Abstract
There are few reports on the pathogenesis of scrapie (Sc) and Visna/maedi virus (VMV) coinfections. The aim of this work was to study in vivo as well as post mortem both diseases in 91 sheep. Diagnosis of Sc and VMV infections allowed the distribution of animals into five groups according to the presence (+) or absence (−) of infection by Sc and VMV: Sc−/VMV−, Sc−/VMV+, Sc+/VMV− and Sc+/VMV+. The latter was divided into two subgroups, with and without VMV-induced lymphoid follicle hyperplasia (LFH), respectively. In both the lung and mammary gland, PrPSc deposits were found in the germinal center of hyperplasic lymphoid follicles in the subgroup of Sc+/VMV+ having VMV-induced LFH. This detection was always associated with (and likely preceded by) PrPSc observation in the corresponding lymph nodes. No PrPSc was found in other VMV-associated lesions. Animals suffering from scrapie had a statistically significantly lower mean age than the scrapie free animals at the time of death, with no apparent VMV influence. ARQ/ARQ genotype was the most abundant among the 91 ewes and the most frequent in scrapie-affected sheep. VMV infection does not seem to influence the scrapie risk group distribution among animals from the five groups established in this work. Altogether, these data indicate that certain VMV-induced lesions can favor PrPSc deposits in Sc non-target organs such as the lung and the mammary gland, making this coinfection an interesting field that warrants further research for a better comprehension of the pathogenesis of both diseases.
Keywords: Visna, maedi, scrapie, sheep, coinfection
1. INTRODUCTION
Scrapie and Visna/maedi (VM) are small ruminant infections that are characterised by a long incubation period and that are also classified as slow diseases [46]. The etiology of scrapie is the misfolded isoform of the prion protein (PrPSc) that derives from the normal cellular isoform of the prion protein (PrPC) [39], whereas VM is caused by a lentivirus called Visna/maedi virus (VMV) [17] that is similar to other lentiviruses such as the human immunodeficiency virus [12]. Scrapie is a neurodegenerative disease that belongs to the group of Transmissible Spongiform Encephalopathies (TSE) like Bovine Spongiform Encephalopathy (BSE) and the variant of Creutzfeldt Jakob disease (vCJD). Scrapie mainly causes neuronal vacuolation and gliosis of the central nervous system (CNS) due to the deposition of PrPSc [18]. However PrPSc can also be found in the lymphoreticular system (LRS) prior to the accumulation in the CNS. Apparently, PrPSc does not cause lesions in this system [19]. VM affects mainly the lungs [45], mammary gland [35], CNS [47] and joints [9], with the most characteristic lesions being interstitial pneumonia (IP) and lymphoid follicle hyperplasia (LFH) in the lungs [34], interstitial mastitis [10], non suppurative encephalomyelitis and leukoencephalomalacia [8] and proliferative arthritis [9]. At the moment there is neither treatment nor vaccine for these two diseases.
Scrapie and VM are both endemic diseases in Spain. Scrapie was first diagnosed in 1987 [14] and since the implementation of the active and passive surveillance systems in 2001, 170 outbreaks were detected until 2006, and about 527 positive animals have been diagnosed out of 390 941 animals tested1. VMV was first reported in Spain in 1984 [15] and studies on seroprevalence have demonstrated a variable level of infection that in certain locations includes almost all flocks and up to 50% of individuals [38].
PrPC is widely distributed throughout ovine tissues [21] and its concentration in the lungs and mammary gland is about 20 and 90-fold less compared with that in the brain [33], while PrPSc is mostly located in the nervous system and LRS [22, 41]. VMV infection, however, induces hyperplasia of the LRS in the CNS, lung and mammary gland. It is therefore possible to expect an interaction between PrPSc and VMV at the lymphoid tissue level in scrapie target (CNS) and non-target organs (lungs and mammary gland). Actually, an unexpected/abnormal PrPSc presence associated with chronic inflammations has already been reported either in mice suffering from nephritis, pancreatitis and hepatitis [20] and sheep affected by chronic mastitis induced by VMV infection [6, 25, 27].
The aim of this work was to study the PrPSc presence and possible interaction with VMV in VM target organs (mainly lungs, mammary gland and CNS) in naturally scrapie and VM coinfected sheep.
2. MATERIALS AND METHODS
2.1. Animals and antemortem studies
A total of 91 Rasa Aragonesa ewes, ranging in age from 1 to 13 years and belonging to the experimental flock from the University of Zaragoza were used in this study [32]. Scrapie was diagnosed in vivo by immunohistochemical analysis for PrPSc detection in lymphoid tissue from biopsies of the 3rd eyelid [36] or rectum [16] using mAb L42 antibody (R-Biopharm, Darmstadt, Germany; 1:500) according to previously-described methods [52]. Infection by VMV was diagnosed by serum antibody detection using a recombinant protein based ELISA (ELITEST™, Hyphen Biomed, Neuville sur Oise, France) [42]. The animals were clinically examined for scrapie and VM through periodic examinations for the presence of clinical signs related to both infections [35, 45, 51]. Genotype analysis for scrapie-related codons (136, 154, 171) was performed [1] and genotypes were assigned to risk groups predefined by the National Scrapie Plan [11].
2.2. Postmortem diagnosis, pathological and PCR studies
Scrapie infected animals were killed at the end stage of the disease when showing terminal clinical signs such as extreme ataxia, tremors and/or pruritus. VMV infected animals were culled if respiratory distress and/or cachexia were observed. Four sheep in the preclinical status were put down due to unrelated diseases such as bacterial pneumonia and pregnancy toxemia. Sheep were given an intravenous barbiturate overdose (Dolethal, Vetoquinol S.A., Lure, France) and exsanguinated.
Samples from all tissues including mammary gland, lung, CNS, mediastinal and mammary lymph node were fixed in 10% formalin for histopathological processing. Samples from the same tissues were also stored at −80 °C for scrapie rapid tests and VMV PCR evaluation. Scrapie confirmation was performed by immunohistochemical detection of PrPSc in CNS and lymphoid tissues following previously-described methods [2, 32]. A subjective assessment of the intensities of PrPSc signals obtained by immunohistochemistry (IHC) in the CNS and lymphoid tissue in all coinfected animals (Sc+/VM+) was performed. The PrPSc intensity was classified from − to +++ following previously-described scores [5, 49].
The presence of VMV-related lesions in target organs [8, 30] was studied in all 91 ewes and they were only found in the lungs and mammary gland but not in the CNS. Scoring of histological VMV-related lesions in the lungs and mammary gland was performed on all coinfected animals. The parameters scored were the presence of IP (presence of fibrosis, mononuclear cell infiltration and smooth muscle hyperplasia), LFH (+: < 2 follicles; ++: 3–6 follicles; +++: > 6 follicles per 40× field) and bronchial epithelisation. VMV infection was verified only in the coinfected group by PCR amplification of LTR (300 nt) and gag (490 nt) regions of the VMV genome using previously described primers and conditions [40]. For amplification, genomic DNA was extracted from tissue samples (mammary gland and lungs) with a QIAamp® DNA Blood Mini Kit (Qiagen, Hilden, Germany).
An animal was finally classified as scrapie positive (Sc+) if PrPSc was detected immunohistochemically in the CNS and/or LRS, whereas an animal was classified as VMV positive (VMV+) if the animal was seropositive for ELITEST™. On the basis of the results obtained, 5 groups were defined: Double negative (Sc−/VM−), VMV positive only (VM+), scrapie positive only (Sc+) and double positive (Sc+/VM+). The Sc+/VM+ animals were subdivided into two further groups depending on the presence or absence of LFH induced by VMV in the lungs and/or mammary gland (Tab. I).
Table I.
Number of sheep and mean age in each group. Percentage of animals in brackets.
| Group | Sc1−/VM2− | Sc−/VM+ | Sc+/VM− | Sc+/VM+ |
Total | |
|---|---|---|---|---|---|---|
| With VMV-associated LFH | Without VMV-associated LFH | |||||
| n | 10 (10.9) | 26 (28.6) | 30 (33.0) | 14 (15.4) | 11 (12.1) | 91 (100) |
| Mean age (years) | 8.50 | 7.46 | 4.23 | 5 | 5.18 | 5.85 |
Sc: scrapie.
VM: Visna/maedi, Sc+/VM+ group was divided into two groups depending on the presence of lymphoid follicle hyperplasia (LFH) associated to VM infection in the lung or mammary gland.
2.3. Additional tests in the lungs and mammary gland
In order to prove the presence of PrPSc in the lungs and mammary gland, four additional tests were performed to the 14 VMV-scrapie double positive sheep showing LFH and in 3 ARQ/ARQ sheep from each of the other four groups (Tab. II): (1) IHC for PrPSc detection, as applied in the CNS and LRS [2, 32], (2) Western blotting (WB; Prionics®-Check WESTERN SR, Zurich-Schlieren, Prionics AG, Switzerland) using mAb P4 as the primary antibody (R-Biopharm, Darmstadt Germany; 1:2500) [5], processing the sample as described for retropharyngeal lymph node [26] and (3) two ELISA tests (BioRad TeSeE™ sheep/goat, Hercules, CA, USA, and IDEXX HerdCheck™, Westbrook, ME, USA). For these ELISA, an amount of 350 and 300 mg respectively were taken from the lungs and mammary gland. Tissues were homogenised using kit gridding tubes and 4 or 5 agitation cycles in both ELISA until the tissue was correctly homogenised. For BioRad ELISA, the procedure followed the manufacturer’s instructions and for the IDEXX ELISA, the “Standard protocol” (as per kit definition) was selected. Based on the results of these four tests, a “Final Result” was established: a sample was negative if all four tests were negative but a sample was considered positive if IHC and/or WB were positive, since they are the reference standard techniques for the World Organisation for Animal Health (OIE).
Table II.
VM and scrapie detection in the lung and mammary gland in Sc+/VM+ infected sheep.
| Sc+/VM+ with VMV-induced lymphoid follicle hyperplasia (LFH) |
Sc+/VM+ without VMV-induced LFH |
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sheep1 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
|||||||||||||||||||
| Organ2 | L | M | L | M | L | M | L | M | L | M | L | M | L | M | L | M | L | M | L | M | L | M | L | M | L | M | L | M | L | M | L | M | L | M | ||
| VM | Lesion3 | ++ | + | − | + | + | + | + | − | − | + | + | + | ++ | + | + | + | − | + | + | + | − | + | − | + | + | + | + | − | − | − | − | − | − | − | |
| PCR4 | + | − | − | − | + | + | + | + | − | − | − | + | + | + | + | + | + | − | + | + | + | + | − | + | + | − | − | + | + | − | + | + | − | − | ||
| Sc | IHC5 | + | + | − | − | + | − | + | − | − | − | + | + | + | + | − | − | − | − | − | − | − | + | − | + | − | − | + | − | − | − | − | − | − | − | |
| WB6 | + | − | − | − | + | + | + | − | − | − | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| BioRad7 | + | − | − | − | − | − | + | − | − | − | + | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| IDEXX8 | − | − | − | − | + | + | + | − | − | − | + | + | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| Final Result9 | + | + | − | − | + | + | + | − | − | − | + | + | + | + | + | − | − | − | − | − | − | + | − | + | − | − | + | − | − | − | − | − | − | − | ||
| Ln10 | + | + | − | − | + | + | + | + | − | − | + | + | + | + | + | + | − | + | − | − | + | + | − | + | − | − | + | + | + | + | + | + | − | − | ||
All animals were positive for detection of VMV antibodies (ELITEST™, Hyphen Biomed).
Organ: L = lung, M = mammary gland.
Lesion: presence of VMV-related LFH.
PCR: detection VMV-LTR sequences.
IHC: detection of PrPSc by immunohistochemistry.
WB: detection of PrPSc by Western blotting (Prionics®-Check WESTERN SR, Prionics AG).
BioRad: detection of PrPSc by BioRad ELISA (BioRad TeSeE™ sheep/goat).
IDEXX: detection of PrPSc by IDEXX ELISA (IDEXX HerdCheck™).
Final Result: negative (−): all techniques for PrPSc detection were negative; Positive (+): IHC and/or WB were positive.
Ln: IHC for PrPSc in the associated lymph node (l: mediastinal lymph node, m: mammary lymph node).
2.4. Statistical analysis
In order to detect differences in the mean age between groups (n = 5), an analysis using the Duncan test was performed. The four animals killed in the preclinical status were not included in this study.
3. RESULTS
3.1. Scrapie and VM status
Final classification of sheep was achieved only after the post-mortem analysis and the animals were classified into 5 groups (Tab. I). Regarding in vivo diagnosis, 41 out of 55 scrapie positive animals (74.5%) were detected by the 3rd eyelid and/or rectal biopsies.
All the scrapie positive sheep (Sc+/VMV− and Sc+/VMV+ groups) showed a significantly decreased mean age (P value: < 0.001) compared to non-scrapie sheep (Sc−/VMV− and Sc−/VMV+ groups), independently of the presence of VMV infection or VM-related lesions. There were no statistically significant differences between the Sc+ groups regarding mean age (Sc+/VMV− and Sc+/VMV+ groups). Furthermore, no differences in PrPSc IHC staining intensities in CNS and LRS were observed between Sc+/VM+ sheep with and without VMV related LFH (data not shown).
VM lesions in the lung and mammary gland consisted of LFH and diffuse interstitial inflammation that involved mononuclear inflammatory cells, such as lymphocytes, macrophages and plasma cells. The lung and mammary gland also presented smooth muscle hyperplasia and fibrosis, respectively. In the Sc+/VMV+ group with VM-induced LFH (n = 14), 2 animals showed LFH only in the lung (Nos. 4 and 14), 5 only in the mammary gland (Nos. 2, 5, 9, 11 and 12) and 7 in both organs (Nos. 1, 3, 6, 7, 8, 10 and 13) (Tab. II). The presence of LFH in the lung and mammary gland was always related with the presence of interstitial inflammation. Taken together, among the 28 organs analysed from the 14 Sc+/VMV+ with VM induced LFH ewes (14 lungs and 14 mammary glands sampled), 21 showed VM-induced LFH. According to the PCR results, 18 of these organs (9 lungs and 9 mammary glands) were positive for the VMV genome. In the group Sc+/VMV+ without VM-induced LFH, the lesions consisted of IP only, interstitial mastitis only or both, and 3 of the 6 organs were positive for VMV provirus for VMV in the PCR (Tab. II).
Regarding scrapie, four additional tests were used to detect PrPSc presence in the lung and the mammary gland and the results are detailed in Table II. IHC confirmed the presence of PrPSc in both organs in 3 sheep (Nos. 1, 6 and 7), only in the lung in 3 sheep (Nos. 3, 4 and 14) (Fig. 1A) and only in the mammary gland in 2 sheep (Nos. 11 and 12) (Fig. 1B). WB yielded positive results in both organs in one sheep (No. 3), and only in the lung in 4 sheep (Nos. 1, 4, 6 and 7). BioRad ELISA was positive in both organs in one sheep (No. 7) and only in the lung in 3 sheep (Nos. 1, 4 and 6). Finally, IDEXX ELISA was positive in both organs in 2 sheep (Nos. 3 and 6) and in the lung in 3 sheep (Nos. 4, 7 and 8). The “Final Result” showed that 4 sheep were positive for PrPSc in both organs (Nos. 1, 3, 6 and 7), 3 ewes were positive for PrPSc in the lung (Nos. 4, 8 and 14) and 2 animals were positive for PrPSc in the mammary gland (Nos. 11, 12).
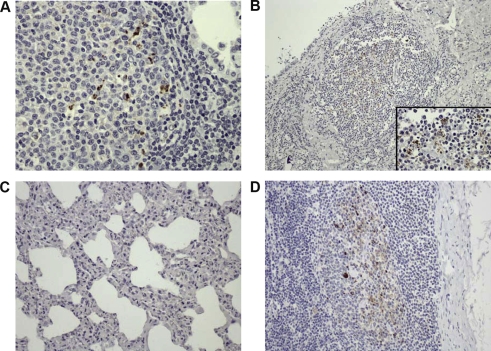
Figure 1.
Detection of PrPSc by immunohistochemistry. (A) Lung, scrapie-VM coinfected animal. Scattered PrPSc deposition within the germinal center of a lymphoid follicle close to an alveolar space, 40×. (B) Mammary gland, scrapie-VM coinfected animal. PrPSc deposition within the germinal center of a lymphoid follicle close to a lactiferous duct, 10×. Insert: Detail of the same image, 60×. (C) Lung, scrapie-VM coinfected animal. Lack of PrPSc detection within the IP, 20×. (D) Mediastinal lymph node, scrapie-VM coinfected animal. PrPSc presence within the germinal centers, 20×. (A color version of this figure is available at www.vetres.org.)
3.2. Location of PrPSc in the lung, mammary gland and lymph nodes of coinfected animals
Positive reactions to PrPSc were always observed within the germinal center of lymphoid follicles as scattered aggregates (Fig. 1A and 1B) but not all cases with LFH in tissue showing the simultaneous presence of PrPSc (Tab. II). Interestingly, the presence of PrPSc was never observed in other VMV-related lesions such as interstitial inflammation in the lung or mammary gland (Fig. 1C). PrPSc in the regional lymph node was also observed within the germinal center of cortical follicles (Fig. 1D). Finally, PrPSc positive results in the lung or mammary gland were always seen together with PrPSc presence in the corresponding regional lymph nodes (Tab. II) but a positive result in those lymph nodes did not necessarily imply a positive detection in the lung or mammary gland.
3.3. PRNP genotypes
A summary of the genotype results is shown in Table III. The 91 animals of this study were genotyped for PRNP and they were included in one of the predefined risk groups [11]. Overall, genotypes from scrapie negative sheep belonged to risk groups from 2, 3, 4 and 5, whereas scrapie positive animals were always included in risk groups 3 and 5. The most frequent genotype was ARQ/ARQ (61 sheep, 67.03%), with 46 ewes being scrapie positive and 15 animals scrapie negative.
Table III.
Classification of the 91 sheep included in this work per risk group (as defined by the National Scrapie Plan [11]) and scrapie and VM status.
| Risk group (R) | Genotype | Sc−/VM− | Sc−/VM+ | Sc+/VM− | Sc+/VM+ |
Total | |
|---|---|---|---|---|---|---|---|
| With VMV LFH | Without VMV LFH | ||||||
| R2 | ARR/ARQ | 1 | 9 | 0 | 0 | 0 | 10 (11) |
| R3 | AHQ/ARQ | 2 | 1 | 0 | 0 | 0 | 3 (3.3) |
| R3 | ARH/ARH | 0 | 1 | 0 | 0 | 0 | 1 (1.1) |
| R3 | ARH/ARQ | 0 | 4 | 0 | 1 | 1 | 6 (6.6) |
| R3 | ARQ/ARQ | 7 | 8 | 24 | 13 | 9 | 61 (67) |
| R4 | ARR/VRQ | 0 | 1 | 0 | 0 | 0 | 1 (1.1) |
| R5 | ARQ/VRQ | 0 | 2 | 4 | 0 | 1 | 7 (7.7) |
| R5 | VRQ/VRQ | 0 | 0 | 2 | 0 | 0 | 2 (2.2) |
| Total | 10 (10.9) | 26 (28.6) | 30 (33.0) | 14 (15.4) | 11 (12.1) | 91 (100.00) | |
4. DISCUSSION
Scrapie and VM are worldwide ovine diseases with a relative high prevalence in some geographical areas. Scrapie and VM are representative models for diseases caused by prions and lentiviruses, respectively. Whether the presence of one disease affects the time of onset of the other has not yet been fully studied.
Chronic inflammations can lead to PrPSc deposition in the affected organs both in experimental conditions in mice and sheep2 [20, 44] and in natural infections in sheep [6, 25, 27]. Moreover, the incubation period for TSE decreases if there is a previous or concurrent inflammatory process such as enteritis and encephalitis [13, 48]. The inflammatory process that takes place in the gut causes a local upregulation of PrPC that favors gut-associated lymphoid tissue invasion by PrPSc and lymphatic dissemination of the scrapie agent [48]. In the CNS, the inflammatory reaction produces activated infected immune cells that infiltrate the tissue and facilitate the process of PrPSc neuroinvasion [13]. Actually, in vitro small-ruminant lentivirus infection in microglial cells enhances PrPC conversion to PrPSc [50]. The global interpretation of the main results obtained in the present work (Tab. II) indicates that the presence of VMV-related lesions in non-scrapie target organs (lung and mammary gland) can favor PrPSc deposition within the hyperplasic lymphoid follicles induced by the lentivirus. The presence of PrPSc in non-target tissues seems to be preceded by PrPSc deposition in the associated lymph node (mediastinal or mammary) since no case showed PrPSc in the lung and/or the mammary gland without PrPSc in the corresponding lymph node. Likely, both PrPSc lymphoinvasion in the lymph node and VM lesions in the organ must be present for the detection of PrPSc in that organ. The presence of PrPSc in LRS does not have any relevant clinical or pathological features but the lymphoinvasion is usually the previous phase to the neuroinvasion and the appearance of clinical signs [53]. However, lymphoinvasion is not always a prerequisite: in ARQ/ARQ Sarda sheep there is neuroinvasion in preclinical animals with lack of deposition of PrPSc in the LRS [28]. In this work, IHC and WB techniques have been used as reference techniques since they are official confirmation tests for the OIE, whereas ELISA have been used for studying their performance in tissues other than the CNS and LRS. If IHC and/or WB were positive, the animal was classified as positive, independently of the results obtained by the two ELISA used.
In this study, 10 of 13 PrPSc positive lungs and mammary glands were positive for VMV by PCR, pointing out the possible role of VMV in up-regulating the PrP conversion [50] or spread in body tissues. PCR positive results may have been underestimated, since, VMV provirus detected by PCR was found only in about half the number of organs with VMV-related lesions. Possibly, the presence of the virus in the VMV-related lesions was not constant, the sample obtained did not contain provirus or the primer sequence was inadequate for a mutated proviral DNA template in the animal tissue under study. VMV proviral sequences may have been detected by PCR in the blood but unfortunately blood was not available from any of the sheep studied.
In scrapie, both the lung and mammary gland are classified as “Lower infectivity tissues” sharing the same category as lymphoreticular organs such as the spleen, tonsil and lymph nodes3. Regarding the lung, neither the presence of PrPSc nor infectivity has been proven so far. In the mammary gland, PrPSc has been found only when there is a concurrent inflammatory process, but infectivity has not been detected3. Here, we demonstrate the presence of PrPSc in the lung with VMV-related lesions. The PrPSc presence in the lung and mammary gland does not seem to have any additional pathological role in these organs but it could modify the epidemiology and/or transmission of scrapie by increasing infectivity of these tissues and – for the mammary gland – by increasing the possibility of infection of the lambs through scrapie infected colostrum/milk. Recently, it has been demonstrated that milk from naturally-infected scrapie sheep without mastitis and with no PrPSc in the mammary gland is infective for mice in experimental conditions [25]. Furthermore, PrPSc has been amplified by PMCA in milk from clinically normal scrapie-exposed sheep [31]. Recent work has also shown that lambs fed with milk from experimentally co-infected scrapie-VMV ewes develop scrapie clinical signs faster than lambs fed with milk from only scrapie experimentally-infected sheep, indicating that VMV could play an important role in scrapie transmission4.
Interestingly, we did not find any VMV-induced inflammatory process in the CNS. This finding agrees with previous observations indicating that the nervous form of VM is almost absent from the Rasa Aragonesa breed5 and as a consequence, we can not draw any conclusion about the putative interaction between scrapie and VMV at the CNS level. However, this form is frequently observed in other regions of Spain [4]. This difference may be explained by genetic strain variability, breed genetic background or factors related with the rearing system. There is a report on a single case of concomitant non-suppurative encephalitis and Nor98-like atypical scrapie without PrPSc deposits in the ectopic lymphoproliferative process, which is explained by lymphotropism differences between prion strains [54].
Mean age results show that scrapie-affected sheep either infected or not infected with VM had significantly lower age at the time of death when compared with the non-scrapie-affected sheep. This difference may be related to the genetic make up of scrapie. Moreover, the results between the Sc+ groups show that VM infection or the development of LFH induced by VM do not influence the course of scrapie infection. Additionally, clinical status of scrapie positive animals at the time of death is similar independently from VMV status. To our understanding, these results indicate that neither VMV infection nor the presence of VMV-induced lesions affect the course of scrapie infection. Overall, these observations were in agreement with previous work on experimentally scrapie-VMV coinfected animals, where the clinical evolution of scrapie was independent of VM infection in spite of the development of VMV-related lesions in the lung and mammary gland2. In addition, PRNP genotype frequencies among the 91 sheep included in this work were in agreement with those previously-reported in Rasa Aragonesa breed, where scrapie-affected sheep had only genotypes belonging to groups R3, R4 or R5 [1]. The ARQ/ARQ genotype was the most relevant genotype among scrapie affected sheep (75.4%) as previously described [1]; these results were in agreement with other reports indicating that VRQ and ARQ are the most susceptible haplotypes for scrapie infection [3]. Therefore, the presence of VM infection does not seem to influence the specific phenotypes affected by scrapie.
The pathogenesis associated to PrPSc deposition is not completely understood but recruitment of lymphoid cells in the inflamed organs enables prion replication in atypical places by the up-regulation of lymphotoxin-α produced by B lymphocytes and an ectopic induction of lymphotoxin-α receptor by the FDC-M1+ expressing normal prion protein [20]. Moreover, PrPC expression is increased in some inflammatory processes such as inclusion-body myositis, inflammatory myophathies and skin tumoral or inflammatory diseases [37, 43, 55], possibly due to the role of PrPC in general stress-response [24]. This increase of PrPC can favor the extraneural conversion of PrPC to PrPSc [23]. Inflammations caused by some viruses like small ruminant lentiviruses, human adenovirus 5 and vesicular stomatitis virus may increase local PrPC expression [7, 29, 50]. Altogether, it is reasonable to expect the presence of PrPSc in organs/tissues not previously regarded as targets for scrapie but being targets for other pathogens such as VMV, making the study of VM-scrapie coinfected animals relevant for the understanding of the pathogenesis of both diseases.
Acknowledgments
This work has been funded by a grant of Ministerio de Ciencia e Innovación (ref. AGL2007-66874-C04 GAN). We are indebted to Belén Marín and the technicians at the Research Center on Encephalopaties and Emerging Diseases (University of Zaragoza). We thank Dr Ignacio de Blas for statistical help and we also thank Santiago Becerra and Rosario Puyó for technical help. E. Salazar was a PhD student funded by grant B073/2006 from Gobierno de Aragón.
Footnotes
Ministerio de medio ambiente y medio rural y marino (MARM), Ganadería. Sanidad animal, Encefalopatías espongiformes transmisibles de los pequeños rumiantes, Información específica respecto a la tembladera o scrapie en pequeños rumiantes [on line] http://www.mapa.es/es/ganaderia/pags/sanidad_animal/caprino/carino.htm#documentos [consulted 20 January 2010].
Ligios C., Sigurdson C., Carta A., Carcassola M., Cancedda M.G., Madau L., et al., PrPSc deposits in lungs and mammary glands in sheep experimentally coinfected with scrapie and maedi-visna virus, Neuroprion 2006, Torino, 2006, p. 261.
WHO Guidelines on tissue infectivity distribution in transmissible spongiform encephalopathies [on line] (2007) http://www.who.int/biologicals/BS 2078 TSE.pdf [consulted 20 January 2010].
Ligios C., Cancedda G.M., Carta A., Santucciu C., Maestrale C., Demontis F., et al., Prion infectivity in milk from ARQ/ARQ sheep experimentally infected with scrapie and MAEDI-VISNA virus, Neuroprion 2009, Thessaloniki-Chalkidiki, 2009, p. 118.
Luján L., et al., personal observations.
References
- 1.Acín C., Martín-Burriel I., Goldmann W., Lyahyai J., Monzón M., Bolea R., et al., Prion protein gene polymorphisms in healthy and scrapie-affected Spanish sheep, J. Gen. Virol. (2004) 85:2103–2110 [DOI] [PubMed] [Google Scholar]
- 2.Badiola J.J., Monleón E., Monzón M., Acín C., Luján L., Fernández D., et al., Description of the first cases of BSE in Spain, Vet. Rec. (2002) 151:509–510 [DOI] [PubMed] [Google Scholar]
- 3.Baylis M., Chihota C., Stevenson E., Goldmann W., Smith A., Sivam K., et al., Risk of scrapie in British sheep of different prion protein genotype, J. Gen. Virol. (2004) 85:2735–2740 [DOI] [PubMed] [Google Scholar]
- 4.Benavides J., Gómez N., Gelmetti D., Ferreras M.C., García-Pariente C., Fuertes M., et al., Diagnosis of the nervous form of Maedi-Visna infection with a high frequency in sheep in Castilla y León, Spain, Vet. Rec. (2006) 158:230–235 [DOI] [PubMed] [Google Scholar]
- 5.Bolea R., Monleón E., Schiller I., Raeber A.J., Acín C., Monzón M., et al., Comparison of immunohistochemistry and two rapid tests for detection of abnormal prion protein in different brain regions of sheep with typical scrapie, J. Vet. Diagn. Invest. (2005) 17:467–469 [DOI] [PubMed] [Google Scholar]
- 6.Caplazi P., O’Rourke K., Wolf C., Shaw D., Timothy V., Baszler T.V., Biology of PrPSc accumulation in two natural scrapie-infected sheep flocks, J. Vet. Diagn. Invest. (2004) 16:489–496 [DOI] [PubMed] [Google Scholar]
- 7.Caruso P., Burla R., Piersanti S., Cherubini G., Remoli C., Martina Y., Saggio I., Prion expression is activated by Adenovirus 5 infection and affects the adenoviral cycle in human cells, Virology (2009) 385:343–350 [DOI] [PubMed] [Google Scholar]
- 8.Cultip R.C., Jackson T.A., Lehmkuhl H.D., Lesions of ovine progressive pneumonia: interstitial pneumonitis and encephalitis, Am. J. Vet. Res. (1979) 40:1370–1374 [PubMed] [Google Scholar]
- 9.Cultip R.C., Lehmkuhl H.D., Wood R.L., Brodgen K.A., Arthritis associated with ovine progressive pneumonia, Am. J. Vet. Res. (1985) 46:65–68 [PubMed] [Google Scholar]
- 10.Cultip R.C., Lehmkuhl H.D., Mastitis associated with ovine progressive pneumonia virus infection in sheep, Am. J. Vet. Res. (1985) 46:326–328 [PubMed] [Google Scholar]
- 11.Dawson M., Hoinville L.J., Hosie B.D., Hunter N., Guidance on the use of PrP genotyping as an aid to the control of clinical scrapie. Scrapie Information Group, Vet. Rec. (1998) 142:623–625 [PubMed] [Google Scholar]
- 12.Forsman A., Weiss R.A., Why is HIV a pathogen?, Trends Microbiol. (2008) 16:555–560 [DOI] [PubMed] [Google Scholar]
- 13.Friedman-Levi Y., Ovadia H., Hoftberger R., Einstein O., Abramsky O., Budka H., Gabizon R., Fatal neurological disease in scrapie-infected mice induced for experimental autoimmune encephalomyelitis, J. Virol. (2007) 31:9942–9949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García de Jalón J.A., De las Heras M., Balaguer L., Badiola J.J., Enfermedad del prurigo lumbar (scrapie) en la oveja: Diagnóstico en 5 rebaños, Med. Vet. (1987) 4:5–6 [Google Scholar]
- 15.González L., Badiola J.J., Gelabert J.L., Neumonía progresiva (MAEDI) en el ganado ovino del País Vasco, Med. Vet. (1984) 1:277–282 [Google Scholar]
- 16.González L., Dagleish M.P., Bellworthy S.J., Sisó S., Stack M.J., Chaplin M.J., et al., Postmortem diagnosis of preclinical and clinical scrapie in sheep by the detection of disease-associated PrP in their rectal mucosa, Vet. Rec. (2006) 158:325–331 [DOI] [PubMed] [Google Scholar]
- 17.Gudnadottir M., Visna-Maedi in sheep, Progr. Med. Virol. (1974) 18:336–349 [PubMed] [Google Scholar]
- 18.Hadlow G., To a better understanding of natural scrapie, in: Bradley R., Savey M., Marchant B. (Eds.), Subacute Spongiform encephalopathies, Kluwer Academic Publishers, Boston, 1961, pp. 117–130 [Google Scholar]
- 19.Hadlow W.J., Kennedy R.C., Race R.E., Natural infection of Suffolk sheep with scrapie virus, J. Infect. Dis. (1982) 146:657–664 [DOI] [PubMed] [Google Scholar]
- 20.Heikenwalder M., Zeller N., Seeger H., Prinz M., Klöhn P.C., Schwarz P., et al., Chronic lymphocytic inflammation specifies the organ tropism of prions, Science (2005) 307:1107. [DOI] [PubMed] [Google Scholar]
- 21.Horiuchi M., Yamazaki N., Ikeda T., Ishiguro N., Shinagawa M., A cellular form of prion protein (PrPC) exists in many nonneuronal tissues of sheep, J. Gen. Virol. (1995) 76:2583–2587 [DOI] [PubMed] [Google Scholar]
- 22.Ikegami Y., Ito M., Isomura H., Momotani E., Sasaki K., Muramatsu Y., et al., Pre-clinical and clinical diagnosis of scrapie by detection of PrP protein in tissues of sheep, Vet. Rec. (1991) 128:271–275 [DOI] [PubMed] [Google Scholar]
- 23.Kovács G.G., Lindeck-Pozza E., Chimelli L., Araújo A.Q.C., Gabbai A.A., Strobel T., et al., Creutzfeldt-Jakob disease and inclusion body myositis: abundant disease-associated prion protein in muscle, Ann. Neurol. (2004) 55:121–125 [DOI] [PubMed] [Google Scholar]
- 24.Kovács G.G., Kalev O., Gelpi E., Haberler C., Wanschitz J., Strohschneider M., et al., The prion protein in human neuromuscular diseases, J. Pathol. (2004) 204:241–247 [DOI] [PubMed] [Google Scholar]
- 25.Lacroux C., Simon S., Benestad S.L., Maillet S., Mathey J., Lugan S., et al., Prions in milk from ewes incubating natural scrapie, PloS Pathog. (2008) 4:e1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langeveld J.P.M., Jacobs J.G., Erkens J.H.F., Bossers A., van Zijderveld F.G., van Keulen L.J.M., Rapid and discriminatory diagnosis of scrapie and BSE in retro-pharyngeal lymph nodes of sheep, BMC Vet. Res. (2006) 2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ligios C., Sigurdson C.J., Santucciu C., Carcassola G., Manco G., Basagni M., et al., PrPSc in mammary glands of sheep affected by scrapie and mastitis, Nat. Med. (2005) 11:1137–1138 [DOI] [PubMed] [Google Scholar]
- 28.Ligios C., Cancedda M.G., Madau L., Santucciu C., Maestrale C., Agrimi U., et al., PrP(Sc) deposition in nervous tissues without lymphoid tissue involvement is frequently found in ARQ/ARQ Sarda breed sheep preclinically affected with natural scrapie, Arch. Virol. (2006) 151:2007–2020 [DOI] [PubMed] [Google Scholar]
- 29.Lötscher M., Recher M., Hunziker L., Klein M.A., Immunologically induced, complement-dependent up-regulation of the prion protein in the mouse spleen: follicular dendritic cells versus capsule and trabeculae, J. Immunol. (2003) 170:6040–6047 [DOI] [PubMed] [Google Scholar]
- 30.Luján L., García-Marín J.F., Fernández de Luco D., Vargas A., Badiola J.J., Pathological changes in the lungs and mammary glands of sheep and relationship with maedi-visna infection, Vet. Rec. (1991) 129:51–54 [DOI] [PubMed] [Google Scholar]
- 31.Maddison B.C., Baker C.A., Rees H.C., Terry L.A., Thorne L., Bellworthy S.J., et al., Prions are secreted in milk from clinically normal scrapie-exposed sheep, J. Virol. (2009) 83:8283–8286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monleón E., Monzón M., Hortells P., Vargas A., Acín C., Badiola J.J., Detection of PrPSc on lymphoid tissues from naturally affected scrapie animals: comparison of three visualization systems, J. Histochem. Cytochem. (2004) 52:145–151 [DOI] [PubMed] [Google Scholar]
- 33.Moudjou M., Frobert Y., Grassi J., La Bonnardière C., Cellular prion protein status in sheep: tissue-specific biochemical signatures, J. Gen. Virol. (2001) 82:2017–2024 [DOI] [PubMed] [Google Scholar]
- 34.Narayan O., Clements J.E., Biology and pathogenesis of lentiviruses, J. Gen. Virol. (1989) 70:1617–1639 [DOI] [PubMed] [Google Scholar]
- 35.Oliver R.E., Gorham J.R., Parish S.F., Hadlow W.J., Naravan O., Ovine progressive pneumonia: pathologic and virologic studies on the naturally occurring disease, Am. J. Vet. Res. (1981) 42:1554–1559 [PubMed] [Google Scholar]
- 36.O’Rourke K.I., Baszler T.V., Parish S.M., Knowles D.P., Preclinical detection of PrPSc in nictitating membrane lymphoid tissue of sheep, Vet. Rec. (1998) 142:489–491 [DOI] [PubMed] [Google Scholar]
- 37.Pammer J., Weninger W., Tschachler E., Human keratinocytes express cellular prion-related protein in vitro and during inflammatory skin diseases, Am. J. Pathol. (1998) 153:1353–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pérez M., Biescas E., de Andrés X., Leginagoikoa I., Salazar E., Berriatua E., et al., Visna/maedi virus serology in sheep: survey, risk factors and implementation of a successful control programme in Aragón, Vet. J. (2009) doi:10.1016/j.tvjl.2009.07.031 [DOI] [PubMed] [Google Scholar]
- 39.Prusiner S.B., Novel proteinaceous infectious particles cause scrapie, Science (1982) 216:136–144 [DOI] [PubMed] [Google Scholar]
- 40.Reina R., Mora M.I., Glaria I., García I., Solano C., Luján L., et al., Molecular characterization and phylogenetic study of Maedi Visna and Caprine Arthritis Encephalitis viral sequences in sheep and goats from Spain, Virus Res. (2006) 121:189–198 [DOI] [PubMed] [Google Scholar]
- 41.Rubenstein R., Merz P.A., Kascsak R.J., Carp R.I., Scalici C.L., Fama C.L., Wisniewski H.M., Detection scrapie-associated fibrils (SAF) and SAF proteins from scrapie-affected sheep, J. Infect. Dis. (1987) 156:36–42 [DOI] [PubMed] [Google Scholar]
- 42.Saman E., van Eynde G., Luján L., Extramiana B., Harkiss G., Tolari F., et al., A new sensitive serological assay for detection of lentivirus infections in small ruminants, Clin. Diagn. Lab. Immunol. (1999) 6:734–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarkozi E., Askanas V., Engel W.K., Abnormal accumulation of prion protein mRNA in muscle fibers of patients with sporadic inclusion-body myositis and hereditary inclusion-body myopathy, Am. J. Pathol. (1994) 145:1280–1284 [PMC free article] [PubMed] [Google Scholar]
- 44.Seeger H., Heikenwalder M., Zeller N., Kranich J., Schwarz P., Gaspert A., et al., Coincident scrapie infection and nephritis lead to urinary prion excretion, Science (2005) 310:324–326 [DOI] [PubMed] [Google Scholar]
- 45.Sigurdsson B., Grimsson H., Palsson P.A., Maedi, a chronic progressive infection of sheep’s lungs, J. Infect. Dis. (1952) 90:233–241 [DOI] [PubMed] [Google Scholar]
- 46.Sigurdsson B., Rida, a chronic encephalitis of sheep with general remarks on infections which develop slowly and some of their special characteristics, Br. Vet. J. (1954) 110:341–354 [Google Scholar]
- 47.Sigurdsson B., Palsson P.A., Grimsson H., Pathology of Visna. Transmissible demyelinating disease in sheep in Iceland, Acta Neuropathol. (1962) 1:343–362 [Google Scholar]
- 48.Sigurdson C.J., Heikenwalder M., Manco G., Barthel M., Schwarz P., Stecher B., et al., Bacterial colitis increases susceptibility to oral prion disease, J. Infect. Dis. (2009) 199:243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spraker T.R., VerCauteren K.C., Gidlewski T., Schneider D.A., Munger R., Balachandran A., O’Rourke K.I., Antemortem detection of PrPCWD in preclinical, ranch-raised Rocky Mountain elk (Cervus elaphus nelsoni) by biopsy of the rectal mucosa, J. Vet. Diagn. Invest. (2009) 21:15–24 [DOI] [PubMed] [Google Scholar]
- 50.Stanton J.B., Knowles D.P., O’Rourke K.I., Herrmann-Hoesing L.M., Mathison B.A., Baszlen T.V., Small-Ruminant Lentivirus enhances PrPSc accumulation in cultured sheep microglial cells, J. Virol. (2008) 82:9839–9847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vargas F., Bolea R., Monleón E., Acín C., Vargas A., De Blas I., et al., Clinical characterisation of natural scrapie in a native Spanish breed of sheep, Vet. Rec. (2005) 156:318–320 [DOI] [PubMed] [Google Scholar]
- 52.Vargas F., Luján L., Bolea R., Monleón E., Martín-Burriel I., Fernández A., et al., Detection and clinical evolution of scrapie in sheep by 3rd eyelid biopsy, J. Vet. Intern. Med. (2006) 20:187–193 [DOI] [PubMed] [Google Scholar]
- 53.Van Keulen L.J., Vromans M.E., van Zijderveld F.G., Early and late pathogenesis of natural scrapie infection in sheep, APMIS (2002) 110:23–32 [DOI] [PubMed] [Google Scholar]
- 54.Vidal E., Tortosa R., Costa C., Benavides J., Francino O., Sánchez-Robert E., et al., Lack of PrPSc immunostaining in intracranial ectopic lymphoid follicles in a sheep with concomitant non-suppurative encephalitis and Nor98-like atypical scrapie: a case report, Vet. J. (2008) 177:283–288 [DOI] [PubMed] [Google Scholar]
- 55.Zanusso G., Vattemi G., Ferrari S., Tabaton M., Pecini E., Cavallaro T., et al., Increased expression of the normal cellular isoform of prion protein in inclusion-body myositis, inflammatory myopathies and denervation atrophy, Brain Pathol. (2001) 11:182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]