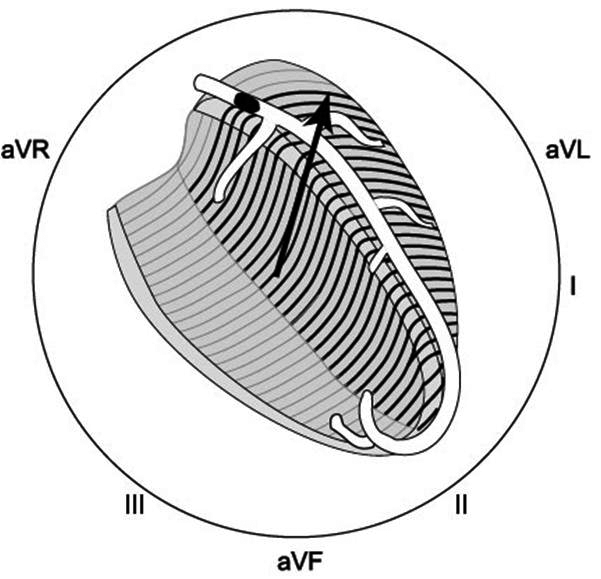
An example of the ST-segment deviation behaviour in a patient with an acute anterior wall myocardial infarction because of an occlusion in the left anterior descending coronary artery proximal to the first septal and the first diagonal branch. On the left the 12-lead ECG, on the right the ST-segment deviation axis in the frontal plane. The axis goes from the leads showing ST-segment depression to the leads showing ST-segment elevation.
The culprit lesion sites suggested by the algorithm are: left main; proximal left anterior descending (LAD), either proximal to the first septal branch, the first diagonal branch or proximal to both; distal LAD, distal to first septal and first diagonal; proximal right coronary artery (RCA), proximal to the right ventricular branch; distal RCA, distal to the right ventricular branch; circumflex coronary artery (Cx); multivessel disease; or no localisation possible. The characteristics of ST-segment deviations in these different coronary occlusion sites are given in references 16 and 19.
The coronary angiogram, taken within one hour after the ECG, was analysed by two investigators, unaware of the ECG findings. For this study the exact sites of occlusion or severe coronary narrowing (>70%) were described.
Results
A total of 400 patients entered the study. In 14 patients the ECG could not be accepted because of artifacts (13) or lead misplacement (1). Of the remaining 386 patients, 280 were men (mean age 61.8±13.2 years), and 106 women (mean age 69.3±13.4 years). Forty-four patients had undergone previous coronary artery bypass grafting (CABG) or a percutaneous coronary intervention (PCI), and 53 patients had a QRS width of more than 120 ms. The 289 patients with a narrow QRS during sinus rhythm were divided into 185 with a total ST-segment deviation score of ≥5 mm, and 104 patients with a score of ≤4 mm.
Table 1 gives the findings in the 185 patients with a total ST-deviation score of at least 5 mm (group 1). The ECG algorithm correctly predicted the occlusion site in almost all patients with a proximal occlusion (left main, proximal LAD, proximal RCA). Seven out of the 185 patients with ≥5 mm ST deviation (varying from 6 to 14 mm) had no coronary heart disease. All were men, aged 27 to 50 years. One had severe hypertension, two a dilated cardiomyopathy and four early repolarisation changes on the ECG. Therefore, in patients with acute chest pain and an ECG with a total ST-deviation score of ≥5 mm the coronary occlusion site was identified correctly in 156 of the 185 patients (84.3%).
Table 1.
Correlation between coronary angiographic and ECG algorithm findings in 185 patients with ST-deviation score of 5 mm or more.
| Site of coronary occlusion according to ECG algorithm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LM | Prox LAD | Dist LAD | Prox RCA | Dist RCA | CX | Multi-vessel | No location possible | Correct diagnosis | % | |
| CA diagnosis | ||||||||||
| Left main | 13 | 1 | 13/14 | 93 | ||||||
| Prox LAD | 1 | 54 | 1 | 54/56 | 96 | |||||
| Dist LAD | 1 | 22 | 1 | 22/24 | 92 | |||||
| Prox RCA | 36 | 36/36 | 100 | |||||||
| Dist RCA | 1 | 1 | 10 | 10/12 | 83 | |||||
| CX | 1 | 1 | 1 | 12 | 1 | 12/16 | 75 | |||
| Multi-vessel | 2 | 1 | 1 | 9 | 9/13 | 69 | ||||
| Coronary narrowing | 3 | 1 | 2 | 1 | 0/7 | 0 | ||||
| No occlusion | 2 | 3 | 1 | 1 | 0/7 | 0 | ||||
CA=coronary angiogram, LM=left main artery, LAD=left anterior descending, RCA=right coronary artery, CX=circumflex artery.
In contrast, in the 104 patients with ≤4 mm (group 2), the location of the culprit lesion could not be predicted from the ST-segment deviation. Table 2 gives the ECG and coronary angiographic findings.
Table 2.
Coronary angiographic and ECG findings in 104 patients with ST-segment deviation of 4 mm or less.
| Coronary angiographic findings | |||
|---|---|---|---|
| No CAD | Coronary occlusion | Coronary narrowing >70% | |
| ECG findings | |||
| Q wave MI | 18 | 20 | |
| Negative T-waves | 6 | 11 | |
| ST depression | 1 | 2 | |
| No abnormalities | 25 | 6 | 15 |
| Total | 25 | 31 | 48 |
CAD=coronary artery disease, MI=myocardial infarction.
In 53 patients a widened QRS complex (of ≥120 ms) was present. Twelve patients had both a previous CABG/PCI and a widened QRS. Right bundle branch block (RBBB) was present in 24 patients, with two patients having no coronary artery disease. In 11 out of 13 RBBB patients with a total ST-segment deviation score of ≥5 mm the coronary occlusion site was correctly predicted; all patients had a proximal LAD occlusion.
Left bundle branch block (LBBB) was present in 25 patients, with 22 having severe, usually multivessel, CAD. Three LBBB patients had no CAD (hypertension in one, severe aortic stenosis in one, and a dilated cardiomyopathy in one). In three patients the wide QRS was caused by frequent episodes of non-sustained ventricular tachycardia. One patient had a paced QRS complex.
Therefore, in LBBB patients the culprit lesion could not be predicted.
Forty-four patients had a previous CABG or PCI. In 27 patients the ST-segment deviation score was ≤4 mm. In eight of the 17 patients with an ST-deviation score of ≥5 mm the occlusion site in the coronary artery was correctly predicted by the algorithm.
Discussion
The early ECG changes during cardiac ischaemia take place in the ST segment. When thrombolytic agents were introduced, they were most successful when given during the stage of ST-segment deviation before the development of Q waves and T-wave inversion.20 That reperfusion therapy should be applied as soon as possible, preferably during the stage of ST-segment deviation, is clearly stressed in the current guidelines.21,22 When possible, ECG information should already be obtained during the first patient contact outside hospital.23
Unfortunately, in many countries this is currently possible in only a limited number of patients.24 By determining the pattern of ST-segment deviations in the different ECG leads one may be able to risk stratify the chest pain patient according to the size of the jeopardised area, and a computerised ECG analysis to obtain that information has been suggested.25 However, validation of this concept requires a short interval between ECG and coronary arteriogram, as in our study.
So far, no information was available on how much 12-lead ST-segment deviation is required to use the ST-segment deviation direction to correctly predict the location of the culprit lesion. We also investigated the value of the ECG in chest pain patients with bundle branch block or a previous CABG or PCI.
Our results suggest that four patient categories can be recognised for rapid decision-making in acute chest pain: 1) patients with a narrow QRS and an ST-segment deviation score of ≥5 mm; 2) patients with a narrow QRS and an ST-segment deviation score of ≤4 mm; 3) patients with a widened QRS (≥120 ms); and 4) patients with a previous CABG or PCI.
Narrow QRS and ST-segment deviation score of ≥5 mm
Present in 185 of the 386 patients (47%), narrow QRS and an ST-segment deviation score of ≥5 mm allowed recognition of the high-risk patients because of a proximal coronary occlusion; 93% of left main, 96% of proximal LAD, and 100% of proximal RCA occlusion were correctly identified. In inferoposterior MI lead V4R was very helpful to differentiate between a proximal RCA, distal RCA or a circumflex occlusion. Of interest is the finding that all seven of the 184 patients without coronary heart disease were men, five patients were ≤40 years with four having an early repolarisation pattern on their ECG.
Narrow QRS and ST-segment deviation score of ≤4 mm
Of the 104 patients with a narrow QRS and an ST-segment deviation score of ≤4 mm (29% of the 386), 25 had no coronary artery disease. As shown by Macfarlane, a limited amount of ST-segment elevation may be present in healthy people.26 In 31 patients an occluded coronary artery and in 48 patients coronary narrowing was present. Most patients with CAD had Q waves or inverted T waves in two or more consecutive leads. In 12 out of the 14 patients with a coronary occlusion in whom the time interval between onset of chest pain and ECG recording was known that value exceeded 12 hours. Therefore, in most patients with coronary heart disease and an ST-deviation score of ≤4 mm the culprit lesion can not be localised using the 12-lead ECG. Q waves and/or inverted T waves suggest either an old myocardial infarction or a long interval between onset of chest pain and the ECG. Those patients are less likely to benefit from a rapid reperfusion attempt.
Widened QRS (≥120 ms)
Fifty-three patients (13 % of the 386) fell into the widened QRS category. Forty-nine patients had either right or left bundle branch block. There were four important findings: 1) 11 had a previous CABG/PCI; 2) severe CAD was present in 44 of the 49; 3) in case of RBBB the culprit lesion could be localised if a total ST-segment deviation score of ≥5 mm was present; and 4) in LBBB patients the coronary occlusion site could not be identified. This is not surprising because LBBB, in contrast to RBBB, results in marked changes in the left ventricular depolarisation pattern and therefore also in an abnormal pattern of repolarisation.
Previous CABG or PCI
Of the 44 patients with previous CABG or PCI (11% of the 386), 27 had an ST-deviation score of ≤4 mm, making localisation of the culprit lesion impossible in the majority of these patients, in spite of extensive coronary artery disease.
Limitations
There are many different non-coronary causes leading to ST-segment deviation, stressing the importance of using the algorithm diagnosis only in case of acute chest pain.
Unfortunately we do not have information about the time interval between the onset of chest pain and the ECG for most patients. Less or absent ST-segment deviation suggests a long interval between the coronary occlusion and the ECG. Also, as pointed out already, in coronary-related chest pain ST-segment deviation may not indicate the culprit coronary artery during abnormal ventricular depolarisation, previous cardiac damage or a previous coronary intervention.
The practical approach
After being questioned about the duration of pain and presence or absence of a previous myocardial infarction, CABG or PCI, patients with acute chest pain can be divided into four groups: 1) Patients with a 12-lead ECG with a narrow QRS and an ST-segment deviation score of ≥5 mm. In those patients, the ECG algorithm is able to identify those patients with a very proximal coronary occlusion, at a time that they will benefit from an early reperfusion intervention. Preferably they should go to a PCI centre, even after fibrinolysis has been given, because of a long distance to the PCI centre.27 2) Patients with a narrow QRS and a total ST-segment deviation score of ≤4 mm. Then the ECG is usually not helpful to indicate the location of the culprit lesion. These patients have either no coronary artery disease, coronary narrowing or in a number of cases a coronary occlusion of longer duration. The specific lead location of Q waves and inverted T waves is a better marker for the site of cardiac damage. 3) Patients with a QRS width of ≥120 ms. Especially in patients with LBBB the presence of abnormal left ventricular activation and repolarisation make the pattern of ST-segment deviation unreliable to indicate the location of the culprit lesion. Since most of these patients were found to have severe coronary artery disease, rapid referral to a PCI centre, where therapy can be based upon the coronary angiogram, is advisable.
There is a fourth group of patients in whom the ECG usually does not indicate the culprit lesion. These are the patients with known coronary artery disease who have had a previous myocardial infarction or a previous intervention, either CABG or PCI. Coronary angiography will be required to determine the culprit lesion and rapid referral to a PCI centre should be the preferred mode of management.28
Conclusion
In the patient with acute chest pain the ECG, using the ST-segment deviation direction, is of value to accelerate decision-making outside hospital by identifying patients most likely to profit from a rapid percutaneous coronary intervention, such as those with a proximal coronary lesion, or bundle branch block. Culprit lesion location can not usually be predicted by the ECG in patients with a previous coronary occlusion, PCI or CABG.
References
- 1.Birnbaum Y, Sclarovky S, Solodky A, et al. Prediction of the level of left anterior descending coronary artery obstruction during anterior wall acute myocardial infarction by the admission electrocardiogram. Am J Cardiol. 1993;72;823-6. [DOI] [PubMed] [Google Scholar]
- 2.Herz I, Assali AR, Adler Y, et al. New electrocardiographic criteria for predicting either the right or left circumflex artery as the culprit coronary artery in inferior wall acute myocardial infarction. Am J Cardiol. 1997;80;1343-5. [DOI] [PubMed] [Google Scholar]
- 3.Zimetbaum PJ, Krishnan S, Gold A, et al. Usefulness of ST-segment elevation in lead III exceeding that of lead II for identifying the location of the totally occluded coronary artery in inferior wall myocardial infarction. Am J Cardiol. 1998;81;918-9. [DOI] [PubMed] [Google Scholar]
- 4.Sclarovsky S. Electrocardiography of acute myocardial ischemic syndromes. London, Martin Dunitz Ltd; 1999. [Google Scholar]
- 5.Engelen DJ, Gorgels AP, Cheriex EC, et al. Value of the electrocardiogram in localizing the occlusion site in the left anterior descending coronary artery in acute anterior myocardial infarction. J Am Cardiol. 1999;34;389-95. [DOI] [PubMed] [Google Scholar]
- 6.Yamaji H, Iwasachi S, Kusachi S, et al. Prediction of acute left main coronary obstruction by 12-lead electrocardiogram: aVR ST-elevation with less V1 ST-segment elevation. J Am Coll Cardiol. 2001;38;1348-54. [DOI] [PubMed] [Google Scholar]
- 7.Birnbaum Y, Drew BJ. The electrocardiogram in ST elevation acute myocardial infarction: correlation with coronary anatomy and prognosis. Postgrad Med J. .2003;79;490-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiol M, Cygankiewicz I, Carillo A, et al. Value of electrocardiographic algorithm based on “ups and downs” of ST in assessment of a culprit artery in evolving inferior wall acute myocardial infarction Am J Cardiol. 2004;94;709-14. [DOI] [PubMed] [Google Scholar]
- 9.Erhardt LR, Sjogren A, Wahlberg I. Single right sided precordial lead in the diagnosis of right ventricular involvement in inferior myocardial infarction. Am Heart J. 1976;91;571-6. [DOI] [PubMed] [Google Scholar]
- 10.Braat SH, Brugada P, De Zwaan C, et al. Value of the electrocardiogram in diagnosing right ventricular involvement in patients with an acute inferior wall myocardial infarction. Br Heart J. 1983;49;368-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saski K, Yotsukura M, Sakata K, et al. Relation of ST-segment changes in inferior leads during anterior wall acute myocardial infarction to length and occlusion site of the left anterior descending coronary artery. Am J Cardiol. 2001;87;1340-6. [DOI] [PubMed] [Google Scholar]
- 12.Zimetbaum PJ, Josephson ME. Use of the electrocardiogram in acute myocardial infarction. N Engl J Med. 2003;348;933-40. [DOI] [PubMed] [Google Scholar]
- 13.Atar S, Barbagelata A, Birnbaum Y. Electrocardiographic diagnosis of ST-elevation myocardial infarction. Cardiol Clin. 2006;24;343-65. [DOI] [PubMed] [Google Scholar]
- 14.Eskola MJ, Nikus KC, Holmvang L, et al. Value of the 12-lead electrocardiogram to define the level of obstruction in acute anterior wall myocardial infarction: correlation to coronary angiography and clinical outcome in the DANAMI-2 trial. Int J Cardiol. 2009;131;378-83. [DOI] [PubMed] [Google Scholar]
- 15.Wang SS, Paynter l, Kelly RV, et al. Electrocardiographic determination of culprit lesion site in patients with acute coronary events. J Electrocardol. 2009;42;46-51. [DOI] [PubMed] [Google Scholar]
- 16.Wellens HJJ, Conover M. The ECG in Emergency Decision Making, 2nd edition, St Louis: Elsevier/Saunders; 2006: p 3-16. [Google Scholar]
- 17.Foerster JM, Vera Z, Janzen DA, et al. Evaluation of precordial orthogonal vector cardiographic lead ST segment magnitude in the assessment of myocardial ischemia injury. Circulation. 1977;55;728-35. [DOI] [PubMed] [Google Scholar]
- 18.Hurst JW. Methods used to interpret the 12-lead electrocardiogram: pattern memorization vs the use of the vector concept. Clin Cardiol.2004;24;4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorgels APM. Chapter 3 Electrocardiography. In: Willerson JT, Cohn JN, Wellens HJJ, Holmes DR Jr, eds. Cardiovascular Medicine, 3d ed. London: Springer; 2007: 60-7. [Google Scholar]
- 20.Bar FW, Vermeer F, De Zwaan C, et al. Value of the admission electrocardiogram in predicting outcome of thrombolytic therapy in acute myocardial infarction. Am J Cardiol. 1987;59;6-13. [DOI] [PubMed] [Google Scholar]
- 21.Antman EM, Hand M, Armstrong PW, et al. 2007 Focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51;1210-47. [DOI] [PubMed] [Google Scholar]
- 22.Van de Werf F, Bax J, Betriu A, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation. The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology. Eur Heart J. 2008;29;2909-45. [DOI] [PubMed] [Google Scholar]
- 23.Antman EM. Time is muscle. Translation into practice. J Am Coll Cardiol. 2008; 52; 1216-21. [DOI] [PubMed] [Google Scholar]
- 24.Diercks DB, Kontos CK, Chen AY, et al. Utilization and impact of pre-hospital electrocardiograms for patients with acute ST-segment elevation myocardial infarction. Data from the NCDR ( National Cardiovascular Data Registry) ACTION ( Acute Coronary Treatment and Intervention Outcomes Network) registry. J Am Coll Cardiol. 2009;53;161-6. [DOI] [PubMed] [Google Scholar]
- 25.Eskola MJ, Nikus KC, Voipio-Pulkki LM, et al. Detection of proximal coronary occlusion in acute coronary syndrome: a feasibility study using computerized electrocardiographic analysis. Ann Noninvasive Electrocardiol. 2007;12;301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macfarlane PW. Age, sex and the ST amplitude in health and disease. J Electrocardiol. 2001;34;S35-S41. [DOI] [PubMed] [Google Scholar]
- 27.Cantor WJ, Fitchett D, Borgundvaag B, et al, for the TRANSFER-AMI Trial Investigators. Routine early angioplasty after fibrinolysis for acute myocardial Infarction. N Engl J Med. 2009;360;2705-18. [DOI] [PubMed] [Google Scholar]
- 28.Berry C, Pieper KS, White HD, et al. Patients with prior coronary bypass grafting have a poor outcome after myocardial infarction: an analysis of the VALsartan in acute myocardial infarction trial (VALIANT). Eur Heart J. 2009;30;1450-6. [DOI] [PubMed] [Google Scholar]


