Abstract
Background
Bone mass density (BMD) may be influenced by the general dietary pattern and the potential renal acid load (PRAL).
Objective
To evaluate the association between PRAL and BMD.
Methods
We evaluated BMD using computed tomography and dietary intake using the EPIC questionnaire in 543 community-living women aged 60 years and older. We grouped the participants according to tertiles of total, trabecular and cortical BMD, and compared demographic, anthropometric and nutritional characteristics across groups. Analyses were repeated using tertiles of BMD variation over a 6-years follow-up.
Results
Total BMD was inversely associated with age, time since menopause, and creatinine clearance. The intake of PUFAs was slightly higher among women with the highest total BMD, none of the other nutrients taken into account nor PRAL was associated with total BMD. Similar results were found for trabecular BMD, with the exception that alcohol intake was associated with lower bone density. Cortical BMD was associated with vitamin D intake (1.6, 1.8, and 1.8 mcg/day in first, second and third tertile, respectively) and serum 25-OH vitamin D (38.8, 43.2, and 49.5 nmol/L in the first, second, and third tertile, respectively). In the longitudinal analysis, a lower BMI was associated with greater loss of total BMD, while lower serum 25-hydroxy vitamin D at baseline was associated with smaller loss of cortical BMD.
Conclusions
We found no relationship between dietary acid load and BMD. We also confirmed the role of well recognized risk factor for osteoporosis and found a possible protective effect of PUFA intake on BMD.
Introduction
Dietary intake of calcium and vitamin D plays an important role in preserving bone mass, but other dietary factor also seem to be related to bone mineral density (BMD) (1). For example, the Dietary Approaches to Stop Hypertension (DASH) diet - a low fat, calcium, and mineral-rich diet that is high in fruits, vegetables, and dairy products - significantly reduced bone turnover in adults (2). Furthermore, data coming from 14,563 subjects aged 42–82 years enrolled in the EPIC-Norfolk cohort study show that in women the acid dietary load is inversely associated with BMD measured using calcaneal broadband ultrasound attenuation (3). Similarly, the dietary acid load has been reported to be weakly and inversely correlated with calcaneal broadband ultrasound attenuation in a female population aged over 75 years or older, but it was not different in participants with and without history of fracture (4). These findings are physiologically founded because the bone matrix behaves as a base contributing to buffer the acid load (5). Accordingly, the intake of animal proteins, which account for the majority of the alimentary acid load, should be counterbalanced by consumption of alkali-rich foods such as fruits and vegetables to fully exploit its beneficial effect on bone growth and maintenance (6). Further supporting this interpretation is the finding that the alimentary acid load is inversely associated with bone area and cortical width of the radius in a population of children and adolescents, while daily protein intake is directly associated with several indexes of bone health (7). Thus, the dietary protein intake seems to optimally guarantee for bone growth and maintenance in the context of an alkali-rich diet, i. e. rich in potassium, magnesium and calcium salts.
These observations are of special interest for the elderly, given that their diet frequently is poorly equilibrated. While a formal computation of the alimentary acid load has never been made in the elderly, the frequently reported insufficient intake of folate, magnesium and calcium (8–10) suggests that the diet has a poor alkalinizing power.
On these bases, we hypothesized that the general dietary pattern, more than the intake of selected nutrients, contributes to the age-related decline in BMD. In particular, in this study we evaluated whether dietary acid load is associated with lower BMD in women aged 60 years or older recruited in the InChianti study.
Subjects and Methods
Study population
We used data from the InCHIANTI study, which was designed to investigate the factors contributing to the decline of mobility in older persons (11). The participants in the study were randomly selected from the populations of two town areas in the Chianti region: Greve in Chianti and Bagno a Ripoli. The Italian National Institute of Research and Care on Aging ethical committee ratified the study protocol. Participants received an extensive description of the study and signed an informed participation consent that included permission to conduct analyses on the biological specimens collected and stored. For those unable to fully consent because of cognitive or physical problems, surrogate consent was also obtained from a close relative. The eligible participants were interviewed at their homes by trained study researcher using a structured questionnaire aimed at investigating their heath status, their physical and cognitive performance, and other factors possibly related to loss of independence in late life. The interview was followed by a physical examination at the study clinic. Evaluation of patients was repeated at 3 and 6 years from baseline; for the present study we only used the data coming from the 6-years follow-up.
Assessment of the nutritional intake
The InCHIANTI study included an evaluation of the dietary intake estimated using the questionnaire developed by the European Prospective Investigation into Cancer and Nutrition (EPIC) (12). The EPIC questionnaire is divided into two parts: the first investigates the general dietary pattern and the frequency of meals consumed away from home. The second investigates the intake frequency of 236 specific foods, along with the average size of the serving selected from a range as shown in photographs. The EPIC questionnaire was developed as a self-administered instrument. However, a preliminary study showed that older persons were prone to misunderstanding some of the questions. Therefore, trained interviewers administered the questionnaire. The information derived from the questionnaire was automatically converted into data on energy, micro- and macronutrient intake, by software specifically designed for the EPIC study. The EPIC nutritional assessment has been successfully validated in the population being studied, by comparing the dietary intake estimated by this method with the dietary intake estimated with a direct method of measure, the weighing and recording of seven day food consumption (13).
Estimation of bone mass density
Bone mass density was estimated using peripheral quantitative computed tomography (pQCT) using a XCT 2000 device (Stratec Medizin-technik, Pforzheim, Germany). The tibio-talar joint was identified using a pQCT longitudinal scout and used as an anatomic marker for the identification of measurements sites. Standard (2.5 mm thickness) transverse scans were obtained at 4% and 38 of tibial length to measure trabecular and cortical bone density, respectively. The cross-sectional images obtained by pQCT were analyzed using BonAlyse software (BonAlyse Oy, Jyvaskyla, Finland) that automatically identifies cortical and trabecular bone and assesses its density. Areas with density values > 710 mg/cm3 were considered as cortical bone (14), whereas areas with density between 180 and 710 mg/cm3 were considered as trabecular bone.
Analytic approach
From the initial sample of 726 female participants with both interview and clinical data we excluded those not having pQCT (N=64) or nutritional assessment (N=4), and those younger than 60 years (N=115). We then grouped patients on the basis of the distribution tertiles for total and trabecular bone density measured at 4% of tibial length and cortical bone density measured at 38% of tibial length, and compared demographic, clinical, and nutritional characteristics of participants in the 3 groups. Total calories intake and intake of all macro-nutrients were expressed relative to the ideal weight, calculated using the formula proposed by Lorenz: kilograms of ideal weight (Kgi) in males = height – 100 – (height-150)/4; Kgi (women) = height – 100 – (height-150)/2. Potential renal acid load (PRAL) was calculated according to Remer (15) using the following formula: (0.49 * total protein intake) + (0.037 * phosphorus intake) – (0.021 * potassium intake) – (0.026 * magnesium intake) – (0.013 * calcium intake). This variable was analyzed using both absolute and weight-adjusted values.
We included in the analysis an estimate of the level of physical activity between ages 40 and 60, and considered sedentary those performing less the 1–2 hours/week of moderate or less then 4 hours/week of light physical exercise. We also analyzed renal function evaluated using creatinine clearance (directly measured with the 24-h urine collection method), serum concentrations of 25-OH vitamin D, and serum concentration of a generic marker of inflammation (IL-6). Finally, we compared groups with regards to prevalence of hormone replacement therapy.
The probability of findings being spurious was evaluated using analysis of variance for continuous variables and chi-square test for categorical variables. The analyses were repeated using tertiles of the variation of BMD over 6 years. All analyses were performed using SAS V9 for Windows (SAS Institute, Cary, NC).
Results
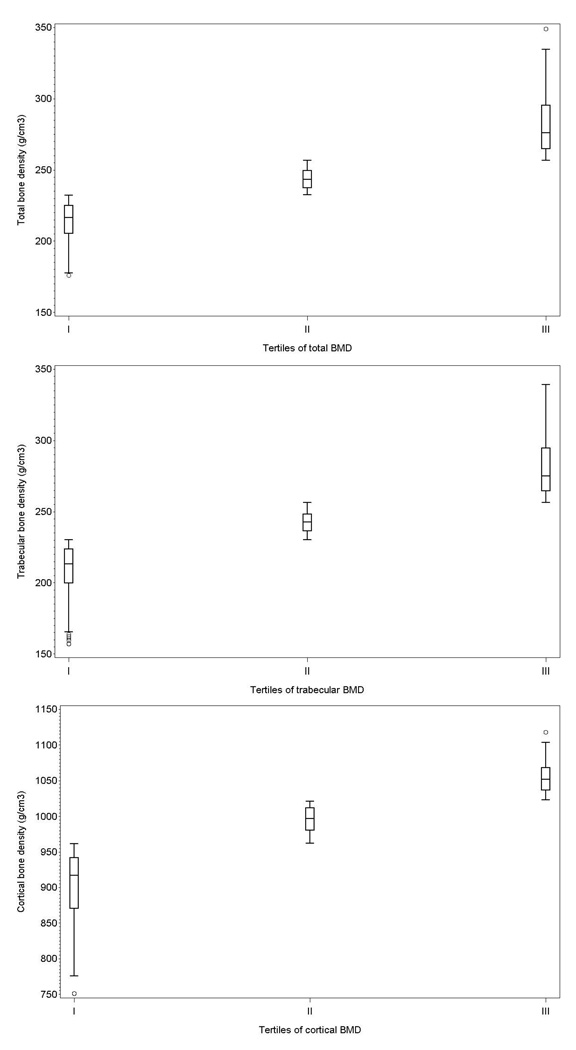
The sample size was 543, with a mean age of 74.7 years (SD: 7.4; range: 60 – 96); 46% of participants were aged 75 years or older. Only a small fraction (8%) of participants had restriction in mobility needing help in walking for 400 meters, and most of them (80%) reported to go out of home more than two times/day when the weather was nice. The calculated cut-off for total BMD tertiles (g/cm3) were 232.5 and 256.9, 230.1 and 256.5 for trabecular BMD, and 962.1 and 1022.7 for cortical BMD . Figure 1 shows the distribution of BMD in the different tertiles. Only 8 participants were on corticosteroid treatment, 7 suffered from hyperthyroidism, and 32 were on hormone replacement therapy.
Figure 1.
Distribution of baseline total (upper panel), trabecular (middle panel), and cortical (lower panel) BMD according to distribution tertiles. Box edges are at 25th and 75th percentile, the middle horizontal line is at the median, whiskers span 1.5 interquartile range.
The average energy intake was 33.7 Kcal/Kgi, with about 50% of caloric intake coming from carbohydrates. The average protein intake was 1.4 grams/Kgi, and it was strongly related with the intake of calories of non-proteic origin (Spearman's r=0.83). The intake of dairy products was limited, with on average 125 g/day of milk, 19 g/day of yogurt, and 41 g/day of cheese. The average PRAL was 1.9 (SD: 10.6, range: −34.7 – 36.1).
As shown in table 1, total BMD was – as expected – inversely associated with age, time since menopause, and creatinine clearance. Also expected was the direct association with BMI. Except for the intake of PUFAs, that was slightly higher among women with the higher total BMD, none of the nutrients taken into account, including calcium and vitamin D, was associated with total BMD. Furthermore, no association was found between total BMD and PRAL. Most participants had had a low level of physical activity between 40 and 60 years of age, with higher proportion (83.8%) shown among women in the first tertile of total BMD compared with those in the second (72.6%) and third tertile (74.1%). Serum concentration of IL-6 was lower in women with lower total BMD, although on average the concentration was in the normal range.
Table 1.
Characteristics of participants according to tertiles of total BMD.
| Tertiles of total BMD | ||||
|---|---|---|---|---|
| I | II | III | P | |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age | 77.0 (7.83) | 74.5 (6.99) | 72.7 (6.80) | <0.001 |
| Years since menopause | 27.0 (9.03) | 25.0 (9.11) | 22.9 (8.94) | <0.001 |
| BMI | 26.3 (4.38) | 27.6 (3.93) | 29.5 (4.99) | <0.001 |
| Cigarette smoking (pack-years) | 3.1 (10.20) | 2.9 (9.10) | 2.4 (6.80) | 0.720 |
| Calories/Kgi | 33.7 (9.05) | 33.4 (8.47) | 34.2 (9.40) | 0.732 |
| Dietary Fibre (g/day) | 17.9 (5.67) | 18.7 (5.43) | 18.9 (5.46) | 0.180 |
| Calories from carbohydrates/ideal weight |
17.7 (5.60) | 17.6 (5.09) | 17.7 (5.48) | 0.990 |
| Calories from lipids/ideal weight | 10.5 (3.18) | 10.7 (3.24) | 11.1 (3.45) | 0.247 |
| Proteins (g)/ideal weight | 1.3 (0.34) | 1.3 (0.36) | 1.39 (0.38) | 0.434 |
| Animal proteins (g)/ideal weight | 0.87 (0.26) | 0.9 (0.27) | 0.90 (0.28) | 0.344 |
| Vegetal proteins (g)/ideal weight | 0.48 (0.17) | 0.49 (0.14) | 0.49 (0.16) | 0.962 |
| Non protein calories/g of nitrogen | 131.5 (20.62) | 131.7 (21.31) | 129.3 (19.17) | 0.460 |
| Calcium (mg/day) | 792.9 (285.21) | 803.5 (332.15) | 808.3 (328.34) | 0.893 |
| Phosphorous (mg/day) | 1093.3 (285.98) | 1109.8 (321.08) | 1134.1 (331.63) | 0.458 |
| Vitamin D (mcg/day) | 1.69 (1.00) | 1.67 (0.68) | 1.83 (0.87) | 0.155 |
| Magnesium (mg/day) | 222.2 (57.46) | 225.3 (65.26) | 229.9 (68.14) | 0.493 |
| Folic Acid (mcg/day) | 236.8 (71.48) | 243.7 (77.12) | 251.9 (75.63) | 0.156 |
| Total PUFA (g/day) | 6.38 (1.83) | 6.84 (2.37) | 6.96 (2.38) | 0.031 |
| PRAL | 2.96 (9.74) | 0.71 (11.24) | 2.20 (10.77) | 0.124 |
| PRAL/ideal weight | 0.06 (0.19) | 0.01 (0.22) | 0.04 (0.21) | 0.135 |
| Alcohol (g/day) | 8.27 (11.07) | 5.99 (8.04) | 6.29 (10.44) | 0.061 |
| Creatinine clearance (mL/min) | 64.7 (21.88) | 72.3 (24.74) | 73.9 (22.63) | <0.001 |
| 25-hydroxyvitamin D (nmol/L) | 41.0 (31.12) | 47.8 (45.21) | 43.0 (25.98) | 0.181 |
| Interleukin-6 (pg/mL) | 1.6 (1.27) | 1.82 (1.92) | 1.96 (2.30) | 0.231 |
| % | % | % | ||
| Low physical activity | 83.8 | 72.6 | 74.0 | 0.017 |
| Hormone replacement therapy | 5.6 | 8.4 | 3.8 | 0.386 |
In table 2 are shown the results relative to trabecular BMD. Overall, there were no differences compared to what was observed for total BMD, with the exception that we found a significant association with alcohol intake, that was higher in women with the lowest trabecular BMD compared to those with higher trabecular BMD (8.4 g/day vs. 6 g/day, respectively, P=0.032).
Table 2.
Characteristics of participants according to tertiles of trabecular BMD.
| Tertiles of trabecular BMD | ||||
|---|---|---|---|---|
| I | II | III | P | |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age | 77.1 (7.77) | 74.4 (7.00) | 72.6 (6.81) | <0.001 |
| Years since menopause | 27.0 (8.91) | 25.0 (9.11) | 23.0 (9.07) | <0.001 |
| BMI | 26.5 (4.56) | 27.5 (3.83) | 29.2 (4.95) | <0.001 |
| Cigarette smoking (pack-years) | 3.48 (11.43) | 2.70 (7.82) | 2.28 (6.40) | 0.417 |
| Calories/Kgi | 33.8 (9.09) | 33.3 (8.38) | 34.1 (9.42) | 0.688 |
| Dietary Fibre (g/day) | 18.1 (5.78) | 18.5 (5.30) | 19.0 (5.49) | 0.301 |
| Calories from carbohydrates/ideal weight |
17.7 (5.59) | 17.5 (5.06) | 17.7 (5.52) | 0.941 |
| Calories from lipids/ideal weight | 10.6 (3.26) | 10.6 (3.18) | 11.1 (3.44) | 0.307 |
| Proteins (g)/ideal weight | 1.36 (0.34) | 1.35 (0.36) | 1.39 (0.38) | 0.506 |
| Animal proteins (g)/ideal weight | 0.87 (0.26) | 0.87 (0.27) | 0.90 (0.28) | 0.444 |
| Vegetal proteins (g)/ideal weight | 0.48 (0.17) | 0.48 (0.14) | 0.49 (0.16) | 0.964 |
| Non protein calories/g of nitrogen | 131.7 (20.91) | 131.3 (20.9)8 | 129.6 (19.23) | 0.568 |
| Calcium (mg/day) | 796.2 (289.85) | 802.7 (327.90) | 805.8 (328.65) | 0.957 |
| Phosphorous (mg/day) | 1096.7 (289.82) | 1109.9 (319.02) | 1130.8 (330.70) | 0.578 |
| Vitamin D (mcg/day) | 1.69 (1.00) | 1.68 (0.67) | 1.82 (0.87) | 0.217 |
| Magnesium (mg/day) | 223.7 (59.55) | 224.7 (63.81) | 228.90 (67.86) | 0.712 |
| Folic Acid (mcg/day) | 236.4 (70.62) | 244.6 (77.85) | 251.3 (75.71) | 0.164 |
| Total Polyunsaturated Fatty Acids (g/day) |
6.40 (1.89) | 6.81 (2.33) | 6.96 (2.37) | 0.046 |
| PRAL | 2.77 (9.61) | 0.92 (11.33) | 2.18 (10.83) | 0.243 |
| PRAL/ideal weight | 0.05 (0.19) | 0.02 (0.22) | 0.04 (0.21) | 0.256 |
| Alcohol (g/day) | 8.44 (11.16) | 6.03 (8.02) | 6.08 (10.34) | 0.032 |
| Creatinine clearance (mL/min) | 64.1 (21.78) | 72.1 (24.69) | 74.6 (22.50) | <0.001 |
| 25-hydroxyvitamin D (nmol/L) | 42.6 (32.92) | 44.0 (41.18) | 45.0 (30.10) | 0.813 |
| Interleukin-6 (pg/mL) | 1.63 (1.30) | 1.85 (1.90) | 1.94 (2.31) | 0.293 |
| % | % | % | ||
| Low physical activity | 82.7 | 72.6 | 75.1 | 0.048 |
| Hormone replacement therapy | 5.6 | 8.4 | 3.8 | 0.367 |
As shown in table 3, also cortical BMD was associated with age, BMI, and renal function. It was not associated, however, with PUFA and alcohol intake, nor with physical activity, while it showed an association with vitamin D intake (1.6, 1.8, and 1.8 mcg/day in first, second and third tertile of cortical BMD, respectively, P=0.033) and serum concentration of 25-OH vitamin D (38.8, 43.2, and 49.5 nmol/L in the first, second, and third tertile of cortical BMD, respectively, P=0.017).
Table 3.
Characteristics of participants according to tertiles of cortical BMD.
| Tertiles of cortical BMD | ||||
|---|---|---|---|---|
| I | II | III | P | |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age | 76.3 (7.70) | 74.0 (7.72) | 72.8 (6.33) | <0.001 |
| Years since menopause | 26.2 (9.20) | 25.5 (9.63) | 23.1 (8.46) | 0.005 |
| BMI | 26.8 (4.57) | 28.1 (4.85) | 28.2 (4.31) | 0.007 |
| Cigarette smoking (pack-years) | 2.69 (8.49) | 3.29 (10.60) | 2.50 (7.01) | 0.677 |
| Calories/Kgi | 33.7 (8.71) | 34.2 (9.07) | 33.5 (9.13) | 0.747 |
| Dietary Fibre (g/day) | 17.8 (5.08) | 18.8 (5.69) | 19.1 (5.74) | 0.069 |
| Calories from carbohydrates/ideal weight |
17.9 (5.10) | 17.8 (5.61) | 17.4 (5.44) | 0.633 |
| Calories from lipids/ideal weight | 10.6 (3.30) | 11.0 (3.31) | 10.8 (3.29) | 0.491 |
| Proteins (g)/ideal weight | 1.35 (0.37) | 1.38 (0.33) | 1.37 (0.38) | 0.598 |
| Animal proteins (g)/ideal weight | 0.86 (0.29) | 0.89 (0.25) | 0.89 (0.27) | 0.591 |
| Vegetal proteins (g)/ideal weight | 0.49 (0.15) | 0.49 (0.15) | 0.49 (0.17) | 0.998 |
| Non protein calories/g of nitrogen | 133.4 (21.66) | 130.3 (20.96) | 128.8 (18.34) | 0.094 |
| Calcium (mg/day) | 795.8 (329.57) | 812.4 (294.62) | 800.4 (322.59) | 0.878 |
| Phosphorous (mg/day) | 1089.1 (322.65) | 1120.9 (284.46) | 1131.7 (330.19) | 0.408 |
| Vitamin D (mcg/day) | 1.60 (0.68) | 1.81 (1.10) | 1.79 (0.73) | 0.033 |
| Magnesium (mg/day) | 220.5 (63.77) | 227.9 (59.01) | 229.7 (67.93) | 0.351 |
| Folic Acid (mcg/day) | 234.4 (74.62) | 247.9 (71.92) | 250.7 (77.50) | 0.088 |
| Total Polyunsaturated Fatty Acids (g/day) |
6.46 (2.22) | 6.86 (2.21) | 6.87 (2.22) | 0.144 |
| PRAL | 2.26 (10.66) | 2.04 (9.77) | 1.63 (11.45) | 0.843 |
| PRAL/ideal weight | 0.05 (0.21) | 0.04 (0.19) | 0.03 (0.22) | 0.850 |
| Alcohol (g/day) | 6.87 (10.38) | 7.03 (9.77) | 6.71 (9.86) | 0.954 |
| Creatinine clearance (mL/min) | 66.8 (23.50) | 69.3 (23.54) | 74.8 (22.57) | 0.005 |
| 25-hydroxyvitamin D (nmol/L) | 38.8 (31.19) | 43.2 (39.05) | 49.5 (33.23) | 0.017 |
| Interleukin-6 (pg/mL) | 1.71 (1.47) | 2.02 (2.17) | 1.58 (1.41) | 0.042 |
| % | % | % | ||
| Low physical activity | 78.0 | 79.3 | 73.5 | 0.365 |
| Hormone replacement therapy | 3.9 | 8.4 | 5.4 | 0.092 |
Follow-up data were available for 293 participants (54.0%). Participants without follow-up data were older (78.2 vs. 71.7 years, P<0.001) and had a slightly lower total BMD (244.1 g/cm3 vs. 251.0 g/cm3, P=0.026). Daily intake of calories/kg ideal weight and of proteins/kg of ideal weight was similar in the two groups.
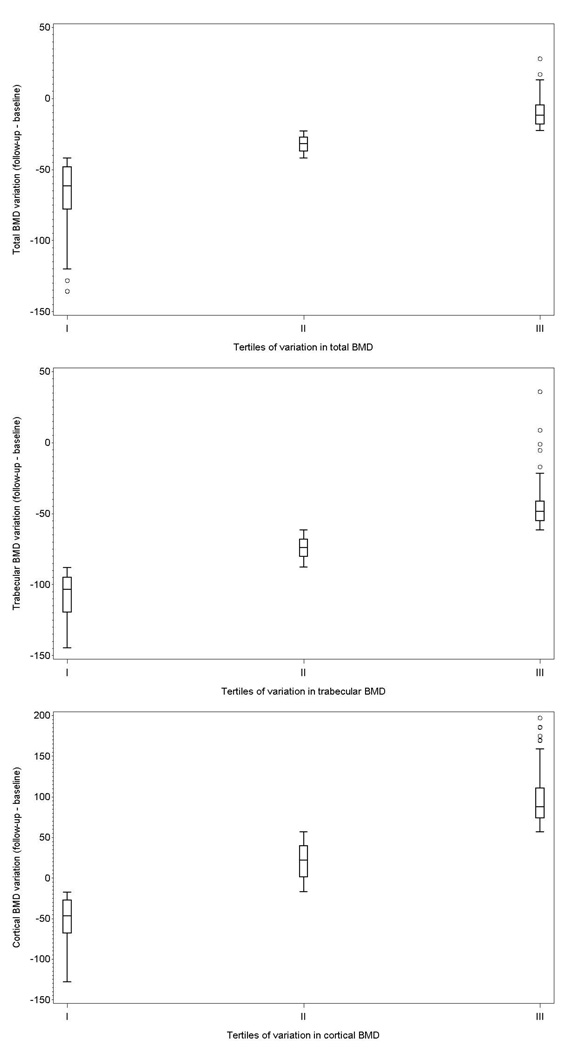
The mean (SD) BMD variation was −36.2 (30.5), −76.1 (36.9), and 26.5 (90.3) mg/cm3 for total, trabecular, and cortical bone, respectively. Figure 2 shows the distribution of the variation across tertiles. Tables 4–6 show the association between the variables of interest and tertiles of the variation in BMD (lower tertiles indicate greater bone loss). Older age and lower BMI were associated with greater total BMD reduction, as well as lower intakes of dietary fibers, folic acid and magnesium. When variation of trabecular BMD was taken into account, we found less strong an association with age, and no association with time since menopause or BMI. The association with folic acid, however, was confirmed. Furthermore, participants with greater loss of trabecular BMD had a higher PRAL (3.42 mEq/day vs. 0.85 mEq/day and 0.13 mEq/day in those in the 2nd and 3rd tertile, respectively, P=0.079).
Figure 2.
Distribution of variation in total (upper panel), trabecular (middle panel), and cortical (lower panel) BMD according to distribution tertiles. Box edges are at 25th and 75th percentile, the middle horizontal line is at the median, whiskers span 1.5 interquartile range.
Table 4.
Baseline characteristics of participants according to tertiles of variation* in total BMD.
| Tertiles of variation in total BMD | ||||
|---|---|---|---|---|
| I | II | III | P | |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age | 74.0 (6.15) | 71.4 (5.09) | 69.7 (5.25) | <.0001 |
| Years since menopause | 25.0 (7.75) | 21.7 (7.76) | 20.6 (8.38) | 0.001 |
| BMI | 26.6 (4.57) | 28.1 (4.24) | 28.7 (4.31) | 0.003 |
| Cigarette smoking (pack-years) | 3.22 (11.94) | 2.81 (7.92) | 2.26 (5.96) | 0.752 |
| Calories/Kg ideal weight | 33.4 (8.26) | 33.9 (8.62) | 35.0 (10.54) | 0.468 |
| Dietary Fibre (g/day) | 17.8 (4.90) | 19.5 (6.19) | 19.7 (5.74) | 0.032 |
| Calories from carbohydrates/ideal weight |
17.4 (4.89) | 17.5 (4.96) | 18.1 (6.29) | 0.659 |
| Calories from lipids/ideal weight | 10.71 (3.18) | 11.06 (3.52) | 11.32 (3.61) | 0.467 |
| Proteins (g)/ideal weight | 1.34 (0.36) | 1.39 (0.35) | 1.40 (0.40) | 0.528 |
| Animal proteins (g)/ideal weight | 0.86 (0.27) | 0.90 (0.26) | 0.90 (0.30) | 0.402 |
| Vegetal proteins (g)/ideal weight | 0.49 (0.15) | 0.48 (0.15) | 0.49 (0.17) | 0.907 |
| Non protein calories/g of nitrogen | 132.6 (20.68) | 128.2 (18.73) | 132.9 (20.86) | 0.193 |
| Calcium (mg/day) | 794.7 (325.37) | 797.0 (275.95) | 866.9 (391.92) | 0.229 |
| Phosphorous (mg/day) | 1090.1 (321.73) | 1142.5 (289.28) | 1183.2 (374.81) | 0.145 |
| Vitamin D (mcg/day) | 1.62 (0.60) | 1.83 (0.83) | 1.89 (1.27) | 0.111 |
| Magnesium (mg/day) | 221.1 (65.88) | 235.2 (60.30) | 243.2 (72.54) | 0.064 |
| Folic Acid (mcg/day) | 234.2 (72.47) | 258.1 (77.00) | 266.1 (82.58) | 0.012 |
| Total Polyunsaturated Fatty Acids (g/day) |
6.76 (2.24) | 7.01 (2.24) | 7.21 (2.53) | 0.416 |
| PRAL | 2.64 (10.61) | 1.52 (10.28) | 0.24 (11.26) | 0.294 |
| PRAL/ideal weight | 0.06 (0.21) | 0.03 (0.20) | 0.00 (0.22) | 0.208 |
| Alcohol (g/day) | 7.08 (9.98) | 6.50 (9.63) | 8.57 (10.72) | 0.335 |
| Creatinine clearance (mL/min) | 71.4 (21.50) | 77.4 (19.77) | 75.6 (21.14) | 0.126 |
| 25-hydroxyvitamin D (nmol/L) | 42.45 (37.75) | 50.0 (40.63) | 52. (33.56) | 0.162 |
| Interleukin-6 (pg/mL) | 1.56 (1.63) | 1.55 (1.51) | 1.55 (1.42) | 0.997 |
| % | % | % | ||
| Low physical activity | 77.1 | 77.3 | 86.0 | 0.201 |
| Hormone replacement therapy | 8.3 | 9.3 | 9.0 | 0.716 |
Calculated as follow-up BMD minus baseline BMD, lower tertile indicates greater BMD loss.
Table 6.
Baseline characteristics of participants according to tertiles of variation* in cortical BMD.
| Tertiles of variation in cortical BMD | ||||
|---|---|---|---|---|
| I | II | III | P | |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age | 73.1 (6.85) | 71.5 (5.10) | 70.5 (5.01) | 0.008 |
| Years since menopause | 22.9 (9.14) | 22.5 (7.45) | 21.8 (7.87) | 0.626 |
| BMI | 27.2 (3.85) | 28.0 (4.32) | 28.2 (5.01) | 0.245 |
| Cigarette smoking (pack-years) | 3.90 (11.89) | 3.14 (8.57) | 1.22 (4.61) | 0.088 |
| Calories/Kg ideal weight | 32.4 (7.46) | 35.6 (9.86) | 34.6 (9.84) | 0.048 |
| Dietary Fibre (g/day) | 19.0 (6.15) | 19.0 (5.59) | 19.1 (5.32) | 0.992 |
| Calories from carbohydrates/ideal weight |
17.0 (4.91) | 18.2 (5.66) | 17.9 (5.61) | 0.237 |
| Calories from lipids/ideal weight | 10.2 (2.73) | 11.7 (3.68) | 11.2 (3.66) | 0.011 |
| Proteins (g)/ideal weight | 1.29 (0.30) | 1.43 (0.39) | 1.41 (0.40) | 0.016 |
| Animal proteins (g)/ideal weight | 0.82 (0.22) | 0.92 (0.28) | 0.92 (0.30) | 0.009 |
| Vegetal proteins (g)/ideal weight | 0.47 (0.15) | 0.51 (0.17) | 0.49 (0.15) | 0.323 |
| Non protein calories/g of nitrogen | 132.7 (21.46) | 131.2 (18.34) | 130.3 (20.73) | 0.704 |
| Calcium (mg/day) | 757.0 (231.40) | 847.0 (356.68) | 857.5 (387.21) | 0.069 |
| Phosphorous (mg/day) | 1063.8 (230.50) | 1184.0 (360.71) | 1170.1 (371.75) | 0.021 |
| Vitamin D (mcg/day) | 1.62 (0.62) | 1.87 (0.75) | 1.87 (1.30) | 0.110 |
| Magnesium (mg/day) | 222.3 (52.26) | 242.2 (74.87) | 236.2 (70.08) | 0.104 |
| Folic Acid (mcg/day) | 239.8 (71.75) | 266.4 (88.12) | 252.6 (72.63) | 0.060 |
| Total Polyunsaturated Fatty Acids (g/day) |
6.58 (1.87) | 7.19 (2.46) | 7.22 (2.56) | 0.0924 |
| PRAL | 0.34 (10.01) | 1.00 (10.53) | 2.86 (11.46) | 0.229 |
| PRAL/ideal weight | 0.01 (0.19) | 0.02 (0.21) | 0.06 (0.22) | 0.248 |
| Alcohol (g/day) | 7.88 (10.53) | 7.54 (11.47) | 7.00 (8.51) | 0.829 |
| Creatinine clearance (mL/min) | 77.8 (22.82) | 73.6 (20.10) | 73.7 (19.78) | 0.278 |
| 25-hydroxyvitamin D (nmol/L) | 59.6 (45.60) | 45.5 (37.47) | 39.9 (22.93) | 0.001 |
| Interleukin-6 (pg/mL) | 1.57 (1.67) | 1.57 (1.68) | 1.50 (1.16) | 0.934 |
| % | % | % | ||
| Low physical activity | 79.4 | 76.3 | 85.1 | 0.280 |
| Hormone replacement therapy | 6.19 | 6.19 | 13.9 | 0.137 |
Calculated as follow-up BMD minus baseline BMD, lower tertile indicates greater BMD loss.
Women with greater loss of cortical BMD were older and had a lower caloric intake (32.4 Kcal/day and 34.6 Kcal/day in the 1st and 3rd tertile of cortical bone loss, respectively, P=0.048). Among macronutrients, greater loss was associated with lower intake of proteins and lipids, while among micronutrients it was associated with lower intake of calcium, phosphorous, and folic acid. The serum concentration of 25-OH vitamin D was 59.6 nmol/L among those in the first tertile of cortical BMD variation, 45.5 nmol/L among those in the second tertile, and 39.9 nmol/L among those in the third tertile (P=0.001).
Discussion
Our results do not support the basic hypothesis that the dietary acid load is inversely associated with BMD in an elderly female population and confirms the role of well recognized risk factors for loss of BMD. Additionally, we have found a weak association between the intake of selected nutrients and BMD.
The lack of association between dietary acid load and BMD is only to some extent surprising. Indeed, our hypothesis was based on the finding of a negative association between PRAL and BMD (3,4) that was weak and observed using a different technique (calcaneal broadband ultrasound attenuation). Furthermore, in those studies the estimation of dietary acid load showed a larger range of values (for example −119.7 to 68.2 mEq/day in the study by Welch et al (3)), with an average value that was in the alkaline range, suggesting a protective role of a negative PRAL rather than a harmful role of a positive one. At any rate, the clear evidence of a bone reabsorbing effect of an acidic environment pertains to chronic metabolic acidosis (16) which is a condition quite different from the one characterized by an increased acid load. Finally, it should be observed that in a Taiwanese population BMD values did not distinguish vegetarians from non vegetarians (17), and only extreme vegetarian habits, such as the vegan diet, have been found to be a risk factor for accelerated bone loss (18). None of the participants in our study was exposed to either a highly acidifying diet or an extreme vegetarian one, and this likely smoothed the correlation between PRAL and BMD. Nevertheless, the finding of a non significant association between lower PRAL and smaller loss of trabecular BMD at the 6 years follow up makes the basic hypothesis worthy of further test over a longer time interval. The association between folic acid intake, a marker of an alkaline diet, and lesser changes of total and trabecular BMD at 6 years supports this conclusion.
Our study confirms that older age and the number of years elapsed from menopause are inversely, whereas BMI and GFR are directly related to BMD. The associations with the GFR, however, was not confirmed in the longitudinal analysis. This result might in part be related with the method chosen to estimate renal function, which seems to be a factor influencing the association between renal function and bone loss. In the Rancho Bernardo Study, for example, creatinine clearance estimated using the Cockroft-Gault formula was a predictor of bone loss, whereas the glomerular filtration rate estimated using the MDRD formula was not (19). Furthermore, in the Cardiovascular Health Study, after adjustment for body weight, renal function estimated using Cystatin C was not associated with bone loss (20).
It is of interest that daily physical activity was positively, though weakly, correlated with baseline total and trabecular BMD. This finding is in line with other studies suggesting that lifestyle as a whole should be considered as a determinant of bone health. For example, in the Tromso Study physically active women experienced substantially lower reduction of BMD over 5 years (21). The protective effects of physical activity have also been shown in an elderly population: women aged 50 years or older who maintained a habitually active lifestyle had a slower progression of BMD loss over a 10-years follow-up (22). We found no association, however, between physical activity in adulthood and changes in BMD at 6 years in our population. This might reflect different representativity of this measure versus the outcomes: overall physical activity in the 40–60 years range of age is a logical correlate of BMD recorded at a mean age of 75 years and, thus, reflecting previous activity, but not of changes in BMD in older age. Furthermore, regular physical activity has been reported to decline dramatically with age up to a 12–15% prevalence in a very old female population (23).
We could find only few significant associations between BMD and nutrient intake such as the positive one between the intake of n-3 polyunsaturated fatty acids (PUFA) and baseline total and trabecular BMD. Although the strength of this association was mild, the finding seems worthy of consideration. Indeed, fish oil rich in PUFA have been reported to slow inflammation-induced bone loss in chronic inflammatory diseases by inhibiting osteoclast activity (24) and have been proved to preserve the BMD of aged ovariectomized rats (25). However, a higher PUFA intake has been reported to characterize elderly subjects with a greater risk of osteoporotic fracture (26). Unfortunately, dietary habits of our population, with a very high mono- to poly-unsatured fatty acid ratio and a narrow range of PUFA intake, likely smoothed the association between PUFA and BMD. However, such an association seems biologically plausible and worthy to be tested in a population with a wider distribution of daily PUFA intake. On the other hand, the weak inverse association between alcohol intake and baseline trabecular BMD might be a chance finding. In fact, also the average alcohol intake of the lowest BMD tertile was in the range reported to protect against bone loss (27).
Calcium intake of our population met the old RDA of 800 mg , but not the current suggested adequate intake of 1200 mg (28), which has been set based on the evidence for the higher protective effect against fracture in the elderly (29). Thus, our population may be considered to diffusely suffer from a deficit of Calcium intake because only 8% met the new RDA. This is not surprising: to achieve the desired amount of calcium it is required to consume a relatively large amount of dairy products, or to use special attentions in cooking to make calcium available also from other sources such as vegetables.
Furthermore, low fat diets are frequently prescribed against hypercholesterolemia and might dramatically affect dietary calcium intake (30). Thus, the new adequate intake seems too ambitious, at least for an elderly population, and even more dramatic was the gap between RDA (5 mcg) and actual intake of Vitamin D. This finding, which reflects the low intake of dairy products should be interpreted with some caution, since the vast majority of our subjects was active and able to spent time out of home and, thus, could benefit from endogenous production of D vitamin. At any rate, the average serum concentration of 25-OH vitamin D was 43.9 nmol/L, and serum levels below 75 nmol/L are associated with an increased risk of myocardial infarction and with PTH serum level greter than 30 pg/ml, which is the threshold value from which the association between PTH and several adverse events becomes evident (31,32). Thus, serum Vitamin D levels in our population is not adequate to protect the bone, as well as to achieve other important biological effects. However, the only significant associations found for vitamin D level were a positive one with basal cortical BMD and a counterintuitive negative one with 6 year change in cortical BMD. This latter result was unexpected, and we can only provide speculative interpretation. Over the 6 years of follow-up time, cortical BMD increased in this population, and it is conceivable that women with higher cortical BMD at baseline (who had the higher 25-OH vitamin D concentration at baseline) were those in which this increment was less evident, leading to a spurious inverse association between vitamin D concentration and difference in cortical BMD between baseline and follow-up.
It is of interest that a higher intake of protein and a greater caloric intake protected against the loss of cortical, but not trabecular BMD as if an anabolic diet selectively benefited the cortical bone. While hard to interpret on the basis of current knowledge, this finding might open new horizons in the assessment of the relationship between dietary pattern and bone health.
This study has some limitations. First, the fact that the observed correlations between dietary pattern and BMD were weak might partly reflect the average good quality of diet of our population. Indeed, the vast majority of our probands met the RDA for macronutrients, which likely prevented the diet from affecting BMD. Second, the 6 year interval between basal and final BMD measurements is likely too short to fully appreciate the effects of dietary pattern on BMD. This relatively short time-frame, however, makes the bias linked to modification of diet over time less likely, although we cannot exclude that our results are at least in part affected by modifications of diet. Third, a good diet might be a marker of a healthier life style, which the available variables could not completely capture.
In conclusion, we found no relationship between dietary acid load and BMD in an elderly female population with a well balanced diet. However, this relationship cannot be definitively excluded, given the low average dietary acid load of our population. We also confirmed the role of well recognized risk factor for osteoporosis and found a possible protective effect of PUFA intake on trabecular BMD and of the average caloric and proteic intake on cortical BMD. These last findings seems worthy of assessment in dedicated intervention studies. Thus, our data provide some clue to future research on modifiable risk factors for osteoporosis.
Table 5.
Baseline characteristics of participants according to tertiles of variation* in trabecular BMD.
| Tertiles of variation in trabecular BMD | ||||
|---|---|---|---|---|
| I | II | III | P | |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age | 72.8 (5.78) | 70.8 (5.52) | 71.5 (5.89) | 0.054 |
| Years since menopause | 23.7 (8.34) | 22.1 (7.23) | 21.5 (8.75) | 0.170 |
| BMI | 27.5 (4.90) | 28.0 (4.09) | 27.9 (4.34) | 0.706 |
| Cigarette smoking (pack-years) | 2.55 (9.12) | 2.93 (9.95) | 2.77 (7.59) | 0.956 |
| Calories/Kg ideal weight | 33.9 (8.79) | 35.0 (9.91) | 33.6 (8.90) | 0.534 |
| Dietary Fibre (g/day) | 18.2 (5.35) | 19.5 (6.39) | 19.2 (5.24) | 0.255 |
| Calories from carbohydrates/ideal weight |
17.6 (5.13) | 18.1 (5.95) | 17.2 (5.15) | 0.500 |
| Calories from lipids/ideal weight | 11.0 (3.43) | 11.3 (3.58) | 10.8 (3.34) | 0.617 |
| Proteins (g)/ideal weight | 1.36 (0.38) | 1.42 (0.37) | 1.35 (0.36) | 0.335 |
| Animal proteins (g)/ideal weight | 0.88 (0.29) | 0.91 (0.27) | 0.87 (0.27) | 0.482 |
| Vegetal proteins (g)/ideal weight | 0.48 (0.16) | 0.50 (0.16) | 0.48 (0.15) | 0.5304 |
| Non protein calories/g of nitrogen | 132.3 (19.76) | 130.2 (20.88) | 131.3 (20.00) | 0.769 |
| Calcium (mg/day) | 785.5 (338.12) | 843.6 (297.76) | 830.5 (367.29) | 0.453 |
| Phosphorous (mg/day) | 1101.8 (331.51) | 1170.9 (318.25) | 1144.4 (345.29) | 0.347 |
| Vitamin D (mcg/day) | 1.65 (0.66) | 1.96 (1.33) | 1.74 (0.70) | 0.058 |
| Magnesium (mg/day) | 224.2 (67.05) | 238.4 (64.44) | 237.1 (68.85) | 0.266 |
| Folic Acid (mcg/day) | 237.17 (77.46) | 265.36 (80.67) | 256.23 (75.36) | 0.038 |
| Total Polyunsaturated Fatty Acids (g/day) |
6.79 (1.96) | 7.13 (2.68) | 7.06 (2.34) | 0.558 |
| PRAL | 3.42 (10.74) | 0.85 (10.44) | 0.13 (10.85) | 0.079 |
| PRAL/ideal weight | 0.07 (0.21) | 0.02 (0.20) | 0.00 (0.21) | 0.057 |
| Alcohol (g/day) | 6.24 (8.13) | 7.20 (11.11) | 8.70 (10.79) | 0.231 |
| Creatinine clearance (mL/min) | 75.8 (22.44) | 76.7 (20.07) | 72.1 (20.11) | 0.267 |
| 25-hydroxyvitamin D (nmol/L) | 43.5 (25.21) | 48.1 (39.26) | 53.2 (44.41) | 0.207 |
| Interleukin-6 (pg/mL) | 1.47 (1.43) | 1.40 (1.26) | 1.77 (1.79) | 0.193 |
| % | % | % | ||
| Low physical activity | 77.1 | 81.4 | 82.0 | 0.642 |
| Hormone replacement therapy | 10.4 | 9.3 | 7.0 | 0.132 |
Calculated as follow-up BMD minus baseline BMD, lower tertile indicates greater BMD loss.
References
- 1.Kitchin B, Morgan SL. Not just calcium and vitamin D: other nutritional considerations in osteoporosis. Curr Rheumatol Rep. 2007;9:85–92. doi: 10.1007/s11926-007-0027-9. [DOI] [PubMed] [Google Scholar]
- 2.Lin P, Ginty F, Appel LJ, et al. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr. 2003;133:3130–3136. doi: 10.1093/jn/133.10.3130. [DOI] [PubMed] [Google Scholar]
- 3.Welch AA, Bingham SA, Reeve J, Khaw KT. More acidic dietary acid-base load is associated with reduced calcaneal broadband ultrasound attenuation in women but not in men: results from the EPIC-Norfolk cohort study. Am J Clin Nutr. 2007;85:1134–1141. doi: 10.1093/ajcn/85.4.1134. [DOI] [PubMed] [Google Scholar]
- 4.Wynn E, Lanham-New SA, Krieg M, Whittamore DR, Burckhardt P. Low estimates of dietary acid load are positively associated with bone ultrasound in women older than 75 years of age with a lifetime fracture. J Nutr. 2008;138:1349–1354. doi: 10.1093/jn/138.7.1349. [DOI] [PubMed] [Google Scholar]
- 5.Bushinsky DA, Krieger NS, Geisser DI, Grossman EB, Coe FL. Effects of pH on bone calcium and proton fluxes in vitro. Am J Physiol Renal Physiol. 1983;245:F204–F209. doi: 10.1152/ajprenal.1983.245.2.F204. [DOI] [PubMed] [Google Scholar]
- 6.Barzel US, Massey LK. Excess Dietary Protein Can Adversely Affect Bone. J. Nutr. 1998;128:1051–1053. doi: 10.1093/jn/128.6.1051. [DOI] [PubMed] [Google Scholar]
- 7.Alexy U, Remer T, Manz F, Neu CM, Schoenau E. Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am J Clin Nutr. 2005;82:1107–1114. doi: 10.1093/ajcn/82.5.1107. [DOI] [PubMed] [Google Scholar]
- 8.Aghdassi E, McArthur M, Liu B, McGeer A, Simor A, Allard JP. Dietary intake of elderly living in Toronto long-term care facilities: comparison to the dietary reference intake. Rejuvenation Res. 2007;10:301–309. doi: 10.1089/rej.2006.0530. [DOI] [PubMed] [Google Scholar]
- 9.Marshall TA, Stumbo PJ, Warren JJ, Xie XJ. Inadequate nutrient intakes are common and are associated with low diet variety in rural, community-dwelling elderly. J Nutr. 2001;131:2192–2196. doi: 10.1093/jn/131.8.2192. [DOI] [PubMed] [Google Scholar]
- 10.Fritz K, Elmadfa I. Quality of nutrition of elderly with different degrees of dependency: elderly living in private homes. Ann Nutr Metab. 2008;52 Suppl 1:47–50. doi: 10.1159/000115349. [DOI] [PubMed] [Google Scholar]
- 11.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 12.Riboli E, Hunt KJ, Slimani N, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–1124. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 13.Bartali B, Turrini A, Salvini S, et al. Dietary intake estimated using different methods in two Italian older populations. Arch Gerontol Geriatr. 2004;38:51–60. doi: 10.1016/s0167-4943(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 14.Rittweger J, Beller G, Ehrig J, et al. Bone-muscle strength indices for the human lower leg. Bone. 2000;27:319–326. doi: 10.1016/s8756-3282(00)00327-6. [DOI] [PubMed] [Google Scholar]
- 15.Remer T, Dimitriou T, Manz F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am J Clin Nutr. 2003;77:1255–1260. doi: 10.1093/ajcn/77.5.1255. [DOI] [PubMed] [Google Scholar]
- 16.Bushinsky DA. Net calcium efflux from live bone during chronic metabolic, but not respiratory, acidosis. Am J Physiol. 1989;256:F836–F842. doi: 10.1152/ajprenal.1989.256.5.F836. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Chiu J, Chuang M, Chiu J, Lin C. Bone mineral density of vegetarian and non-vegetarian adults in Taiwan. Asia Pac J Clin Nutr. 2008;17:101–106. [PubMed] [Google Scholar]
- 18.Chiu JF, Lan SJ, Yang CY, et al. Long-term vegetarian diet and bone mineral density in postmenopausal Taiwanese women. Calcif Tissue Int. 1997;60:245–249. doi: 10.1007/pl00005812. [DOI] [PubMed] [Google Scholar]
- 19.Jassal SK, von Muhlen D, Barrett-Connor E. Measures of renal function, BMD, bone loss, and osteoporotic fracture in older adults: the Rancho Bernardo study. J Bone Miner Res. 2007;22:203–210. doi: 10.1359/jbmr.061014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried LF, Shlipak MG, Stehman-Breen C, et al. Kidney function predicts the rate of bone loss in older individuals: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2006;61:743–748. doi: 10.1093/gerona/61.7.743. [DOI] [PubMed] [Google Scholar]
- 21.Wilsgaard T, Emaus N, Ahmed LA, et al. Lifestyle Impact on Lifetime Bone Loss in Women and Men: The Tromso Study. Am J Epidemiol. 2009 doi: 10.1093/aje/kwn407. [DOI] [PubMed] [Google Scholar]
- 22.Daly RM, Ahlborg HG, Ringsberg K, Gardsell P, Sernbo I, Karlsson MK. Association between changes in habitual physical activity and changes in bone density, muscle strength, and functional performance in elderly men and women. J Am Geriatr Soc. 2008;56:2252–2260. doi: 10.1111/j.1532-5415.2008.02039.x. [DOI] [PubMed] [Google Scholar]
- 23.Caspersen CJ, Pereira MA, Curran KM. Changes in physical activity patterns in the United States, by sex and cross-sectional age. Med Sci Sports Exerc. 2000;32:1601–1609. doi: 10.1097/00005768-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Rahman MM, Bhattacharya A, Fernandes G. Docosahexaenoic acid is more potent inhibitor of osteoclast differentiation in RAW 264.7 cells than eicosapentaenoic acid. J Cell Physiol. 2008;214:201–209. doi: 10.1002/jcp.21188. [DOI] [PubMed] [Google Scholar]
- 25.Matsushita H, Barrios JA, Shea JE, Miller SC. Dietary fish oil results in a greater bone mass and bone formation indices in aged ovariectomized rats. J Bone Miner Metab. 2008;26:241–247. doi: 10.1007/s00774-007-0815-3. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Ramírez MJ, Palma S, Martí nez-González MA, Delgado-Martínez AD, de la Fuente C, Delgado-Rodríguez M. Dietary fat intake and the risk of osteoporotic fractures in the elderly. Eur J Clin Nutr. 2007;61:1114–1120. doi: 10.1038/sj.ejcn.1602624. [DOI] [PubMed] [Google Scholar]
- 27.Ilich JZ, Brownbill RA, Tamborini L, Crncevic-Orlic Z. To drink or not to drink: how are alcohol, caffeine and past smoking related to bone mineral density in elderly women? J Am Coll Nutr. 2002;21:536–544. doi: 10.1080/07315724.2002.10719252. [DOI] [PubMed] [Google Scholar]
- 28.A Report of the Subcommittees on Interpretation and Uses of Dietary Reference Intakes and Upper Reference Levels of Nutrients, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board. Dietary Reference Intakes: Applications in Dietary Assessment.
- 29.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride.
- 30.Varenna M, Binelli L, Zucchi F, Ghiringhelli D, Sinigaglia L. Unbalanced diet to lower serum cholesterol level is a risk factor for postmenopausal osteoporosis and distal forearm fracture. Osteoporos Int. 2001;12:296–301. doi: 10.1007/s001980170119. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]