Abstract
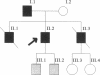
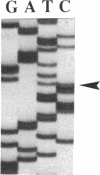
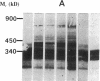
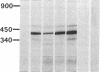
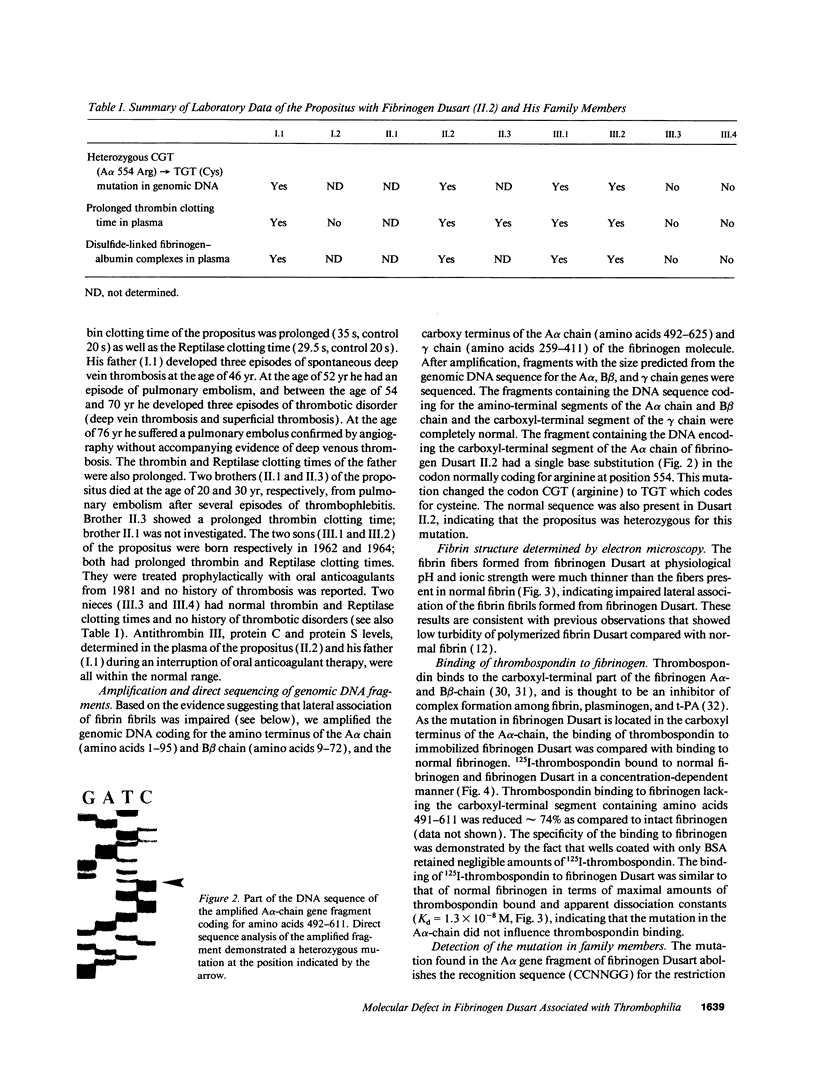
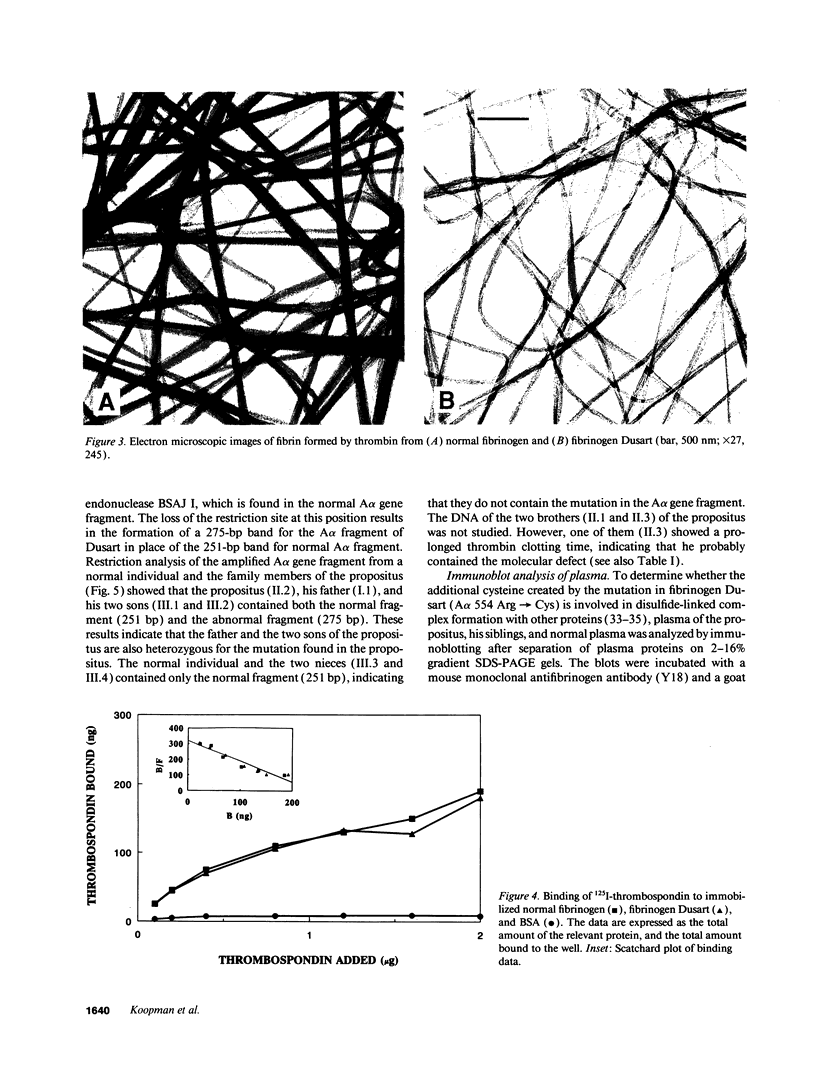
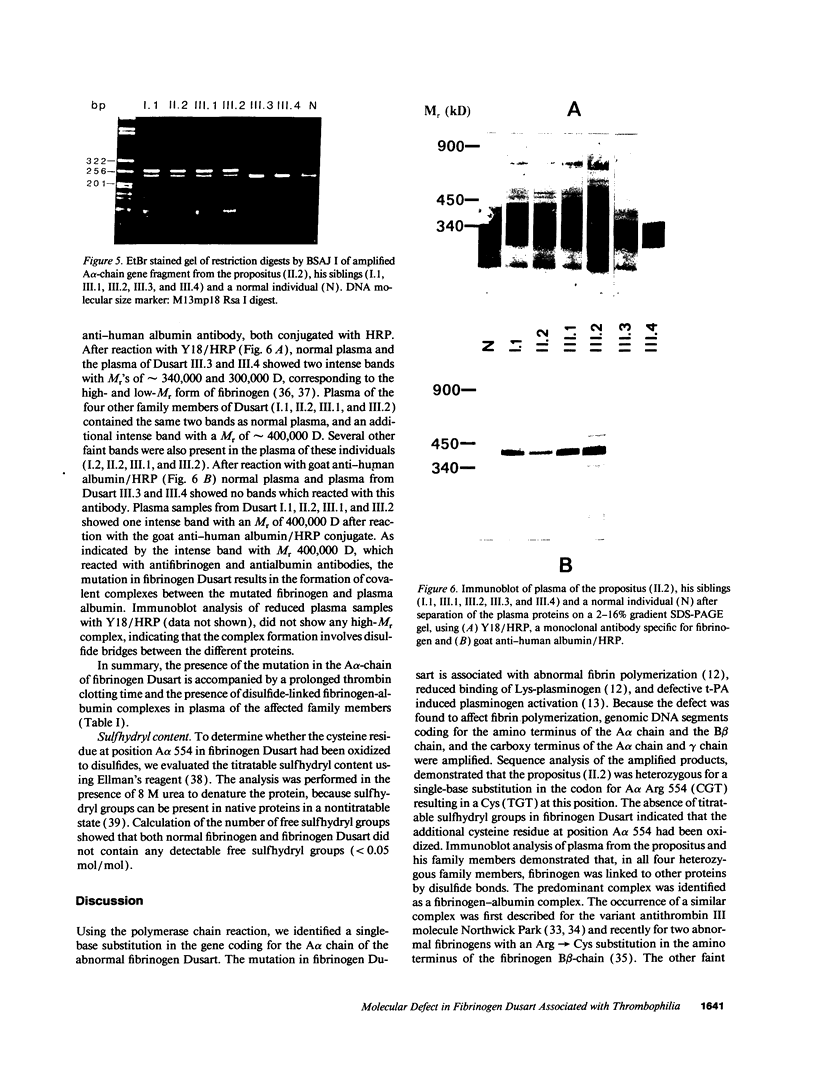
The molecular defect in the abnormal fibrinogen Dusart (Paris V) that is associated with thrombophilia was determined by sequence analysis of genomic DNA that had been amplified using the polymerase chain reaction. The propositus was heterozygous for a single base change (C-->T) in the A alpha-chain gene, resulting in the amino acid substitution A alpha 554 Arg-->Cys. Restriction analysis of the amplified DNA derived from the family members showed that his father and his two sons were also heterozygous. Electron microscopic studies on fibrin formed from purified fibrinogen Dusart demonstrated fibers that were much thinner than in normal fibrin. In contrast to the previously observed defective binding of plasminogen, the binding of thrombospondin to immobilized fibrinogen Dusart was similar to that of normal fibrinogen. Immunoblot analysis of plasma fibrinogen demonstrated that a substantial part of the fibrinogen Dusart molecules were disulfide-linked to albumin. The plasma of the affected family members also contained fibrinogen-albumin complexes. Furthermore, small amounts of high molecular weight complexes containing fibrinogen were detected in all the heterozygous individuals. These data indicate that the molecular abnormality in fibrinogen Dusart (A alpha 554 Arg-->Cys) results in defective lateral association of the fibrin fibers and disulfide-linked complex formation with albumin, and is associated with a family history of recurrent thrombosis in the affected individuals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon-Baguley T., Kudryk B. J., Walz D. A. Thrombospondin interaction with fibrinogen. Evidence for binding to the A alpha- and B beta-chains of fibrinogen. J Biol Chem. 1987 Feb 15;262(5):1927–1930. [PubMed] [Google Scholar]
- Budzynski A. Z., Olexa S. A., Pandya B. V. Fibrin polymerization sites in fibrinogen and fibrin fragments. Ann N Y Acad Sci. 1983 Jun 27;408:301–314. doi: 10.1111/j.1749-6632.1983.tb23253.x. [DOI] [PubMed] [Google Scholar]
- CLAUSS A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. Acta Haematol. 1957 Apr;17(4):237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- Carr M. E., Jr, Shen L. L., Hermans J. Mass-length ratio of fibrin fibers from gel permeation and light scattering. Biopolymers. 1977 Jan;16(1):1–15. doi: 10.1002/bip.1977.360160102. [DOI] [PubMed] [Google Scholar]
- Chung D. W., Que B. G., Rixon M. W., Mace M., Jr, Davie E. W. Characterization of complementary deoxyribonucleic acid and genomic deoxyribonucleic acid for the beta chain of human fibrinogen. Biochemistry. 1983 Jun 21;22(13):3244–3250. doi: 10.1021/bi00282a032. [DOI] [PubMed] [Google Scholar]
- Dubernard V., Legrand C. Characterization of the binding of thrombospondin to human platelets and its association with the platelet cytoskeleton. J Lab Clin Med. 1991 Nov;118(5):446–457. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Erdjument H., Lane D. A., Ireland H., Panico M., Di Marzo V., Blench I., Morris H. R. Formation of a covalent disulfide-linked antithrombin-albumin complex by an antithrombin variant, antithrombin "Northwick Park". J Biol Chem. 1987 Oct 5;262(28):13381–13384. [PubMed] [Google Scholar]
- Erdjument H., Lane D. A., Panico M., Di Marzo V., Morris H. R. Single amino acid substitutions in the reactive site of antithrombin leading to thrombosis. Congenital substitution of arginine 393 to cysteine in antithrombin Northwick Park and to histidine in antithrombin Glasgow. J Biol Chem. 1988 Apr 25;263(12):5589–5593. [PubMed] [Google Scholar]
- Gustafson S., Proper J. A., Bowie E. J., Sommer S. S. Parameters affecting the yield of DNA from human blood. Anal Biochem. 1987 Sep;165(2):294–299. doi: 10.1016/0003-2697(87)90272-7. [DOI] [PubMed] [Google Scholar]
- Hantgan R. R., Hermans J. Assembly of fibrin. A light scattering study. J Biol Chem. 1979 Nov 25;254(22):11272–11281. [PubMed] [Google Scholar]
- Hasegawa N., Sasaki S. Location of the binding site "b" for lateral polymerization of fibrin. Thromb Res. 1990 Jan 15;57(2):183–195. doi: 10.1016/0049-3848(90)90318-7. [DOI] [PubMed] [Google Scholar]
- Hong C. S., Stadler B. M., Wälti M., De Weck A. L. Dot-immunobinding assay with monoclonal anti-IgE antibodies for the detection and quantitation of human IgE. J Immunol Methods. 1986 Dec 24;95(2):195–202. doi: 10.1016/0022-1759(86)90406-0. [DOI] [PubMed] [Google Scholar]
- Koopman J., Haverkate F., Grimbergen J., Engesser L., Nováková I., Kerst A. F., Lord S. T. Abnormal fibrinogens IJmuiden (B beta Arg14----Cys) and Nijmegen (B beta Arg44----Cys) form disulfide-linked fibrinogen-albumin complexes. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3478–3482. doi: 10.1073/pnas.89.8.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppert P. W., Huijsmans C. M., Nieuwenhuizen W. A monoclonal antibody, specific for human fibrinogen, fibrinopeptide A-containing fragments and not reacting with free fibrinopeptide A. Blood. 1985 Sep;66(3):503–507. [PubMed] [Google Scholar]
- Kudryk B. J., Collen D., Woods K. R., Blombäck B. Evidence for localization of polymerization sites in fibrinogen. J Biol Chem. 1974 May 25;249(10):3322–3325. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Soria J., Soria C., Collen D., Caen J. P. Dysfibrinogenemia (fibrinogen Dusard) associated with impaired fibrin-enhanced plasminogen activation. Thromb Haemost. 1984 Feb 28;51(1):108–109. [PubMed] [Google Scholar]
- Lipinska I., Lipinski B., Gurewich V. Fibrinogen heterogeneity in human plasma. Electrophoretic demonstration and characterization of two major fibrinogen components. J Lab Clin Med. 1974 Oct;84(4):509–516. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lawler J. W., Slayter H. S. Physical characterization of platelet thrombospondin. J Biol Chem. 1981 Jul 25;256(14):7495–7500. [PubMed] [Google Scholar]
- Mosesson M. W., Alkjaersig N., Sweet B., Sherry S. Human fibrinogen of relatively high solubility. Comparative biophysical, biochemical, and biological studies with fibrinogen of lower solubility. Biochemistry. 1967 Oct;6(10):3279–3287. doi: 10.1021/bi00862a038. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W. Fibrin polymerization and its regulatory role in hemostasis. J Lab Clin Med. 1990 Jul;116(1):8–17. [PubMed] [Google Scholar]
- Mosesson M. W., Galanakis D. K., Finlayson J. S. Comparison of human plasma fibrinogen subfractions and early plasmic fibrinogen derivatives. J Biol Chem. 1974 Jul 25;249(14):4656–4664. [PubMed] [Google Scholar]
- Odegard O. R., Lie M., Abildgaard U. Heparin cofactor activity measured with an amidolytic method. Thromb Res. 1975 Apr;6(4):287–294. doi: 10.1016/0049-3848(75)90078-x. [DOI] [PubMed] [Google Scholar]
- Olexa S. A., Budzynski A. Z., Doolittle R. F., Cottrell B. A., Greene T. C. Structure of fragment E species from human cross-linked fibrin. Biochemistry. 1981 Oct 13;20(21):6139–6145. doi: 10.1021/bi00524a035. [DOI] [PubMed] [Google Scholar]
- Olexa S. A., Budzynski A. Z. Evidence for four different polymerization sites involved in human fibrin formation. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1374–1378. doi: 10.1073/pnas.77.3.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixon M. W., Chung D. W., Davie E. W. Nucleotide sequence of the gene for the gamma chain of human fibrinogen. Biochemistry. 1985 Apr 9;24(8):2077–2086. doi: 10.1021/bi00329a041. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shainoff J. R., Dardik B. N. Fibrinopeptide B and aggregation of fibrinogen. Science. 1979 Apr 13;204(4389):200–202. doi: 10.1126/science.155308. [DOI] [PubMed] [Google Scholar]
- Shainoff J. R., Dardik B. N. Fibrinopeptide B in fibrin assembly and metabolism: physiologic significance in delayed release of the peptide. Ann N Y Acad Sci. 1983 Jun 27;408:254–268. doi: 10.1111/j.1749-6632.1983.tb23249.x. [DOI] [PubMed] [Google Scholar]
- Silverstein R. L., Leung L. L., Harpel P. C., Nachman R. L. Complex formation of platelet thrombospondin with plasminogen. Modulation of activation by tissue activator. J Clin Invest. 1984 Nov;74(5):1625–1633. doi: 10.1172/JCI111578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria J., Soria C., Caen P. A new type of congenital dysfibrinogenaemia with defective fibrin lysis--Dusard syndrome: possible relation to thrombosis. Br J Haematol. 1983 Apr;53(4):575–586. doi: 10.1111/j.1365-2141.1983.tb07309.x. [DOI] [PubMed] [Google Scholar]
- Suenson E., Petersen L. C. Fibrin and plasminogen structures essential to stimulation of plasmin formation by tissue-type plasminogen activator. Biochim Biophys Acta. 1986 Apr 22;870(3):510–519. doi: 10.1016/0167-4838(86)90260-8. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Hirose M. Determination of sulfhydryl groups and disulfide bonds in a protein by polyacrylamide gel electrophoresis. Anal Biochem. 1990 Aug 1;188(2):359–365. doi: 10.1016/0003-2697(90)90621-f. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski G. P., Srivastava S., Switalska H. I., Holt J. C., Cierniewski C. S., Niewiarowski S. The interaction of human platelet thrombospondin with fibrinogen. Thrombospondin purification and specificity of interaction. J Biol Chem. 1985 Oct 5;260(22):12240–12245. [PubMed] [Google Scholar]